Interstitial High-Dose-Rate Brachytherapy of Liver Metastases in Oligometastatic Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Brachytherapy
2.3. Follow-Up and Treatment Complications
2.4. Statistics
3. Results
3.1. Patients
3.2. Brachytherapy
3.3. Treatment Complications
3.4. Follow-Up
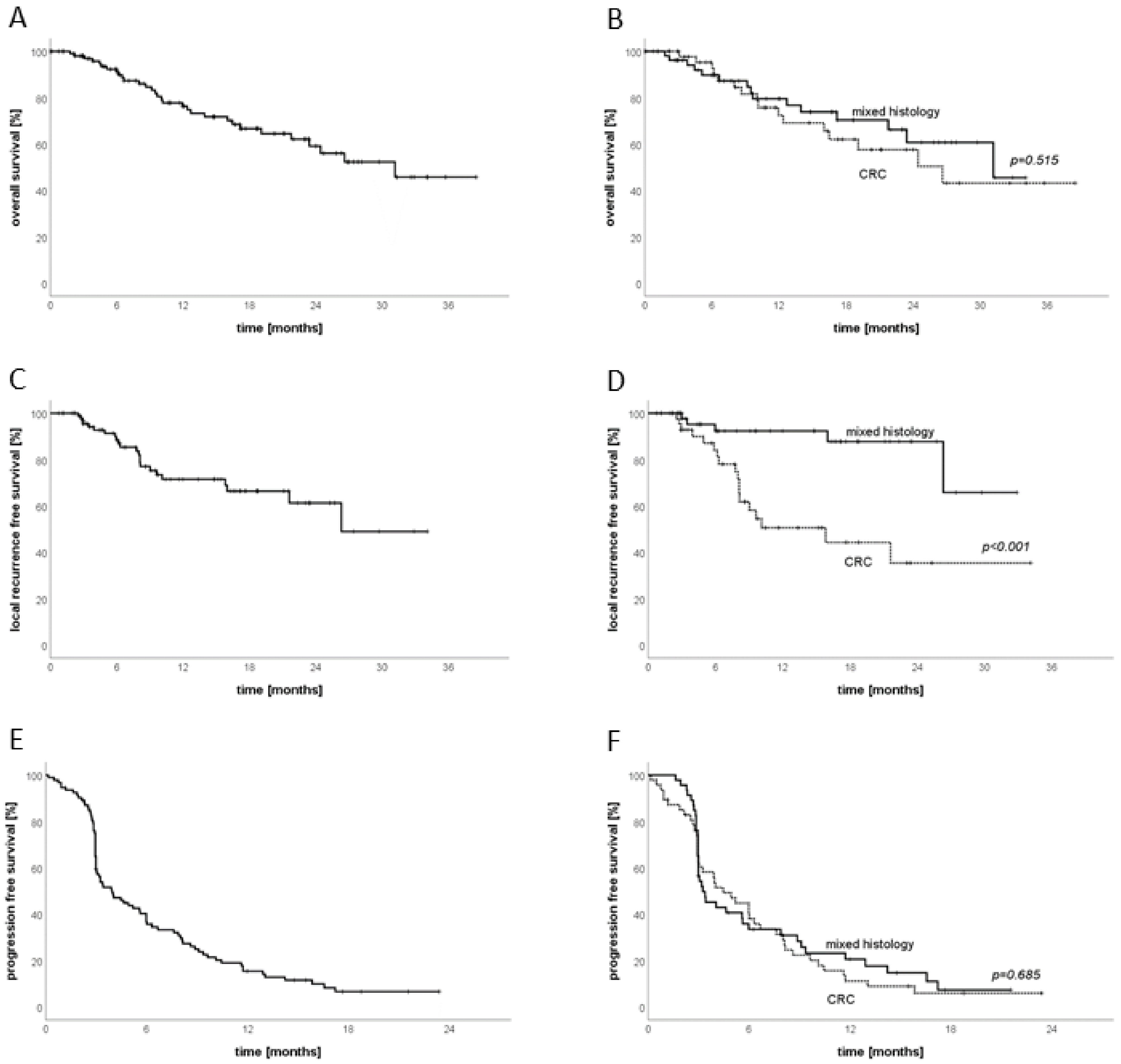
3.5. Sub-Group Analysis CRC
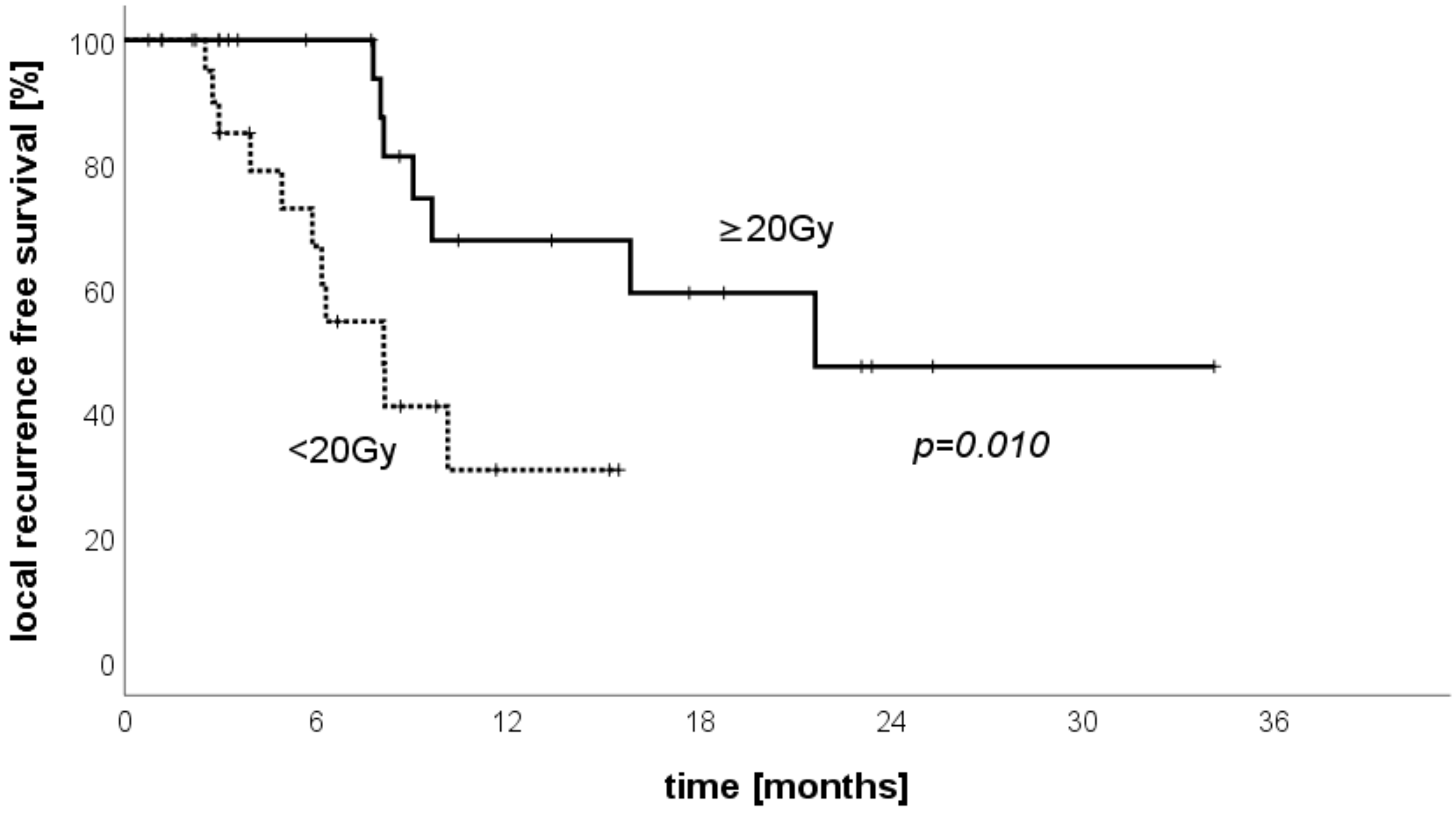
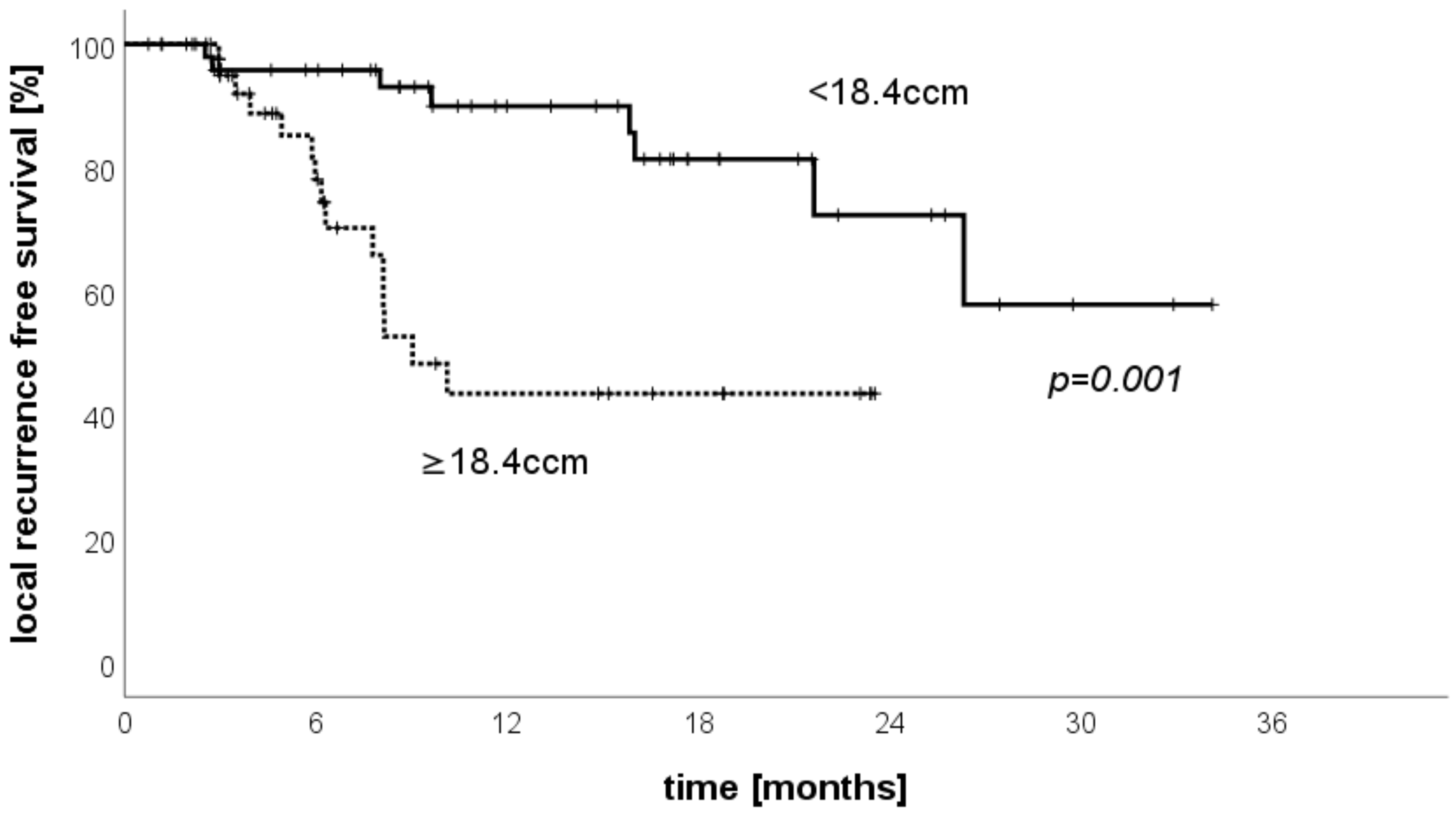
3.6. LRFS Stratified by Treated Volume per Patient
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guckenberger, M.; Lievens, Y.; Bouma, A.B.; Collette, L.; Dekker, A.; Desouza, N.M.; Dingemans, A.-M.C.; Fournier, B.; Hurkmans, C.; Lecouvet, F.E.; et al. Characterisation and classification of oligometastatic disease: A European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020, 21, e18–e28. [Google Scholar] [CrossRef] [Green Version]
- Lievens, Y.; Guckenberger, M.; Gomez, D.; Hoyer, M.; Iyengar, P.; Kindts, I.; Romero, A.M.; Nevens, D.; Palma, D.; Park, C.; et al. Defining oligometastatic disease from a radiation oncology perspective: An ESTRO-ASTRO consensus document. Radiother. Oncol. 2020, 148, 157–166. [Google Scholar] [CrossRef]
- Ost, P.; Reynders, D.; Decaestecker, K.; Fonteyne, V.; Lumen, N.; De Bruycker, A.; Lambert, B.; Delrue, L.; Bultijnck, R.; Claeys, T.; et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J. Clin. Oncol. 2018, 36, 446–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, P.G.B.; Yaremko, B.P.; et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): A randomised, phase 2, open-label trial. Lancet 2019, 393, 2051–2058. [Google Scholar] [CrossRef]
- Iyengar, P.; Wardak, Z.; Gerber, D.; Tumati, V.; Ahn, C.; Hughes, R.S.; Dowell, J.E.; Cheedella, N.; Nedzi, L.; Westover, K.; et al. Consolidative Radiotherapy for Limited Metastatic Non–Small-Cell Lung Cancer. JAMA Oncol. 2018, 4, e173501. [Google Scholar] [CrossRef]
- Gomez, D.R.; Tang, C.; Zhang, J.; Blumenschein, G.R.; Hernandez, M.; Lee, J.J.; Ye, R.; Palma, D.A.; Louie, A.V.; Camidge, D.R.; et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients with Oligometastatic Non–Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J. Clin. Oncol. 2019, 37, 1558–1565. [Google Scholar] [CrossRef]
- Heinemann, V.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.-E.; Heintges, T.; Lerchenmüller, C.; Kahl, C.; Seipelt, G.; et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 1065–1075. [Google Scholar] [CrossRef]
- Kopetz, S.; Chang, G.J.; Overman, M.J.; Eng, C.; Sargent, D.; Larson, D.W.; Grothey, A.; Vauthey, J.-N.; Nagorney, D.M.; McWilliams, R.R. Improved Survival in Metastatic Colorectal Cancer Is Associated With Adoption of Hepatic Resection and Improved Chemotherapy. J. Clin. Oncol. 2009, 27, 3677–3683. [Google Scholar] [CrossRef]
- Nordlinger, B.; Sorbye, H.; Glimelius, B.; Poston, G.J.; Schlag, P.M.; O Rougier, P.; Bechstein, W.; Primrose, J.N.; Walpole, E.; Finch-Jones, M.; et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): Long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013, 14, 1208–1215. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; van Krieken, J.H.; Aderka, D.; Aguilar, E.A.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- Ruers, T.; Van Coevorden, F.; Punt, C.J.A.; Pierie, J.-P.E.N.; Borel-Rinkes, I.; Ledermann, J.A.; Poston, G.; Bechstein, W.; Lentz, M.-A.; Mauer, M.; et al. Local Treatment of Unresectable Colorectal Liver Metastases: Results of a Randomized Phase II Trial. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef] [PubMed]
- Walter, F.; Nierer, L.; Rottler, M.; Duque, A.S.; Weingandt, H.; Well, J.; Shpani, R.; Landry, G.; Seidensticker, M.; Streitparth, F.; et al. Comparison of liver exposure in CT-guided high-dose rate (HDR) interstitial brachytherapy versus SBRT in hepatocellular carcinoma. Radiat. Oncol. 2021, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Bodensohn, R.; Kaempfel, A.-L.; Fleischmann, D.F.; Hadi, I.; Hofmaier, J.; Garny, S.; Reiner, M.; Forbrig, R.; Corradini, S.; Thon, N.; et al. Simultaneous stereotactic radiosurgery of multiple brain metastases using single-isocenter dynamic conformal arc therapy: A prospective monocentric registry trial. Strahlenther. Onkol. 2021, 197, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Klement, R.; Hoerner-Rieber, J.; Adebahr, S.; Andratschke, N.; Blanck, O.; Boda-Heggemann, J.; Duma, M.; Eble, M.; Eich, H.; Flentje, M.; et al. Stereotactic body radiotherapy (SBRT) for multiple pulmonary oligometastases: Analysis of number and timing of repeat SBRT as impact factors on treatment safety and efficacy. Radiother. Oncol. 2018, 127, 246–252. [Google Scholar] [CrossRef]
- Rogowski, P.; von Bestenbostel, R.; Walter, F.; Straub, K.; Nierer, L.; Kurz, C.; Landry, G.; Reiner, M.; Auernhammer, C.; Belka, C.; et al. Feasibility and Early Clinical Experience of Online Adaptive MR-Guided Radiotherapy of Liver Tumors. Cancers 2021, 13, 1523. [Google Scholar] [CrossRef]
- Giaj-Levra, N.; Niyazi, M.; Figlia, V.; Napoli, G.; Mazzola, R.; Nicosia, L.; Corradini, S.; Ruggieri, R.; Minniti, G.; Alongi, F. Feasibility and preliminary clinical results of linac-based Stereotactic Body Radiotherapy for spinal metastases using a dedicated contouring and planning system. Radiat. Oncol. 2019, 14, 184–188. [Google Scholar] [CrossRef]
- Ricke, J.; Mohnike, K.; Pech, M.; Seidensticker, M.; Rühl, R.; Wieners, G.; Gaffke, G.; Kropf, S.; Felix, R.; Wust, P. Local Response and Impact on Survival After Local Ablation of Liver Metastases From Colorectal Carcinoma by Computed Tomography–Guided High-Dose-Rate Brachytherapy. Int. J. Radiat. Oncol. 2010, 78, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Böning, G.; Büttner, L.; Jonczyk, M.; Lüdemann, W.M.; Denecke, T.; Schnapauff, D.; Wieners, G.; Wust, P.; Gebauer, B. Complications of Computed Tomography-Guided High-Dose-Rate Brachytherapy (CT-HDRBT) and Risk Factors: Results from More than 10 Years of Experience. Cardiovasc. Interv. Radiol. 2020, 43, 284–294. [Google Scholar] [CrossRef]
- Mohnike, K.; Wieners, G.; Schwartz, F.; Seidensticker, M.; Pech, M.; Ruehl, R.; Wust, P.; Lopez-Hänninen, E.; Gademann, G.; Peters, N.; et al. Computed Tomography–Guided High-Dose-Rate Brachytherapy in Hepatocellular Carcinoma: Safety, Efficacy, and Effect on Survival. Int. J. Radiat. Oncol. 2010, 78, 172–179. [Google Scholar] [CrossRef]
- Mohnike, K.; Sauerland, H.; Seidensticker, M.; Hass, P.; Kropf, S.; Seidensticker, R.; Friebe, B.; Fischbach, F.; Fischbach, K.; Powerski, M.; et al. Haemorrhagic Complications and Symptomatic Venous Thromboembolism in Interventional Tumour Ablations: The Impact of Peri-interventional Thrombosis Prophylaxis. Cardiovasc. Interv. Radiol. 2016, 39, 1716–1721. [Google Scholar] [CrossRef]
- Mohnike, K.; Wolf, S.; Damm, R.; Seidensticker, M.; Seidensticker, R.; Fischbach, F.; Peters, N.; Hass, P.; Gademann, G.; Pech, M.; et al. Radioablation of liver malignancies with interstitial high-dose-rate brachytherapy. Strahlenther. Onkol. 2016, 192, 288–296. [Google Scholar] [CrossRef]
- Wieners, G.; Pech, M.; Hildebrandt, B.; Peters, N.; Nicolaou, A.; Mohnike, K.; Seidensticker, M.; Sawicki, M.; Wust, P.; Ricke, J. Phase II Feasibility Study on the Combination of Two Different Regional Treatment Approaches in Patients with Colorectal “Liver-Only” Metastases: Hepatic Interstitial Brachytherapy Plus Regional Chemotherapy. Cardiovasc. Interv. Radiol. 2009, 32, 937–945. [Google Scholar] [CrossRef]
- Collettini, F.; Lutter, A.; Schnapauff, D.; Hildebrandt, B.; Puhl, G.; Denecke, T.; Wust, P.; Gebauer, B. Unresectable Colorectal Liver Metastases: Percutaneous Ablation Using CT-Guided High-Dose-Rate Brachytherapy (CT-HDBRT). RöFo Fortschr. Dem Geb. Röntgenstrahlen Bildgeb. Verfahr. 2014, 186, 606–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wieners, G.; Mohnike, K.; Peters, N.; Bischoff, J.; Kleine-Tebbe, A.; Seidensticker, R.; Seidensticker, M.; Gademann, G.; Wust, P.; Pech, M.; et al. Treatment of hepatic metastases of breast cancer with CT-guided interstitial brachytherapy—A phase II-study. Radiother. Oncol. 2011, 100, 314–319. [Google Scholar] [CrossRef]
- Geisel, D.; Collettini, F.; Denecke, T.; Grieser, C.; Flörcken, A.; Wust, P.; Hamm, B.; Gebauer, B. Treatment for liver metastasis from renal cell carcinoma with computed-tomography-guided high-dose-rate brachytherapy (CT-HDRBT): A case series. World J. Urol. 2012, 31, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- Geisel, D.; Denecke, T.; Collettini, F.; Grieser, C.; Wust, P.; Thuss-Patience, P.; Hamm, B.; Gebauer, B. Treatment of hepatic metastases from gastric or gastroesophageal adenocarcinoma with computed tomography-guided high-dose-rate brachytherapy (CT-HDRBT). Anticancer Res. 2012, 32, 5453–5458. [Google Scholar] [PubMed]
- Collettini, F.; Golenia, M.; Schnapauff, D.; Poellinger, A.; Denecke, T.; Wust, P.; Riess, H.; Hamm, B.; Gebauer, B. Percutaneous Computed Tomography–guided High-Dose-Rate Brachytherapy Ablation of Breast Cancer Liver Metastases: Initial Experience with 80 Lesions. J. Vasc. Interv. Radiol. 2012, 23, 618–626. [Google Scholar] [CrossRef]
- Wieners, G.; Schippers, A.C.; Collettini, F.; Schnapauff, D.; Hamm, B.; Wust, P.; Riess, H.; Gebauer, B. CT-guided high-dose-rate brachytherapy in the interdisciplinary treatment of patients with liver metastases of pancreatic cancer. Hepatobiliary Pancreat. Dis. Int. 2015, 14, 530–538. [Google Scholar] [CrossRef]
- Schippers, A.C.; Collettini, F.; Steffen, I.G.; Wieners, G.; Denecke, T.; Pavel, M.; Wust, P.; Gebauer, B. Initial Experience with CT–Guided High-Dose-Rate Brachytherapy in the Multimodality Treatment of Neuroendocrine Tumor Liver Metastases. J. Vasc. Interv. Radiol. 2017, 28, 672–682. [Google Scholar] [CrossRef]
- Omari, J.; Drewes, R.; Matthias, M.; Mohnike, K.; Seidensticker, M.; Seidensticker, R.; Streitparth, T.; Ricke, J.; Powerski, M.; Pech, M. Treatment of metastatic, imatinib refractory, gastrointestinal stroma tumor with image-guided high-dose-rate interstitial brachytherapy. Brachytherapy 2019, 18, 63–70. [Google Scholar] [CrossRef]
- Kieszko, D.; Cisek, P.; Kordzińska-Cisek, I.; Grzybowska-Szatkowska, L. Treatment of hepatic metastases with computed tomography-guided interstitial brachytherapy. Oncol. Lett. 2018, 15, 8717–8722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omari, J.; Drewes, R.; Othmer, M.; Hass, P.; Pech, M.; Powerski, M.; Orthmer, M. Treatment of metastatic gastric adenocarcinoma with image-guided high-dose rate, interstitial brachytherapy as second-line or salvage therapy. Diagn. Interv. Radiol. 2019, 25, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Omari, J.; Heinze, C.; Damm, R.; Hass, P.; Janitzky, A.; Wendler, J.J.; Seidensticker, M.; Ricke, J.; Powerski, M.J.; Pech, M. Radioablation of Hepatic Metastases from Renal Cell Carcinoma with Image-guided Interstitial Brachytherapy. Anticancer Res. 2019, 39, 2501–2508. [Google Scholar] [CrossRef]
- Drewes, R.; Omari, J.; Manig, M.; Seidensticker, M.; Hass, P.; Ricke, J.; Powerski, M.; Pech, M. Treatment of hepatic pancreatic ductal adenocarcinoma metastases with high-dose-rate image-guided interstitial brachytherapy: A single center experience. J. Contemp. Brachyther. 2019, 11, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Heinze, C.; Omari, J.; Damm, R.; Hass, P.; Brunner, T.; Surov, A.; Seidesticker, R.; Seidensticker, M.; Ricke, J.; Powerski, M.; et al. Interstitial Brachytherapy for Limited (/=4 cm) Hepatic Metastases from Rare and Less Common Cancers. Anticancer. Res. 2020, 40, 4281–4289. [Google Scholar] [CrossRef] [PubMed]
- Duque, A.S.; Corradini, S.; Kamp, F.; Seidensticker, M.; Streitparth, F.; Kurz, C.; Walter, F.; Parodi, K.; Verhaegen, F.; Ricke, J.; et al. The dosimetric impact of replacing the TG-43 algorithm by model based dose calculation for liver brachytherapy. Radiat. Oncol. 2020, 15, 60. [Google Scholar] [CrossRef] [Green Version]
- Duque, A.S.; van Wagenberg, T.; Seidensticker, M.; Streitparth, F.; Walter, F.; Parodi, K.; Verhaegen, F.; Ricke, J.; Belka, C.; Fonseca, G.P.; et al. Validation of the collapsed cone algorithm for HDR liver brachytherapy against Monte Carlo simulations. Brachyther. 2021, 20, 936–947. [Google Scholar] [CrossRef]
- Girard, P.; Gossot, D.; Mariolo, A.; Caliandro, R.; Seguin-Givelet, A.; Girard, N. Oligometastases for Clinicians: Size Matters. J. Clin. Oncol. 2021, 39, 2643–2646. [Google Scholar] [CrossRef]
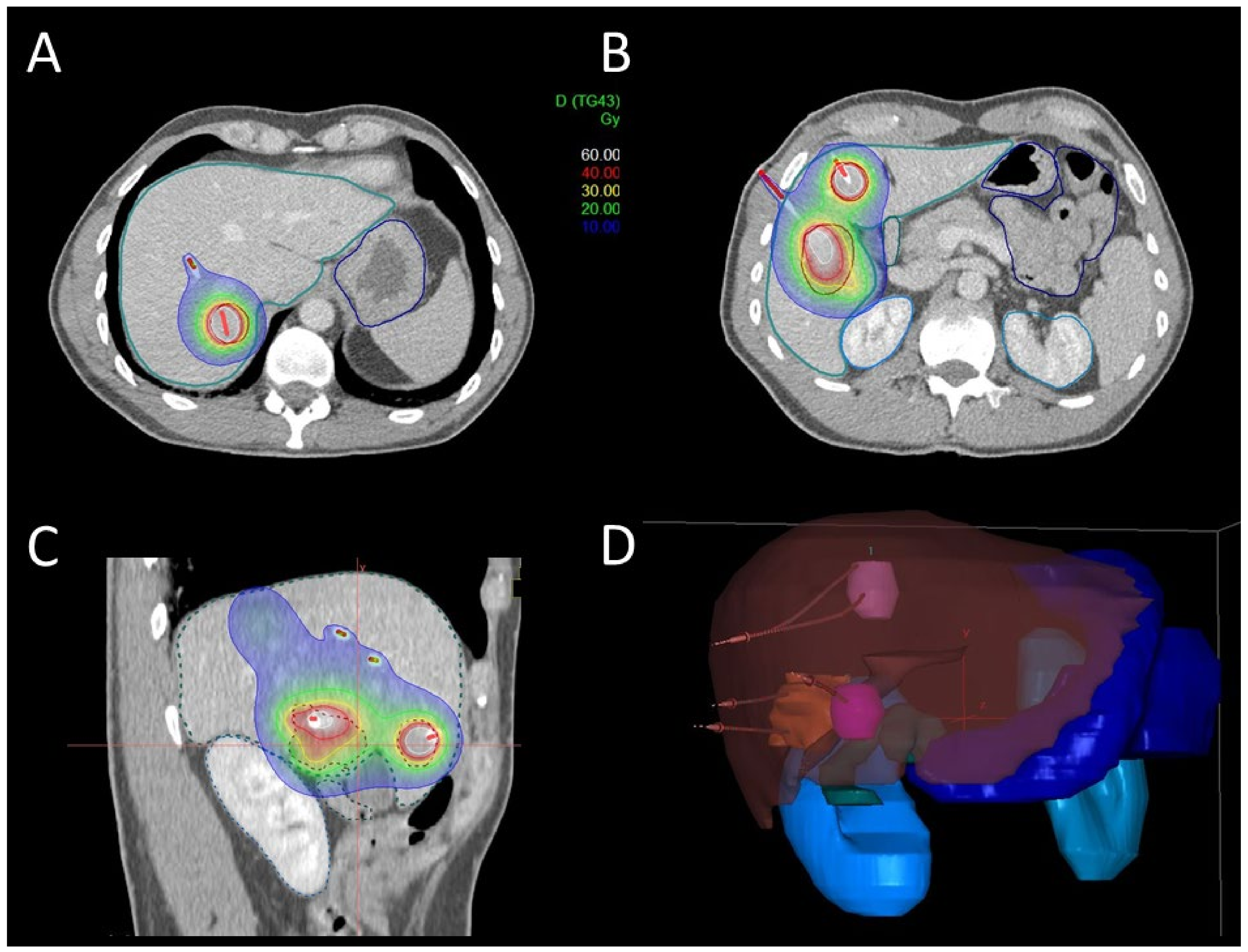
| Patient Characteristics | n = 106 | Treatment Characteristics | n = 141 |
|---|---|---|---|
| Age (years) | 66 ± 12 | Treated metastases | |
| Gender | 1 | 72 | |
| Female | 54 | 2 | 44 |
| Male | 52 | 3 | 17 |
| Histology | 4 | 7 | |
| Sarcoma | 4 | 5 | 1 |
| NET | 7 | Liver volume (mean, SD) | 1466 ± 336 ccm |
| GIST | 1 | Liver exposure (mean, SD) | |
| Pituitary gland carcinoma | 1 | 5 Gy | 385 ± 310 ccm (26%) |
| Breast cancer | 11 | 10 Gy | 184 ± 169 ccm (13%) |
| Pancreatic cancer | 10 | 15 Gy | 110 ± 107 ccm (8%) |
| Gastric cancer | 4 | Catheters (number) | |
| Intestinal | 1 | 1 | 35 |
| Colon cancer | 15 | 2 | 49 |
| Rectosigmoid | 39 | 3 | 22 |
| Prostate cancer | 2 | 4 | 21 |
| Renal cell carcinoma | 1 | 5 | 7 |
| Adrenal gland | 1 | 6 | 4 |
| Gall bladder cancer | 1 | >6 | 3 |
| NSCLC | 5 | PTV diameter (mean, SD) | 3.2 ± 1.9 cm |
| Ovarian cancer | 3 | PTV volume (mean, SD) | 23.3 ± 58.1 ccm |
| Other metastatic sites | 45 | PTV dose coverage (mean, SD) | |
| Pulmonary | 21 | V100 | 23 ± 58 ccm |
| Bone | 11 | V98 | 23 ± 57 ccm |
| Lymph nodes | 18 | V95 | 22 ± 55 ccm |
| Others | 6 | V90 | 21 ± 52 ccm |
| Prior local treatment liver | 56 | D100 | 19 ± 5 Gy |
| Multiple | 14 | D98 | 23 ± 6 Gy |
| Surgery | 37 | D95 | 25 ± 6 Gy |
| Other LAT | 33 | D90 | 28 ± 7 Gy |
| Study (Year) | Entity | Patients | Lesion Count | Lesion Size (cm) | Applied Dose (D100) | LTCR at FU (Months) | OS Median |
|---|---|---|---|---|---|---|---|
| Ricke (2010) [17] | CRC | 73 | 200 | 3.6 (1–13.5) | 3 dose groups 12.8–18.8 Gy | 74.9% at 34 m | 23.4 m (26 m with CTX) |
| Wieners (2009) [22] | CRC | 33 | 138 | 4.6 (1–12) | 3 dose groups 14.2–21.2 Gy | 87% at 6 m 76% at 12 m 69% at 24 m | n/a |
| Collettini (2014) [23] | CRC | 80 | 179 | 2.85 (0.8–10.7) | 19.1 Gy (15–20 Gy) | 88.3% at 12 m81.2% at 24 m68% at 36 m | 18 m |
| Wieners (2011) [24] | Breast cancer | 41 | 115 | 4.4 (1.0–11) | 18.5 Gy (12–25 Gy) | 97% at 6 m 93.5% at 18 m | n/a |
| Geisel (2013) [25] | Renal cell carcinoma | 10 | 16 | 3.8 (1–8.2) | n/a | 93.8% at avg. FU 21.6 m ±13.7 m) | n/a |
| Geisel (2012) [26] | Gastro-esophageal cancer | 8 | 12 | 4.6 (1.4–6.8) | 21 Gy (5–29 Gy) | 100% at 8.4 m (±6.8 m FU) | n/a |
| Collettini (2012) [27] | Breast cancer | 37 | 80 | 2.6 (0.8–7.4) | 18.6 Gy (±2.27 Gy) | 94.6% at 12 m | 18 m (3–39 m) |
| Wieners (2015) [28] | Pancreatic cancer | 41 | 49 | 2 (1.0–7.3) | 18.1 Gy | 91% at 12 m | 8.6 m (1.5–55.3 m) |
| Schippers (2016) [29] | NET | 27 | 52 | 2.1 (0.7–11.0) | 15–20 Gy (target) | 92% at 1 y 83% at 3 y | 36 m (4.2–106.1 m) |
| Omari (2018) [30] | GIST | 10 | 40 | 2.4 (0.6–11.2) | 15 Gy (6.7–21.96 Gy) | 97.5% at 25 m median FU | 37.3 m (11.4–89.7 m) |
| Heinze (2018) | Anal cancer | 7 | 38 | 1.2 (0.4–6.2) | 16.2 (12.0–32.6 Gy) | 97.4% at 15 m | 25.2 m (6.5–51 m) |
| Kieszko (2018) [31] | Mixed histologies | 61 | 73 | <8 | 13 Gy (7–20 Gy) | 88.7% at 6 m 70.7% at 12 m | 96.7% at 6 m 79.6% at 12 m |
| Omari (2019) [32] | Gastric cancer | 12 | 36 | 2 (1–10.2) | 19.9 Gy (5.4–22.5 Gy) | 89% at 8.3 m median FU | 11.4 m (4.3–47 m) |
| Omari (2019) [33] | Renal cell carcinoma | 14 | 54 | 1.8 (0.5–13.9) | 16.1 Gy | 92.6% avg. FU 10.2 (range 2.4–73.6 m) | 51.2 m (1.0–27.8 m) |
| Drewes (2019) [34] | Pancreatic cancer | 16 | 45 | 2.2 (1.0–11.2) | 21 Gy (5–29.1 Gy) | 87% at 3.3 m median FU | 8.9 m (3.1–29.3 m) |
| Heinze (2020) [35] | Mixed histologies | 59 | 194 | A:1.6 (0.4–3.8) B:6 (4–13.9) | 17.1 Gy (5–29.1 Gy) | 90.2% at 6 m median FU | n/a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walter, F.; Rottler, M.; Nierer, L.; Landry, G.; Well, J.; Rogowski, P.; Mohnike, K.; Seidensticker, M.; Ricke, J.; Belka, C.; et al. Interstitial High-Dose-Rate Brachytherapy of Liver Metastases in Oligometastatic Patients. Cancers 2021, 13, 6250. https://doi.org/10.3390/cancers13246250
Walter F, Rottler M, Nierer L, Landry G, Well J, Rogowski P, Mohnike K, Seidensticker M, Ricke J, Belka C, et al. Interstitial High-Dose-Rate Brachytherapy of Liver Metastases in Oligometastatic Patients. Cancers. 2021; 13(24):6250. https://doi.org/10.3390/cancers13246250
Chicago/Turabian StyleWalter, Franziska, Maya Rottler, Lukas Nierer, Guillaume Landry, Justus Well, Paul Rogowski, Konrad Mohnike, Max Seidensticker, Jens Ricke, Claus Belka, and et al. 2021. "Interstitial High-Dose-Rate Brachytherapy of Liver Metastases in Oligometastatic Patients" Cancers 13, no. 24: 6250. https://doi.org/10.3390/cancers13246250
APA StyleWalter, F., Rottler, M., Nierer, L., Landry, G., Well, J., Rogowski, P., Mohnike, K., Seidensticker, M., Ricke, J., Belka, C., & Corradini, S. (2021). Interstitial High-Dose-Rate Brachytherapy of Liver Metastases in Oligometastatic Patients. Cancers, 13(24), 6250. https://doi.org/10.3390/cancers13246250






