Simple Summary
The incidence of nonalcoholic fatty liver disease (NAFLD)-related hepatocellular carcinoma (HCC) is increasing. However, an effective screening or surveillance method is not established. Recently, the NAFLD/nonalcoholic steatohepatitis (NASH) guidelines of Japan were revised to incorporate new strategies and evidence for the management and surveillance of NAFLD/NASH. Advanced fibrosis and lifestyle-related and metabolic comorbidities, especially obesity and diabetes mellitus, are associated with HCC development. At the first screening, serum markers of hepatic fibrosis (hyaluronic acid, type IV collagen 7S, and mac-2 binding protein), or the fibrosis (FIB)-4 index or the nonalcoholic fatty liver disease fibrosis score (NFS), or a platelet count should be evaluated. When liver fibrosis is indicated, consultation with a gastroenterology specialist should be considered for the second screening. The risk of HCC should be stratified using the FIB-4 index or the NFS. Liver stiffness should be measured using vibration-controlled transient elastography in those at intermediate or high risk. Blood tests and imaging should be performed every 6–12 months in patients with advanced fibrosis for HCC surveillance. We review here what is known about NAFLD-HCC and provide perspectives for future research.
Abstract
Nonalcoholic fatty liver disease (NAFLD) is the hepatic manifestation of metabolic syndrome and can develop into hepatocellular carcinoma (HCC). The incidence of NAFLD-related HCC, which is accompanied by life-threatening complications, is increasing. Advanced fibrosis and lifestyle-related and metabolic comorbidities, especially obesity and diabetes mellitus, are associated with HCC development. However, HCC is also observed in the non-cirrhotic liver. Often, diagnosis is delayed until the tumor is relatively large and the disease is advanced; an effective screening or surveillance method is urgently required. Recently, the NAFLD/nonalcoholic steatohepatitis (NASH) guidelines of Japan were revised to incorporate new strategies and evidence for the management and surveillance of NAFLD/NASH. Fibrosis must be tested for noninvasively, and the risk of carcinogenesis must be stratified. The treatment of lifestyle-related diseases is expected to reduce the incidence of NAFLD and prevent liver carcinogenesis.
1. Introduction
The prevalence of nonalcoholic fatty liver disease (NAFLD) has been growing worldwide, and the incidence of this disease in Japan is estimated to be 29.7% [1]. NAFLD may give rise to hepatocellular carcinoma (HCC); such HCC is becoming more common [2]. However, the characteristics of, risk factors for, and prognosis of NAFLD-HCC have not been elucidated fully. The American Association for the Study of Liver Diseases [3], European Association for the Study of the Liver [4], and American Gastroenterological Association (AGA) [5] have published practice guidelines for NAFLD management. Recently, the Japanese NAFLD/nonalcoholic steatohepatitis (NASH) guidelines [6] were revised to include new evidence and strategies for NAFLD/NASH management and surveillance. Here, we review what is known about NAFLD-HCC and provide perspectives for future research.
2. Prevalence of NAFLD-HCC
In a multicenter survey performed in Japan, Tateishi et al. [2] examined 7370 patients with HCC and other liver diseases and reported that the incidence of non-B non-C liver cancer increased from 10% in 1991 to 32.5% in 2015. Such liver cancer was induced by alcoholic liver disease (ALD) in 675 (32.3%) patients, NAFLD/NASH in 315 (15.1%) patients, and unknown causes in 911 (43.7%) patients. Such “unknown cause(s)” may include “burned out” NASH [7]. Thus, the prevalence of NAFLD-HCC may be underestimated.
Reports on HCC development after cirrhosis caused by NAFLD are listed in Table 1. Yatsuji et al. [8] described cancers that developed in 7 of 68 patients with NASH and cirrhosis (average age 63 years, 57% male) over an average follow-up period of 3.4 years. The five-year cumulative carcinogenesis rate was 11.3%, 30.5% that of the control group [patients with hepatitis C virus (HCV)-related cirrhosis]. Ascha et al. [9] studied 195 patients with NASH cirrhosis (mean age 56.6 years, 44.1% male) for a median of 3.2 years, and found liver cancer in 25 (12.8%) of these patients; the annual rate was 2.6%. In another report, the annual HCC rate in patients with liver cirrhosis was 1–3% [10]. The annual HCC rate in patients with liver cirrhosis caused by HCV was 6% [11]; the rate of HCC associated with NAFLD was lower. In one large study, the NAFLD-HCC rate was 0.44/1000 person-years [95% confidence interval (CI) 0.29–0.66]; it was 5.29/1000 person-years (95% CI 0.75–37.56) in patients with NASH [12]. As mentioned above, the HCC rate is not high, but the number of patients with cancer is increasing, given the large population with NAFLD. In Japan, about 23 million persons are thought to have NAFLD and 100,000 will develop HCC over the next 10 years. This situation cannot be ignored.

Table 1.
Prevalence of NAFLD-HCC in patients with cirrhosis.
Cancer in non-cirrhotic subjects has also been reported (Table 2). Kawada et al. [13] evaluated 1168 surgically resected HCCs; 6 were not associated with cirrhosis. In a study of 1562 patients, Bengtsson et al. [14] found that 83 of 225 NAFLD-HCCs were not associated with cirrhosis. HCC arising in non-cirrhotic livers constituted 10–50% of all cases and was thus more common than HCV-related HCC [15]. A multicenter study of 209 patients with liver cirrhosis reported that F4 HCC developed in 72.7% of females and 37.6% of males; a sex difference was thus evident [16]. In contrast, the frequency of HCC in non-cirrhosis was reported to be significantly higher in female than male [17]. Thus, HCC also should be noticed in non-cirrhotic cases.

Table 2.
Prevalence of NAFLD-HCC in non-cirrhotic patients.
3. Characteristics and Pathogenesis of NAFLD-HCC
The “multiple parallel hits” hypothesis has been advanced to explain NASH onset [42]. Adipose tissue lipotoxicity, insulin resistance, lifestyle-related diseases, alcohol consumption, growth and sex hormone–related factors, menopause, aging, oxidative stress (disruption of iron and free fatty acid levels), and changes in the profiles of gut and oral bacteria and Helicobacter pylori are thought to contribute to NASH pathology and carcinogenesis. The hyperinsulinemia associated with insulin resistance may promote cell proliferation and trigger carcinogenesis. Cellular stress responses, including autophagy and endoplasmic reticulum-associated activity, may trigger NAFLD cytotoxicity [43]. In addition, innate immune reactions between the products of intestinal bacteria and the liver increase deoxycholic acid levels, triggering the senescence of hepatic stellate cells [44]. The senescence-associated secretory phenotype, which features the extracellular secretion of inflammatory cytokines (tumor necrosis factor-α and interleukin-6), chemokines, and extracellular matrix–degrading enzymes from senescent cells, may trigger liver carcinogenesis [45]. Furthermore, changes in the profiles of hepatokines, including angiopoietin-like factors and fibroblast growth factors [46], and acylcarnitine may be involved in carcinogenesis [47]
Genetic influences on NASH and carcinogenesis have been reported. The gene encoding the patatin-like phospholipase domain-containing 3 (PNPLA3) protein is widely accepted to affect disease susceptibility. The frequency of “risky” alleles is elevated in patients with liver cancer; the gene is thus presumed to be associated with carcinogenesis [48]. Additionally, 17-beta hydroxysteroid dehydrogenase 13 (HSD17B13) was identified as having the protective role of NAFLD [49] and protecting against HCC development [50]. Lower HSD17B13 in non-cancerous tissues was associated with worse recurrence-free survival and overall survival in HCC patients. Although they mainly studied viral hepatitis-associated HCC, further study is required.
Like similar liver diseases, NAFLD-HCC is characterized by a trabecular phenotype [15]. In patients with a common subtype of NAFLD-HCC, Salomao et al. [51] reported cancers with the morphological characteristics of steatohepatitis and termed this phenotype steatohepatitic HCC (SH-HCC). The expression of markers of hepatocellular adenoma is not specific to NAFLD-HCC; but is evident in some patients with NAFLD-HCC [52]. Notably, the levels of serum amyloid A and c-reactive protein were increased significantly in patients with SH-HCC. In addition, HCC tumors in non-cirrhotic livers are large, and may have arisen from hepatocellular adenomas [31].
Turning to tumor markers, in a study of 209 cases in Japan, the α-fetoprotein (AFP) positivity rate was about 50% (AFP 10–100 ng/mL, 27.9%; ≥100 ng/mL, 20.6%), accompanied by an abnormal prothrombin [des-gammacarboxy prothrombin (DCP)]. The DCP positivity rate was about 68% (DCP 40–100 mAU/mL, 11.8%; ≥100 mAU/mL, 56.4%). This rate was slightly higher in patients with NAFLD-HCC than in others [16].
In terms of HCC in the non-cirrhotic liver, Mohamad et al. [37] found that patients with HCC who lacked cirrhosis were older than those with cirrhosis [67.5 ± 12.3 vs. 62.7 ± 8.1 years], less likely to be obese (52% vs. 83%) or to have type 2 diabetes (38% vs. 83%), more likely to have single nodules (80.6% vs. 52.2%) or larger (>5 cm) nodules (77.8% vs. 10.6%) and to undergo hepatic resection (66.7% vs. 17%), and less likely to receive locoregional therapy (22.3% vs. 61.7%) or deceased-donor liver transplantation (LT; 0% vs. 72.3%). Kodama et al. [39] described HCCs in non-cirrhotic livers as large; recurrence after curative surgical treatment was less common than in patients with HCC and cirrhosis.
4. Risk Factors for NAFLD-HCC
In a study of 622 patients with decompensated cirrhosis, Muto et al. [53] found that the risk factors for liver cancer were male sex, diabetes, high body mass index (BMI), AFP level ≥ 20 ng/mL, and low serum albumin level. A 16-year prospective follow-up study of 900,000 persons in the United States showed that among patients with BMIs ≥35 kg/m2, the relative risk of death from HCC was 4.52 (95% CI 1.13–2.05) in males and 1.62 (95% CI 1.40–1.87) in females [54]. Obesity is a risk factor for HCC and HCC-related death. Kawamura et al. [55] evaluated 6508 patients aged ≥ 60 years with NAFLD, aspartate aminotransferase (AST) levels ≥40 IU/L, and platelet counts <15 × 104/μL, and found that diabetic complications were independent risk factors for HCC [hazard ratio (HR) 3.21, 95% CI 1.09–9.50, p = 0.035] (Table 3). In a study of combined lifestyle-related diseases, the presence of both hypertension and dyslipidemia was associated with a 1.8-fold increase in HCC risk (HR 1.8, 95% CI 1.59–2.06); in the presence of diabetes, obesity, and dyslipidemia, the increase was 2.6-fold (HR 2.6, 95% CI 2.3–2.9) [41]. Kogiso et al. [56] demonstrated that risk factors for new—onset HCC were AST level (HR 1.021, 95% CI 1.009–1.034, p = 0.001), platelet count (HR 0.881, 95% CI 0.801–0.970, p = 0.010), and treatment for hypertension (HR 4.986, 95% CI 1.223–20.329, p = 0.025). Patients with platelet counts <11.5 × 104/μL exhibited significantly greater mortality and a greater risk of HCC development (p < 0.01), and the above-listed factors were predictive of HCC development in patients with NAFLD.

Table 3.
Summary of NAFLD-HCC predictors.
In a systematic review, NAFLD and NASH cohorts lacking cirrhosis were at minimal risk of HCC development (cumulative HCC mortality 0–3% over study periods of up to 20 years) [59]. Cohorts with NASH and cirrhosis were at consistently higher risk (cumulative incidence 12.8% over three years and 2.4% over seven years). Tokushige et al. [57] reported that risk factors for NAFLD-HCC in patients with cirrhosis were Child-Pugh score (HR 3.09, 95% CI 1.374–6.934), serum γ-glutamyltranspeptidase level (HR 1.01, 95% CI 1.002–1.022), and older age (HR 1.12, 95% CI: 1.014–1.226). Thus, the evaluation of cirrhosis is important when considering the risk of HCC development in patients with NAFLD.
As risk factors for HCC development in non-cirrhotic livers, Tobari et al. [40] identified male sex [odds ratio (OR) 7.774, 95% CI 2.176–27.775], light drinking (OR 4.893, 95% CI 1.923–12.449), and a high Fibrosis-4 (FIB-4) index (OR 2.634, 95% CI 1.787–3.884). The recurrence rate was significantly lower in patients with NAFLD-HCC who lacked cirrhosis (p < 0.01) [39]. The risk factors for recurrence were male sex, lower serum albumin levels, and advanced fibrosis. In females, NAFLD-HCC develops only in cirrhotic livers; it also develops in non-cirrhotic livers in males.
5. Treatment of NAFLD-HCC
NAFLD-HCC treatment is determined by liver function and the extent of HCC progression, in accordance with the Liver Cancer Practice Guidelines [60,61] and Barcelona Clinic Liver Cancer staging [62]. However, postoperative mortality is higher in patients with NAFLD-HCC than in those with HCV-HCC [62]. Vascular lesions (complications of lifestyle-related diseases) may be involved.
In patients with end-stage liver disease, NAFLD-HCC is an indicator for LT; NAFLD/NASH has been the fastest growing indicator for LT over the past 20 years [63,64]. Wong et al. [24] studied 61,868 patients who had undergone LT, including 10,061 HCC cases; the incidence of NASH-HCC increased from 8.3% in 2002 to 13.5% in 2012. About 50% of transplant recipients with NASH develop recurrent NAFLD, but the post-LT outcomes of patients with NAFLD are similar to those of patients without NAFLD [65].
6. Prognosis of NAFLD-HCC
In a large NAFLD cohort study, the liver-related death rate was 0.77/1000 person-years (95% CI 0.33–1.77) and the overall mortality rate was 15.44/1000 person-years (95% CI 11.72–20.34) [12]. In a NASH cohort study, the liver-related death rate was 11.77/1000 person-years (95% CI 7.10–19.53) and the overall mortality rate was 25.56/1000 person-years (95% CI 6.29–103.8). Eguchi et al. [66] reported a mortality rate of 40.0% over 2.7 years in a Japanese cohort. On long-term follow-up (7.7 years), mortality was higher in F3/F4 than in F0/F2 patients (25.0% vs. 0.0%) [66]. Younossi et al. [27] reported that NAFLD-HCC had a poor prognosis because HCC was diagnosed at an advanced stage. In contrast, NAFLD-HCC–related mortality did not differ significantly by HCC stage in another study [67]. The prognosis of NASH-HCC is reportedly similar to that of ALD-HCC (5-year survival rates, 49.1% and 43.7%) [16]. Notably, the overall survival rate was significantly higher for patients with NAFLD-HCC than for those with hepatitis B virus–HCC and HCV-HCC (HR 0.35, 95% CI 0.15–0.80 and HR 0.37, 95% CI 0.17–0.77, respectively) [68]. The five-year recurrence rate was 69.6% for NAFLD-HCC; DCP was a risk factor [16]. Summary of NAFLD-HCC outcomes after curative therapies were shown in Table 4. Thus, the prognosis of NAFLD-HCC does not differ significantly from those of other HCCs, but early diagnosis and treatment are essential.

Table 4.
Summary of NAFLD-HCC outcomes after curative therapies.
7. Prevention of NAFLD-HCC
A meta-analysis of the effects of drugs used to treat lifestyle-related conditions on carcinogenesis showed that metformin taken by patients with type 2 diabetes was associated with a reduced risk of HCC [73]. In a large national cohort study, Kaplan et al. [74] found that metformin use was associated independently with a decline in overall mortality, but not liver-related mortality, HCC, or decompensation, in patients with cirrhosis. A large meta-analysis of 4298 HCC cases showed that statins reduced the HCC incidence by 37%, and that the treatment of lifestyle-related diseases may suppress HCC [75].
Recent analysis demonstrated that bariatric surgery was reduced NASH-HCC [76]. Bariatric group showed lower incidence of new-onset HCC (0.05% vs. 0.34%, p = 0.03) comparing to the propensity-matched control group. It might become an option for HCC prevention.
8. NAFLD-HCC Screening and Surveillance
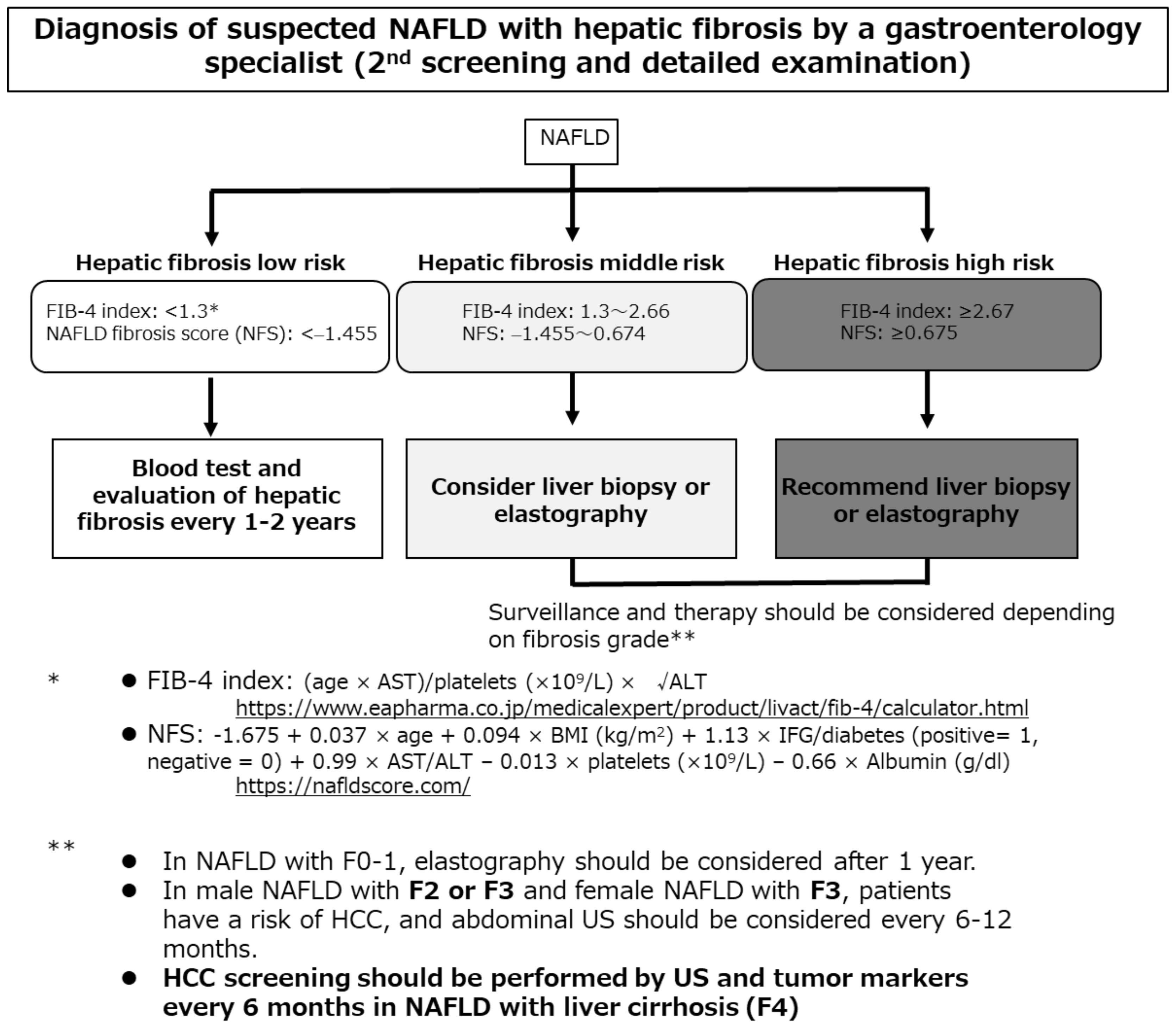
NAFLD/NASH-HCC is often diagnosed when the tumor is relatively large and the disease is thus advanced [15]. Bertot et al. [77] reported that patients with incidentally diagnosed NAFLD cirrhosis exhibited decreased platelet counts and international normalized ratios (both p < 0.05) and were more likely to have HCC (12%). In contrast, a surveillance group (who underwent liver ultrasound every six months) had no HCC complication. Screening and surveillance are necessary. As the cancer risk associated with NAFLD/NASH is minimal, regular surveillance of low-risk groups is inefficient and uneconomical. The risk factor for liver carcinogenesis in patients with NAFLD/NASH is advanced liver fibrosis; such patients require strict follow-up by a general physician applying the algorithm of the revised Japanese guidelines (Figure 1) [6]. At the first screening, serum markers of hepatic fibrosis (hyaluronic acid, type IV collagen 7S, and mac-2 binding protein), or the FIB-4 index or the nonalcoholic fatty liver disease fibrosis score (NFS), or a platelet count should be evaluated. When liver fibrosis is indicated, consultation with a gastroenterology specialist should be considered for the second screening.
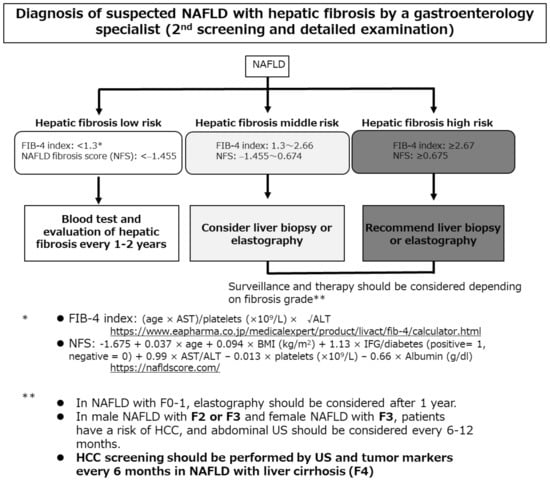
Figure 1.
The algorithm for hepatocellular carcinoma screening and surveillance contained in the revised Japanese guidelines [6]. AST, aspartate aminotransferase; ALT, alanine aminotransferase; BMI, body mass index; FIB-4, Fibrosis-4; NFS, nonalcoholic fatty liver disease fibrosis score; IFG, impaired fasting glucose; NAFLD, nonalcoholic fatty liver disease; HCC, hepatocellular carcinoma; US, ultrasonography.
Castera et al. [78] found that such simple, inexpensive, and widely available assays had high negative predictive values, effectively ruling out advanced fibrosis. Patients at low risk of advanced fibrosis (FIB-4 score < 1.3 or the NFS score < −1.455) do not require further assessment. Liver stiffness should be measured using vibration-controlled transient elastography in those at intermediate (FIB-4 score 1.3 to 2.66 or NFS score −1.455 to 0.674) or high (FIB-4 score ≥ 2.67 or NFS score ≥ 0.675) risk. Although no NAFLD/NASH-HCC surveillance method has been established, blood tests and imaging should be performed every 6–12 months in patients with advanced fibrosis.
Recently, the AGA proposed an HCC surveillance method for patients with NAFLD and liver cirrhosis, or severe fibrosis revealed by noninvasive testing [5]. When the quality of ultrasonography is suboptimal for screening of HCC, future screening by either computed tomography (CT) or magnetic resonance imaging (MRI) every six months are recommended. The GALAD score (based on age, sex, and AFP, AFP-L3, and DCP levels) is also used to estimate fibrosis [79]. In terms of imaging, abdominal ultrasonography is cost effective and noninvasive, but its diagnostic sensitivity is poor in obese patients [80]. Although CT is useful, patients with NAFLD often have renal disorders contraindicating the use of contrast media, and antidiabetic drugs (especially biguanide) must be stopped a few days before contrast enhancement. Although MRI detects HCC with high sensitivity, it is costly and its contraindications include pacemaker presence and renal dysfunction. The establishment of an efficient, cost-effective method for the screening of those at high risk of HCC is desirable.
9. Perspectives for Future Research
The development of tyrosine kinase inhibitors and immune checkpoint inhibitors affected a paradigm shift in HCC treatment [81,82]. However, the molecular and biological mechanisms underlying NAFLD-HCC development are not fully understood. Shimada et al. [83] classified HCCs by reference to genetic and immune profiles. Proteins that play key roles in HCC development would be valuable biomarkers for NAFLD-HCC screening. Molecular profiling might reveal target molecules for NAFLD-HCC therapy. It might be able to apply for personalized therapy and also when loco-regional treatment is not feasible. In addition, extracellular vesicles (EVs) [84,85,86] and CRISPR-Cas9– mediated genomic editing [87] are new tools for cancer therapy. EVs have shown great potential as drug delivery systems; exosomal micro-RNAs from HCC cells enhance transformed cell-like growth of recipient cells. Genomic editing of genes such as PNPLA3 may potentially treat NAFLD-HCC.
In terms of NAFLD-HCC prevention, metformin and pioglitazone reduced NAFLD-HCC development in patients with type 2 diabetes; statins were effective in those with dyslipidemia. Several novel NAFLD treatment agents are under evaluation. Future studies will explore new monotherapies and combination therapies.
10. Conclusions
The incidence of NAFLD-HCC will increase as obesity increases. Efficient surveillance is essential. Fibrosis should be evaluated noninvasively and the risk of cancer should be stratified. The treatment of lifestyle-related diseases prevents liver carcinogenesis. Further studies should seek to identify more noninvasive markers of fibrosis and NAFLD-HCC.
Author Contributions
Conception, and design: T.K. Drafting of the manuscript: T.K. and K.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of TWMU.
Informed Consent Statement
Informed consent was obtained from the patient for the publication of our study.
Data Availability Statement
The datasets used and/or analyzed in this study are available from the corresponding author upon reasonable request.
Acknowledgments
We would like to thank Etsuko Hashimoto of Seibu Railway Company Health Support Center for supervision. We also thank Yuri Ogasawara, Takaomi Sagawa, Yuichi Ikarashi, Kazuhisa Kodama, and Makiko Taniai of the Institute of Gastroenterology, Department of Internal Medicine, TWMU, for treating patients with NAFLD.
Conflicts of Interest
K.T. has received research funding from Sumitomo Dainippon Pharma Co., Ltd.; Astellas Pharma Inc.; EA Pharma Co., Ltd.; Eisai Co., Ltd.; Taiho Pharmaceutical Co., Ltd.; Chugai Pharmaceutical Co., Ltd.; Shionogi & Co., Ltd.; AbbVie GK; Takeda Pharmaceutical Co., Ltd.; and Otsuka Pharmaceutical Co., Ltd. T.K.: Nothing to declare.
References
- Eguchi, Y.; Hyogo, H.; Ono, M.; Mizuta, T.; Ono, N.; Fujimoto, K.; Chayama, K.; Saibara, T. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: A multicenter large retrospective study. J. Gastroenterol. 2012, 47, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, R.; Uchino, K.; Fujiwara, N.; Takehara, T.; Okanoue, T.; Seike, M.; Yoshiji, H.; Yatsuhashi, H.; Shimizu, M.; Torimura, T.; et al. A nationwide survey on non-B, non-C hepatocellular carcinoma in Japan: 2011–2015 update. J. Gastroenterol. 2019, 54, 367–376. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; LaVine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver; European Association for the Study of Diabetes. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Loomba, R.; Lim, J.K.; Patton, H.; El-Serag, H.B. AGA Clinical Practice Update on Screening and Surveillance for Hepatocellular Carcinoma in Patients with Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology 2020, 158, 1822–1830. [Google Scholar] [CrossRef]
- The Japanese Society of Gastroenterology; Japan Society of Hepatology. Evidence-Based Clinical Practice Guidelinefor Nonalcoholic Fatty liver Diseases/Nonalcoholic Steatohepatitis 2020 (Japanese), 2nd ed.; Nankodo Co., Ltd.: Tokyo, Japan, 2020. [Google Scholar]
- Margini, C.; Dufour, J.F. The story of HCC in NAFLD: From epidemiology, across pathogenesis, to prevention and treatment. Liver Int. 2016, 36, 317–324. [Google Scholar] [CrossRef]
- Yatsuji, S.; Hashimoto, E.; Tobari, M.; Taniai, M.; Tokushige, K.; Shiratori, K. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J. Gastroenterol. Hepatol. 2009, 24, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Ascha, M.S.; Hanouneh, I.A.; Lopez, R.; Tamimi, T.A.; Feldstein, A.F.; Zein, N.N. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010, 51, 1972–1978. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Saitoh, S.; Koida, I.; Arase, Y.; Tsubota, A.; Chayama, K.; Kumada, H.; Kawanishi, M. A multivariate analysis of risk factors for hepatocellular carcinogenesis: A prospective observation of 795 patients with viral and alcoholic cirrhosis. Hepatology 1993, 18, 47–53. [Google Scholar] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Kawada, N.; Imanaka, K.; Kawaguchi, T.; Tamai, C.; Ishihara, R.; Matsunaga, T.; Gotoh, K.; Yamada, T.; Tomita, Y. Hepatocellular carcinoma arising from non-cirrhotic nonalcoholic steatohepatitis. J. Gastroenterol. 2009, 44, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, B.; Stål, P.; Wahlin, S.; Björkström, N.K.; Hagström, H. Characteristics and outcome of hepatocellular carcinoma in patients with NAFLD without cirrhosis. Liver Int. 2019, 39, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Piscaglia, F.; Svegliati-Baroni, G.; Barchetti, A.; Pecorelli, A.; Marinelli, S.; Tiribelli, C.; Bellentani, S.; HCC-NAFLD Italian Study Group. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology 2016, 63, 827–838. [Google Scholar] [CrossRef]
- Tokushige, K.; Hyogo, H.; Nakajima, T.; Ono, M.; Kawaguchi, T.; Honda, K.; Eguchi, Y.; Nozaki, Y.; Kawanaka, M.; Tanaka, S.; et al. Hepatocellular carcinoma in Japanese patients with nonalcoholic fatty liver disease and alcoholic liver disease: Multicenter survey. J. Gastroenterol. 2016, 51, 586–596. [Google Scholar] [CrossRef]
- Phipps, M.; Livanos, A.; Guo, A.; Pomenti, S.; Yeh, J.; Dakhoul, L.; Burney, H.; Kettler, C.; Liu, H.; Miller, E.; et al. Gender Matters: Characteristics of Hepatocellular Carcinoma in Women from a Large, Multicenter Study in the United States. Am. J. Gastroenterol. 2020, 115, 1486–1495. [Google Scholar]
- Bugianesi, E.; Leone, N.; Vanni, E.; Marchesini, G.; Brunello, F.; Carucci, P.; Musso, A.; De Paolis, P.; Capussotti, L.; Salizzoni, M.; et al. Expanding the natural history of nonalcoholic steatohepatitis: From cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology 2002, 123, 134–140. [Google Scholar] [CrossRef]
- Marrero, J.A.; Fontana, R.J.; Su, G.L.; Conjeevaram, H.S.; Emick, D.M.; Lok, A.S. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology 2002, 36, 1349–1354. [Google Scholar] [CrossRef]
- Malik, S.M.; Gupte, P.A.; De Vera, M.E.; Ahmad, J. Liver Transplantation in Patients with Nonalcoholic Steatohepatitis-Related Hepatocellular Carcinoma. Clin. Gastroenterol. Hepatol. 2009, 7, 800–806. [Google Scholar] [CrossRef]
- Shimada, M.; Hashimoto, E.; Taniai, M.; Hasegawa, K.; Okuda, H.; Hayashi, N.; Takasaki, K.; Ludwig, J. Hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. J. Gastroenterol. 2009, 44, 89–95. [Google Scholar]
- Tokushige, K.; Hashimoto, E.; Horie, Y.; Taniai, M.; Higuchi, S. Hepatocellular carcinoma in Japanese patients with nonalcoholic fatty liver disease, alcoholic liver disease, and chronic liver disease of unknown etiology: Report of the nationwide survey. J. Gastroenterol. 2011, 46, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Kodama, K.; Tojjar, D.; Yamada, S.; Toda, K.; Patel, C.J.; Butte, A.J. Ethnic differences in the relationship between insulin sensitivity and insulin response: A systematic review and meta-analysis. Diabetes Care 2013, 36, 1789–1796. [Google Scholar] [PubMed]
- Wong, R.J.; Cheung, R.; Ahmed, A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 2014, 59, 2188–2195. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, R.; Okanoue, T.; Fujiwara, N.; Okita, K.; Kiyosawa, K.; Omata, M.; Kumada, H.; Hayashi, N.; Koike, K. Clinical characteristics, treatment, and prognosis of non-B, non-C hepatocellular carcinoma: A large retrospective multicenter cohort study. J. Gastroenterol. 2015, 50, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Sada, Y.H.; El-Serag, H.B.; Kanwal, F.; Duan, Z.; Temple, S.; May, S.B.; Kramer, J.R.; Richardson, P.A.; Davila, J.A. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the veteran affairs population. Clin. Gastroenterol. Hepatol. 2015, 13, 594–601.e591. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Otgonsuren, M.; Henry, L.; Venkatesan, C.; Mishra, A.; Erario, M.; Hunt, S. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology 2015, 62, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Kramer, J.R.; Mapakshi, S.; Natarajan, Y.; Chayanupatkul, M.; Richardson, P.A.; Li, L.; Desiderio, R.; Thrift, A.P.; Asch, S.M.; et al. Risk of Hepatocellular Cancer in Patients with Non-Alcoholic Fatty Liver Disease. Gastroenterology 2018, 155, 1828–1837.e1822. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Bertot, L.C.; Wong, V.W.-S.; Castellanos, M.; La Fuente, R.A.-D.; Metwally, M.; Eslam, M.; Gonzalez-Fabian, L.; Sanz, M.A.-Q.; Conde-Martín, A.F.; et al. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients with Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology 2018, 155, 443–457.e417. [Google Scholar] [CrossRef]
- Guzman, G.; Brunt, E.M.; Petrovic, L.M.; Chejfec, G.; Layden, T.J.; Cotler, S.J. Does nonalcoholic fatty liver disease predispose patients to hepatocellular carcinoma in the absence of cirrhosis? Arch. Pathol. Lab. Med. 2008, 132, 1761–1766. [Google Scholar]
- Paradis, V.; Zalinski, S.; Chelbi, E.; Guedj, N.; Degos, F.; Vilgrain, V.; Bedossa, P.; Belghiti, J. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: A pathological analysis. Hepatology 2008, 49, 851–859. [Google Scholar] [CrossRef]
- Yasui, K.; Hashimoto, E.; Komorizono, Y.; Koike, K.; Arii, S.; Imai, Y.; Shima, T.; Kanbara, Y.; Saibara, T.; Mori, T.; et al. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin. Gastroenterol. Hepatol. 2011, 9, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Ertle, J.; Dechêne, A.; Sowa, J.P.; Penndorf, V.; Herzer, K.; Kaiser, G.; Schlaak, J.F.; Gerken, G.; Syn, W.-K.; Canbay, A. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int. J. Cancer 2011, 128, 2436–2443. [Google Scholar] [CrossRef] [PubMed]
- Dyson, J.; Jaques, B.; Chattopadyhay, D.; Lochan, R.; Graham, J.; Das, D.; Aslam, T.; Patanwala, I.; Gaggar, S.; Cole, M.; et al. Hepatocellular cancer: The impact of obesity, type 2 diabetes and a multidisciplinary team. J. Hepatol. 2014, 60, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Perumpail, R.B.; Wong, R.J.; Ahmed, A.; Harrison, S.A. Hepatocellular Carcinoma in the Setting of Non-cirrhotic Nonalcoholic Fatty Liver Disease and the Metabolic Syndrome: US Experience. Dig. Dis. Sci. 2015, 60, 3142–3148. [Google Scholar] [CrossRef]
- Mittal, S.; El-Serag, H.B.; Sada, Y.H.; Kanwal, F.; Duan, Z.; Temple, S.; May, S.B.; Kramer, J.R.; Richardson, P.A.; Davila, J.A. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans is Associated with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2016, 14, 124–131.e121. [Google Scholar] [CrossRef]
- Mohamad, B.; Shah, V.; Onyshchenko, M.; Elshamy, M.; Aucejo, F.; Lopez, R.; Hanouneh, I.A.; Alhaddad, R.; Alkhouri, N. Characterization of hepatocellular carcinoma (HCC) in non-alcoholic fatty liver disease (NAFLD) patients without cirrhosis. Hepatol. Int. 2016, 10, 632–639. [Google Scholar] [CrossRef]
- Gawrieh, S.; Dakhoul, L.; Miller, E.; Scanga, A.; DeLemos, A.; Kettler, C.; Burney, H.; Liu, H.; Abu-Sbeih, H.; Chalasani, N.; et al. Characteristics, aetiologies and trends of hepatocellular carcinoma in patients without cirrhosis: A United States multicentre study. Aliment. Pharmacol. Ther. 2019, 50, 809–821. [Google Scholar] [CrossRef]
- Kodama, K.; Kawaguchi, T.; Hyogo, H.; Nakajima, T.; Ono, M.; Seike, M.; Takahashi, H.; Nozaki, Y.; Kawanaka, M.; Tanaka, S.; et al. Clinical features of hepatocellular carcinoma in nonalcoholic fatty liver disease patients without advanced fibrosis. J. Gastroenterol. Hepatol. 2019, 34, 1626–1632. [Google Scholar] [CrossRef]
- Tobari, M.; Hashimoto, E.; Taniai, M.; Kodama, K.; Kogiso, T.; Tokushige, K.; Yamamoto, M.; Takayoshi, N.; Satoshi, K.; Tatsuo, A. The characteristics and risk factors of hepatocellular carcinoma in nonalcoholic fatty liver disease without cirrhosis. J. Gastroenterol. Hepatol. 2020, 35, 862–869. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.R.; Li, L.; Dai, J.; Natarajan, Y.; Yu, X.; Asch, S.M.; El-Serag, H.B. Effect of Metabolic Traits on the Risk of Cirrhosis and Hepatocellular Cancer in Nonalcoholic Fatty Liver Disease. Hepatology 2020, 71, 808–819. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.E.; Moschen, A.R. Multiple Parallel Hits Hypothesis in NAFLD—Revisited After a Decade. Hepatology 2020. [Google Scholar] [CrossRef]
- Ikejima, K.; Kon, K.; Yamashina, S. Nonalcoholic fatty liver disease and alcohol-related liver disease: From clinical aspects to pathophysiological insights. Clin. Mol. Hepatol. 2020, 26, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, S.; Loo, T.M.; Atarashi, K.; Kanda, H.; Sato, S.; Oyadomari, S.; Iwakura, Y.; Oshima, K.; Morita, H.; Hattori, M.; et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 2013, 499, 97–101. [Google Scholar] [PubMed]
- Martin, N.; Ziegler, D.V.; Parent, R.; Bernard, D. Hepatic Stellate Cell Senescence in Liver Tumorigenesis. Hepatology 2020. [Google Scholar] [CrossRef] [PubMed]
- Kucukoglu, O.; Sowa, J.P.; Mazzolini, G.D.; Syn, W.K.; Canbay, A. Hepatokines and adipokines in NASH-related hepatocellular carcinoma. J. Hepatol. 2020, 74, 442–457. [Google Scholar] [CrossRef]
- Enooku, K.; Nakagawa, H.; Fujiwara, N.; Kondo, M.; Minami, T.; Hoshida, Y.; Shibahara, J.; Tateishi, R.; Koike, K. Altered serum acylcarnitine profile is associated with the status of nonalcoholic fatty liver disease (NAFLD) and NAFLD-related hepatocellular carcinoma. Sci. Rep. 2019, 9, 10663. [Google Scholar] [CrossRef]
- Valenti, L.; Motta, B.M.; Soardo, G.; Iavarone, M.; Donati, B.; SanGiovanni, A.; Carnelutti, A.; Dongiovanni, P.; Rametta, R.; Bertelli, C.; et al. PNPLA3 I148M Polymorphism, Clinical Presentation, and Survival in Patients with Hepatocellular Carcinoma. PLoS ONE 2013, 8, e75982. [Google Scholar] [CrossRef]
- Su, W.; Wang, Y.; Jia, X.; Wu, W.; Li, L.; Tian, X.; Li, S.; Wang, C.; Xu, H.; Cao, J.; et al. Comparative proteomic study reveals 17β-HSD13 as a pathogenic protein in nonalcoholic fatty liver disease. Proc. Natl. Acad. Sci. USA 2014, 111, 11437–11442. [Google Scholar] [CrossRef]
- Chen, J.; Zhuo, J.Y.; Yang, F.; Liu, Z.K.; Zhou, L.; Xie, H.Y.; Xu, X.; Zheng, S.S. 17-beta-hydroxysteroid dehydrogenase 13 inhibits the progression and recurrence of hepatocellular carcinoma. Hepatobiliary Pancreat. Dis. Int. 2018, 17, 220–226. [Google Scholar]
- Salomao, M.; Woojin, M.Y.; Brown, R.S., Jr.; Emond, J.C.; Lefkowitch, J.H. Steatohepatitic hepatocellular carcinoma (SH-HCC): A distinctive histological variant of HCC in hepatitis C virus-related cirrhosis with associated NAFLD/NASH. Am. J. Surg. Pathol. 2010, 34, 1630–1636. [Google Scholar]
- Taniai, M.; Hashimoto, E.; Tobari, M.; Kodama, K.; Tokushige, K.; Yamamoto, M.; Takayama, T.; Sugitani, M.; Sano, K.; Kondo, F.; et al. Clinicopathological investigation of steatohepatitic hepatocellular carcinoma: A multicenter study using immunohistochemical analysis of adenoma-related markers. Hepatol. Res. 2018, 48, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Muto, Y.; Sato, S.; Watanabe, A.; Moriwaki, H.; Suzuki, K.; Kato, A.; Kato, M.; Nakamura, T.; Higuchi, K.; Nishiguchi, S.; et al. Overweight and obesity increase the risk for liver cancer in patients with liver cirrhosis and long-term oral supplementation with branched-chain amino acid granules inhibits liver carcinogenesis in heavier patients with liver cirrhosis. Hepatol. Res. 2006, 35, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, Y.; Arase, Y.; Ikeda, K.; Seko, Y.; Imai, N.; Hosaka, T.; Masahiro, K.; Satoshi, S.; Hitomi, S.; Norio, A.; et al. Large-scale long-term follow-up study of Japanese patients with non-alcoholic Fatty liver disease for the onset of hepatocellular carcinoma. Am. J. Gastroenterol. 2012, 107, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Kogiso, T.; Sagawa, T.; Kodama, K.; Taniai, M.; Hashimoto, E.; Tokushige, K. Long-term outcomes of non-alcoholic fatty liver disease and the risk factors for mortality and hepatocellular carcinoma in a Japanese population. J. Gastroenterol. Hepatol. 2020, 35, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Tokushige, K.; Hashimoto, E.; Kodama, K. Hepatocarcinogenesis in non-alcoholic fatty liver disease in Japan. J. Gastroenterol. Hepatol. 2013, 28, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Seko, Y.; Sumida, Y.; Tanaka, S.; Mori, K.; Taketani, H.; Ishiba, H.; Hara, T.; Okajima, A.; Umemura, A.; Nishikawa, T.; et al. Development of hepatocellular carcinoma in Japanese patients with biopsy-proven non-alcoholic fatty liver disease: Association between PNPLA3 genotype and hepatocarcinogenesis/fibrosis progression. Hepatol. Res. 2017, 47, 1083–1092. [Google Scholar] [CrossRef]
- White, D.L.; Kanwal, F.; El-Serag, H.B. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin. Gastroenterol. Hepatol. 2012, 10, 1342–1359.e1342. [Google Scholar] [CrossRef]
- Kudo, M.; Matsui, O.; Izumi, N.; Iijima, H.; Kadoya, M.; Imai, Y.; Okusaka, T.; Miyayama, S.; Tsuchiya, K.; Ueshima, K.; et al. JSH Consensus-Based Clinical Practice Guidelines for the Management of Hepatocellular Carcinoma: 2014 Update by the Liver Cancer Study Group of Japan. Liver Cancer 2014, 3, 458–468. [Google Scholar] [CrossRef]
- Clinical Practice Guidelines for Liver Cancer 2017 Version, the Japan Society of Hepatology HCC Guidelines 2017; Kanehara & Co., LTD: Bunkyo-ku, Tokyo, 2017.
- Bruix, J.; Sherman, M. Diseases AAftSoL. Management of hepatocellular carcinoma: An update. Hepatology 2011, 53, 1020–1022. [Google Scholar] [CrossRef]
- Adam, R.; Karam, V.; Cailliez, V.; Grady, J.G.O.; Mirza, D.; Cherqui, D.; Klempnauer, J.; Salizzoni, M.; Pratschke, J.; Jamieson, N.; et al. 2018 Annual Report of the European Liver Transplant Registry (ELTR)—50-year evolution of liver transplantation. Transpl. Int. 2018, 31, 1293–1317. [Google Scholar] [CrossRef]
- Cotter, T.G.; Charlton, M. Nonalcoholic Steatohepatitis after Liver Transplantation. Liver Transplant. 2020, 26, 141–159. [Google Scholar] [CrossRef] [PubMed]
- Charlton, M.R.; Burns, J.M.; Pedersen, R.A.; Watt, K.D.; Heimbach, J.K.; Dierkhising, R.A. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology 2011, 141, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, Y.; Wong, G.; Lee, I.H.; Akhtar, O.; Lopes, R.; Sumida, Y. Hepatocellular carcinoma and other complications of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in Japan: A structured literature review article. Hepatol. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.K.; Steel, J.L.; Chen, H.-W.; DeMateo, D.J.; Cardinal, J.S.; Behari, J.; Humar, A.; Marsh, J.W.; Geller, D.A.; Tsung, A. Outcomes of curative treatment for hepatocellular cancer in nonalcoholic steatohepatitis versus hepatitis C and alcoholic liver disease. Hepatology 2012, 55, 1809–1819. [Google Scholar] [CrossRef] [PubMed]
- Benhammou, J.N.; Aby, E.S.; Shirvanian, G.; Manansala, K.; Hussain, S.K.; Tong, M.J. Improved survival after treatments of patients with nonalcoholic fatty liver disease associated hepatocellular carcinoma. Sci. Rep. 2020, 10, 9902. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Alejandro, R.; Croome, K.P.; Drage, M.; Sela, N.; Parfitt, J.; Chandok, N.; Marotta, P.; Dale, C.; Wall, W.; Quan, D. A comparison of survival and pathologic features of non-alcoholic steatohepatitis and hepatitis C virus patients with hepatocellular carcinoma. World J. Gastroenterol. 2012, 18, 4145–4149. [Google Scholar] [CrossRef]
- Cauchy, F.; Zalinski, S.; Dokmak, S.; Fuks, D.; Farges, O.; Castera, L.; Paradis, V.; Belghiti, J. Surgical treatment of hepatocellular carcinoma associated with the metabolic syndrome. Br. J. Surg. 2013, 100, 113–121. [Google Scholar] [CrossRef]
- Wakai, T.; Shirai, Y.; Sakata, J.; Korita, P.V.; Ajioka, Y.; Hatakeyama, K. Surgical Outcomes for Hepatocellular Carcinoma in Nonalcoholic Fatty Liver Disease. J. Gastrointest. Surg. 2011, 15, 1450–1458. [Google Scholar] [CrossRef]
- Takuma, Y.; Nouso, K.; Makino, Y.; Gotoh, T.; Toshikuni, N.; Morimoto, Y.; Shimomura, H.; Yamamoto, H. Outcomes after curative treatment for cryptogenic cirrhosis-associated hepatocellular carcinoma satisfying the Milan criteria. J. Gastroenterol. Hepatol. 2011, 26, 1417–1424. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Zheng, Z.J.; Shi, R.; Su, Q.; Jiang, Q.; Kip, K.E. Metformin for liver cancer prevention in patients with type 2 diabetes: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2012, 97, 2347–2353. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.E.; Serper, M.; John, B.V.; Tessiatore, K.M.; Lerer, R.; Mehta, R.; Fox, R.; Aytaman, A.; Baytarian, M.; Hunt, K.; et al. Effects of Metformin Exposure on Survival in a Large National Cohort of Patients with Diabetes and Cirrhosis. Clin. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, P.P.; Singh, A.G.; Murad, M.H.; Sanchez, W. Statins are associated with a reduced risk of hepatocellular cancer: A systematic review and meta-analysis. Gastroenterology 2013, 144, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.; Mehaffey, J.H.; Hawkins, R.B.; Hsu, A.; Schirmer, B.; Hallowell, P.T. Bariatric surgery is associated with reduction in non-alcoholic steatohepatitis and hepatocellular carcinoma: A propensity matched analysis. Am. J. Surg. 2020, 219, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Bertot, L.C.; Jeffrey, G.P.; Wallace, M.; MacQuillan, G.; Garas, G.; Ching, H.L.; Adams, L.A. Nonalcoholic fatty liver disease-related cirrhosis is commonly unrecognized and associated with hepatocellular carcinoma. Hepatol. Commun. 2017, 1, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Castera, L.; Friedrich-Rust, M.; Loomba, R. Noninvasive Assessment of Liver Disease in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1264–1281. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.; Berhane, S.; Teng, M.; Cox, T.; Tada, T.; Toyoda, H.; Kumada, T.; Kagebayashi, C.; Satomura, S.; Johnson, P.J. Biomarker-based prognosis in hepatocellular carcinoma: Validation and extension of the BALAD model. Br. J. Cancer 2014, 110, 2090–2098. [Google Scholar] [CrossRef]
- Mottin, C.C.; Moretto, M.; Padoin, A.V.; Swarowsky, A.M.; Toneto, M.G.; Glock, L.; Repetto, G. The Role of Ultrasound in the Diagnosis of Hepatic Steatosis in Morbidly Obese Patients. Obes. Surg. 2004, 14, 635–637. [Google Scholar] [CrossRef]
- Nishida, N.; Kudo, M. Immune checkpoint blockade for the treatment of human hepatocellular carcinoma. Hepatol. Res. 2018, 48, 622–634. [Google Scholar] [CrossRef]
- Bangaru, S.; Marrero, J.A.; Singal, A.G. Review article: New therapeutic interventions for advanced hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2020, 51, 78–89. [Google Scholar]
- Shimada, S.; Mogushi, K.; Akiyama, Y.; Furuyama, T.; Watanabe, S.; Ogura, T.; Ogawa, K.; Ono, H.; Mitsunori, Y.; Ban, D.; et al. Comprehensive molecular and immunological characterization of hepatocellular carcinoma. EBioMedicine 2019, 40, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Julich-Haertel, H.; Urban, S.K.; Krawczyk, M.; Willms, A.; Jankowski, K.; Patkowski, W.; Kruk, B.; Krasnodębski, M.; Ligocka, J.; Schwab, R.; et al. Cancer-associated circulating large extracellular vesicles in cholangiocarcinoma and hepatocellular carcinoma. J. Hepatol. 2017, 67, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Li, S.; Li, G.; Zhang, S.; Tang, X.; Ni, S.; Jian, X.; Xu, C.; Zhu, J.; Lu, M. The role of extracellular vesicles in mediating progression, metastasis and potential treatment of hepatocellular carcinoma. Oncotarget 2017, 8, 3683–3695. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, A.; Ishiguro, K.; Yan, I.K.; Patel, T. Extracellular Vesicle-Based Therapeutic Targeting of β-Catenin to Modulate Anticancer Immune Responses in Hepatocellular Cancer. Hepatol. Commun. 2019, 3, 525–541. [Google Scholar] [CrossRef]
- Rosenblum, D.; Gutkin, A.; Kedmi, R.; Ramishetti, S.; Veiga, N.; Jacobi, A.M.; Schubert, M.S.; Friedmann-Morvinski, D.; Cohen, Z.R.; Behlke, M.A.; et al. CRISPR-Cas9 genome editing using targeted lipid nanoparticles for cancer therapy. Sci. Adv. 2020, 6, eabc9450. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).