SALL Proteins; Common and Antagonistic Roles in Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Essential Roles of SALL Genes during Development
3. Common Cellular Functions and Targets of the SALL Proteins in Cancer
3.1. Cell Proliferation
3.2. Apoptosis and Cell Survival
3.3. Cell Migration and Invasion
3.4. Stemmess
4. Common Regulatory Mechanisms for SALL Proteins in Cancer
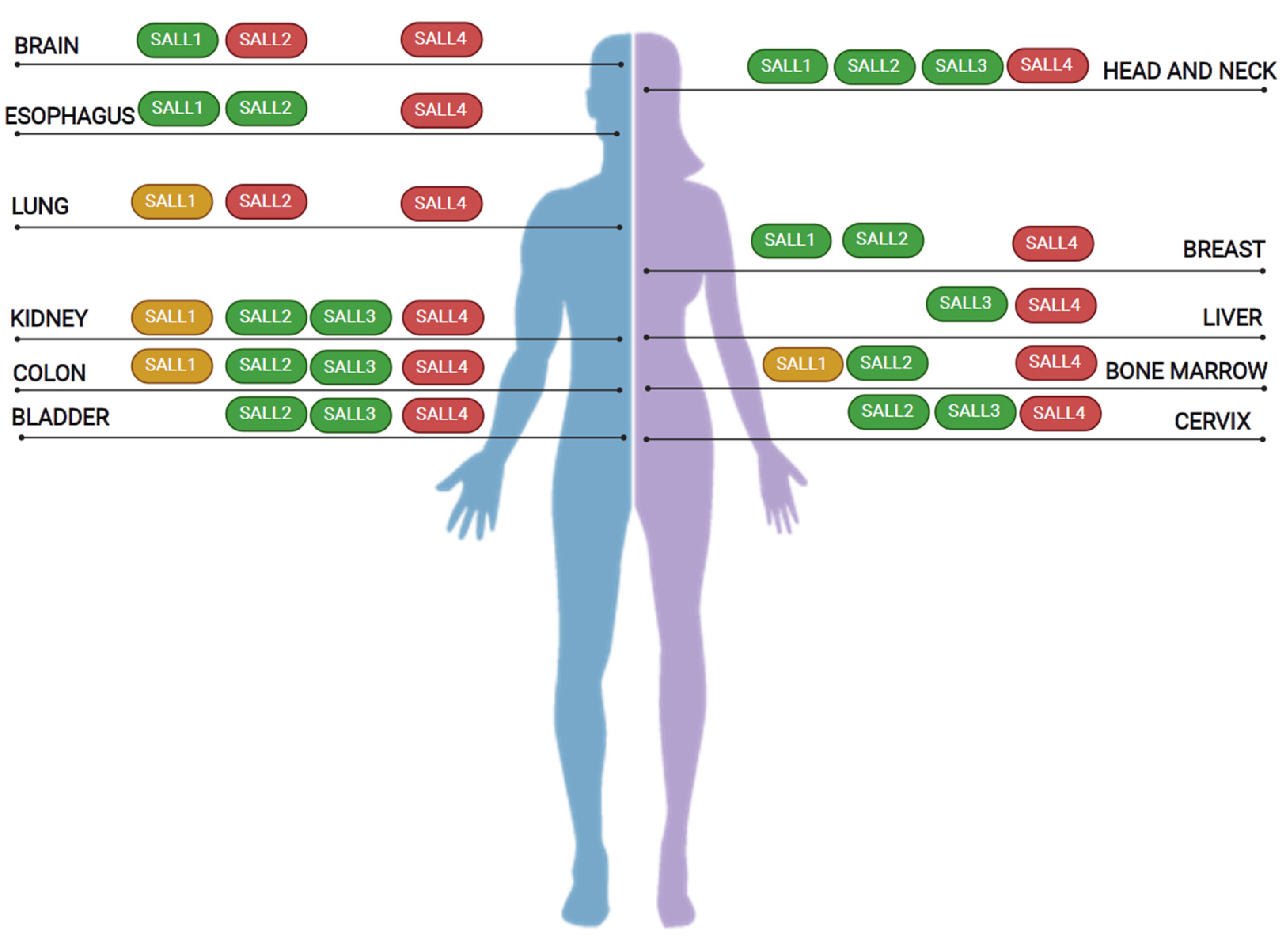
5. SALL Proteins in Cancer
5.1. Breast Cancer
5.2. Brain Tumors
5.3. Blood Cancers
5.4. Colorectal Cancer
5.5. Hepatocellular Carcinoma
5.6. HNSCC and Cervical Cancer
6. Targeting SALLs for Cancer Therapy
7. Concluding Remarks/Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- De Celis, J.F.; Barrio, R. Regulation and function of Spalt proteins during animal development. Int. J. Dev. Biol. 2009, 53, 1385–1398. [Google Scholar] [CrossRef]
- Sweetman, D.; Munsterberg, A. The vertebrate spalt genes in development and disease. Dev. Biol. 2006, 293, 285–293. [Google Scholar] [CrossRef]
- Lorente-Sorolla, J.; Truchado-Garcia, M.; Perry, K.J.; Henry, J.Q.; Grande, C. Molecular, phylogenetic and developmental analyses of Sall proteins in bilaterians. EvoDevo 2018, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Hermosilla, V.; Hepp, M.I.; Escobar, D.; Farkas, C.; Riffo, E.N.; Castro, A.F.; Pincheira, R. Developmental SALL2 transcription factor: A new player in cancer. Carcinogenesis 2017, 38, 680–690. [Google Scholar] [CrossRef]
- Lauberth, S.; Rauchman, M. A Conserved 12-Amino Acid Motif in Sall1 Recruits the Nucleosome Remodeling and Deacetylase Corepressor Complex. J. Biol. Chem. 2006, 281, 23922–23931. [Google Scholar] [CrossRef]
- Farkas, C.; Quiroz, A.; Alvarez, C.; Hermosilla, V.; Aylwin, C.F.; Lomniczi, A.; Castro, A.F.; Hepp, M.I.; Pincheira, R. Characterization of SALL2 Gene Isoforms and Targets Across Cell Types Reveals Highly Conserved Networks. Front. Genet. 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Kohlhase, J.; Schuh, R.; Dowe, G.; Kühnlein, R.P.; Jäckle, H.; Schroeder, B.; Schulz-Schaeffer, W.; Kretzschmar, H.A.; Köhler, A.; Müller, U.; et al. Isolation, Characterization, and Organ-Specific Expression of Two Novel Human Zinc Finger Genes Related to theDrosophilaGenespalt. Genomics 1996, 38, 291–298. [Google Scholar] [CrossRef]
- Ma, Y.; Cui, W.; Yang, J.; Qu, J.; Di, C.; Amin, H.M.; Lai, R.; Ritz, J.; Krause, D.S.; Chai, L. SALL4, a novel oncogene, is constitutively expressed in human acute myeloid leukemia (AML) and induces AML in transgenic mice. Blood 2006, 108, 2726–2735. [Google Scholar] [CrossRef]
- Salman, H.; Shuai, X.; Nguyen-Lefebvre, A.T.; Giri, B.; Ren, M.; Rauchman, M.; Robbins, L.; Hou, W.; Korkaya, H.; Ma, Y. SALL1 expression in acute myeloid leukemia. Oncotarget 2018, 9, 7442–7452. [Google Scholar] [CrossRef]
- Böhm, J.; Sustmann, C.; Wilhelm, C.; Kohlhase, J. SALL4 is directly activated by TCF/LEF in the canonical Wnt signaling pathway. Biochem. Biophys. Res. Commun. 2006, 348, 898–907. [Google Scholar] [CrossRef]
- Nicolè, L.; Sanavia, T.; Veronese, N.; Cappellesso, R.; Luchini, C.; Dabrilli, P.; Fassina, A. Oncofetal gene SALL4 and prognosis in cancer: A systematic review with meta-analysis. Oncotarget 2017, 8, 22968–22979. [Google Scholar] [CrossRef]
- Tatetsu, H.; Kong, N.R.; Chong, G.; Amabile, G.; Tenen, D.G.; Chai, L. SALL4, the missing link between stem cells, development and cancer. Gene 2016, 584, 111–119. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, X.; Zhu, W.; Qian, H.; Xu, W. SALL4: An emerging cancer biomarker and target. Cancer Lett. 2015, 357, 55–62. [Google Scholar] [CrossRef]
- Sung, C.K.; Yim, H. Roles of SALL2 in tumorigenesis. Arch. Pharmacal Res. 2016, 40, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Sung, C.K.; Yim, H. The tumor suppressor protein p150Sal2 in carcinogenesis. Tumor Biol. 2015, 36, 489–494. [Google Scholar] [CrossRef]
- Kawakami, Y.; Uchiyama, Y.; Esteban, C.R.; Inenaga, T.; Koyano-Nakagawa, N.; Kawakami, H.; Marti, M.; Kmita, M.; Monaghan-Nichols, P.; Nishinakamura, R.; et al. Sall genes regulate region-specific morphogenesis in the mouse limb by modulating Hox activities. Development 2009, 136, 585–594. [Google Scholar] [CrossRef]
- Kohlhase, J.; Heinrich, M.; Schubert, L.; Liebers, M.; Kispert, A.; Laccone, F.; Turnpenny, P.; Winter, R.M.; Reardon, W. Okihiro syndrome is caused by SALL4 mutations. Hum. Mol. Genet. 2002, 11, 2979–2987. [Google Scholar] [CrossRef]
- Kelberman, D.; Islam, L.; Lakowski, J.; Bacchelli, C.; Chanudet, E.; Lescai, F.; Patel, A.; Stupka, E.; Buck, A.; Wolf, S.; et al. Mutation of SALL2 causes recessive ocular coloboma in humans and mice. Hum. Mol. Genet. 2014, 23, 2511–2526. [Google Scholar] [CrossRef]
- Dostal, A.; Nemeckova, J.; Gaillyova, R. The 18q deletion syndrome and analysis of the critical region for orofacial cleft at 18q22.3. J. Cranio-Maxillofac. Surg. 2009, 37, 272–275. [Google Scholar] [CrossRef]
- Kiefer, S.M.; Ohlemiller, K.K.; Yang, J.; McDill, B.W.; Kohlhase, J.; Rauchman, M. Expression of a truncated Sall1 transcriptional repressor is responsible for Townes-Brocks syndrome birth defects. Hum. Mol. Genet. 2003, 12, 2221–2227. [Google Scholar] [CrossRef]
- Parrish, M.; Ott, T.; Lance-Jones, C.; Schuetz, G.; Schwaeger-Nickolenko, A.; Monaghan, A.P. Loss of the Sall3 Gene Leads to Palate Deficiency, Abnormalities in Cranial Nerves, and Perinatal Lethality. Mol. Cell. Biol. 2004, 24, 7102–7112. [Google Scholar] [CrossRef][Green Version]
- Warren, M.; Wang, W.; Spiden, S.; Chen-Murchie, D.; Tannahill, D.; Steel, K.P.; Bradley, A. ASall4 mutant mouse model useful for studying the role ofSall4 in early embryonic development and organogenesis. Genes 2007, 45, 51–58. [Google Scholar] [CrossRef]
- Sato, A.; Matsumoto, Y.; Koide, U.; Kataoka, Y.; Yoshida, N.; Yokota, T.; Asashima, M.; Nishinakamura, R. Zinc Finger Protein Sall2 Is Not Essential for Embryonic and Kidney Development. Mol. Cell. Biol. 2003, 23, 62–69. [Google Scholar] [CrossRef]
- Böhm, J.; Buck, A.; Borozdin, W.; Mannan, A.U.; Matysiak-Scholze, U.; Adham, I.; Schulz-Schaeffer, W.; Floss, T.; Wurst, W.; Kohlhase, J.; et al. Sall1, Sall2, and Sall4 Are Required for Neural Tube Closure in Mice. Am. J. Pathol. 2008, 173, 1455–1463. [Google Scholar] [CrossRef]
- Chai, L. The role of HSAL (SALL) genes in proliferation and differentiation in normal hematopoiesis and leukemogenesis. Transfusion 2011, 51, 87S–93S. [Google Scholar] [CrossRef]
- Hermosilla, V.; Salgado, G.; Riffo, E.; Escobar, D.; Hepp, M.; Farkas, C.; Galindo, M.; Morín, V.; García-Robles, M.A.; Castro, A.F.; et al. SALL2 represses cyclins D1 and E1 expression and restrains G1/S cell cycle transition and cancer-related phenotypes. Mol. Oncol. 2018, 12, 1026–1046. [Google Scholar] [CrossRef]
- Basta, J.M.; Singh, A.P.; Robbins, L.; Stout, L.; Pherson, M.; Rauchman, M. The core SWI/SNF catalytic subunit Brg1 regulates nephron progenitor cell proliferation and differentiation. Dev. Biol. 2020, 464, 176–187. [Google Scholar] [CrossRef]
- Basta, J.M.; Robbins, L.; Denner, D.R.; Kolar, G.R.; Rauchman, M. Sall1-NuRD interaction regulates multipotent nephron progenitors and is required for loop of Henle formation. Development 2017, 144, 3080–3094. [Google Scholar] [CrossRef]
- Basta, J.; Rauchman, M. The Nucleosome Remodeling and Deacetylase (NuRD) Complex in Development and Disease. Transl. Res. 2015, 165, 36–47. [Google Scholar] [CrossRef]
- Tahara, N.; Kawakami, H.; Chen, K.Q.; Anderson, A.; Peterson, M.Y.; Gong, W.; Shah, P.; Hayashi, S.; Nishinakamura, R.; Nakagawa, Y.; et al. Sall4 regulates neuromesodermal progenitors and their descendants during body elongation in mouse embryos. Development 2019, 146, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.Y.; Wade, P.A. Cancer biology and NuRD: A multifaceted chromatin remodelling complex. Nat. Rev. Cancer 2011, 11, 588–596. [Google Scholar] [CrossRef]
- Lu, J.; Jeong, H.; Kong, N.; Yang, Y.; Carroll, J.; Luo, H.R.; Silberstein, L.E.; Ma, Y.; Chai, L. Stem Cell Factor SALL4 Represses the Transcriptions of PTEN and SALL1 through an Epigenetic Repressor Complex. PLoS ONE 2009, 4, e5577. [Google Scholar] [CrossRef]
- Chan, A.-L.; La, H.M.; Legrand, J.; Mäkelä, J.-A.; Eichenlaub, M.; De Seram, M.; Ramialison, M.; Hobbs, R.M. Germline Stem Cell Activity Is Sustained by SALL4-Dependent Silencing of Distinct Tumor Suppressor Genes. Stem Cell Rep. 2017, 9, 956–971. [Google Scholar] [CrossRef]
- Miller, A.; Ralser, M.; Kloet, S.; Loos, R.; Nishinakamura, R.; Bertone, P.; Vermeulen, M.; Hendrich, B. Sall4 controls differentiation of pluripotent cells independently of the Nucleosome Remodelling and Deacetylation (NuRD) complex. Development 2016, 143, 3074–3084. [Google Scholar] [CrossRef]
- Lauberth, S.M.; Bilyeu, A.C.; Firulli, B.A.; Kroll, K.L.; Rauchman, M. A Phosphomimetic Mutation in the Sall1 Repression Motif Disrupts Recruitment of the Nucleosome Remodeling and Deacetylase Complex and Repression of Gbx2. J. Biol. Chem. 2007, 282, 34858–34868. [Google Scholar] [CrossRef]
- Ma, C.; Wang, F.; Han, B.; Zhong, X.; Si, F.; Ye, J.; Hsueh, E.C.; Robbins, L.; Kiefer, S.M.; Zhang, Y.; et al. SALL1 functions as a tumor suppressor in breast cancer by regulating cancer cell senescence and metastasis through the NuRD complex. Mol. Cancer 2018, 17, 1–21. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Li, D.; Tian, Y.; Ma, Y.; Benjamin, T. p150 Sal2 Is a p53-Independent Regulator of p21 WAF1/CIP. Mol. Cell. Biol. 2004, 24, 3885–3893. [Google Scholar] [CrossRef]
- Wu, Z.; Cheng, K.; Shi, L.; Li, Z.; Negi, H.; Gao, G.; Kamle, S.; Li, D. Sal-like protein 2 upregulates p16 expression through a proximal promoter element. Cancer Sci. 2015, 106, 253–261. [Google Scholar] [CrossRef]
- Miao, F.; Zhang, X.; Cao, Y.; Wang, Y.; Zhang, X. Effect of siRNA-silencing of SALL2 gene on growth, migration and invasion of human ovarian carcinoma A2780 cells. BMC Cancer 2017, 17, 838. [Google Scholar] [CrossRef]
- Chen, M.; Li, L.; Zheng, P. SALL4 promotes the tumorigenicity of cervical cancer cells through activation of the Wnt/β-catenin pathway viaCTNNB1. Cancer Sci. 2019, 110, 2794–2805. [Google Scholar] [CrossRef]
- He, J.; Zhou, M.; Chen, X.; Yue, D.; Yang, L.; Qin, G.; Zhang, Z.; Gao, Q.; Wang, D.; Zhang, C.; et al. Inhibition of SALL4 reduces tumorigenicity involving epithelial-mesenchymal transition via Wnt/β-catenin pathway in esophageal squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2016, 35, 1–13. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Tao, X.; You, Q.; Tao, Z.; He, Z.; Ou, J. Knockdown of SALL4 inhibits the proliferation, migration, and invasion of human lung cancer cells in vivo and in vitro. Ann. Transl. Med. 2020, 8, 1678. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, H.; Li, Y.; Shen, L.; Yu, R.; Yin, H.; Sun, T.; Sun, C.; Zhou, Y.; Du, Z. SALL4 suppresses PTEN expression to promote glioma cell proliferation via PI3K/AKT signaling pathway. J. Neuro-Oncol. 2017, 135, 263–272. [Google Scholar] [CrossRef]
- Liu, C.; Yao, F.; Mao, X.; Li, W.; Chen, H. Effect of SALL4 on the Proliferation, Invasion and Apoptosis of Breast Cancer Cells. Technol. Cancer Res. Treat. 2020, 19, 1–10. [Google Scholar] [CrossRef]
- Sung, C.K.; Yim, H.; Gu, H.; Li, D.; Andrews, E.; Duraisamy, S.; Li, C.; Drapkin, R.; Benjamin, T. The Polyoma Virus Large T Binding Protein p150 Is a Transcriptional Repressor of c-MYC. PLoS ONE 2012, 7, e46486. [Google Scholar] [CrossRef]
- Sato, A.; Kishida, S.; Tanaka, T.; Kikuchi, A.; Kodama, T.; Asashima, M.; Nishinakamura, R. Sall1, a causative gene for Townes–Brocks syndrome, enhances the canonical Wnt signaling by localizing to heterochromatin. Biochem. Biophys. Res. Commun. 2004, 319, 103–113. [Google Scholar] [CrossRef]
- Yang, J.; Chai, L.; Gao, C.; Fowles, T.C.; Alipio, Z.; Dang, H.; Xu, D.; Fink, L.M.; Ward, D.C.; Ma, Y. SALL4 is a key regulator of survival and apoptosis in human leukemic cells. Blood 2008, 112, 805–813. [Google Scholar] [CrossRef]
- Gu, H.; Li, D.; Sung, C.K.; Yim, H.; Troke, P.; Benjamin, T. DNA-binding and regulatory properties of the transcription factor and putative tumor suppressor p150Sal2. Biochim. Biophys. Acta (BBA)-Bioenerg. 2011, 1809, 276–283. [Google Scholar] [CrossRef]
- Escobar, D.; Hepp, M.; Farkas, C.; Campos, T.M.C.; Sodir, N.M.; Morales, M.M.; Alvarez, C.; Swigart, L.B.; Evan, G.I.; Gutierrez, J.; et al. Sall2 is required for proapoptotic Noxa expression and genotoxic stress-induced apoptosis by doxorubicin. Cell Death Dis. 2015, 6, e1816. [Google Scholar] [CrossRef]
- Hepp, M.I.; Escobar, D.; Farkas, C.; Hermosilla, V.; Álvarez, C.; Amigo, R.; Gutiérrez, J.L.; Castro, A.F.; Pincheira, R. A Trichostatin A (TSA)/Sp1-mediated mechanism for the regulation of SALL2 tumor suppressor in Jurkat T cells. Biochim. Biophys. Acta (BBA)-Bioenerg. 2018, 1861, 623–636. [Google Scholar] [CrossRef]
- Hopkins, B.D.; Hodakoski, C.; Barrows, D.; Mense, S.M.; Parsons, R.E. PTEN function: The long and the short of it. Trends Biochem. Sci. 2014, 39, 183–190. [Google Scholar] [CrossRef]
- Deng, G.; Zhu, L.; Huang, F.; Nie, W.; Huang, W.; Xu, H.; Zheng, S.; Yi, Z.; Wan, T. Knockdown of Sall4 inhibits intrahepatic cholangiocarcinoma cell migration and invasion in ICC-9810 cells. OncoTargets Ther. 2016, 9, 5297–5305. [Google Scholar] [CrossRef]
- Ye, L.; Lin, C.; Wang, X.; Li, Q.; Li, Y.; Wang, M.; Zhao, Z.; Wu, X.; Shi, D.; Xiao, Y.; et al. Epigenetic silencing of SALL 2 confers tamoxifen resistance in breast cancer. EMBO Mol. Med. 2019, 11, e10638. [Google Scholar] [CrossRef]
- Luo, J.; Wang, W.; Tang, Y.; Zhou, D.; Gao, Y.; Zhang, Q.; Zhou, X.; Zhu, H.; Xing, L.; Yu, J. mRNA and methylation profiling of radioresistant esophageal cancer cells: The involvement of Sall2 in acquired aggressive phenotypes. J. Cancer 2017, 8, 646–656. [Google Scholar] [CrossRef]
- Yang, J.; Aguila, J.R.; Alipio, Z.; Lai, R.; Fink, L.M.; Ma, Y. Enhanced self-renewal of hematopoietic stem/progenitor cells mediated by the stem cell gene Sall4. J. Hematol. Oncol. 2011, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Hoggatt, J.; Singh, P.; Abe, M.; Speth, J.; Hu, P.; Conway, E.; Nucifora, G.; Yamaguchi, S.; Pelus, L.M. Survivin modulates genes with divergent molecular functions and regulates proliferation of hematopoietic stem cells through Evi-1. Leukemia 2014, 29, 433–440. [Google Scholar] [CrossRef]
- Zhou, Q.; Guo, Y.; Zheng, B.; Shao, B.; Jiang, M.; Wang, G.; Zhou, T.; Wang, L.; Zhou, Z.; Guo, X.; et al. Establishment of a proteome profile and identification of molecular markers for mouse spermatogonial stem cells. J. Cell. Mol. Med. 2014, 19, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Andersen, P.; Hotta, A.; Tsukahara, Y.; Sasagawa, N.; Hayashida, N.; Koga, C.; Nishikawa, M.; Saga, Y.; Evans, S.M.; et al. Sall1 transiently marks undifferentiated heart precursors and regulates their fate. J. Mol. Cell. Cardiol. 2016, 92, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Osafune, K.; Takasato, M.; Kispert, A.; Asashima, M.; Nishinakamura, R. Identification of multipotent progenitors in the embryonic mouse kidney by a novel colony-forming assay. Development 2006, 133, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xiao, Y.; Lin, C.; Zhang, Q.; Zhang, S.; Pei, F.; Liu, H.; Chen, Z. SALL1 regulates commitment of odontoblast lineages by interacting with RUNX2 to remodel open chromatin regions. Stem Cells 2020, 39, 196–209. [Google Scholar] [CrossRef]
- Hobbs, R.; Fagoonee, S.; Papa, A.; Webster, K.; Altruda, F.; Nishinakamura, R.; Chai, L.; Pandolfi, P.P. Functional Antagonism between Sall4 and Plzf Defines Germline Progenitors. Cell Stem Cell 2012, 10, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Gao, C.; Chai, L.; Ma, Y. A Novel SALL4/OCT4 Transcriptional Feedback Network for Pluripotency of Embryonic Stem Cells. PLoS ONE 2010, 5, e10766. [Google Scholar] [CrossRef]
- Pantier, R.; Chhatbar, K.; Quante, T.; Skourti-Stathaki, K.; Cholewa-Waclaw, J.; Alston, G.; Alexander-Howden, B.; Lee, H.Y.; Cook, A.G.; Spruijt, C.G.; et al. SALL4 controls cell fate in response to DNA base composition. Mol. Cell 2021, 81, 845–858.e8. [Google Scholar] [CrossRef]
- Suvà, M.L.; Rheinbay, E.; Gillespie, S.M.; Patel, A.P.; Wakimoto, H.; Rabkin, S.D.; Riggi, N.; Chi, A.S.; Cahill, D.P.; Nahed, B.V.; et al. Reconstructing and Reprogramming the Tumor-Propagating Potential of Glioblastoma Stem-like Cells. Cell 2014, 157, 580–594. [Google Scholar] [CrossRef]
- Karantzali, E.; Lekakis, V.; Ioannou, M.; Hadjimichael, C.; Papamatheakis, J.; Kretsovali, A. Sall1 Regulates Embryonic Stem Cell Differentiation in Association with Nanog. J. Biol. Chem. 2011, 286, 1037–1045. [Google Scholar] [CrossRef]
- Quevedo, M.; Meert, L.; Dekker, M.R.; Dekkers, D.H.W.; Brandsma, J.H.; Berg, D.L.C.V.D.; Ozgür, Z.; Van Ijcken, W.F.J.; Demmers, J.; Fornerod, M.; et al. Mediator complex interaction partners organize the transcriptional network that defines neural stem cells. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Argos, M.; Kibriya, M.G.; Jasmine, F.; Olopade, O.I.; Su, T.; Hibshoosh, H.; Ahsan, H. Genomewide scan for loss of heterozygosity and chromosomal amplification in breast carcinoma using single-nucleotide polymorphism arrays. Cancer Genet. Cytogenet. 2008, 182, 69–74. [Google Scholar] [CrossRef][Green Version]
- Mathew, R.; Arora, S.; Mathur, M.; Ralhan, R.; Chattopadhyay, T.K. Esophageal squamous cell carcinomas with DNA replication errors (RER +) are associated with p16/pRb loss and wild-type p53. J. Cancer Res. Clin. Oncol. 2001, 127, 603–612. [Google Scholar] [CrossRef]
- Chang, W.Y.; Cairns, P.; Schoenberg, M.P.; Polascik, T.J.; Sidransky, D. Novel suppressor loci on chromosome 14q in primary bladder cancer. Cancer Res. 1995, 55, 3246–3249. [Google Scholar]
- Abujiang, P.; Mori, T.J.; Takahashi, T.; Tanaka, F.; Kasyu, I.; Hitomi, S.; Hiai, H. Loss of heterozygosity (LOH) at 17q and 14q in human lung cancers. Oncogene 1998, 17, 3029–3033. [Google Scholar] [CrossRef]
- Al-Mulla, F.; Alfadhli, S.; Al-Hakim, A.H.; Going, J.J.; Bitar, M.S. Metastatic recurrence of early-stage colorectal cancer is linked to loss of heterozygosity on chromosomes 4 and 14q. J. Clin. Pathol. 2006, 59, 624–630. [Google Scholar] [CrossRef]
- Beder, L.B.; Gunduz, M.; Ouchida, M.; Fukushima, K.; Gunduz, E.; Ito, S.; Sakai, A.; Nagai, N.; Nishizaki, K.; Shimizu, K. Genome-Wide Analyses on Loss of Heterozygosity in Head and Neck Squamous Cell Carcinomas. Lab. Investig. 2003, 83, 99–105. [Google Scholar] [CrossRef]
- Lee, D.J.; Koch, W.M.; Yoo, G.; Lango, M.; Reed, A.; Califano, J.; Brennan, J.A.; Westra, W.H.; Zahurak, M.; Sidransky, D. Impact of chromosome 14q loss on survival in primary head and neck squamous cell carcinoma. Clin. Cancer Res. 1997, 3, 501–505. [Google Scholar]
- Pehlivan, D.; Gunduz, E.; Gunduz, M.; Nagatsuka, H.; Beder, L.B.; Cengiz, B.; Rivera, R.S.; Fukushima, K.; Palanduz, S.; Ozturk, S.; et al. Loss of heterozygosity at chromosome 14q is associated with poor prognosis in head and neck squamous cell carcinomas. J. Cancer Res. Clin. Oncol. 2008, 134, 1267–1276. [Google Scholar] [CrossRef]
- Nishizuka, S.; Tamura, G.; Terashima, M.; Satodate, R. Loss of heterozygosity during the development and progression of differentiated adenocarcinoma of the stomach. J. Pathol. 1998, 185, 38–43. [Google Scholar] [CrossRef]
- Takebayashi, S.; Hickson, A.; Ogawa, T.; Jung, K.-Y.; Mineta, H.; Ueda, Y.; Grénman, R.; Fisher, S.G.; Carey, T.E. Loss of chromosome arm 18q with tumor progression in head and neck squamous cancer. Genes Chromosomes Cancer 2004, 41, 145–154. [Google Scholar] [CrossRef]
- Jen, J.; Kim, H.; Piantadosi, S.; Liu, Z.-F.; Levitt, R.C.; Sistonen, P.; Kinzler, K.W.; Vogelstein, B.; Hamilton, S.R. Allelic Loss of Chromosome 18q and Prognosis in Colorectal Cancer. N. Engl. J. Med. 1994, 331, 213–221. [Google Scholar] [CrossRef]
- Savelieva, E.; Belair, C.D.; Newton, M.A.; Devries, S.; Gray, J.W.; Waldman, F.; Reznikoff, C.A. 20q gain associates with immortalization: 20q13.2 amplification correlates with genome instability in human papillomavirus 16 E7 transformed human uroepithelial cells. Oncogene 1997, 14, 551–560. [Google Scholar] [CrossRef]
- Werner, M.; Mattis, A.; Aubele, M.; Cummings, M.; Zitzelsberger, H.; Hutzler, P.; Höfler, H. 20q13.2 Amplification in intraductal hyperplasia adjacent to in situ and invasive ductal carcinoma of the breast. Virchows Arch. 1999, 435, 469–472. [Google Scholar] [CrossRef]
- Huang, H.-N.; Huang, W.-C.; Lin, C.-H.; Chiang, Y.-C.; Huang, H.-Y.; Kuo, K.-T. Chromosome 20q13.2 ZNF217 locus amplification correlates with decreased E-cadherin expression in ovarian clear cell carcinoma with PI3K-Akt pathway alterations. Hum. Pathol. 2014, 45, 2318–2325. [Google Scholar] [CrossRef]
- Briand-Suleau, A.; Martinovic, J.; Tosca, L.; Tou, B.; Brisset, S.; Bouligand, J.; Delattre, V.; Giurgea, I.; Bachir, J.; Folliot, P.; et al. SALL4 and NFATC2: Two major actors of interstitial 20q13.2 duplication. Eur. J. Med. Genet. 2014, 57, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, A.; Sehouli, J.; Yanaihara, N.; Hirata, Y.; Braicu, I.; Kim, B.-G.; Takakura, S.; Saito, M.; Yanagida, S.; Takenaka, M.; et al. Somatic Copy Number Alterations Associated with Japanese or Endometriosis in Ovarian Clear Cell Adenocarcinoma. PLoS ONE 2015, 10, e0116977. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.M.; Jang, B.G.; Hyun, C.L.; Kim, Y.S.; Hyun, J.W.; Chang, W.Y.; Maeng, Y.H. Aurora Kinase A Is a Prognostic Marker in Colorectal Adenocarcinoma. J. Pathol. Transl. Med. 2017, 51, 32–39. [Google Scholar] [CrossRef]
- Morikawa, A.; Hayashi, T.; Kobayashi, M.; Kato, Y.; Shirahige, K.; Itoh, T.; Urashima, M.; Okamoto, A.; Akiyama, T. Somatic copy number alterations have prognostic impact in patients with ovarian clear cell carcinoma. Oncol. Rep. 2018, 40, 309–318. [Google Scholar] [CrossRef]
- Sung, C.K.; Dahl, J.; Yim, H.; Rodig, S.; Benjamin, A.T.L. Transcriptional and post-translational regulation of the quiescence factor and putative tumor suppressor p150Sal2. FASEB J. 2011, 25, 1275–1283. [Google Scholar] [CrossRef]
- Ma, Y.; Li, D.; Chai, L.; Luciani, A.M.; Ford, D.; Morgan, J.; Maizel, A.L. Cloning and Characterization of Two Promoters for the Human HSAL2 Gene and Their Transcriptional Repression by the Wilms Tumor Suppressor Gene Product. J. Biol. Chem. 2001, 276, 48223–48230. [Google Scholar] [CrossRef]
- Farkas, C.; Martins, C.P.; Escobar, D.; Hepp, M.I.; Donner, D.B.; Castro, A.F.; Evan, G.; Gutiérrez, J.L.; Warren, R.; Pincheira, R. Wild Type p53 Transcriptionally Represses the SALL2 Transcription Factor under Genotoxic Stress. PLoS ONE 2013, 8, e73817. [Google Scholar] [CrossRef][Green Version]
- Pecce, V.; Verrienti, A.; Fiscon, G.; Sponziello, M.; Conte, F.; Abballe, L.; Durante, C.; Farina, L.; Filetti, S.; Paci, P. The role of FOSL1 in stem-like cell reprogramming processes. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Bard, J.D.; Gelebart, P.; Amin, H.M.; Young, L.C.; Ma, Y.; Lai, R. Signal transducer and activator of transcription 3 is a transcriptional factor regulating the gene expression ofSALL4. FASEB J. 2009, 23, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Yoshihashi, K.; Suzuki, H.; Tsutsumi, S.; Mutoh, H.; Maeda, S.; Yamagata, Y.; Seto, Y.; Aburatani, H.; Hatakeyama, M. CDX1 confers intestinal phenotype on gastric epithelial cells via induction of stemness-associated reprogramming factors SALL4 and KLF5. Proc. Natl. Acad. Sci. USA 2012, 109, 20584–20589. [Google Scholar] [CrossRef]
- Hill, V.K.; Hesson, L.B.; Dansranjavin, T.; Dallol, A.; Bieche, I.; Vacher, S.; Tommasi, S.; Dobbins, T.; Gentle, D.; Euhus, D.; et al. Identification of 5 novel genes methylated in breast and other epithelial cancers. Mol. Cancer 2010, 9, 51. [Google Scholar] [CrossRef]
- Wang, C.; Pu, W.; Zhao, D.; Zhou, Y.; Lu, T.; Chen, S.; He, Z.; Feng, X.; Wang, Y.; Li, C.; et al. Identification of Hyper-Methylated Tumor Suppressor Genes-Based Diagnostic Panel for Esophageal Squamous Cell Carcinoma (ESCC) in a Chinese Han Population. Front. Genet. 2018, 9, 356. [Google Scholar] [CrossRef]
- Imai, A.; Mochizuki, D.; Misawa, Y.; Nakagawa, T.; Endo, S.; Mima, M.; Yamada, S.; Kawasaki, H.; Kanazawa, T.; Misawa, K. SALL2 Is a Novel Prognostic Methylation Marker in Patients with Oral Squamous Carcinomas: Associations with SALL1 and SALL3 Methylation Status. DNA Cell Biol. 2019, 38, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Misawa, K.; Kanazawa, T.; Mochizuki, D.; Imai, A.; Mima, M.; Yamada, S.; Morita, K.; Misawa, Y.; Shinmura, K.; Mineta, H. Genes Located on 18q23 Are Epigenetic Markers and Have Prognostic Significance for Patients with Head and Neck Cancer. Cancers 2019, 11, 401. [Google Scholar] [CrossRef]
- Misawa, K.; Mochizuki, D.; Imai, A.; Misawa, Y.; Endo, S.; Mima, M.; Kawasaki, H.; Carey, T.E.; Kanazawa, T. Epigenetic silencing of SALL3 is an independent predictor of poor survival in head and neck cancer. Clin. Epigenet. 2017, 9, 1–12. [Google Scholar] [CrossRef]
- Misawa, K.; Misawa, Y.; Imai, A.; Mochizuki, D.; Endo, S.; Mima, M.; Ishikawa, R.; Kawasaki, H.; Yamatodani, T.; Kanazawa, T. Epigenetic modification of SALL1 as a novel biomarker for the prognosis of early stage head and neck cancer. J. Cancer 2018, 9, 941–949. [Google Scholar] [CrossRef]
- Lin, J.; Qian, J.; Yao, D.-M.; Qian, W.; Yang, J.; Wang, C.-Z.; Chai, H.-Y.; Ma, J.-C.; Deng, Z.-Q.; Li, Y.; et al. Aberrant hypomethylation of SALL4 gene in patients with myelodysplastic syndrome. Leuk. Res. 2013, 37, 71–75. [Google Scholar] [CrossRef]
- Ma, J.-C.; Qian, J.; Lin, J.; Qian, W.; Yang, J.; Wang, C.-Z.; Chai, H.-Y.; Li, Y.; Chen, Q.; Qian, Z. Aberrant hypomethylation of SALL4 gene is associated with intermediate and poor karyotypes in acute myeloid leukemia. Clin. Biochem. 2013, 46, 304–307. [Google Scholar] [CrossRef]
- Liu, J.; Sauer, M.A.; Hussein, S.G.; Yang, J.; Tenen, D.G.; Chai, L. SALL4 and microRNA: The Role of Let-7. Genes 2021, 12, 1301. [Google Scholar] [CrossRef]
- Yang, C.M.; Chiba, T.; Brill, B.; Delis, N.; von Manstein, V.; Vafaizadeh, V.; Oellerich, T.; Groner, B. Expression of the mi R-302/367 cluster in glioblastoma cells suppresses tumorigenic gene expression patterns and abolishes transformation related phenotypes. Int. J. Cancer 2015, 137, 2296–2309. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Hu, C.; Jiang, Y. MicroRNA-16 inhibits the proliferation, migration and invasion of glioma cells by targeting Sal-like protein 4. Int. J. Mol. Med. 2016, 38, 1768–1776. [Google Scholar] [CrossRef]
- Chen, L.-P.; Zhang, N.-N.; Ren, X.-Q.; He, J.; Li, Y. miR-103/miR-195/miR-15b Regulate SALL4 and Inhibit Proliferation and Migration in Glioma. Molecules 2018, 23, 2938. [Google Scholar] [CrossRef]
- He, J.; Zhang, W.; Zhou, Q.; Zhao, T.; Song, Y.; Chai, L.; Li, Y. Low-expression of microRNA-107 inhibits cell apoptosis in glioma by upregulation of SALL4. Int. J. Biochem. Cell Biol. 2013, 45, 1962–1973. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, Y.; Liu, M.; Jiang, Y. MicroRNA-181b Inhibits Cellular Proliferation and Invasion of Glioma Cells via Targeting Sal-Like Protein 4. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2017, 25, 947–957. [Google Scholar] [CrossRef]
- Wang, M.; Qiu, R.; Gong, Z.; Zhao, X.; Wang, T.; Zhou, L.; Lu, W.; Shen, B.; Zhu, W.; Xu, W. miR-188-5p emerges as an oncomiRNA to promote gastric cancer cell proliferation and migration via upregulation of SALL4. J. Cell. Biochem. 2019, 120, 15027–15037. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, Z. miR-16 targets SALL4 to repress the proliferation and migration of gastric cancer. Oncol. Lett. 2018, 16, 3005–3012. [Google Scholar] [CrossRef]
- Kondelova, A.; Alburquerque-González, B.; Vychytilova-Faltejskova, P.; García-Solano, J.; Prochazka, V.; Kala, Z.; Pérez, F.; Slaby, O.; Conesa-Zamora, P. miR-181a-2* expression is different amongst carcinomas from the colorectal serrated route. Mutagenesis 2020, 35, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Deng, R.; Zhang, P.; Wu, C.; Wu, K.; Shi, L.; Liu, X.; Bai, J.; Deng, M.; Shuai, X.; et al. miR-219-5p plays a tumor suppressive role in colon cancer by targeting oncogene Sall4. Oncol. Rep. 2015, 34, 1923–1932. [Google Scholar] [CrossRef]
- Chang, S.; Sun, G.; Zhang, D.; Li, Q.; Qian, H. MiR-3622a-3p acts as a tumor suppressor in colorectal cancer by reducing stemness features and EMT through targeting spalt-like transcription factor 4. Cell Death Dis. 2020, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Rahnama, M.A.; Movassaghpour, A.A.; Soleimani, M.; Atashi, A.; Anbarlou, A.; Asenjan, K.S. MicroRNA-15b target Sall4 and diminish in vitro UCB-derived HSCs expansion. EXCLI J. 2015, 14, 601–610. [Google Scholar] [CrossRef]
- Melton, C.; Judson, R.L.; Blelloch, R. Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nat. Cell Biol. 2010, 463, 621–626. [Google Scholar] [CrossRef]
- Liu, X.; Cao, Y.; Zhang, Y.; Zhou, H.; Li, H. Regulatory effect of MiR103 on proliferation, EMT and invasion of oral squamous carcinoma cell through SALL4. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9931–9938. [Google Scholar]
- Yuan, J.H.; Li, W.X.; Hu, C.; Zhang, B. Upregulation of SNHG12 accelerates cell proliferation, migration, invasion and restrain cell apoptosis in breast cancer by enhancing regulating SALL4 expression via sponging miR-15a-5p. Neoplasma 2020, 67, 861–870. [Google Scholar] [CrossRef]
- Chen, Y.; He, J.; Su, C.; Wang, H.; Chen, Y.; Guo, W.; Li, Y.; Ding, G. LINC00461 affects the survival of patients with renal cell carcinoma by acting as a competing endogenous RNA for microRNA-942. Oncol. Rep. 2019, 42, 1924–1934. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, S.; Wang, H.; Zhang, B.; Zhang, P. miR-4286 promotes prostate cancer progression via targeting the expression of SALL1. J. Gene Med. 2019, e3127. [Google Scholar] [CrossRef]
- Xia, H.; Niu, Q.; Ding, Y.; Zhang, Z.; Yuan, J.; Jin, W. Long noncoding HOXA11-AS knockdown suppresses the progression of non-small cell lung cancer by regulating miR-3619-5p/SALL4 axis. J. Mol. Histol. 2021, 52, 729–740. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, N.; Li, M.-Y.; Du, M.-F. Long non-coding RNA ZEB2-AS1 regulates osteosarcoma progression by acting as a molecular sponge of miR-107 to modulate SALL4 expression. Am. J. Transl. Res. 2021, 13, 1140–1154. [Google Scholar]
- Shi, D.-M.; Shi, X.-L.; Xing, K.-L.; Zhou, H.-X.; Lu, L.-L.; Wu, W.-Z. miR-296-5p suppresses stem cell potency of hepatocellular carcinoma cells via regulating Brg1/Sall4 axis. Cell. Signal. 2020, 72, 109650. [Google Scholar] [CrossRef]
- Ma, Y.-S.; Liu, J.-B.; Lin, L.; Zhang, H.; Wu, J.-J.; Shi, Y.; Jia, C.-Y.; Zhang, D.-D.; Yu, F.; Wang, H.-M.; et al. Exosomal microRNA-15a from mesenchymal stem cells impedes hepatocellular carcinoma progression via downregulation of SALL4. Cell Death Discov. 2021, 7, 1–11. [Google Scholar] [CrossRef]
- Misawa, K.; Misawa, Y.; Mima, M.; Yamada, S.; Imai, A.; Mochizuki, D.; Nakagawa, T.; Kurokawa, T.; Endo, S.; Kawasaki, H.; et al. Overexpression of Sal-like protein 4 in head and neck cancer: Epigenetic effects and clinical correlations. Cell. Oncol. 2020, 43, 631–641. [Google Scholar] [CrossRef]
- Zhang, D.-L.; Qu, L.-W.; Ma, L.; Zhou, Y.-C.; Wang, G.-Z.; Zhao, X.-C.; Zhang, C.; Zhang, Y.-F.; Wang, M.; Zhang, M.-Y.; et al. Genome-wide identification of transcription factors that are critical to non-small cell lung cancer. Cancer Lett. 2018, 434, 132–143. [Google Scholar] [CrossRef]
- Gautam, A.K.; Wang, C.; Zeng, J.; Wang, J.; Lu, J.; Wei, J.; Huang, G.; Mo, B.; Luo, M.; Mo, B. Expression and clinical significance of SALL4 and LGR5 in patients with lung cancer. Oncol. Lett. 2015, 10, 3629–3634. [Google Scholar] [CrossRef]
- Yanagihara, N.; Kobayashi, D.; Kuribayashi, K.; Tanaka, M.; Hasegawa, T.; Watanabe, N. Significance of SALL4 as a drug-resistant factor in lung cancer. Int. J. Oncol. 2015, 46, 1527–1534. [Google Scholar] [CrossRef]
- Abnet, C.C.; Arnold, M.; Wei, W.-Q. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology 2018, 154, 360–373. [Google Scholar] [CrossRef]
- Forghanifard, M.M.; Khales, S.A.; Javdani-Mallak, A.; Rad, A.; Farshchian, M.; Abbaszadegan, M.R. Stemness state regulators SALL4 and SOX2 are involved in progression and invasiveness of esophageal squamous cell carcinoma. Med. Oncol. 2014, 31, 1–8. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, T.; Wang, Z.; Zhang, H.; Qian, Z.; Xu, H.; Gao, B.; Wang, W.; Gu, L.; Meng, J.; et al. A Novel Set of DNA Methylation Markers in Urine Sediments for Sensitive/Specific Detection of Bladder Cancer. Clin. Cancer Res. 2007, 13, 7296–7304. [Google Scholar] [CrossRef]
- Van Der Heijden, A.G.; Mengual, L.; Ingelmo, M.; Lozano, J.J.; Westerlo, C.C.M.V.R.-V.D.; Baixauli, M.; Geavlete, B.; Moldoveanud, C.; Ene, C.; Dinney, C.P.; et al. Urine cell-based DNA methylation classifier for monitoring bladder cancer. Clin. Epigenet. 2018, 10, 71. [Google Scholar] [CrossRef]
- Cao, D.; Humphrey, P.A.; Allan, R.W. SALL4 is a novel sensitive and specific marker for metastatic germ cell tumors, with particular utility in detection of metastatic yolk sac tumors. Cancer 2009, 115, 2640–2651. [Google Scholar] [CrossRef]
- Ma, Y.; Singer, D.B.; Gozman, A.; Ford, D.; Chai, L.; Steinhoff, M.M.; Hansen, K.; Maizel, A.L. Hsal 1 is related to kidney and gonad development and is expressed in Wilms tumor. Pediatr. Nephrol. 2001, 16, 701–709. [Google Scholar] [CrossRef]
- Brown, K.W.; Charles, A.; Dallosso, A.; White, G.; Charlet, J.; Standen, G.R.; Malik, K. Characterization of 17.94, a novel anaplastic Wilms’ tumor cell line. Cancer Genet. 2012, 205, 319–326. [Google Scholar] [CrossRef]
- Li, C.-M.; Guo, M.; Borczuk, A.; Powell, C.A.; Wei, M.; Thaker, H.M.; Friedman, R.; Klein, U.; Tycko, B. Gene Expression in Wilms’ Tumor Mimics the Earliest Committed Stage in the Metanephric Mesenchymal-Epithelial Transition. Am. J. Pathol. 2002, 160, 2181–2190. [Google Scholar] [CrossRef]
- Artemov, A.V.; Zhigalova, N.; Zhenilo, S.; Mazur, A.M.; Prokhortchouk, E.B. VHL inactivation without hypoxia is sufficient to achieve genome hypermethylation. Sci. Rep. 2018, 8, 10667. [Google Scholar] [CrossRef]
- Deisch, J.; Raisanen, J.; Rakheja, D. Immunoexpression of SALL4 in Wilms Tumors and Developing Kidney. Pathol. Oncol. Res. 2011, 17, 639–644. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef]
- Liu, L.-Y.; Chang, L.-Y.; Kuo, W.-H.; Hwa, H.-L.; Chang, K.-J.; Hsieh, F.-J. A Supervised Network Analysis on Gene Expression Profiles of Breast Tumors Predicts a 41-Gene Prognostic Signature of the Transcription FactorMYBacross Molecular Subtypes. Comput. Math. Methods Med. 2014, 2014, 1–19. [Google Scholar] [CrossRef]
- Zuo, Y.; Cui, Y.; Yu, G.; Li, R.; Ressom, H.W. Incorporating prior biological knowledge for network-based differential gene expression analysis using differentially weighted graphical LASSO. BMC Bioinform. 2017, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Müller-Decker, K.; Flechtenmacher, C.; Zhang, F.; Shahmoradgoli, M.; Mills, G.B.; Hoheisel, J.D.; Boettcher, M. An in vivo RNAi screen identifies SALL1 as a tumor suppressor in human breast cancer with a role in CDH1 regulation. Oncogene 2014, 33, 4273–4278. [Google Scholar] [CrossRef] [PubMed]
- Watanabe; Kobayashi, D.; Kuribayshi, K.; Tanaka, M. SALL4 is essential for cancer cell proliferation and is overexpressed at early clinical stages in breast cancer. Int. J. Oncol. 2011, 38, 933–939. [Google Scholar] [CrossRef][Green Version]
- Itou, J.; Matsumoto, Y.; Yoshikawa, K.; Toi, M. Sal-like 4(SALL4) suppressesCDH1expression and maintains cell dispersion in basal-like breast cancer. FEBS Lett. 2013, 587, 3115–3121. [Google Scholar] [CrossRef]
- Itou, J.; Tanaka, S.; Li, W.; Iida, A.; Sehara-Fujisawa, A.; Sato, F.; Toi, M. The Sal-like 4—Integrin α6β1 network promotes cell migration for metastasis via activation of focal adhesion dynamics in basal-like breast cancer cells. Biochim. Biophys. Acta (BBA)-Bioenerg. 2017, 1864, 76–88. [Google Scholar] [CrossRef]
- Chen, T.; Tsang, J.Y.S.; Su, X.; Li, P.; Sun, W.; Wong, I.L.K.; Choy, K.; Yang, Q.; Tse, G.M.K.; Chan, T.H.; et al. SALL4 promotes tumor progression in breast cancer by targeting EMT. Mol. Carcinog. 2020, 59, 1209–1226. [Google Scholar] [CrossRef] [PubMed]
- Dirican, E.; Akkiprik, M. Functional and clinical significance of SALL4 in breast cancer. Tumor Biol. 2016, 37, 11701–11709. [Google Scholar] [CrossRef]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar]
- Sung, C.K.; Li, D.; Andrews, E.; Drapkin, R.; Benjamin, T. Promoter methylation of the SALL2 tumor suppressor gene in ovarian cancers. Mol. Oncol. 2012, 7, 419–427. [Google Scholar] [CrossRef]
- Parroche, P.; Touka, M.; Mansour, M.; Bouvard, V.; Thépot, A.; Accardi, R.; Carreira, C.; Roblot, G.G.; Sylla, B.S.; Hasan, U.; et al. Human papillomavirus type 16 E6 inhibits p21WAF1 transcription independently of p53 by inactivating p150Sal2. Virology 2011, 417, 443–448. [Google Scholar] [CrossRef]
- Yu, D.; Khan, O.F.; Suvà, M.L.; Dong, B.; Panek, W.K.; Xiao, T.; Wu, M.; Han, Y.; Ahmed, A.U.; Balyasnikova, I.V.; et al. Multiplexed RNAi therapy against brain tumor-initiating cells via lipopolymeric nanoparticle infusion delays glioblastoma progression. Proc. Natl. Acad. Sci. USA 2017, 114, E6147–E6156. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, Y.; Jiang, Y.; Cui, Y.; Zou, Y.; Qian, J.; Luo, C.; Lu, Y.; Wu, X. The expression of SALL4 in patients with gliomas: High level of SALL4 expression is correlated with poor outcome. J. Neuro-Oncol. 2014, 121, 261–268. [Google Scholar] [CrossRef]
- Chi, D.; Zhang, W.; Jia, Y.; Cong, D.; Hu, S. Spalt-Like Transcription Factor 1 (SALL1) Gene Expression Inhibits Cell Proliferation and Cell Migration of Human Glioma Cells Through the Wnt/β-Catenin Signaling Pathway. Med. Sci. Monit. Basic Res. 2019, 25, 128–138. [Google Scholar] [CrossRef]
- Hu, D.; Shilatifard, A. Epigenetics of hematopoiesis and hematological malignancies. Genes Dev. 2016, 30, 2021–2041. [Google Scholar] [CrossRef]
- Tong, W.-G.; Wierda, W.G.; Lin, E.; Kuang, S.-Q.; Bekele, B.N.; Estrov, Z.; Wei, Y.; Yang, H.; Keating, M.J.; Garcia-Manero, G. Genome-wide DNA methylation profiling of chronic lymphocytic leukemia allows identification of epigenetically repressed molecular pathways with clinical impact. Epigenetics 2010, 5, 499–508. [Google Scholar] [CrossRef]
- Kuang, S.-Q.; Tong, W.-G.; Yang, H.; Lin, W.; Lee, M.K.; Fang, Z.H.; Wei, Y.; Jelinek, J.; Issa, J.-P.; Garcia-Manero, G. Genome-wide identification of aberrantly methylated promoter associated CpG islands in acute lymphocytic leukemia. Leukemia 2008, 22, 1529–1538. [Google Scholar] [CrossRef]
- Yang, J. SALL4 as a transcriptional and epigenetic regulator in normal and leukemic hematopoiesis. Biomark. Res. 2018, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shuai, X.; Zhou, D.; Shen, T.; Wu, Y.; Zhang, J.; Wang, X.; Li, Q. Overexpression of the novel oncogene SALL4 and activation of the Wnt/β-catenin pathway in myelodysplastic syndromes. Cancer Genet. Cytogenet. 2009, 194, 119–124. [Google Scholar] [CrossRef]
- Gao, C.; Dimitrov, T.; Yong, K.J.; Tatetsu, H.; Jeong, H.-W.; Luo, H.R.; Bradner, J.E.; Tenen, D.; Chai, L. Targeting transcription factor SALL4 in acute myeloid leukemia by interrupting its interaction with an epigenetic complex. Blood 2013, 121, 1413–1421. [Google Scholar] [CrossRef]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, H.; Li, J.; Liu, H.; Wang, F.; Wei, Y.; Su, J.; Zhang, N.; Liu, T.; Zhang, Y. The Identification of Specific Methylation Patterns across Different Cancers. PLoS ONE 2015, 10, e0120361. [Google Scholar] [CrossRef]
- Zheng, W.; Lu, Y.; Feng, X.; Yang, C.; Qiu, L.; Deng, H.; Xue, Q.; Sun, K. Improving the overall survival prognosis prediction accuracy: A 9-gene signature in CRC patients. Cancer Med. 2021, 10, 5998–6009. [Google Scholar] [CrossRef]
- Forghanifard, M.M.; Moghbeli, M.; Raeisossadati, R.; Tavassoli, A.; Mallak, A.J.; Boroumand-Noughabi, S.; Abbaszadegan, M.R. Role of SALL4 in the progression and metastasis of colorectal cancer. J. Biomed. Sci. 2013, 20, 6. [Google Scholar] [CrossRef]
- Cheng, J.; Deng, R.; Wu, C.; Zhang, P.; Wu, K.; Shi, L.; Liu, X.; Bai, J.; Deng, M.; Gao, J.; et al. Inhibition of SALL4 suppresses carcinogenesis of colorectal cancer via regulating Gli1 expression. Int. J. Clin. Exp. Pathol. 2015, 8, 10092–10101. [Google Scholar]
- Wu, H.-K.; Liu, C.; Fan, X.-X.; Wang, H.; Zhou, L. Spalt-like transcription factor 4 as a potential diagnostic and prognostic marker of colorectal cancer. Cancer Biomark. 2017, 20, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Zhao, Y.; Wang, Z.; Yin, H.; Zhang, X.; He, T.; Song, S.; Sun, S.; Wang, B.; Li, Z.; et al. Expression and clinical significance of SALL4 and β-catenin in colorectal cancer. J. Mol. Histol. 2016, 47, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Bahadori, M.; Baharara, J.; Amini, E. Anticancer Properties of Chrysin on Colon Cancer Cells, In vitro and In vivo with Modulation of Caspase-3, -9, Bax and Sall4. Iran. J. Biotechnol. 2016, 14, 177–184. [Google Scholar] [CrossRef]
- Ma, T.; Shi, S.; Jiang, H.; Chen, X.; Xu, D.; Ding, X.; Zhang, H.; Xi, Y. A pan-cancer study of spalt-like transcription factors 1/2/3/4 as therapeutic targets. Arch. Biochem. Biophys. 2021, 711, 109016. [Google Scholar] [CrossRef]
- Kim, S.; Park, S.; Lee, J.; Park, Y.; Kim, H. 201 Transcriptome analysis of CD133-positive stem cells and prognostic value of survivin in colorectal cancer. Eur. J. Cancer 2014, 50, 65. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, C.; Sui, X.; Zhong, X.; Yang, W.; Hu, X.; Li, Y. Association between gene methylation and HBV infection in hepatocellular carcinoma: A meta-analysis. J. Cancer 2019, 10, 6457–6465. [Google Scholar] [CrossRef] [PubMed]
- Shikauchi, Y.; Saiura, A.; Kubo, T.; Niwa, Y.; Yamamoto, J.; Murase, Y.; Yoshikawa, H. SALL3 Interacts with DNMT3A and Shows the Ability To Inhibit CpG Island Methylation in Hepatocellular Carcinoma. Mol. Cell. Biol. 2009, 29, 1944–1958. [Google Scholar] [CrossRef]
- Yang, X.-X.; Sun, J.-Z.; Li, F.-X.; Wu, Y.-S.; Du, H.-Y.; Zhu, W.; Li, X.-H.; Li, M. Aberrant methylation and downregulation ofsall3in human hepatocellular carcinoma. World J. Gastroenterol. 2012, 18, 2719–2726. [Google Scholar] [CrossRef]
- Jee, B.A.; Choi, J.-H.; Rhee, H.; Yoon, S.; Kwon, S.M.; Nahm, J.H.; Yoo, J.E.; Jeon, Y.; Choi, G.H.; Woo, H.G.; et al. Dynamics of Genomic, Epigenomic, and Transcriptomic Aberrations during Stepwise Hepatocarcinogenesis. Cancer Res. 2019, 79, 5500–5512. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Cui, Z.; Zhang, H.; Mani, S.K.K.; Diab, A.; Lefrancois, L.; Fares, N.; Merle, P.; Andrisani, O. DNA demethylation induces SALL4 gene re-expression in subgroups of hepatocellular carcinoma associated with Hepatitis B or C virus infection. Oncogene 2016, 36, 2435–2445. [Google Scholar] [CrossRef]
- Oikawa, T.; Kamiya, A.; Zeniya, M.; Chikada, H.; Hyuck, A.D.; Yamazaki, Y.; Wauthier, E.; Tajiri, H.; Miller, L.; Wang, X.W.; et al. Sal-like protein 4 (SALL4), a stem cell biomarker in liver cancers. Hepatology 2013, 57, 1469–1483. [Google Scholar] [CrossRef]
- Yin, F.; Han, X.; Yao, S.-K.; Wang, X.-L.; Yang, H.-C. Importance of SALL4 in the development and prognosis of hepatocellular carcinoma. World J. Gastroenterol. 2016, 22, 2837–2843. [Google Scholar] [CrossRef]
- Zeng, S.S.; Yamashita, T.; Kondo, M.; Nio, K.; Hayashi, T.; Hara, Y.; Nomura, Y.; Yoshida, M.; Hayashi, T.; Oishi, N.; et al. The transcription factor SALL4 regulates stemness of EpCAM-positive hepatocellular carcinoma. J. Hepatol. 2014, 60, 127–134. [Google Scholar] [CrossRef]
- Yong, K.J.; Gao, C.; Lim, J.S.; Yan, B.; Yang, H.; Dimitrov, T.; Kawasaki, A.; Ong, C.W.; Wong, K.-F.; Lee, S.; et al. Oncofetal Gene SALL4 in Aggressive Hepatocellular Carcinoma. N. Engl. J. Med. 2013, 368, 2266–2276. [Google Scholar] [CrossRef]
- Sideras, K.; Bots, S.J.; Biermann, K.; Sprengers, D.; Polak, W.G.; Ijzermans, J.N.M.; de Man, R.A.; Pan, Q.; Sleijfer, S.; Bruno, M.J.; et al. Tumour antigen expression in hepatocellular carcinoma in a low-endemic western area. Br. J. Cancer 2015, 112, 1911–1920. [Google Scholar] [CrossRef]
- Wang, J.; Huang, J.; Ma, Q.; Liu, G. Association between quantitative parameters of CEUS and Sall4/Wnt/β-catenin signaling in patients with hepatocellular carcinoma. Cancer Manag. Res. 2019, 11, 3339–3347. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, Y.; Tan, X.; Ke, K.; Zheng, X.; Wang, F.; Lan, S.; Liao, N.; Cai, Z.; Shi, Y.; et al. Inflammatory Micro-environment Contributes to Stemness Properties and Metastatic Potential of HCC via the NF-κB/miR-497/SALL4 Axis. Mol. Ther.-Oncolytics 2019, 15, 79–90. [Google Scholar] [CrossRef]
- Yin, C.; Han, Q.; Xu, D.; Zheng, B.; Zhao, X.; Zhang, J. SALL4-mediated upregulation of exosomal miR-146a-5p drives T-cell exhaustion by M2 tumor-associated macrophages in HCC. OncoImmunology 2019, 8, e1601479. [Google Scholar] [CrossRef]
- Cheng, Y.; He, C.; Wang, M.; Ma, X.; Mo, F.; Yang, S.; Han, J.; Wei, X. Targeting epigenetic regulators for cancer therapy: Mechanisms and advances in clinical trials. Signal Transduct. Target. Ther. 2019, 4, 62. [Google Scholar] [CrossRef] [PubMed]
- Boda, D.; Docea, A.O.; Calina, D.; Ilie, M.A.; Caruntu, C.; Zurac, S.; Neagu, M.; Constantin, C.; Branisteanu, D.E.; Voiculescu, V.; et al. Human papilloma virus: Apprehending the link with carcinogenesis and unveiling new research avenues (Review). Int. J. Oncol. 2018, 52, 637–655. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, S.; Cao, D.; Zhao, M.; Zhang, Q.; Zhao, J.; Yang, T.; Pei, M.; Wang, L.; Li, Y.; et al. Aberrant Hypermethylation of SALL3 with HPV Involvement Contributes to the Carcinogenesis of Cervical Cancer. PLoS ONE 2015, 10, e0145700. [Google Scholar] [CrossRef]
- Yong, K.J.; Li, A.; Ou, W.-B.; Hong, C.K.Y.; Zhao, W.; Wang, F.; Tatetsu, H.; Yan, B.; Qi, L.; Fletcher, J.A.; et al. Targeting SALL4 by entinostat in lung cancer. Oncotarget 2016, 7, 75425–75440. [Google Scholar] [CrossRef]
- Liu, B.H.; Jobichen, C.; Chia, C.S.B.; Chan, T.H.M.; Tang, J.P.; Chung, T.X.Y.; Li, J.; Poulsen, A.; Hung, A.W.; Koh-Stenta, C.X.; et al. Targeting cancer addiction for SALL4 by shifting its transcriptome with a pharmacologic peptide. Proc. Natl. Acad. Sci. USA 2018, 115, E7119–E7128. [Google Scholar] [CrossRef]
- Sievers, Q.L.; Petzold, G.; Bunker, R.D.; Renneville, A.; Słabicki, M.; Liddicoat, B.J.; Abdulrahman, W.; Mikkelsen, T.; Ebert, B.L.; Thomä, N.H. Defining the human C2H2 zinc finger degrome targeted by thalidomide analogs through CRBN. Science 2018, 362, eaat0572. [Google Scholar] [CrossRef]
- Donovan, K.A.; An, J.; Nowak, R.P.; Yuan, J.C.; Fink, E.C.; Berry, B.C.; Ebert, B.L.; Fischer, E.S. Thalidomide promotes degradation of SALL4, a transcription factor implicated in Duane Radial Ray syndrome. eLife 2018, 7, 1–25. [Google Scholar] [CrossRef]
- Akiyama, R.; Kawakami, H.; Wong, J.; Oishi, I.; Nishinakamura, R.; Kawakami, Y. Sall4-Gli3 system in early limb progenitors is essential for the development of limb skeletal elements. Proc. Natl. Acad. Sci. USA 2015, 112, 5075–5080. [Google Scholar] [CrossRef]
- Chen, K.Q.; Tahara, N.; Anderson, A.; Kawakami, H.; Kawakami, S.; Nishinakamura, R.; Pandolfi, P.P.; Kawakami, Y. Development of the Proximal-Anterior Skeletal Elements in the Mouse Hindlimb Is Regulated by a Transcriptional and Signaling Network Controlled by Sall4. Genetics 2020, 215, 129–141. [Google Scholar] [CrossRef]
- Shen, L.; Shi, Q.; Wang, W. Double agents: Genes with both oncogenic and tumor-suppressor functions. Oncogenesis 2018, 7, 1–14. [Google Scholar] [CrossRef]
- Kishibuchi, R.; Kondo, K.; Soejima, S.; Tsuboi, M.; Kajiura, K.; Kawakami, Y.; Kawakita, N.; Sawada, T.; Toba, H.; Yoshida, M.; et al. DNA methylation of GHSR, GNG4, HOXD9 and SALL3 is a common epigenetic alteration in thymic carcinoma. Int. J. Oncol. 2019, 56, 315–326. [Google Scholar] [CrossRef]


| Cancer Type/Cellular Model | microRNA | Target | SALL Status/Key Findings | Experimental Approach | Ref. |
|---|---|---|---|---|---|
| Glioma/Glioblastoma | miR-302/367 cluster | SALL2 | miR-302/367 cluster can reprogram tumor cells, generating more benign phenotypes by suppressing OCT3/4, SOX2, KLF4, c-MYC, POU3F2, OLIG2, and SALL2 | qRT-PCR, cytokine array analysis | [101] |
| Glioma/Glioblastoma | miR-16 | SALL4 | miR-16 inhibits proliferation, migration, and invasion in glioma cells by directly targeting SALL4 | qRT-PCR and Luciferase reporter assay | [102] |
| Glioma/Glioblastoma | miR-103/miR-195/miR-15-B | SALL4 | miR-103, miR-195, and miR-15-B inhibit proliferation, migration, and invasion and promote apoptosis in glioma by directly targeting SALL4 | qRT-PCR, Western blot, and Luciferase reporter assay | [103] |
| Glioma/Glioblastoma | miR-107 | SALL4 | miR-107 inhibits proliferation and promotes apoptosis in glioma cells by directly targeting SALL4 | qRT-PCR, Western blot, and Luciferase reporter assay | [104] |
| Glioma/Glioblastoma | miR-181b | SALL4 | miR-181b inhibits proliferation, migration, and invasion and promotes apoptosis in glioma by directly targeting SALL4 | qRT-PCR, Western blot, and Luciferase reporter assay | [105] |
| Gastric cancer | miR188-5p | SALL4 | miR-188-5p promotes proliferation and migration by upregulating SALL4 expression, nuclear translocation, and transcription | qRT-PCR, Western blot, and Luciferase reporter assay | [106] |
| Gastric cancer | miR-16 | SALL4 | miR-16 inhibits proliferation and migration in GC by directly targeting SALL4 | qRT-PCR and Luciferase reporter assay | [107] |
| Colorectal cancer | miR-181a-2 * | SALL1 | miR-181a-2 * correlates with a trend of repression of SALL1 and high methylation status of the SALL1 promoter | qRT-PCR and bisulfite modification followed by quantitative methylation- specific PCR (qMSP) | [108] |
| Colorectal cancer | miR-219-5p | SALL4 | miR-219-5p inhibits proliferation, migration, and invasion, reduces drug resistance, and promotes apoptosis in CRC by directly targeting SALL4 | qRT-PCR, Western blot, and Luciferase reporter assay | [109] |
| Colorectal cancer | miR-3622a-3p | SALL4 | miR-3622a-3p inhibits proliferation, cell cycle, migration, invasion, and stemness features and promotes apoptosis by targeting SALL4 | qRT-PCR, Luciferase assay, RNA immunoprecipitation (RIP) assay, and pull-down assay | [110] |
| Embryonic stem cell | miR15-B | SALL4 | Anti-miR-15b enhances expansion of HSC in vitro by targeting SALL4 | qRT-PCR | [111] |
| Embryonic stem cell | miR-294 and let-7 miRNAs | SALL4 | Let-7 miR family inhibits self-renewal genes, and miR-294 indirectly induces self-renewal genes, including SALL4 | qRT-PCR, Western blot, and Luciferase reporter assay | [112] |
| Oral squamous cell carcinoma | miR-103 | SALL4 | miR-103 inhibits cell proliferation and invasion by downregulating SALL4 mRNA in Tca8113 cells | Luciferase reporter assay | [113] |
| Breast cancer | SNHG12 and miR-15a-5p | SALL4 | Long non-coding RNA (lncRNA) small nucleolar RNA host gene 12 (SNHG12) promotes proliferation, migration, and invasion and inhibits apoptosis in breast cancer by upregulating SALL4 expression via sponging miR-15a-5p; SALL4 is a direct target of miR-15a-5p | qRT-PCR, Western blot, and Luciferase reporter assay | [114] |
| Renal cell carcinoma | miR-942 | SALL1 | miR-942 affects survival of patients with renal cell carcinoma by negatively regulating the expression of SALL1 | RNA-seq and qRT-PCR | [115] |
| Prostate cancer | miR-4286 | SALL1 | miR-4286 regulates proliferation and apoptosis in PCa cells by directly targeting the 3′UTR of SALL1 mRNA | qRT-PCR and Luciferase reporter assay | [116] |
| Lung cancer | HOXA11-AS and miR-3619-5p | SALL4 | lncRNA homeobox A11 antisense (HOXA11-AS) promotes proliferation, migration, invasion, and glycolysis in non-small cell lung cancer (NSCLC) cells by upregulating SALL4 expression via sponging miR-3619-5p; SALL4 is a direct target of miR-3619-5p | qRT-PCR, Western blot, and Luciferase reporter assay | [117] |
| Osteosarcoma | ZEB2-AS1 and miR-107 | SALL4 | lncRNA ZEB2-AS1 (ZEB2-AS1) promotes proliferation, invasion, and metastasis and inhibits apoptosis in osteosarcoma cells by upregulating SALL4 expression via sponging miR-107; SALL4 is a direct target of miR-107 | qRT-PCR, Luciferase assay, and RNA pull-down assay | [118] |
| Hepatocellular carcinoma | miR-296-5p | SALL4 | miR-296-5p inhibits stemness potency of hepatocellular carcinoma (HCC) cells via the Brg1/Sall4 axis; Brg1 binds to the SALL4 promoter | qRT-PCR, Western blot, Luciferase reporter assay, and Chromatin immunoprecipitation (ChIP) assay | [119] |
| Hepatocellular carcinoma | miR-15a | SALL4 | Exosomal miR-15a reduces proliferation, migration, invasion, and survival by directly targeting SALL4 | qRT-PCR, Western blot, and Luciferase reporter assay | [120] |
| Cancer Type | SALL Member | Expression Levels | Genetic Alteration/Regulation | Association With Cancer/Biological Process | Proposed Cancer Role | Ref. |
|---|---|---|---|---|---|---|
| Lung | SALL1 | High | Undescribed | Expression correlated with lower overall survival of NSCLC patients | Oncogene | [122] |
| Lung | SALL2 | Low | LOH | Undescribed | Undescribed | [71] |
| Lung | SALL4 | High | Undescribed | Expressed in 88% of the lung cancer samples May be used as a diagnostic marker | Oncogene | [123] |
| Lung | SALL4 | High | Undescribed | SALL4 knockdown inhibits cell proliferation by cell cycle arrest at the GO/G1 phase Loss of SALL4 function inhibits migration, invasion and reduces the transplanted tumors size in an in vivo model | Oncogene | [43] |
| Lung | SALL4 | High | Undescribed | SALL4 silencing sensitizes cells to cisplatin, carboplatin, and paclitaxel treatment | Oncogene | [124] |
| Esophageal | SALL1 | Low | Hypermethylation | SALL1, ADHFE1, EOMES, and TFPI2 are proposed as part of a tumor suppressors panel with diagnostic relevance | Tumor suppressor | [93,125] |
| Esophageal | SALL2 | Low in radioresistant ESCC cell lines | Hypermethylation | SALL2 overexpression enhances apoptosis after radiation and decreases migration, viability, and cisplatin resistance in TE-1/R and Eca-109/R cell lines | Tumor suppressor | [55] |
| Esophageal | SALL4 | High | Undescribed | SALL4 silencing in ESCC cells is associated with suppressing cell migration, invasion, viability, and drug resistance in vivo SALL4 knockdown reduces epithelial-mesenchymal transition by targeting the Wnt/β-catenin signaling pathway | Oncogene | [42,126] |
| Bladder | SALL2 | Low | LOH | Undescribed | Tumor suppressor | [70] |
| Bladder | SALL3 | Low | Hypermethylation | SALL3, CFTR, and TWIST1 are proposed as disease recurrence predictors | Tumor suppressor | [127,128] |
| Testicular tumors | SALL4 | High | Undescribed | SALL4 is a novel sensitive and specific marker for testicular germ cell tumors | Oncogene | [129] |
| Kidney | SALL1 | Low | miR-942 | SALL1 inhibition plays a potential role in sunitinib resistance in RCC patients | Tumor suppressor | [115] |
| Wilms’ tumor | SALL1 | High | Undescribed | Undescribed | Oncogene | [130,131] |
| Wilms’ tumor | SALL2 | High | Undescribed | SALL2 was identified as one of the 27 signature genes highly expressed by comparing tumor samples with normal fetal kidneys | Oncogene | [132] |
| Kidney | SALL3 | Low | Methylation | SALL3 downregulation may contribute to genome hypermethylation similar to VHL | Tumor suppressor | [133] |
| Wilms’ tumor | SALL4 | High | Undescribed | Undescribed | Oncogene | [134] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez, C.; Quiroz, A.; Benítez-Riquelme, D.; Riffo, E.; Castro, A.F.; Pincheira, R. SALL Proteins; Common and Antagonistic Roles in Cancer. Cancers 2021, 13, 6292. https://doi.org/10.3390/cancers13246292
Álvarez C, Quiroz A, Benítez-Riquelme D, Riffo E, Castro AF, Pincheira R. SALL Proteins; Common and Antagonistic Roles in Cancer. Cancers. 2021; 13(24):6292. https://doi.org/10.3390/cancers13246292
Chicago/Turabian StyleÁlvarez, Claudia, Aracelly Quiroz, Diego Benítez-Riquelme, Elizabeth Riffo, Ariel F. Castro, and Roxana Pincheira. 2021. "SALL Proteins; Common and Antagonistic Roles in Cancer" Cancers 13, no. 24: 6292. https://doi.org/10.3390/cancers13246292
APA StyleÁlvarez, C., Quiroz, A., Benítez-Riquelme, D., Riffo, E., Castro, A. F., & Pincheira, R. (2021). SALL Proteins; Common and Antagonistic Roles in Cancer. Cancers, 13(24), 6292. https://doi.org/10.3390/cancers13246292






