Single-Fraction Adjuvant Electronic Brachytherapy after Resection of Conjunctival Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patient’s Features
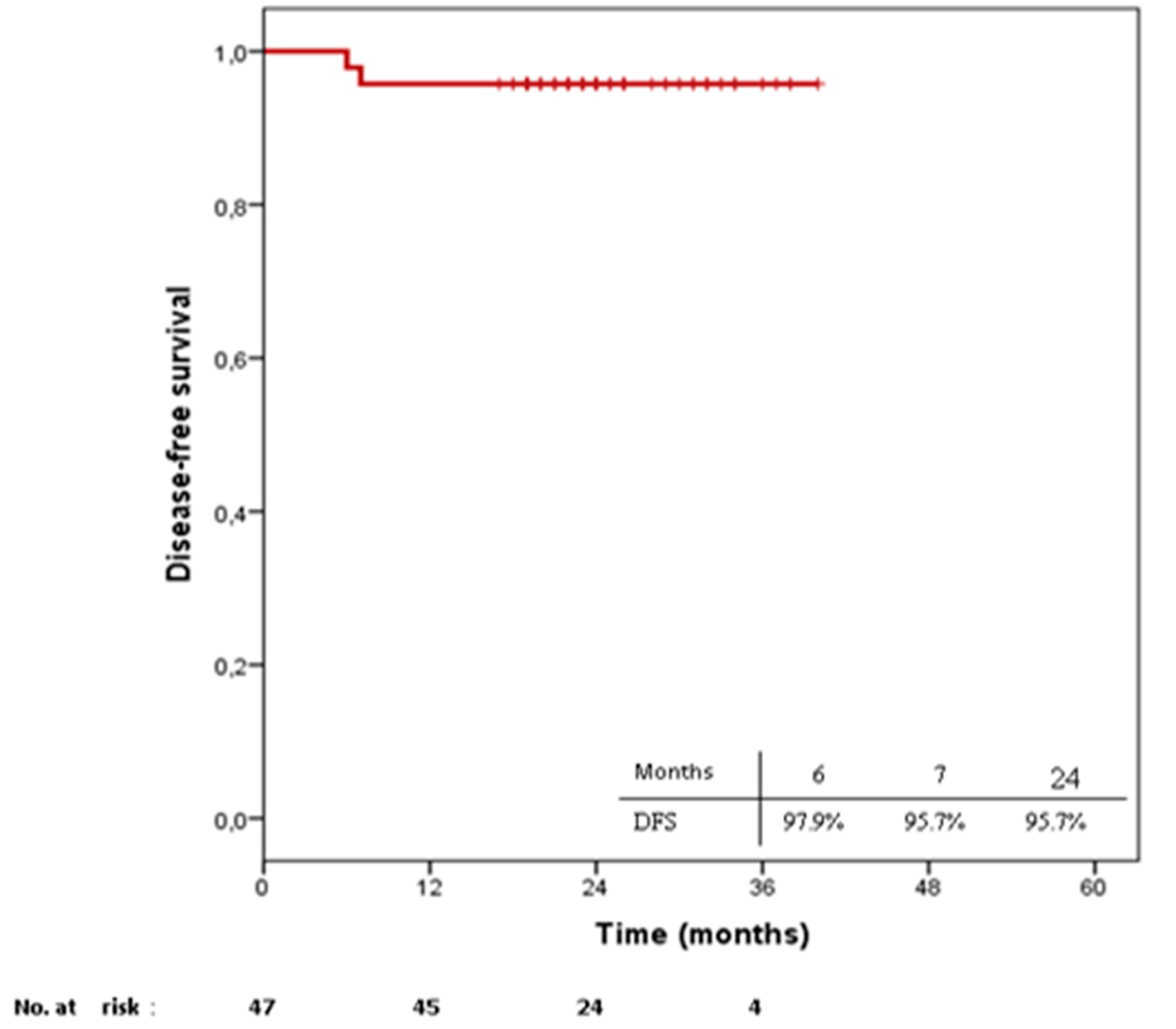
2.2. Treatment Outcomes
3. Discussion
4. Materials and Methods
4.1. Collective
4.2. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, C.V.; Wolf, A.; Klingenstein, A.; Decker, C.; Garip, A.; Kampik, A.; Hintschich, C. Clinical outcome of advanced squamous cell carcinoma of the conjunctiva. Eye (Lond.) 2014, 28, 962–967. [Google Scholar] [CrossRef]
- Shields, C.L.; Alset, A.E.; Boal, N.S.; Casey, M.G.; Knapp, A.N.; Sugarman, J.A.; Schoen, M.A.; Gordon, P.S.; Douglass, A.M.; Sioufi, K.; et al. Conjunctival Tumors in 5002 Cases. Comparative Analysis of Benign Versus Malignant Counterparts. The 2016 James, D. Allen Lecture. Am. J. Ophthalmol. 2017, 173, 106–133. [Google Scholar] [CrossRef] [PubMed]
- Newton, R.; Ziegler, J.; Ateenyi-Agaba, C.; Bousarghin, L.; Casabonne, D.; Beral, V.; Mbidde, E.; Carpenter, L.; Reeves, G.; Parkin, D.M.; et al. The epidemiology of conjunctival squamous cell carcinoma in Uganda. Br. J. Cancer 2002, 87, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Gichuhi, S.; Sagoo, M.S.; Weiss, H.A.; Burton, M.J. Epidemiology of ocular surface squamous neoplasia in Africa. Trop. Med. Int. Health 2013, 18, 1424–1443. [Google Scholar] [CrossRef] [PubMed]
- Sun, E.C.; Fears, T.R.; Goedert, J.J. Epidemiology of squamous cell conjunctival cancer. Cancer Epidemiol. Biomark. Prev. 1997, 6, 73–77. [Google Scholar]
- Guech-Ongey, M.; Engels, E.A.; Goedert, J.J.; Biggar, R.J.; Mbulaiteye, S.M. Elevated risk for squamous cell carcinoma of the conjunctiva among adults with AIDS in the United States. Int. J. Cancer 2008, 122, 2590–2593. [Google Scholar] [CrossRef]
- Yousef, Y.A.; Finger, P.T. Squamous carcinoma and dysplasia of the conjunctiva and cornea: An analysis of 101 cases. Ophthalmology 2012, 119, 233–240. [Google Scholar] [CrossRef]
- Cervantes, G.; Rodríguez Abelardo, A.; Gómez Leal, A. Squamous cell carcinoma of the conjunctiva: Clinicopathological features in 287 cases. Can. J. Ophthalmol. 2002, 37, 14–20. [Google Scholar] [CrossRef]
- Kiire, C.A.; Dhillon, B. The aetiology and associations of conjunctival intraepithelial neoplasia. Br. J. Ophthalmol. 2006, 90, 109–113. [Google Scholar] [CrossRef]
- Tabin, G.; Levin Samantha, S.G.; Loughnan, M.; Taylor, H. Late Recurrences and the Necessity for Long-term Follow-up in Corneal and Conjunctival Intraepithelial Neoplasia. Ophthalmology 1997, 104, 485–492. [Google Scholar] [CrossRef]
- Shields, C.L.; Shields, J.A. Tumors of the conjunctiva and cornea. Surv. Ophthalmol. 2004, 49, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.A.; Shields, C.L.; De Potter, P. Surgical management of conjunctival tumors. The 1994 Lynn B. McMahan Lecture. Arch. Ophthalmol. 1997, 115, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Lee Graham, A.; Hirst Lawrence, W. Ocular surface squamous neoplasia. Surv. Ophthalmol. 1995, 39, 429–450. [Google Scholar]
- Galor, A.; Karp, C.L.; Oellers, P.; Kao, A.A.; Abdelaziz, A.; Feuer, W.; Dubovy, S.R. Predictors of ocular surface squamous neoplasia recurrence after excisional surgery. Ophthalmology 2012, 119, 1974–1981. [Google Scholar] [CrossRef] [PubMed]
- Cicinelli, M.V.; Marchese, A.; Bandello, F.; Modorati, G. Clinical Management of Ocular Surface Squamous Neoplasia: A Review of the Current Evidence. Ophthalmol. Ther. 2018, 7, 247–262. [Google Scholar] [CrossRef]
- Gichuhi, S.; Macharia, E.; Kabiru, J.; Zindamoyen, A.M.; Rono, H.; Ollando, E.; Wachira, J.; Munene, R.; Maina, J.; Onyuma, T.; et al. Topical fluorouracil after surgery for ocular surface squamous neoplasia in Kenya: A randomised, double-blind, placebo-controlled trial. Lancet Global Health 2016, 4, e378–e85. [Google Scholar] [CrossRef]
- Sturges, A.; Butt, A.L.; Lai, J.E.; Chodosh, J. Topical interferon or surgical excision for the management of primary ocular surface squamous neoplasia. Ophthalmology 2008, 115, 1297–1302.e1. [Google Scholar] [CrossRef]
- Buuns Delyse, R.; Tse David, T.; Folberg, R. Microscopically Controlled Excision of Conjunctival Squamous Cell Carcinoma. Am. J. Ophthalmol. 1994, 117, 97–102. [Google Scholar] [CrossRef]
- Al Bayyat, G.; Arreaza-Kaufman, D.; Venkateswaran, N.; Galor, A.; Karp, C.L. Update on pharmacotherapy for ocular surface squamous neoplasia. Eye Vis. (Lond.) 2019, 6, 24. [Google Scholar] [CrossRef]
- Arepalli, S.; Kaliki, S.; Shields, C.L.; Emrich, J.; Komarnicky, L.; Shields, J.A. Plaque radiotherapy in the management of scleral-invasive conjunctival squamous cell carcinoma: An analysis of 15 eyes. JAMA Ophthalmol. 2014, 132, 691–696. [Google Scholar] [CrossRef]
- Schneider, F.; Clausen, S.; Tholking, J.; Wenz, F.; Abo-Madyan, Y. A novel approach for superficial intraoperative radiotherapy (IORT) using a 50 kV X-ray source: A technical and case report. J. Appl. Clin. Med. Phys. 2014, 15, 4502. [Google Scholar] [CrossRef] [PubMed]
- Sethi, A.; Emami, B.; Small, W., Jr.; Thomas, T.O. Intraoperative Radiotherapy With INTRABEAM: Technical and Dosimetric Considerations. Front. Oncol. 2018, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Sarria, G.R.; Sarria, G.J.; Rivera, P.F.; Zaharia, M.; Serpa, S.; Buitrago, M. Phase I/II study on kilovoltage surface brachytherapy in conjunctival cancer: Preliminary results. Ecancermedicalscience 2018, 12, 835. [Google Scholar] [CrossRef] [PubMed]
- Sarria, G.J.; Sarria, G.R.; Pinillos, L.V. The Inca Trail to the Present: The Development of Radiation Therapy in Peru. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Giordano, F.A.; Brehmer, S.; Murle, B.; Welzel, G.; Sperk, E.; Keller, A.; Abo-Madyan, Y.; Scherzinger, E.; Clausen, S.; Schneider, F.; et al. Intraoperative Radiotherapy in Newly Diagnosed Glioblastoma (INTRAGO): An Open-Label, Dose-Escalation Phase I/II Trial. Neurosurgery 2019, 84, 41–49. [Google Scholar] [CrossRef]
- Sarria, G.R.; Petrova, V.; Wenz, F.; Abo-Madyan, Y.; Sperk, E.; Giordano, F.A. Intraoperative radiotherapy with low energy x-rays for primary and recurrent soft-tissue sarcomas. Radiat. Oncol. 2020, 15, 110. [Google Scholar] [CrossRef]
- Vaidya, J.S.; Bulsara, M.; Saunders, C.; Flyger, H.; Tobias, J.S.; Corica, T.; Massarut, S.; Wenz, F.; Pigorsch, S.; Alvarado, M.; et al. Effect of Delayed Targeted Intraoperative Radiotherapy vs Whole-Breast Radiotherapy on Local Recurrence and Survival: Long-term Results From the TARGIT-A Randomized Clinical Trial in Early Breast Cancer. JAMA Oncol. 2020, 6, e200249. [Google Scholar] [CrossRef]
- Potemin, S.; Kubler, J.; Uvarov, I.; Wenz, F.; Giordano, F. Intraoperative radiotherapy as an immediate adjuvant treatment of rectal cancer due to limited access to external-beam radiotherapy. Radiat. Oncol. 2020, 15, 11. [Google Scholar] [CrossRef]
- Viani, G.A.; Fendi, L.I. Adjuvant treatment or primary topical monotherapy for ocular surface squamous neoplasia: A systematic review. Arq. Bras. Oftalmol. 2017, 80, 131–136. [Google Scholar] [CrossRef]
- Singh, S.; Mohamed, A.; Kaliki, S. Ocular surface squamous neoplasia: Analysis based on the 8th American Joint Committee on Cancer classification. Int. Ophthalmol. 2019, 39, 1283–1291. [Google Scholar] [CrossRef]
- Shields, C.L.; Chien, J.L.; Surakiatchanukul, T.; Sioufi, K.; Lally, S.E.; Shields, J.A. Conjunctival Tumors: Review of Clinical Features, Risks, Biomarkers, and Outcomes—The 2017 J. Donald M. Gass Lecture. Asia Pac. J. Ophthalmol. (Phila) 2017, 6, 109–120. [Google Scholar]
- Peksayar, G.; Altan-Yaycioglu, R.; Onal, S. Excision and cryosurgery in the treatment of conjunctival malignant epithelial tumours. Eye (Lond.) 2003, 17, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Kaliki, S.; Kim, H.J.; Al-Dahmash, S.; Shah, S.U.; Lally, S.E.; Shields, J.A. Interferon for ocular surface squamous neoplasia in 81 cases: Outcomes based on the American Joint Committee on Cancer classification. Cornea 2013, 32, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Ip, M.H.; Robert George, C.R.; Naing, Z.; Perlman, E.M.; Rawlinson, W.; Coroneo, M.T. Topical Cidofovir for Treatment-Refractory Ocular Surface Squamous Neoplasia. Ophthalmology 2018, 125, 617–619. [Google Scholar] [CrossRef] [PubMed]
- Birkholz, E.S.; Goins, K.M.; Sutphin, J.E.; Kitzmann, A.S.; Wagoner, M.D. Treatment of ocular surface squamous cell intraepithelial neoplasia with and without mitomycin C. Cornea 2011, 30, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Nanji, A.A.; Moon, C.S.; Galor, A.; Sein, J.; Oellers, P.; Karp, C.L. Surgical versus medical treatment of ocular surface squamous neoplasia: A comparison of recurrences and complications. Ophthalmology 2014, 121, 994–1000. [Google Scholar] [CrossRef]
- Sayed-Ahmed, I.O.; Palioura, S.; Galor, A.; Karp, C.L. Diagnosis and Medical Management of Ocular Surface Squamous Neoplasia. Expert. Rev. Ophthalmol. 2017, 12, 11–19. [Google Scholar] [CrossRef]
- Venkateswaran, N.; Mercado, C.; Galor, A.; Karp, C.L. Comparison of Topical 5-Fluorouracil and Interferon Alfa-2b as Primary Treatment Modalities for Ocular Surface Squamous Neoplasia. Am. J. Ophthalmol. 2019, 199, 216–222. [Google Scholar] [CrossRef]
- Arnaud, P. Les différents interférons: Pharmacologie, mécanismes d’action, tolérance et effets secondaires. Rev. Med. Interne 2002, 23, 449S–458S. [Google Scholar] [CrossRef]
- Parrozzani, R.; Frizziero, L.; Trainiti, S.; Testi, I.; Miglionico, G.; Pilotto, E.; Blandamura, S.; Fassina, A.; Midena, E. Topical 1% 5-fluoruracil as a sole treatment of corneoconjunctival ocular surface squamous neoplasia: Long-term study. Br. J. Ophthalmol. 2017, 101, 1094–1099. [Google Scholar] [CrossRef]
- Galor, A.; Karp, C.L.; Chhabra, S.; Barnes, S.; Alfonso, E.C. Topical interferon alpha 2b eye-drops for treatment of ocular surface squamous neoplasia: A dose comparison study. Br. J. Ophthalmol. 2010, 94, 551–554. [Google Scholar] [CrossRef]
- Graue, G.F.; Tena, L.B.; Finger, P.T. Electron beam radiation for conjunctival squamous carcinoma. Ophthalmic. Plast. Reconstr. Surg. 2011, 27, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Jeganathan, V.S.; Wirth, A.; MacManus, M.P. Ocular risks from orbital and periorbital radiation therapy: A critical review. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 650–659. [Google Scholar] [CrossRef]
- Espensen, C.A.; Appelt, A.L.; Fog, L.S.; Gothelf, A.B.; Thariat, J.; Kiilgaard, J.F. Predicting Visual Acuity Deterioration and Radiation-Induced Toxicities after Brachytherapy for Choroidal Melanomas. Cancers 2019, 11, 1124. [Google Scholar] [CrossRef] [PubMed]
- Santoni, A.; Thariat, J.; Maschi, C.; Herault, J.; Baillif, S.; Lassalle, S.; Peyrichon, M.L.; Salleron, J.; Caujolle, J.P. Management of Invasive Squamous Cell Carcinomas of the Conjunctiva. Am. J. Ophthalmol. 2019, 200, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lecuona, K.; Stannard, C.; Hart, G.; Rice, J.; Cook, C.; Wetter, J.; Duffield, M. The treatment of carcinoma in situ and squamous cell carcinoma of the conjunctiva with fractionated strontium-90 radiation in a population with a high prevalence of HIV. Br. J. Ophthalmol. 2015, 99, 1158–1161. [Google Scholar] [CrossRef]
- Busoni, S.; Fedeli, L.; Belli, G.; Genovese, E.; Cannata, V.; Gori, C.; Rossi, F. Pre and post operative radiation protection in Ru-106 brachytherapy ophthalmic plaque surgery and related material shielding properties. Phys. Med. 2019, 57, 245–250. [Google Scholar] [CrossRef]
- Classic, K.L.; Furutani, K.M.; Stafford, S.L.; Pulido, J.S. Radiation dose to the surgeon during plaque brachytherapy. Retina 2012, 32, 1900–1905. [Google Scholar] [CrossRef]
- Van Ginderdeuren, R.; van Limbergen, E.; Spileers, W. 18 years’ experience with high dose rate strontium-90 brachytherapy of small to medium sized posterior uveal melanoma. Br. J. Ophthalmol. 2005, 89, 1306–1310. [Google Scholar] [CrossRef]
- Barbosa, N.A.; da Rosa LA, R.; Facure, A.; Braz, D. Brachytherapy treatment simulation of strontium-90 and ruthenium-106 plaques on small size posterior uveal melanoma using MCNPX code. Radiat. Phys. Chem. 2014, 95, 224–226. [Google Scholar] [CrossRef]
- Kearsley John, H.; Fitchew Robert, S.; Taylor Richard, G.S. Adjunctive radiotherapy with strontium-90 in the treatment of conjunctival squamous cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 1988, 14, 435–443. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events Version 4.03. Available online: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf (accessed on 30 October 2019).
- Muller, K.; Nowak, P.J.; de Pan, C.; Marijnissen, J.P.; Paridaens, D.A.; Levendag, P.; Luyten, G.P. Effectiveness of fractionated stereotactic radiotherapy for uveal melanoma. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Muller, K.; Naus, N.; Nowak, P.J.; Schmitz, P.I.; de Pan, C.; van Santen, C.A.; Marijnissen, J.P.; Paridaens, D.A.; Levendag, P.C.; Luyten, G.P. Fractionated stereotactic radiotherapy for uveal melanoma, late clinical results. Radiother. Oncol. 2012, 102, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Akbaba, S.; Foerster, R.; Nicolay, N.H.; Arians, N.; Bostel, T.; Debus, J.; Hauswald, H. Linear accelerator-based stereotactic fractionated photon radiotherapy as an eye-conserving treatment for uveal melanoma. Radiat. Oncol. 2018, 13, 140. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; Wiley Blackwell: Oxford, UK, 2017. [Google Scholar]
- Liu, Q.; Schneider, F.; Ma, L.; Wenz, F.; Herskind, C. Relative Biologic Effectiveness (RBE) of 50 kV X-rays measured in a phantom for intraoperative tumor-bed irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 1127–1133. [Google Scholar] [CrossRef]

| Toxicity Profiles (%) | |||
|---|---|---|---|
| G0 | G2 | ||
| n = 35 | n = 12 | p | |
| Age, years | |||
| Median (Range) | 69 (29–87) | 72 (37–85) | 0.855 |
| T | |||
| T1 | 15 (42.9) | 4 (33.3) | 1.000 |
| T2 | 20 (57.1) | 7 (58.3) | † 1.000 |
| T3 | 0 (0.0) | 1 (8.3) | |
| Size, cm | |||
| Mean (Range) | 6 (2–20) | 8.5 (1.5–20) | 0.345 |
| Weeks until application | |||
| Median (Range) | 10 (0–37) | 8 (4–11) | 0.354 |
| Doses, Gy | |||
| 18 | 22 (62.9) | 4 (33.3) | 1.000 |
| 20 | 6 (17.1) | 3 (25.0) | † 1.000 |
| 22 | 7 (20.0) | 5 (41.7) | |
| Applicator size | |||
| 1 cm | 32 (91.4) | 11 (91.7) | † 1.000 |
| 2 cm | 3 (8.6) | 1 (8.3) | |
| Doses Profile | ||
|---|---|---|
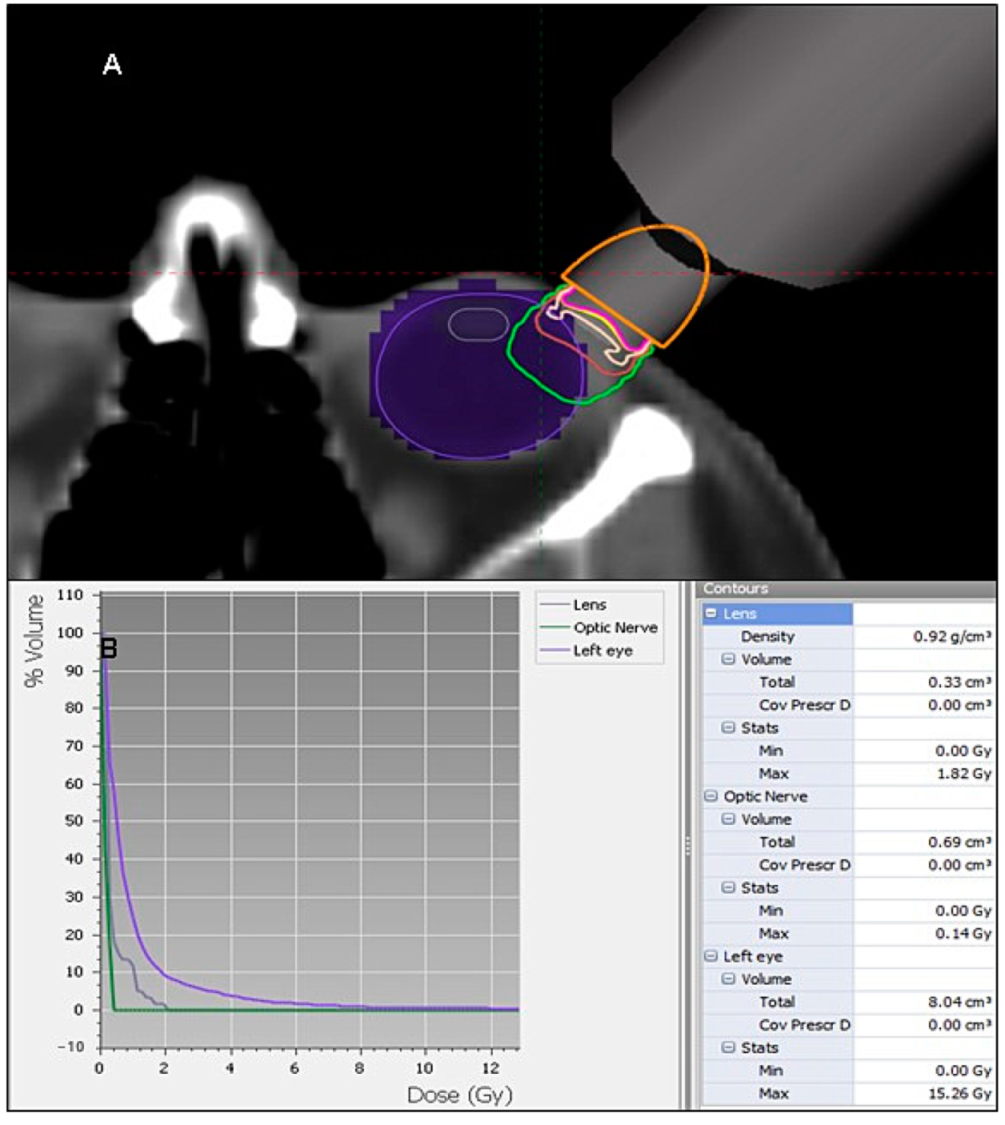
| Structure | Max. Dose (Gy) | Volume (mL) |
| Lens | 1.82 | 0.33 |
| Optic nerve | 0.14 | 0.69 |
| Retina | 5.52 | 2.75 |
| Lacrimal gland | 0.27 | 2.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarria, G.R.; Serpa, S.; Buitrago, M.; Fuentes Rivera, P.; Ramirez, D.; Giordano, F.A.; Sarria, G.J. Single-Fraction Adjuvant Electronic Brachytherapy after Resection of Conjunctival Carcinoma. Cancers 2021, 13, 454. https://doi.org/10.3390/cancers13030454
Sarria GR, Serpa S, Buitrago M, Fuentes Rivera P, Ramirez D, Giordano FA, Sarria GJ. Single-Fraction Adjuvant Electronic Brachytherapy after Resection of Conjunctival Carcinoma. Cancers. 2021; 13(3):454. https://doi.org/10.3390/cancers13030454
Chicago/Turabian StyleSarria, Gustavo R., Solon Serpa, Mario Buitrago, Paola Fuentes Rivera, Diego Ramirez, Frank A. Giordano, and Gustavo J. Sarria. 2021. "Single-Fraction Adjuvant Electronic Brachytherapy after Resection of Conjunctival Carcinoma" Cancers 13, no. 3: 454. https://doi.org/10.3390/cancers13030454
APA StyleSarria, G. R., Serpa, S., Buitrago, M., Fuentes Rivera, P., Ramirez, D., Giordano, F. A., & Sarria, G. J. (2021). Single-Fraction Adjuvant Electronic Brachytherapy after Resection of Conjunctival Carcinoma. Cancers, 13(3), 454. https://doi.org/10.3390/cancers13030454





