Radical Hemithoracic Radiotherapy Induces Systemic Metabolomics Changes That Are Associated with the Clinical Outcome of Malignant Pleural Mesothelioma Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Demographic and Clinical Baseline Patients’ Characteristics
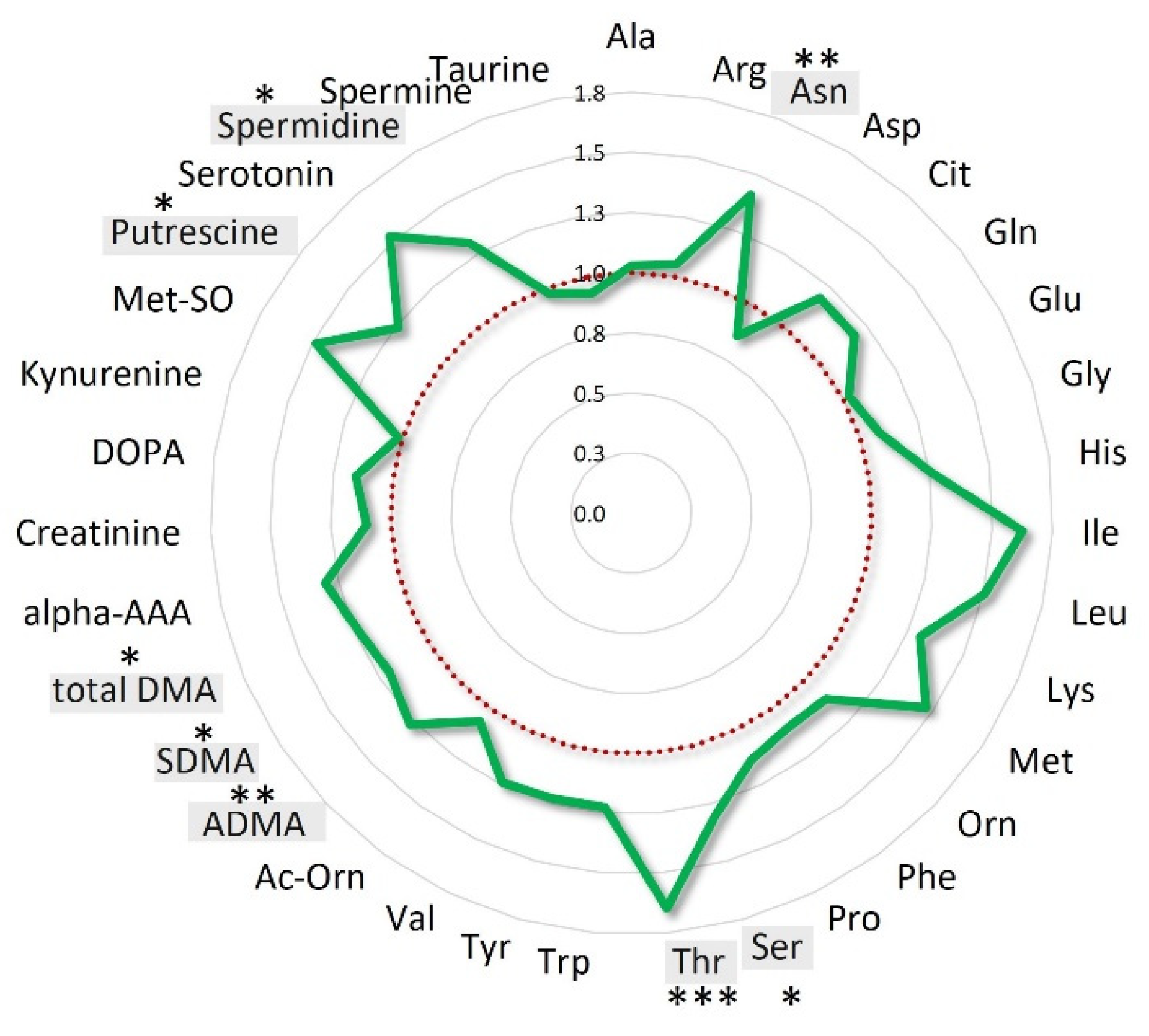
2.2. RHRT Effect on Serum Metabolome
2.3. Metabolic Patterns Influenced by RHRT
2.4. Serum Metabolome Variations as Function of Radiation Dose
2.5. RHRT Metabolomics Alterations and Clinical Outcome
3. Discussion
4. Materials and Methods
4.1. Patients’ Population
4.2. Sample Collection
4.3. Study Design
4.4. Targeted Serum Metabolomics Profile Analysis
4.5. Statistical Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Noonan, C.W. Environmental Asbestos Exposure and Risk of Mesothelioma. Ann. Transl. Med. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Musk, A.W.; Olsen, N.; Alfonso, H.; Reid, A.; Mina, R.; Franklin, P.; Sleith, J.; Hammond, N.; Threlfall, T.; Shilkin, K.B.; et al. Predicting Survival in Malignant Mesothelioma. Eur. Respir. J. 2011, 38, 1420–1424. [Google Scholar] [CrossRef] [PubMed]
- Fahrner, R.; Ochsenbein, A.; Schmid, R.A.; Carboni, G.L. Long Term Survival after Trimodal Therapy in Malignant Pleural Mesothelioma. Swiss Med. Wkly. 2012, 142. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Okada, M.; Tanaka, F.; Yamanaka, T.; Soejima, T.; Kamikonya, N.; Tsujimura, T.; Fukuoka, K.; Yokoi, K.; Nakano, T. Trimodality Strategy for Treating Malignant Pleural Mesothelioma: Results of a Feasibility Study of Induction Pemetrexed plus Cisplatin Followed by Extrapleural Pneumonectomy and Postoperative Hemithoracic Radiation (Japan Mesothelioma Interest Group 0601 Trial). Int. J. Clin. Oncol. 2016, 21, 523–530. [Google Scholar] [CrossRef]
- Rosenzweig, K.E. Malignant Pleural Mesothelioma: Adjuvant Therapy with Radiation Therapy. Ann. Transl. Med. 2017, 5. [Google Scholar] [CrossRef]
- Foroudi, F.; Smith, J.G.; Putt, F.; Wada, M. High-Dose Palliative Radiotherapy for Malignant Pleural Mesothelioma. J. Med. Imaging Radiat. Oncol. 2017, 61, 797–803. [Google Scholar] [CrossRef]
- Parisi, E.; Romeo, A.; Sarnelli, A.; Ghigi, G.; Bellia, S.R.; Neri, E.; Micheletti, S.; Dipalma, B.; Arpa, D.; Furini, G.; et al. High Dose Irradiation after Pleurectomy/Decortication or Biopsy for Pleural Mesothelioma Treatment. Cancer Radiother. 2017, 21, 766–773. [Google Scholar] [CrossRef]
- Minatel, E.; Trovo, M.; Bearz, A.; Maso, M.D.; Baresic, T.; Drigo, A.; Barresi, L.; Furlan, C.; Conte, A.D.; Bruschi, G.; et al. Radical Radiation Therapy after Lung-Sparing Surgery for Malignant Pleural Mesothelioma: Survival, Pattern of Failure, and Prognostic Factors. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, K.E.; Zauderer, M.G.; Laser, B.; Krug, L.M.; Yorke, E.; Sima, C.S.; Rimner, A.; Flores, R.; Rusch, V. Pleural Intensity-Modulated Radiation Therapy (IMRT) for Malignant Pleural Mesothelioma (MPM). Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1278–1283. [Google Scholar] [CrossRef]
- Clish, C.B. Metabolomics: An Emerging but Powerful Tool for Precision Medicine. Cold Spring Harb. Mol. Case Stud. 2015, 1. [Google Scholar] [CrossRef]
- Corona, G.; Rizzolio, F.; Giordano, A.; Toffoli, G. Pharmaco-Metabolomics: An Emerging “Omics” Tool for the Personalization of Anticancer Treatments and Identification of New Valuable Therapeutic Targets. J. Cell. Physiol. 2012, 227, 2827–2831. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S. Metabolomics for Investigating Physiological and Pathophysiological Processes. Physiol. Rev. 2019, 99, 1819–1875. [Google Scholar] [CrossRef] [PubMed]
- Debik, J.; Euceda, L.R.; Lundgren, S.; von der Lippe Gythfeldt, H.; Garred, Ø.; Borgen, E.; Engebraaten, O.; Bathen, T.F.; Giskeødegård, G.F. Assessing Treatment Response and Prognosis by Serum and Tissue Metabolomics in Breast Cancer Patients. J. Proteome Res. 2019, 18, 3649–3660. [Google Scholar] [CrossRef] [PubMed]
- Miolo, G.; Di Gregorio, E.; Saorin, A.; Lombardi, D.; Scalone, S.; Buonadonna, A.; Steffan, A.; Corona, G. Integration of Serum Metabolomics into Clinical Assessment to Improve Outcome Prediction of Metastatic Soft Tissue Sarcoma Patients Treated with Trabectedin. Cancers 2020, 12, 1983. [Google Scholar] [CrossRef]
- Vignoli, A.; Muraro, E.; Miolo, G.; Tenori, L.; Turano, P.; Di Gregorio, E.; Steffan, A.; Luchinat, C.; Corona, G. Effect of Estrogen Receptor Status on Circulatory Immune and Metabolomics Profiles of HER2-Positive Breast Cancer Patients Enrolled for Neoadjuvant Targeted Chemotherapy. Cancers 2020, 12, 314. [Google Scholar] [CrossRef]
- Xu, P.-P.; Xiong, J.; Cheng, S.; Zhao, X.; Wang, C.-F.; Cai, G.; Zhong, H.-J.; Huang, H.-Y.; Chen, J.-Y.; Zhao, W.-L. A Phase II Study of Methotrexate, Etoposide, Dexamethasone and Pegaspargase Sandwiched with Radiotherapy in the Treatment of Newly Diagnosed, Stage IE to IIE Extranodal Natural-Killer/T-Cell Lymphoma, Nasal-Type. EBioMedicine 2017, 25, 41–49. [Google Scholar] [CrossRef]
- Arenas, M.; Rodríguez, E.; García-Heredia, A.; Fernández-Arroyo, S.; Sabater, S.; Robaina, R.; Gascón, M.; Rodríguez-Pla, M.; Cabré, N.; Luciano-Mateo, F.; et al. Metabolite Normalization with Local Radiotherapy Following Breast Tumor Resection. PLoS ONE 2018, 13, e0207474. [Google Scholar] [CrossRef]
- Jelonek, K.; Krzywon, A.; Jablonska, P.; Slominska, E.M.; Smolenski, R.T.; Polanska, J.; Rutkowski, T.; Mrochem-Kwarciak, J.; Skladowski, K.; Widlak, P. Systemic Effects of Radiotherapy and Concurrent Chemo-Radiotherapy in Head and Neck Cancer Patients—Comparison of Serum Metabolome Profiles. Metabolites 2020, 10, 60. [Google Scholar] [CrossRef]
- Jelonek, K.; Pietrowska, M.; Ros, M.; Zagdanski, A.; Suchwalko, A.; Polanska, J.; Marczyk, M.; Rutkowski, T.; Skladowski, K.; Clench, M.R.; et al. Radiation-Induced Changes in Serum Lipidome of Head and Neck Cancer Patients. Int. J. Mol. Sci. 2014, 15, 6609–6624. [Google Scholar] [CrossRef]
- Laiakis, E.C.; Mak, T.D.; Anizan, S.; Amundson, S.A.; Barker, C.A.; Wolden, S.L.; Brenner, D.J.; Fornace, A.J. Development of a Metabolomic Radiation Signature in Urine from Patients Undergoing Total Body Irradiation. Radiat. Res. 2014, 181, 350–361. [Google Scholar] [CrossRef]
- Mörén, L.; Wibom, C.; Bergström, P.; Johansson, M.; Antti, H.; Bergenheim, A.T. Characterization of the Serum Metabolome Following Radiation Treatment in Patients with High-Grade Gliomas. Radiat. Oncol. 2016, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Nalbantoglu, S.; Abu-Asab, M.; Suy, S.; Collins, S.; Amri, H. Metabolomics-Based Biosignatures of Prostate Cancer in Patients Following Radiotherapy. OMICS J. Integr. Biol. 2019, 23, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.S.W.; Jang, G.H.; Kurland, I.J.; Qiu, Y.; Guha, C.; Dawson, L.A. Plasma Metabolomic Profiles in Liver Cancer Patients Following Stereotactic Body Radiotherapy. EBioMedicine 2020, 59, 102973. [Google Scholar] [CrossRef] [PubMed]
- Bentzen, S.M. Preventing or Reducing Late Side Effects of Radiation Therapy: Radiobiology Meets Molecular Pathology. Nat. Rev. Cancer 2006, 6, 702–713. [Google Scholar] [CrossRef]
- Wojakowska, A.; Zebrowska, A.; Skowronek, A.; Rutkowski, T.; Polanski, K.; Widlak, P.; Marczak, L.; Pietrowska, M. Metabolic Profiles of Whole Serum and Serum-Derived Exosomes Are Different in Head and Neck Cancer Patients Treated by Radiotherapy. J. Pers. Med. 2020, 10, 229. [Google Scholar] [CrossRef]
- Ananieva, E.A.; Wilkinson, A.C. Branched-Chain Amino Acid Metabolism in Cancer. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 64–70. [Google Scholar] [CrossRef]
- Thewes, V.; Simon, R.; Hlevnjak, M.; Schlotter, M.; Schroeter, P.; Schmidt, K.; Wu, Y.; Anzeneder, T.; Wang, W.; Windisch, P.; et al. The Branched-Chain Amino Acid Transaminase 1 Sustains Growth of Antiestrogen-Resistant and ERα-Negative Breast Cancer. Oncogene 2017, 36, 4124–4134. [Google Scholar] [CrossRef]
- Mayers, J.R.; Torrence, M.E.; Danai, L.V.; Papagiannakopoulos, T.; Davidson, S.M.; Bauer, M.R.; Lau, A.N.; Ji, B.W.; Dixit, P.D.; Hosios, A.M.; et al. Tissue of Origin Dictates Branched-Chain Amino Acid Metabolism in Mutant Kras-Driven Cancers. Science 2016, 353, 1161–1165. [Google Scholar] [CrossRef]
- Tönjes, M.; Barbus, S.; Park, Y.J.; Wang, W.; Schlotter, M.; Lindroth, A.M.; Pleier, S.V.; Bai, A.H.C.; Karra, D.; Piro, R.M.; et al. BCAT1 Promotes Cell Proliferation through Amino Acid Catabolism in Gliomas Carrying Wild-Type IDH1. Nat. Med. 2013, 19, 901–908. [Google Scholar] [CrossRef]
- Geisler, S.; Gostner, J.M.; Becker, K.; Ueberall, F.; Fuchs, D. Immune Activation and Inflammation Increase the Plasma Phenylalanine-to-Tyrosine Ratio. Pteridines 2013, 24, 27–31. [Google Scholar] [CrossRef]
- Murr, C.; Grammer, T.B.; Meinitzer, A.; Kleber, M.E.; März, W.; Fuchs, D. Immune Activation and Inflammation in Patients with Cardiovascular Disease Are Associated with Higher Phenylalanine to Tyrosine Ratios: The Ludwigshafen Risk and Cardiovascular Health Study. J. Amino Acids 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Neurauter, G.; Grahmann, A.V.; Klieber, M.; Zeimet, A.; Ledochowski, M.; Sperner-Unterweger, B.; Fuchs, D. Serum Phenylalanine Concentrations in Patients with Ovarian Carcinoma Correlate with Concentrations of Immune Activation Markers and of Isoprostane-8. Cancer Lett. 2008, 272, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Ploder, M.; Neurauter, G.; Spittler, A.; Schroecksnadel, K.; Roth, E.; Fuchs, D. Serum Phenylalanine in Patients Post Trauma and with Sepsis Correlate to Neopterin Concentrations. Amino Acids 2008, 35, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Jelonek, K.; Pietrowska, M.; Widlak, P. Systemic Effects of Ionizing Radiation at the Proteome and Metabolome Levels in the Blood of Cancer Patients Treated with Radiotherapy: The Influence of Inflammation and Radiation Toxicity. Int. J. Radiat. Biol. 2017, 93, 683–696. [Google Scholar] [CrossRef]
- McKelvey, K.J.; Hudson, A.L.; Back, M.; Eade, T.; Diakos, C.I. Radiation, Inflammation and the Immune Response in Cancer. Mamm. Genome 2018, 29, 843–865. [Google Scholar] [CrossRef]
- Deloch, L.; Derer, A.; Hartmann, J.; Frey, B.; Fietkau, R.; Gaipl, U.S. Modern Radiotherapy Concepts and the Impact of Radiation on Immune Activation. Front. Oncol. 2016, 6, 141. [Google Scholar] [CrossRef]
- Di Maggio, F.M.; Minafra, L.; Forte, G.I.; Cammarata, F.P.; Lio, D.; Messa, C.; Gilardi, M.C.; Bravatà, V. Portrait of Inflammatory Response to Ionizing Radiation Treatment. J. Inflamm. 2015, 12, 14. [Google Scholar] [CrossRef]
- Huang, J.; Li, J.J. Cell Repopulation, Rewiring Metabolism, and Immune Regulation in Cancer Radiotherapy. Radiat. Med. Prot. 2020, 1, 24–30. [Google Scholar] [CrossRef]
- Muraro, E.; Furlan, C.; Avanzo, M.; Martorelli, D.; Comaro, E.; Rizzo, A.; Fae, D.A.; Berretta, M.; Militello, L.; Del Conte, A.; et al. Local High-Dose Radiotherapy Induces Systemic Immunomodulating Effects of Potential Therapeutic Relevance in Oligometastatic Breast Cancer. Front. Immunol. 2017, 8, 1476. [Google Scholar] [CrossRef]
- Boguszewicz, Ł.; Bieleń, A.; Mrochem-Kwarciak, J.; Skorupa, A.; Ciszek, M.; Heyda, A.; Wygoda, A.; Kotylak, A.; Składowski, K.; Sokół, M. NMR-Based Metabolomics in Real-Time Monitoring of Treatment Induced Toxicity and Cachexia in Head and Neck Cancer: A Method for Early Detection of High Risk Patients. Metabolomics 2019, 15, 110. [Google Scholar] [CrossRef]
- Bartke, N.; Hannun, Y.A. Bioactive Sphingolipids: Metabolism and Function. J. Lipid Res. 2009, 50, S91–S96. [Google Scholar] [CrossRef] [PubMed]
- Eyster, K.M. The Membrane and Lipids as Integral Participants in Signal Transduction: Lipid Signal Transduction for the Non-Lipid Biochemist. Adv. Physiol. Educ. 2007, 31, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.M.; Howe, A.G.; Zaremberg, V. Cell Membranes and Apoptosis: Role of Cardiolipin, Phosphatidylcholine, and Anticancer Lipid Analogues. Biochem. Cell Biol. 2004, 82, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Verheij, M.; van Blitterswijk, W.J.; Bartelink, H. Radiation-Induced Apoptosis: The Ceramide-SAPK Signaling Pathway and Clinical Aspects. Acta Oncol. 1998, 37, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Morris, S.M. Arginine Metabolism: Nitric Oxide and Beyond. Biochem. J. 1998, 336, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Curis, E.; Nicolis, I.; Moinard, C.; Osowska, S.; Zerrouk, N.; Bénazeth, S.; Cynober, L. Almost All about Citrulline in Mammals. Amino Acids 2005, 29, 177. [Google Scholar] [CrossRef]
- Crenn, P.; Messing, B.; Cynober, L. Citrulline as a Biomarker of Intestinal Failure Due to Enterocyte Mass Reduction. Clin. Nutr. 2008, 27, 328–339. [Google Scholar] [CrossRef]
- Blijlevens, N.M.A.; Lutgens, L.C.H.W.; Schattenberg, A.V.M.B.; Donnelly, J.P. Citrulline: A Potentially Simple Quantitative Marker of Intestinal Epithelial Damage Following Myeloablative Therapy. Bone Marrow Transplant. 2004, 34, 193–196. [Google Scholar] [CrossRef]
- Ouaknine Krief, J.; Helly de Tauriers, P.; Dumenil, C.; Neveux, N.; Dumoulin, J.; Giraud, V.; Labrune, S.; Tisserand, J.; Julie, C.; Emile, J.-F.; et al. Role of Antibiotic Use, Plasma Citrulline and Blood Microbiome in Advanced Non-Small Cell Lung Cancer Patients Treated with Nivolumab. J. Immunother. Cancer 2019, 7, 176. [Google Scholar] [CrossRef]
- Bachmayr-Heyda, A.; Aust, S.; Auer, K.; Meier, S.M.; Schmetterer, K.G.; Dekan, S.; Gerner, C.; Pils, D. Integrative Systemic and Local Metabolomics with Impact on Survival in High-Grade Serous Ovarian Cancer. Clin. Cancer Res. 2017, 23, 2081–2092. [Google Scholar] [CrossRef]
- Lutgens, L.C.H.W.; Deutz, N.E.P.; Gueulette, J.; Cleutjens, J.P.M.; Berger, M.P.F.; Wouters, B.G.; von Meyenfeldt, M.F.; Lambin, P. Citrulline: A Physiologic Marker Enabling Quantitation and Monitoring of Epithelial Radiation-Induced Small Bowel Damage. Int. J. Radiat. Oncol. 2003, 57, 1067–1074. [Google Scholar] [CrossRef]
- Onal, C.; Kotek, A.; Unal, B.; Arslan, G.; Yavuz, A.; Topkan, E.; Yavuz, M. Plasma Citrulline Levels Predict Intestinal Toxicity in Patients Treated with Pelvic Radiotherapy. Acta Oncol. 2011, 50, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Akashi, K.; Miyake, C.; Yokota, A. Citrulline, a Novel Compatible Solute in Drought-Tolerant Wild Watermelon Leaves, Is an Efficient Hydroxyl Radical Scavenger. FEBS Lett. 2001, 508, 438–442. [Google Scholar] [CrossRef]
- Ginguay, A.; Regazzetti, A.; Laprevote, O.; Moinard, C.; De Bandt, J.-P.; Cynober, L.; Billard, J.-M.; Allinquant, B.; Dutar, P. Citrulline Prevents Age-Related LTP Decline in Old Rats. Sci. Rep. 2019, 9, 20138. [Google Scholar] [CrossRef]
- Requena, J.R.; Chao, C.-C.; Levine, R.L.; Stadtman, E.R. Glutamic and Aminoadipic Semialdehydes Are the Main Carbonyl Products of Metal-Catalyzed Oxidation of Proteins. PNAS 2001, 98, 69–74. [Google Scholar] [CrossRef]
- Sell, D.R.; Strauch, C.M.; Shen, W.; Monnier, V.M. 2-Aminoadipic Acid Is a Marker of Protein Carbonyl Oxidation in the Aging Human Skin: Effects of Diabetes, Renal Failure and Sepsis. Biochem. J. 2007, 404, 269–277. [Google Scholar] [CrossRef]
- Das, J.; Sil, P.C. Taurine Ameliorates Alloxan-Induced Diabetic Renal Injury, Oxidative Stress-Related Signaling Pathways and Apoptosis in Rats. Amino Acids 2012, 43, 1509–1523. [Google Scholar] [CrossRef]
- Jong, C.J.; Azuma, J.; Schaffer, S. Mechanism Underlying the Antioxidant Activity of Taurine: Prevention of Mitochondrial Oxidant Production. Amino Acids 2012, 42, 2223–2232. [Google Scholar] [CrossRef]
- Sevin, G.; Ozsarlak-Sozer, G.; Keles, D.; Gokce, G.; Reel, B.; Ozgur, H.H.; Oktay, G.; Kerry, Z. Taurine Inhibits Increased MMP-2 Expression in a Model of Oxidative Stress Induced by Glutathione Depletion in Rabbit Heart. Eur. J. Pharmacol. 2013, 706, 98–106. [Google Scholar] [CrossRef]
- Tabassum, H.; Rehman, H.; Banerjee, B.D.; Raisuddin, S.; Parvez, S. Attenuation of Tamoxifen-Induced Hepatotoxicity by Taurine in Mice. Clin. Chim. Acta 2006, 370, 129–136. [Google Scholar] [CrossRef]
- Gao, Y.; Li, X.; Gao, J.; Zhang, Z.; Feng, Y.; Nie, J.; Zhu, W.; Zhang, S.; Cao, J. Metabolomic Analysis of Radiation-Induced Lung Injury in Rats: The Potential Radioprotective Role of Taurine. Dose Response 2019, 17. [Google Scholar] [CrossRef] [PubMed]
- Marini, J.C. Interrelationships between Glutamine and Citrulline Metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Casero, R.A.; Murray Stewart, T.; Pegg, A.E. Polyamine Metabolism and Cancer: Treatments, Challenges and Opportunities. Nat. Rev. Cancer 2018, 18, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.-K.; Yan, S.; Xu, S.; Ho, J.C.-M. Targeting Polyamine as a Novel Therapy in Xenograft Models of Malignant Pleural Mesothelioma. Lung Cancer 2020, 148, 138–148. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | n (%) |
|---|---|
| Age (years), median, range | 70 (33–79) |
| Sex | |
| Female | 2 (11%) |
| Male | 17 (89%) |
| Performance Status * | |
| 0 | 6 (31%) |
| 1 | 10 (53%) |
| 2 | 3 (16%) |
| Histology | |
| Epithelioid | 18 (95%) |
| Nonepithelioid | 1 (5%) |
| Stage | |
| I–II | 9 (47%) |
| III–IV | 10 (53%) |
| Chemotherapy | |
| Pemetrexed, cisplatin | 19 (100%) |
| Surgery | |
| Pleurectomy/decortication (P/D) | 5 (26%) |
| Decortication | 2 (11%) |
| Biopsy | 12 (63%) |
| Class | Name | Mean (µM) ± SD | Fold Change | Trend | p-Value | q-Value | |
|---|---|---|---|---|---|---|---|
| Baseline | Post-HRT | ||||||
| Amino acids and derivatives | Cit | 33.22 ± 7.02 | 22.19 ± 7.15 | 0.67 |  | 9.0 × 10−6 | 0.001 |
| ADMA | 0.57 ± 0.06 | 0.5 ± 0.09 | 0.88 |  | 0.007 | 0.085 | |
| Orn | 71.74 ± 9.56 | 62.89 ± 13.15 | 0.88 |  | 0.017 | 0.113 | |
| Pro | 220.95 ± 36.99 | 178.47 ± 63.76 | 0.81 |  | 0.005 | 0.078 | |
| Putrescine | 0.14 ± 0.03 | 0.13 ± 0.04 | 0.89 |  | 0.027 | 0.149 | |
| Serotonin | 0.48 ± 0.22 | 0.36 ± 0.22 | 0.75 |  | 0.005 | 0.078 | |
| Spermidine | 0.13 ± 0.03 | 0.11 ± 0.03 | 0.86 |  | 0.010 | 0.099 | |
| Spermine | 0.19 ± 0.01 | 0.18 ± 0.01 | 0.96 |  | 0.016 | 0.113 | |
| Taurine | 104.05 ± 18.69 | 74.49 ± 22.79 | 0.72 |  | 3.0 × 10−5 | 0.002 | |
| total DMA | 1.06 ± 0.31 | 0.94 ± 0.34 | 0.88 |  | 0.024 | 0.139 | |
| Phe | 70.96 ± 11.05 | 79.71 ± 8.17 | 1.14 |  | 0.009 | 0.095 | |
| Val | 178.21 ± 38.35 | 202.63 ± 36.56 | 1.12 |  | 0.035 | 0.176 | |
| alpha-AAA | 1.17 ± 0.58 | 1.42 ± 0.34 | 1.21 |  | 0.035 | 0.176 | |
| Trp | 46.96 ± 9.37 | 52.78 ± 10.89 | 1.12 |  | 0.039 | 0.176 | |
| Acyl-carnitines | C10:2 | 0.08 ± 0.01 | 0.06 ± 0.04 | 0.73 |  | 0.012 | 0.104 |
| C14 | 0.06 ± 0.02 | 0.04 ± 0.02 | 0.79 |  | 0.002 | 0.038 | |
| C14:1-OH | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.83 |  | 0.032 | 0.174 | |
| C:16 | 0.15 ± 0.03 | 0.12 ± 0.05 | 0.81 |  | 0.012 | 0.104 | |
| C16:2-OH | 0.01 ± 0.005 | 0.01 ± 0.005 | 0.81 |  | 0.037 | 0.176 | |
| C18:1 | 0.19 ± 0.04 | 0.14 ± 0.06 | 0.77 |  | 0.002 | 0.048 | |
| C18:2 | 0.06 ± 0.02 | 0.04 ± 0.02 | 0.66 |  | 2.8 × 10−4 | 0.015 | |
| C0 | 37.26 ± 6.11 | 41.23 ± 6.00 | 1.11 |  | 0.022 | 0.138 | |
| Phospholipids | lysoPC a C18:0 | 24.71 ± 6.45 | 20.01 ± 6.61 | 0.81 |  | 0.005 | 0.078 |
| PC aa C28:1 | 2.85 ± 0.84 | 2.43 ± 0.95 | 0.85 |  | 0.007 | 0.085 | |
| PC aa C36:2 | 203.53 ± 51.70 | 173.58 ± 42.35 | 0.85 |  | 0.012 | 0.104 | |
| PC aa C38:0 | 3.04 ± 1.00 | 2.44 ± 1.58 | 0.8 |  | 0.024 | 0.139 | |
| PC aa C40:2 | 0.29 ± 0.11 | 0.22 ± 0.12 | 0.76 |  | 0.043 | 0.185 | |
| PC ae C36:3 | 6.23 ± 1.31 | 4.94 ± 2.11 | 0.79 |  | 0.036 | 0.176 | |
| PC ae C38:6 | 6.95 ± 2.27 | 5.92 ± 3.08 | 0.85 |  | 0.046 | 0.195 | |
| PC ae C40:1 | 1.38 ± 0.42 | 1.14 ± 0.60 | 0.82 |  | 0.015 | 0.113 | |
| PC ae C40:4 | 2.22 ± 0.32 | 1.77 ± 0.68 | 0.8 |  | 0.015 | 0.113 | |
| PC ae C42:3 | 0.74 ± 0.23 | 0.61 ± 0.29 | 0.83 |  | 0.039 | 0.176 | |
| PC ae C42:4 | 0.71 ± 0.25 | 0.47 ± 0.41 | 0.67 |  | 0.007 | 0.085 | |
| PC ae C42:5 | 2.33 ± 0.30 | 1.67 ± 0.92 | 0.72 |  | 0.001 | 0.026 | |
| PC ae C44:5 | 2.1 ± 0.42 | 1.37 ± 1.22 | 0.65 |  | 0.001 | 0.026 | |
| PC ae C44:6 | 1.25 ± 0.31 | 0.85 ± 0.65 | 0.68 |  | 3.7 × 10−4 | 0.015 | |
| Sphyngomielyns | SM C24:0 | 18.29 ± 5.87 | 14.75 ± 4.30 | 0.81 |  | 0.018 | 0.113 |
| SM C24:1 | 48.12 ± 13.66 | 41.99 ± 11.09 | 0.87 |  | 0.047 | 0.195 | |
| SM-OH C24:1 | 1.25 ± 0.3 | 0.93 ± 0.49 | 0.74 |  | 0.014 | 0.113 | |
 , down-regulated;
, down-regulated;  , up-regulated.
, up-regulated.Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Gregorio, E.; Miolo, G.; Saorin, A.; Muraro, E.; Cangemi, M.; Revelant, A.; Minatel, E.; Trovò, M.; Steffan, A.; Corona, G. Radical Hemithoracic Radiotherapy Induces Systemic Metabolomics Changes That Are Associated with the Clinical Outcome of Malignant Pleural Mesothelioma Patients. Cancers 2021, 13, 508. https://doi.org/10.3390/cancers13030508
Di Gregorio E, Miolo G, Saorin A, Muraro E, Cangemi M, Revelant A, Minatel E, Trovò M, Steffan A, Corona G. Radical Hemithoracic Radiotherapy Induces Systemic Metabolomics Changes That Are Associated with the Clinical Outcome of Malignant Pleural Mesothelioma Patients. Cancers. 2021; 13(3):508. https://doi.org/10.3390/cancers13030508
Chicago/Turabian StyleDi Gregorio, Emanuela, Gianmaria Miolo, Asia Saorin, Elena Muraro, Michela Cangemi, Alberto Revelant, Emilio Minatel, Marco Trovò, Agostino Steffan, and Giuseppe Corona. 2021. "Radical Hemithoracic Radiotherapy Induces Systemic Metabolomics Changes That Are Associated with the Clinical Outcome of Malignant Pleural Mesothelioma Patients" Cancers 13, no. 3: 508. https://doi.org/10.3390/cancers13030508
APA StyleDi Gregorio, E., Miolo, G., Saorin, A., Muraro, E., Cangemi, M., Revelant, A., Minatel, E., Trovò, M., Steffan, A., & Corona, G. (2021). Radical Hemithoracic Radiotherapy Induces Systemic Metabolomics Changes That Are Associated with the Clinical Outcome of Malignant Pleural Mesothelioma Patients. Cancers, 13(3), 508. https://doi.org/10.3390/cancers13030508