Gynecological Surveillance and Surgery Outcomes in Dutch Lynch Syndrome Carriers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
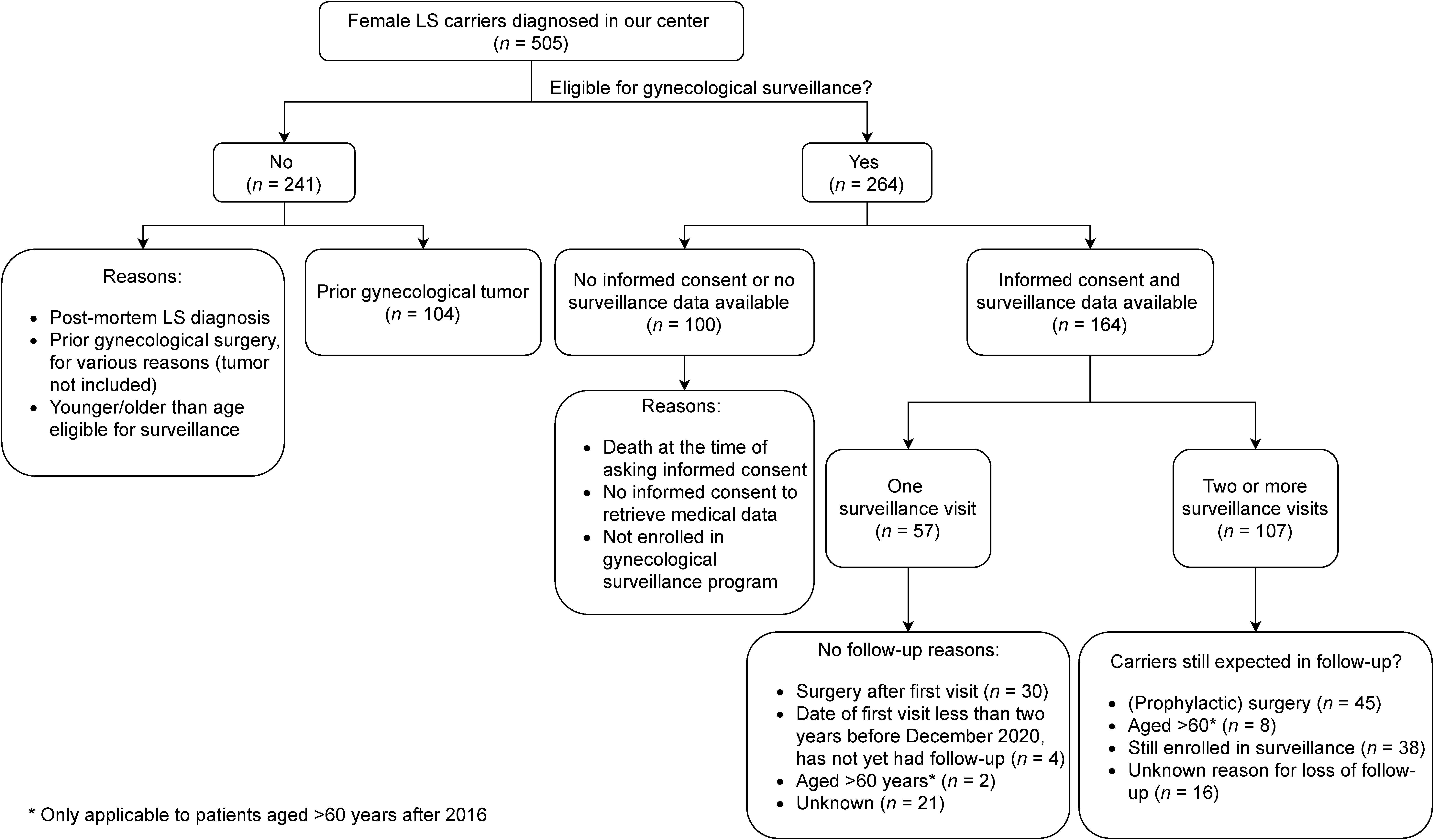
2.1. Patient Selection and Data Extraction
2.2. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Surveillance
3.2.1. Characteristics of Surveillance
3.2.2. First Surveillance Visit
3.2.3. Second or Higher Surveillance Visit
3.2.4. Abnormalities Indicative for (pre)Malignancy
3.3. Risk-Reducing Surgery
4. Discussion
4.1. Surveillance
4.2. Tumors
4.3. Difference in Risk-Reducing Surgery
4.4. Relevance
4.5. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de la Chapelle, A. The incidence of Lynch syndrome. Fam. Cancer 2005, 4, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Fishel, R.; Lescoe, M.K.; Rao, M.R.; Copeland, N.G.; Jenkins, N.A.; Garber, J.; Kane, M.; Kolodner, R. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell 1993, 75, 1027–1038. [Google Scholar] [CrossRef]
- Bronner, C.E.; Baker, S.M.; Morrison, P.T.; Warren, G.; Smith, L.G.; Lescoe, M.K.; Kane, M.; Earabino, C.; Lipford, J.; Lindblom, A.; et al. Mutation in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colon cancer. Nature 1994, 368, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, N.C.; Papadopoulos, N.; Liu, B.; Wei, Y.F.; Carter, K.C.; Ruben, S.M.; Rosen, C.A.; Haseltine, W.A.; Fleischmann, R.D.; Fraser, C.M.; et al. Mutations of two PMS homologues in hereditary nonpolyposis colon cancer. Nature 1994, 371, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Sato, H.; Yamada, T.; Nagasaki, H.; Tsuchiya, A.; Abe, R.; Yuasa, Y. Germ-line mutation of the hMSH6/GTBP gene in an atypical hereditary nonpolyposis colorectal cancer kindred. Cancer Res. 1997, 57, 3920–3923. Available online: https://www.ncbi.nlm.nih.gov/pubmed/9307272 (accessed on 2 November 2020). [PubMed]
- Ligtenberg, M.J.; Kuiper, R.P.; Chan, T.L.; Goossens, M.; Hebeda, K.M.; Voorendt, M.; Lee, T.Y.; Bodmer, D.; Hoenselaar, E.; Hendriks-Cornelissen, S.J.; et al. Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3′ exons of TACSTD1. Nat. Genet. 2009, 41, 112–117. [Google Scholar] [CrossRef]
- Lynch, H.T.; Snyder, C.L.; Shaw, T.G.; Heinen, C.D.; Hitchins, M.P. Milestones of Lynch syndrome: 1895–2015. Nat. Rev. Cancer 2015, 15, 181–194. [Google Scholar] [CrossRef]
- Broeke, S.W.T.; van der Klift, H.M.; Tops, C.M.J.; Aretz, S.; Bernstein, I.; Buchanan, D.D.; de la Chapelle, A.; Capella, G.; Clendenning, M.; Engel, C.; et al. Cancer Risks for PMS2-Associated Lynch Syndrome. J. Clin. Oncol. 2018, 36, 2961–2968. [Google Scholar] [CrossRef]
- Bucksch, K.; Zachariae, S.; Aretz, S.; Buttner, R.; Holinski-Feder, E.; Holzapfel, S.; Huneburg, R.; Kloor, M.; von Doeberitz, M.K.; Morak, M.; et al. Cancer risks in Lynch syndrome, Lynch-like syndrome, and familial colorectal cancer type X: A prospective cohort study. BMC Cancer 2020, 20, 460. [Google Scholar] [CrossRef]
- Dominguez-Valentin, M.; Sampson, J.R.; Seppala, T.T.; Ten Broeke, S.W.; Plazzer, J.P.; Nakken, S.; Engel, C.; Aretz, S.; Jenkins, M.A.; Sunde, L.; et al. Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair variants: Findings from the Prospective Lynch Syndrome Database. Genet. Med. 2020, 22, 15–25. [Google Scholar] [CrossRef]
- Moller, P.; Seppala, T.; Bernstein, I.; Holinski-Feder, E.; Sala, P.; Evans, D.G.; Lindblom, A.; Macrae, F.; Blanco, I.; Sijmons, R.; et al. Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: First report from the prospective Lynch syndrome database. Gut 2017, 66, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Barrow, P.; Khan, M.; Lalloo, F.; Evans, D.G.; Hill, J. Systematic review of the impact of registration and screening on colorectal cancer incidence and mortality in familial adenomatous polyposis and Lynch syndrome. Br. J. Surg. 2013, 100, 1719–1731. [Google Scholar] [CrossRef] [PubMed]
- de Jong, A.E.; Hendriks, Y.M.; Kleibeuker, J.H.; de Boer, S.Y.; Cats, A.; Griffioen, G.; Nagengast, F.M.; Nelis, F.G.; Rookus, M.A.; Vasen, H.F. Decrease in mortality in Lynch syndrome families because of surveillance. Gastroenterology 2006, 130, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Jarvinen, H.J.; Aarnio, M.; Mustonen, H.; Aktan-Collan, K.; Aaltonen, L.A.; Peltomaki, P.; De La Chapelle, A.; Mecklin, J.P. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology 2000, 118, 829–834. [Google Scholar] [CrossRef]
- Crosbie, E.J.; Ryan, N.A.J.; Arends, M.J.; Bosse, T.; Burn, J.; Cornes, J.M.; Crawford, R.; Eccles, D.; Frayling, I.M.; Ghaem-Maghami, S.; et al. The Manchester International Consensus Group recommendations for the management of gynecological cancers in Lynch syndrome. Genet. Med. 2019, 21, 2390–2400. [Google Scholar] [CrossRef]
- Vasen, H.F.; Blanco, I.; Aktan-Collan, K.; Gopie, J.P.; Alonso, A.; Aretz, S.; Bernstein, I.; Bertario, L.; Burn, J.; Capella, G.; et al. Revised guidelines for the clinical management of Lynch syndrome (HNPCC): Recommendations by a group of European experts. Gut 2013, 62, 812–823. [Google Scholar] [CrossRef]
- Ketabi, Z.; Gerdes, A.M.; Mosgaard, B.; Ladelund, S.; Bernstein, I. The results of gynecologic surveillance in families with hereditary nonpolyposis colorectal cancer. Gynecol. Oncol. 2014, 133, 526–530. [Google Scholar] [CrossRef]
- Renkonen-Sinisalo, L.; Butzow, R.; Leminen, A.; Lehtovirta, P.; Mecklin, J.P.; Jarvinen, H.J. Surveillance for endometrial cancer in hereditary nonpolyposis colorectal cancer syndrome. Int. J. Cancer 2007, 120, 821–824. [Google Scholar] [CrossRef]
- VKGN. Oncoline: National Guideline Hereditary Colorectal Cancer. Available online: https://www.oncoline.nl/erfelijke-darmkanker (accessed on 2 November 2020).
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 147: Lynch syndrome. Obstet Gynecol 2014, 124, 1042–1054. [Google Scholar] [CrossRef]
- Provenzale, D.; Gupta, S.; Ahnen, D.J.; Bray, T.; Cannon, J.A.; Cooper, G.; David, D.S.; Early, D.S.; Erwin, D.; Ford, J.M.; et al. Genetic/Familial High-Risk Assessment: Colorectal Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2016, 14, 1010–1030. [Google Scholar] [CrossRef]
- Nieminen, T.T.; Gylling, A.; Abdel-Rahman, W.M.; Nuorva, K.; Aarnio, M.; Renkonen-Sinisalo, L.; Jarvinen, H.J.; Mecklin, J.P.; Butzow, R.; Peltomaki, P. Molecular analysis of endometrial tumorigenesis: Importance of complex hyperplasia regardless of atypia. Clin. Cancer Res. 2009, 15, 5772–5783. [Google Scholar] [CrossRef] [PubMed]
- Auranen, A.; Joutsiniemi, T. A systematic review of gynecological cancer surveillance in women belonging to hereditary nonpolyposis colorectal cancer (Lynch syndrome) families. Acta Obstet. Gynecol. Scand. 2011, 90, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Helder-Woolderink, J.M.; Blok, E.A.; Vasen, H.F.; Hollema, H.; Mourits, M.J.; De Bock, G.H. Ovarian cancer in Lynch syndrome; a systematic review. Eur. J. Cancer 2016, 55, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Amant, F.; Moerman, P.; Neven, P.; Timmerman, D.; Van Limbergen, E.; Vergote, I. Endometrial cancer. Lancet 2005, 366, 491–505. [Google Scholar] [CrossRef]
- Schmeler, K.M.; Lynch, H.T.; Chen, L.M.; Munsell, M.F.; Soliman, P.T.; Clark, M.B.; Daniels, M.S.; White, K.G.; Boyd-Rogers, S.G.; Conrad, P.G.; et al. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N. Engl. J. Med. 2006, 354, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Kempers, M.J.; Kuiper, R.P.; Ockeloen, C.W.; Chappuis, P.O.; Hutter, P.; Rahner, N.; Schackert, H.K.; Steinke, V.; Holinski-Feder, E.; Morak, M.; et al. Risk of colorectal and endometrial cancers in EPCAM deletion-positive Lynch syndrome: A cohort study. Lancet Oncol. 2011, 12, 49–55. [Google Scholar] [CrossRef]
- Creasman, W. Revised FIGO staging for carcinoma of the endometrium. Int. J. Gynaecol. Obstet. 2009, 105, 109. [Google Scholar] [CrossRef]
- Bast, R.C., Jr.; Klug, T.L.; St John, E.; Jenison, E.; Niloff, J.M.; Lazarus, H.; Berkowitz, R.S.; Leavitt, T.; Griffiths, C.T.; Parker, L.; et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N. Engl. J. Med. 1983, 309, 883–887. [Google Scholar] [CrossRef]
- Hendriks, Y.M.; Wagner, A.; Morreau, H.; Menko, F.; Stormorken, A.; Quehenberger, F.; Sandkuijl, L.; Moller, P.; Genuardi, M.; Van Houwelingen, H.; et al. Cancer risk in hereditary nonpolyposis colorectal cancer due to MSH6 mutations: Impact on counseling and surveillance. Gastroenterology 2004, 127, 17–25. [Google Scholar] [CrossRef]
- Ramsoekh, D.; Wagner, A.; van Leerdam, M.E.; Dinjens, W.N.; Steyerberg, E.W.; Halley, D.J.; Kuipers, E.J.; Dooijes, D. A high incidence of MSH6 mutations in Amsterdam criteria II-negative families tested in a diagnostic setting. Gut 2008, 57, 1539–1544. [Google Scholar] [CrossRef]
- Rijcken, F.E.; Mourits, M.J.; Kleibeuker, J.H.; Hollema, H.; van der Zee, A.G. Gynecologic screening in hereditary nonpolyposis colorectal cancer. Gynecol. Oncol. 2003, 91, 74–80. [Google Scholar] [CrossRef]
- Helder-Woolderink, J.M.; De Bock, G.H.; Sijmons, R.H.; Hollema, H.; Mourits, M.J. The additional value of endometrial sampling in the early detection of endometrial cancer in women with Lynch syndrome. Gynecol. Oncol. 2013, 131, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, I.; Gentry-Maharaj, A.; Burnell, M.; Manchanda, R.; Singh, N.; Sharma, A.; Ryan, A.; Seif, M.W.; Amso, N.N.; Turner, G.; et al. Sensitivity of transvaginal ultrasound screening for endometrial cancer in postmenopausal women: A case-control study within the UKCTOCS cohort. Lancet Oncol. 2011, 12, 38–48. [Google Scholar] [CrossRef]
- Gerritzen, L.H.; Hoogerbrugge, N.; Oei, A.L.; Nagengast, F.M.; van Ham, M.A.; Massuger, L.F.; de Hullu, J.A. Improvement of endometrial biopsy over transvaginal ultrasound alone for endometrial surveillance in women with Lynch syndrome. Fam. Cancer 2009, 8, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Dove-Edwin, I.; Boks, D.; Goff, S.; Kenter, G.G.; Carpenter, R.; Vasen, H.F.; Thomas, H.J. The outcome of endometrial carcinoma surveillance by ultrasound scan in women at risk of hereditary nonpolyposis colorectal carcinoma and familial colorectal carcinoma. Cancer 2002, 94, 1708–1712. [Google Scholar] [CrossRef]
- Helder-Woolderink, J.; de Bock, G.; Hollema, H.; van Oven, M.; Mourits, M. Pain evaluation during gynaecological surveillance in women with Lynch syndrome. Fam. Cancer 2017, 16, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Ryan, N.; Nobes, M.; Sedgewick, D.; Teoh, S.N.; Evans, D.G.; Crosbie, E.J. A mismatch in care: Results of a United Kingdom-wide patient and clinician survey of gynaecological services for women with Lynch syndrome. BJOG 2020. [Google Scholar] [CrossRef]
- Yang, K.Y.; Caughey, A.B.; Little, S.E.; Cheung, M.K.; Chen, L.M. A cost-effectiveness analysis of prophylactic surgery versus gynecologic surveillance for women from hereditary non-polyposis colorectal cancer (HNPCC) Families. Fam. Cancer 2011, 10, 535–543. [Google Scholar] [CrossRef]
- Sun, C.C.; Meyer, L.A.; Daniels, M.S.; Bodurka, D.C.; Nebgen, D.R.; Burton-Chase, A.M.; Lu, K.H.; Peterson, S.K. Women’s preferences for cancer risk management strategies in Lynch syndrome. Gynecol. Oncol. 2019, 152, 514–521. [Google Scholar] [CrossRef]
- Lu, K.H.; Dinh, M.; Kohlmann, W.; Watson, P.; Green, J.; Syngal, S.; Bandipalliam, P.; Chen, L.M.; Allen, B.; Conrad, P.; et al. Gynecologic cancer as a “sentinel cancer” for women with hereditary nonpolyposis colorectal cancer syndrome. Obstet. Gynecol. 2005, 105, 569–574. [Google Scholar] [CrossRef]
- Niskakoski, A.; Pasanen, A.; Lassus, H.; Renkonen-Sinisalo, L.; Kaur, S.; Mecklin, J.P.; Butzow, R.; Peltomaki, P. Molecular changes preceding endometrial and ovarian cancer: A study of consecutive endometrial specimens from Lynch syndrome surveillance. Mod. Pathol. 2018, 31, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Dijkhuizen, F.P.H.L.J.; Mol, B.W.J.; Brölmann, H.A.M.; Heintz, A.P.M. The accuracy of endometrial sampling in the diagnosis of patients with endometrial carcinoma and hyperplasia. Cancer 2000, 89, 1765–1772. [Google Scholar] [CrossRef]
- Woolderink, J.M.; De Bock, G.H.; de Hullu, J.A.; Hollema, H.; Zweemer, R.P.; Slangen, B.F.M.; Gaarenstroom, K.N.; van Beurden, M.; van Doorn, H.C.; Sijmons, R.H.; et al. Characteristics of Lynch syndrome associated ovarian cancer. Gynecol. Oncol. 2018, 150, 324–330. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total | MLH1 | MSH2 | MSH6 | PMS2 | p-Value |
|---|---|---|---|---|---|---|
| All patients, N | 164 | 38 | 25 | 82 | 19 | |
| Of which diagnosed before 2016 | 139 | 33 | 23 | 70 | 13 | |
| Of which diagnosed since 2016 | 25 | 5 | 2 | 12 | 6 | |
| Age at first visit, before 2016, median (IQR, range) | 46.0 yr (IQR 37.9–53.6, range 21.5–75.0) | 44.5 yr (IQR 36.2–52.2, range 21.5–65.2) | 43.4 yr (IQR 34.7–50.8, range 26.8–54.6) | 48.2 yr (IQR 39.2–55.6, range 28.6–75.0) | 49.2 yr (IQR 34.9–56.5, range 26.3–70.9) | 0.143 |
| Age at first visit since 2016, median (IQR, range) | 53.8 yr (IQR 42.4–61.1, range 30.0–71.3) | 48.3 yr (IQR 37.1–51.1, range 30.0–61.1) | 57.4 yr (IQR 54.7–60.2, range 54.7–60.2) | 47.6 yr (IQR 42.3–63.0, range 38.5–71.3) | 58.4 yr (IQR 56.5–64.7, range 53.7–65.3) | 0.175 |
| Follow-up years | 685.4 yr | 237.7 yr | 146.2 yr | 238.3 yr | 63.2 yr | |
| Number of follow-up years, median (IQR) | 5.6 yr (IQR 3.0–9.0) | 6.3 yr (IQR 5.2–10.2) | 7.0 yr (IQR 4.5–10.4) | 4.2 yr (IQR 2.4–7.7) | 3.7 yr (IQR 2.0–7.6) | 0.009 |
| Number of visits Δ, median N (IQR) | 3 (IQR 2–6) | 4 (IQR 2–8) | 3 (IQR 2–6) | 3 (IQR 1–5) | 3 (IQR 1–5) | <0.001 |
| Visits with tests performed, N (% of total visits) | ||||||
| Transvaginal ultrasound | 522 (76.8%) | 184 (79.0%) | 79 (72.5%) | 207 (77.8%) | 52 (72.2%) | 0.431 |
| Ca125 | 276 (40.6%) | 128 (54.9%) | 18 (16.5%) | 117 (44.0%) | 13 (18.1%) | <0.001 |
| Endometrial sampling | 244 (35.9%) | 68 (29.2%) | 34 (31.2%) | 99 (37.2%) | 43 (59.7%) | <0.001 |
| Characteristics | Total | MLH1 | MSH2 | MSH6 | PMS2 | p-Value |
|---|---|---|---|---|---|---|
| All patients, N | 164 | 38 | 25 | 82 | 19 | |
| Patients with only one surveillance visit, N | 57 | 8 | 7 | 37 | 5 | 0.040 |
| Surgery after first visit, including for tumor | 30 | 3 | 2 | 21 ‡ | 4 | |
| Recent primary visit (<2 years) | 5 | 0 | 0 | 3 | 1 | |
| Aged >60 years (since 2016) | 2 | 1 | 0 | 1 | 0 | |
| Reason unknown | 20 | 4 | 5 | 12 | 0 | |
| Abnormalities at first surveillance visit, N ** | ||||||
| Ca125 > 35 kU/L | 2 | 1 | 0 | 1 | 0 | |
| Thickened Endometrium * | 5 | 2 | 0 | 3 | 0 | |
| Hyperplasia | 5 | 0 | 0 | 4 | 1 | |
| EC | 1 | 0 | 0 | 1 ‡ | 0 | |
| Patients with ≥2 surveillance visits, N (expected count ∆) | 107 (127) | 30 (34) | 18 (23) | 45 (57) | 14 (14) | |
| Interval between visits before 2016, median (IQR) | 1.0 (IQR 0.9–1.5) | 1.0 (IQR 0.8–1.3) | 1.1 (IQR 1.0–2.0) | 1.0 (IQR 0.6–1.4) | 1.1 (IQR 1.0–1.4) | 0.001 |
| Interval between visits since 2016, median (IQR) | 1.0 (IQR 1.0–1.2) | 1.0 (IQR 1.0–1.3) | 1.1 (IQR 1.0–1.2) | 1.0 (IQR 0.8–1.1) | 1.0 (IQR 1.0–1.1) | 0.277 |
| Subsequent surveillance visit <24 months before 2016, N of total (%) | 343/417 (82.3%) | 131/155 (84.5%) | 47/64 (73.43%) | 139/167 (83.2%) | 26/31 (83.9%) | 0.249 |
| Subsequent surveillance visit <15 months/all visits since 2016, N of total (%) | 80/96 (83.3%) | 26/37 (72.2%) | 11/14 (78.6%) | 24/26 (92.3%) | 18/19 (94.7%) | 0.091 |
| Abnormalities at ≥2 surveillance visit, N (% of carriers with ≥2 surveillance visits) ** | ||||||
| Ca125 > 35 kU/L | 8 (7.5%) | 2 (6.7%) | 1 (5.6%) | 2 (4.4%) | 1 (7.1%) | |
| Thickened Endometrium * | 10 (9.3%) | 0 | 2 (11.1%) | 6 (13.3%) | 2 (14.3%) | |
| Hyperplasia | 3 | 1 | 1 | 1 | 0 | |
| Tumor | 6 | 2 | 3 | 1 | 0 | |
| EC | 5 | 2 | 2 | 1 | 0 | |
| OC | 1 | 0 | 1 | 0 | 0 |
| Tumor (FIGO, Grade) or Hyperplasia | MMR Gene Involved | Age | Surveillance Visits Prior to Hyperplasia or Tumor, N | Years to Previous Visit | Tests Performed at Previous Surveillance Visit | Complaints Prior to Identification of Hyperplasia or Tumor |
|---|---|---|---|---|---|---|
| Endometrial hyperplasia | MSH6 | 46 | N.A. | N.A. | N.A. | Hypermenorrhea |
| Endometrial hyperplasia | MSH6 | 46 | N.A. | N.A. | N.A. | Unknown |
| Endometrial hyperplasia | MSH6 | 57 | N.A. | N.A. | N.A. | Unknown |
| Endometrial hyperplasia | MSH6 | 51 | N.A. | N.A. | N.A. | Unknown |
| Endometrial hyperplasia | PMS2 | 49 | N.A. | N.A. | N.A. | Irregular cycle |
| Endometrial hyperplasia | MSH6 | 52 | 5 | N.A. | N.A. | Hypermenorrhea, hyperplasia identified upon pathological examination after risk-reducing surgery, preoperative endometrial sampling could not rule out hyperplasia |
| Endometrial hyperplasia | MSH2 | 38 | 4 | 1.0 | Transvaginal ultrasound, CA125 (9 kU/L), endometrial sampling (outcome: no premalignancy) | No complaints |
| Endometrial hyperplasia | MLH1 | 48 | 3 | 1.1 | Transvaginal ultrasound | No complaints |
| EC (FIGO IA grade 1) | MSH6 | 64 | N.A. | N.A. | N.A. | No complaints |
| EC (FIGO IB, grade 1) | MSH6 | 58 | 1 | N.A. | N.A. | No complaints, EC identified upon pathological examination after risk-reducing surgery, preoperative normal endometrial sampling |
| EC (FIGO IA grade 1) | MLH1 | 54 | 4 | 1.1 | Transvaginal ultrasound, endometrial sampling (outcome: weakly proliferating endometrium) | No complaints, no anomalies at ultrasound, but endometrial sampling suggestive of pre-malignancy. |
| EC (FIGO I) | MLH1 | 52 | 1 | 0.5 | Transvaginal ultrasound | Complaints: irregular blood loss |
| EC (FIGO IA, grade 2) | MSH2 | 54 | 2 | 1.5 | Transvaginal ultrasound | Complaints: postmenopausal blood loss |
| OC (FIGO IV) | MSH2 | 48 | 1 | 1.0 | Transvaginal ultrasound unknown, no Ca125 or endometrial sampling | Complaints: intermenstrual blood loss, menorrhagia. |
| EC (FIGO IA, grade 1) | MSH2 | 37 | 2 | 1.5 | Transvaginal ultrasound, Ca125 (11 kU/L) | Complaints: blood loss after miscarriage |
| EC (FIGO IA grade 2) | MSH6 | 59 | 3 | 0.1 | Transvaginal ultrasound, Ca125 (outcome unknown), no endometrial sampling | Complaints: postmenopausal blood loss since last visit |
| Characteristics | Total | MLH1 | MSH2 | MSH6 | PMS2 | p-Value |
|---|---|---|---|---|---|---|
| Gynecological surgery ‡, N (% of total carriers) | 73 (45.5%) | 14 (36.8%) | 7 (32.0%) | 44 (53.7%) | 8 (42.1%) | 0.015 |
| Of which performed before 2016 | 54 | 7 | 6 | 35 | 6 | 0.004 |
| Of which performed since 2016 | 19 | 7 | 1 | 9 | 2 | 1.00 |
| Type of surgery | ||||||
| Hysterectomy | 11 | 3 | 3 | 5 | 0 | |
| Ovariectomy ∆ | 4 | 1 | 2 | 1 | 0 | |
| Hysterectomy with bilateral salpingo oophorectomy | 58 | 10 | 2 | 38 | 8 | |
| Age at surgery before 2016, median (IQR, range) | 51 years (IQR 45–54, range 20–72) | 52 years (IQR 42–53, range 35–69) | 45 years (IQR 38–46, range 38–48) | 51 years (IQR 46–56, range 20–72) | 53 years (IQR 50–61, range 35–62) | 0.076 |
| Age at surgery since 2016, median (IQR, range) | 51 years (IQR 47–56, range 42–65) | 49 years (IQR 47–51, range 46–56) | 60 years ∫ | 51 years (IQR 43–52, range 42–61) | 64 years (IQR 62–65, range 62–65) | 0.082 |
| Surveillance visits prior to surgery, median N (IQR) | 3 (IQR 1–5) | 4 (IQR 2–7) | 2 (IQR 1–4) | 3 (IQR 1–5) | 2 (IQR 1–3) | 0.002 |
| Reasons for surgery | 0.158 | |||||
| Risk-reducing surgery, N (% of total per gene) | 53 (71.6%) | 10 (71.4%) | 3 (37.5%) | 34 (77.3%) | 6 (75.0%) | |
| Surgery after abnormal surveillance visit, N (% of total per gene) | 13 (17.6%) | 1 (7.1%) | 2 (25.0%) | 8 (18.2%) | 2 (2.7%) | |
| Other (% of total per gene) | 3 (4.1%) | 2 (14.2%) | 1 (12.5%) | 0 | 0 | |
| Unknown (% per gene) | 5 (6.8%) | 1 (7.1%) | 2 (25.0%) | 2 (4.5%) | 0 | |
| Abnormalities † identified upon pathological assessment, N (% of total per gene) | 3 (4.1%) | 0 | 0 | 3 † (4.1%) | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eikenboom, E.L.; van Doorn, H.C.; Dinjens, W.N.M.; Dubbink, H.J.; Geurts-Giele, W.R.R.; Spaander, M.C.W.; Tops, C.M.J.; Wagner, A.; Goverde, A. Gynecological Surveillance and Surgery Outcomes in Dutch Lynch Syndrome Carriers. Cancers 2021, 13, 459. https://doi.org/10.3390/cancers13030459
Eikenboom EL, van Doorn HC, Dinjens WNM, Dubbink HJ, Geurts-Giele WRR, Spaander MCW, Tops CMJ, Wagner A, Goverde A. Gynecological Surveillance and Surgery Outcomes in Dutch Lynch Syndrome Carriers. Cancers. 2021; 13(3):459. https://doi.org/10.3390/cancers13030459
Chicago/Turabian StyleEikenboom, Ellis L., Helena C. van Doorn, Winand N. M. Dinjens, Hendrikus J. Dubbink, Willemina R. R. Geurts-Giele, Manon C. W. Spaander, Carli M. J. Tops, Anja Wagner, and Anne Goverde. 2021. "Gynecological Surveillance and Surgery Outcomes in Dutch Lynch Syndrome Carriers" Cancers 13, no. 3: 459. https://doi.org/10.3390/cancers13030459
APA StyleEikenboom, E. L., van Doorn, H. C., Dinjens, W. N. M., Dubbink, H. J., Geurts-Giele, W. R. R., Spaander, M. C. W., Tops, C. M. J., Wagner, A., & Goverde, A. (2021). Gynecological Surveillance and Surgery Outcomes in Dutch Lynch Syndrome Carriers. Cancers, 13(3), 459. https://doi.org/10.3390/cancers13030459






