Malignant Vascular Tumors of the Head and Neck—Which Type of Therapy Works Best?
Abstract
Simple Summary
Abstract
1. Introduction
2. Angiosarcomas
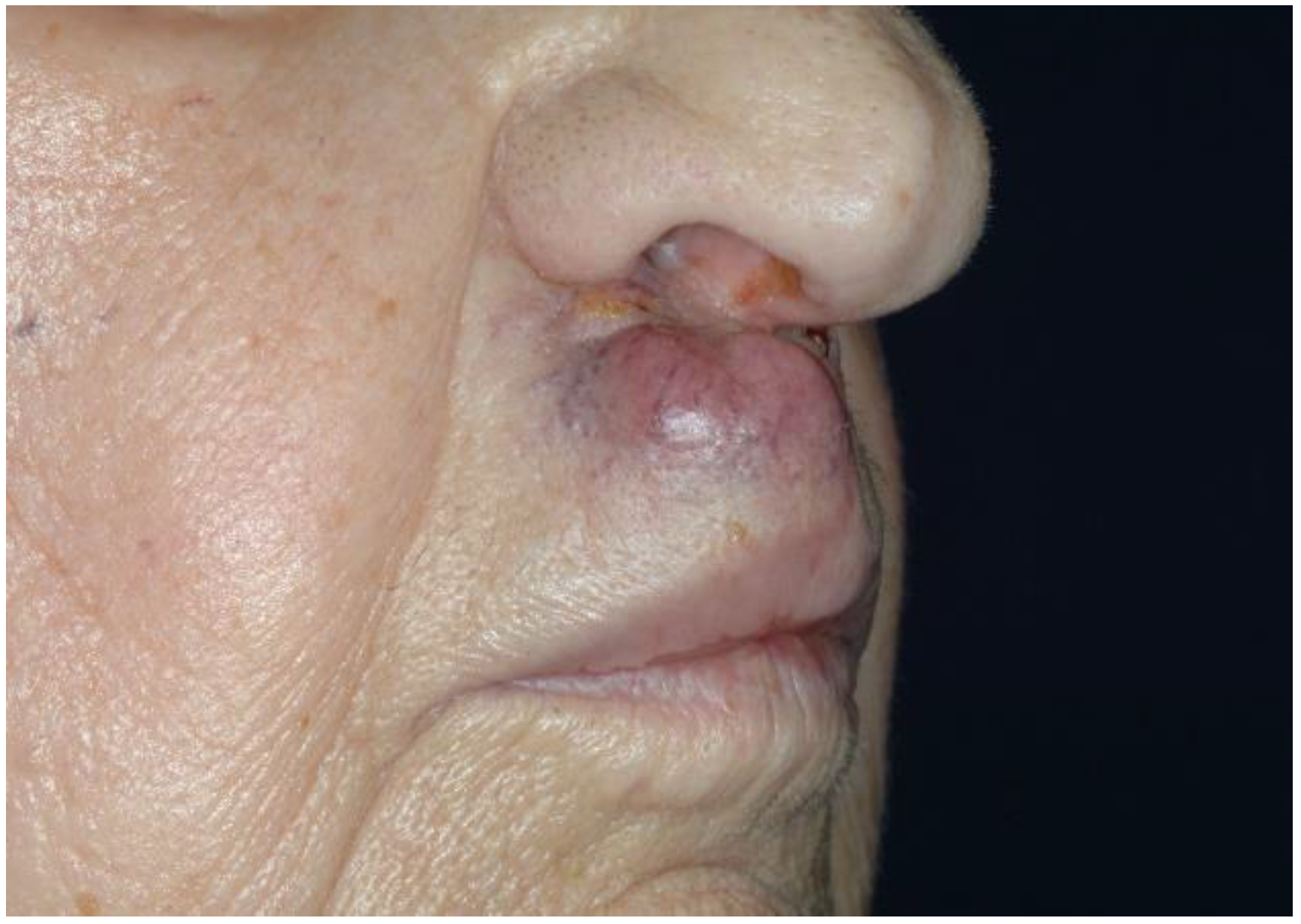
2.1. Clinical Presentation
2.2. Staging
2.3. Histology
2.4. Treatment
2.4.1. Localized Disease
2.4.2. Metastatic Disease
3. Epithelioid Hemangioendothelioma
3.1. Etiology
3.2. Clinical Presentation
3.3. Histology
3.4. Staging
3.5. Therapy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- ISSVA Classification 2018. Available online: http://www.issva.org/UserFiles/file/ISSVA-Classification-2018.pdf (accessed on 7 December 2021).
- Alarhayem, A.Q.; Davis, M.G. Malignant Vascular Tumors: A Nationwide Analysis. J. Vasc. Surg. 2016, 63, 195–196. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Nagasawa, H.; Suzuki, T.; Fujii, E.; Iwaki, H.; Takagi, M.; Amagasa, T. Sarcomas of the oral and maxillofacial region: A review of 32 cases in 25 years. Clin. Oral Investig. 2004, 8, 52–55. [Google Scholar] [CrossRef]
- Antonescu, C. Malignant vascular tumors—An update. MOD. Pathol. 2014, 27, S30–S38. [Google Scholar] [CrossRef] [PubMed]
- Young, R.J.; Brown, N.; Reed, M.W.; Hughes, D.J.; Woll, P. Angiosarcoma. Lancet Oncol. 2010, 11, 983–991. [Google Scholar] [CrossRef]
- Weiss, S.W.; Goldblum, J.R. Enzinger & Weiss’s Soft Tissue Tumors, 5th ed.; Elsevier Health Sciences, Mosby: Philadelphia, PA, USA, 2008. [Google Scholar]
- Abraham, J.A.; Hornicek, F.J.; Kaufman, A.M.; Harmon, D.C.; Springfield, D.S.; Raskin, K.A.; Mankin, H.J.; Kirsch, D.G.; Rosenberg, A.E.; Nielsen, G.P.; et al. Treatment and Outcome of 82 Patients with Angiosarcoma. Ann. Surg. Oncol. 2007, 14, 1953–1967. [Google Scholar] [CrossRef] [PubMed]
- Naka, N.; Ohsawa, M.; Tomita, Y.; Kanno, H.; Uchida, A.; Myoui, A.; Aozasa, K. Prognostic factors in angiosarcoma: A multivariate analysis of 55 cases. J. Surg. Oncol. 1996, 61, 170–176. [Google Scholar] [CrossRef]
- Fayette, J.; Martin, E.; Piperno-Neumann, S.; Le Cesne, A.; Robert, C.; Bonvalot, S.; Ranchère, D.; Pouillart, P.; Coindre, J.M.; Blay, J.Y. Angiosarcomas, a heterogeneous group of sarcomas with specific behavior depending on primary site: A retrospective study of 161 cases. Ann. Oncol. 2007, 18, 2030–2036. [Google Scholar] [CrossRef]
- Von Mehren, M.; Kane, J.M.; Bui, M.M.; Choy, E.; Connelly, M.; Dry, S.; Ganjoo, K.N.; George, S.; Gonzalez, R.J.; Heslin, M.J.; et al. NCCN Guidelines Insights: Soft Tissue Sarcoma, Version 1.2021. J. Natl. Compr. Cancer Netw. 2020, 18, 1604–1612. [Google Scholar] [CrossRef]
- Razek, A.A.; Huang, B.Y. Soft Tissue Tumors of the Head and Neck: Imaging-based Review of the WHO Classification. Radiographics 2011, 31, 1923–1954. [Google Scholar] [CrossRef]
- Ha, S.C.; Oh, J.S.; Roh, J.-L.; Moon, H.; Kim, J.S.; Cho, K.-J.; Choi, S.-H.; Nam, S.Y.; Kim, S.Y. Pretreatment tumor SUVmax predicts disease-specific and overall survival in patients with head and neck soft tissue sarcoma. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Nakamoto, Y.; Ishimori, T.; Saga, T.; Togashi, K. Prognostic Value of Quantitative Parameters of 18F-FDG PET/CT for Patients With Angiosarcoma. Am. J. Roentgenol. 2020, 214, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Italiano, A.; Chen, C.-L.; Thomas, R.; Breen, M.; Bonnet, F.; Sevenet, N.; Longy, M.; Maki, R.G.; Coindre, J.-M.; Antonescu, C.R. Alterations of the p53 and PIK3CA/AKT/mTOR pathways in angiosarcomas. Cancer 2012, 118, 5878–5887. [Google Scholar] [CrossRef]
- Pawlik, T.M.; Paulino, A.F.; McGinn, C.J.; Baker, L.H.; Cohen, D.S.; Morris, J.S.; Rees, R.; Sondak, V.K. Cutaneous angiosarcoma of the scalp. Cancer 2003, 98, 1716–1726. [Google Scholar] [CrossRef]
- Schlemmer, M.; Reichardt, P.; Verweij, J.; Hartmann, J.; Judson, I.; Thyss, A.; Hogendoorn, P.; Marreaud, S.; van Glabbeke, M.; Blay, J.-Y. Paclitaxel in patients with advanced angiosarcomas of soft tissue: A retrospective study of the EORTC soft tissue and bone sarcoma group. Eur. J. Cancer 2008, 44, 2433–2436. [Google Scholar] [CrossRef]
- Guadagnolo, B.A.; Zagars, G.K.; Araujo, D.M.; Ravi, V.; Shellenberger, T.D.; Sturgis, E.M. Outcomes after definitive treatment for cutaneous angiosarcoma of the face and scalp. Head Neck 2010, 33, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Takahashi, K.; Asato, Y.; Yamamoto, Y.; Taira, K.; Matori, S.; Iraha, S.; Yagi, N.; Yogi, A.; Haranaga, S.; et al. Treatment and prognosis of angiosarcoma of the scalp and face: A retrospective analysis of 48 patients. Br. J. Radiol. 2012, 85, e1127–e1133. [Google Scholar] [CrossRef] [PubMed]
- Mark, R.J.; Poen, J.C.; Tran, L.M.; Fu, Y.S.; Juillard, G.F. Angiosarcoma. A report of 67 patients and a review of the literature. Cancer 1996, 77, 2400–2406. [Google Scholar] [CrossRef]
- Scott, M.T.; Portnow, L.H.; Morris, C.G.; Marcus, R.B.; Mendenhall, N.P.; Mendenhall, W.M.; Indelicato, D.J. Radiation Therapy for Angiosarcoma. Am. J. Clin. Oncol. 2013, 36, 174–180. [Google Scholar] [CrossRef]
- Bi, S.; Chen, S.; Wu, B.; Cen, Y.; Chen, J. The Effectiveness of Different Treatment Modalities of Cutaneous Angiosarcoma: Results From Meta-Analysis and Observational Data From SEER Database. Front. Oncol. 2021, 11, 627113. [Google Scholar] [CrossRef]
- Hata, M. Radiation Therapy for Angiosarcoma of the Scalp: Total Scalp Irradiation and Local Irradiation. Anticancer Res. 2018, 38, 1247–1253. [Google Scholar] [CrossRef]
- Suzuki, G.; Yamazaki, H.; Takenaka, H.; Aibe, N.; Masui, K.; Kimoto, T.; Tatekawa, K.; Nakashima, A.; Takenaka, T.; Asai, J.; et al. Definitive Radiation Therapy for Angiosarcoma of the Face and Scalp. Vivo 2016, 30, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Fata, F.; O’Reilly, E.; Ilson, D.; Pfister, D.; Leffel, D.; Kelsen, D.P.; Schwartz, G.K.; Casper, E.S. Paclitaxel in the treatment of patients with angiosarcoma of the scalp or face. Cancer 1999, 86, 2034–2037. [Google Scholar] [CrossRef]
- Budd, G.T. Management of angiosarcoma. Curr. Oncol. Rep. 2002, 4, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Nagano, T.; Yamada, Y.; Ikeda, T.; Kanki, H.; Kamo, T.; Nishigori, C. Docetaxel: A therapeutic option in the treatment of cutaneous angiosarcoma. Cancer 2007, 110, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Fury, M.G.; Antonescu, C.R.; van Zee, K.; Brennan, M.; Maki, R.G. A 14-Year Retrospective Review of Angiosarcoma. Cancer J. 2005, 11, 241–247. [Google Scholar] [CrossRef]
- Ryan, C.W.; von Mehren, M.; Rankin, C.J.; Goldblum, J.R.; Demetri, G.D.; Bramwell, V.H.; Borden, E.C. Phase II intergroup study of sorafenib (S) in advanced soft tissue sarcomas (STS): SWOG 0505. J. Clin. Oncol. 2008, 26, 10532. [Google Scholar] [CrossRef]
- George, S.; Merriam, P.; Maki, R.G.; Abbeele, A.D.V.D.; Yap, J.; Akhurst, T.; Harmon, D.C.; Bhuchar, G.; O’Mara, M.M.; D’Adamo, D.R.; et al. Multicenter Phase II Trial of Sunitinib in the Treatment of Nongastrointestinal Stromal Tumor Sarcomas. J. Clin. Oncol. 2009, 27, 3154–3160. [Google Scholar] [CrossRef]
- Maki, R.G.; D’Adamo, D.R.; Keohan, M.L.; Saulle, M.; Schuetze, S.M.; Undevia, S.D.; Livingston, M.B.; Cooney, M.M.; Hensley, M.L.; Mita, M.M.; et al. Phase II Study of Sorafenib in Patients With Metastatic or Recurrent Sarcomas. J. Clin. Oncol. 2009, 27, 3133–3140. [Google Scholar] [CrossRef] [PubMed]
- Penel, N.; Italiano, A.; Ray-Coquard, I.; Chaigneau, L.; Delcambre, C.; Robin, Y.M.; Bui, B.; Bertucci, F.; Isambert, N.; Cupissol, D.; et al. Metastatic angiosarcomas: Doxorubicin-based regimens, weekly paclitaxel and metastasectomy significantly improve the outcome. Ann. Oncol. 2012, 23, 517–523. [Google Scholar] [CrossRef]
- Stacchiotti, S.; Palassini, E.; Sanfilippo, R.; Vincenzi, B.; Arena, M.; Bochicchio, A.M.; de Rosa, P.; Nuzzo, A.; Turano, S.; Morosi, C.; et al. Gemcitabine in advanced angiosarcoma: A retrospective case series analysis from the Italian Rare Cancer Network. Ann. Oncol. 2012, 23, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Italiano, A.; Bompas, E.; Le Cesne, A.; Robin, Y.; Chevreau, C.; Bay, J.; Bousquet, G.; Piperno-Neumann, S.; Isambert, N.; et al. Sorafenib for Patients with Advanced Angiosarcoma: A Phase II Trial from the French Sarcoma Group (GSF/GETO). Oncologist 2012, 17, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Italiano, A.; Cioffi, A.; Penel, N.; Levra, M.G.; Delcambre, C.; Kalbacher, E.; Chevreau, C.; Bertucci, F.; Isambert, N.; Blay, J.-Y.; et al. Comparison of doxorubicin and weekly paclitaxel efficacy in metastatic angiosarcomas. Cancer 2012, 118, 3330–3336. [Google Scholar] [CrossRef] [PubMed]
- Agulnik, M.; Yarber, J.; Okuno, S.; von Mehren, M.; Jovanovic, B.; Brockstein, B.; Evens, A.; Benjamin, R. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann. Oncol. 2013, 24, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Young, R.; Natukunda, A.; Litière, S.; Woll, P.; Wardelmann, E.; van der Graaf, W. First-line anthracycline-based chemotherapy for angiosarcoma and other soft tissue sarcoma subtypes: Pooled analysis of eleven European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group trials. Eur. J. Cancer 2014, 50, 3178–3186. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Munhoz, R.R.; Kuk, D.; Landa, J.; Hartley, E.W.; Bonafede, M.; Dickson, M.A.; Gounder, M.; Keohan, M.L.; Crago, A.; et al. Outcomes of Systemic Therapy for Patients with Metastatic Angiosarcoma. Oncology 2015, 89, 205–214. [Google Scholar] [CrossRef]
- Ray-Coquard, I.L.; Domont, J.; Tresch-Bruneel, E.; Bompas, E.; Cassier, P.A.; Mir, O.; Piperno-Neumann, S.; Italiano, A.; Chevreau, C.; Cupissol, D.; et al. Paclitaxel Given Once Per Week With or Without Bevacizumab in Patients With Advanced Angiosarcoma: A Randomized Phase II Trial. J. Clin. Oncol. 2015, 33, 2797–2802. [Google Scholar] [CrossRef]
- Kollár, A.; Jones, R.L.; Stacchiotti, S.; Gelderblom, H.; Guida, M.; Grignani, G.; Steeghs, N.; Safwat, A.; Katz, D.; Duffaud, F.; et al. Pazopanib in advanced vascular sarcomas: An EORTC Soft Tissue and Bone Sarcoma Group (STBSG) retrospective analysis. Acta Oncol. 2017, 56, 88–92. [Google Scholar] [CrossRef]
- Lebellec, L.; Bertucci, F.; Tresch-Bruneel, E.; Ray-Coquard, I.; Le Cesne, A.; Bompas, E.; Blay, J.-Y.; Italiano, A.; Mir, O.; Ryckewaert, T.; et al. Prognostic and predictive factors for angiosarcoma patients receiving paclitaxel once weekly plus or minus bevacizumab: An ancillary study derived from a randomized clinical trial. BMC Cancer 2018, 18, 963. [Google Scholar] [CrossRef]
- Agulnik, M.; Schulte, B.; Robinson, S.; Hirbe, A.C.; Kozak, K.; Chawla, S.P.; Attia, S.; Rademaker, A.; Zhang, H.; Abbinanti, S.; et al. An open-label single-arm phase II study of regorafenib for the treatment of angiosarcoma. Eur. J. Cancer 2021, 154, 201–208. [Google Scholar] [CrossRef]
- Wagner, M.J.; Othus, M.; Patel, S.P.; Ryan, C.; Sangal, A.; Powers, B.; Budd, G.T.; Victor, A.I.; Hsueh, C.-T.; Chugh, R.; et al. Multicenter phase II trial (SWOG S1609, cohort 51) of ipilimumab and nivolumab in metastatic or unresectable angiosarcoma: A substudy of dual anti-CTLA-4 and anti-PD-1 blockade in rare tumors (DART). J. Immunother. Cancer 2021, 9, e002990. [Google Scholar] [CrossRef]
- Suppiah, R.; Wood, L.; Elson, P.; Budd, G.T. Phase I/II study of docetaxel, ifosfamide, and doxorubicin in advanced, recurrent, or metastatic soft tissue sarcoma (STS). Investig. New Drugs 2006, 24, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Van der Graaf, W.T.; Blay, J.-Y.; Chawla, S.P.; Kim, D.-W.; Bui-Nguyen, B.; Casali, P.G.; Schöffski, P.; Aglietta, M.; Staddon, A.P.; Beppu, Y.; et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012, 379, 1879–1886. [Google Scholar] [CrossRef]
- Yoo, K.H.; Kim, H.S.; Lee, S.J.; Park, S.H.; Kim, S.J.; Kim, S.H.; La Choi, Y.; Shin, K.-H.; Cho, Y.J.; Lee, J.; et al. Efficacy of pazopanib monotherapy in patients who had been heavily pretreated for metastatic soft tissue sarcoma: A retrospective case series. BMC Cancer 2015, 15, 154. [Google Scholar] [CrossRef] [PubMed]
- Ogata, D.; Yanagisawa, H.; Suzuki, K.; Oashi, K.; Yamazaki, N.; Tsuchida, T. Pazopanib treatment slows progression and stabilizes disease in patients with taxane-resistant cutaneous angiosarcoma. Med. Oncol. 2016, 33, 116. [Google Scholar] [CrossRef]
- Pasquier, E.; André, N.; Street, J.; Chougule, A.; Rekhi, B.; Ghosh, J.; Philip, D.S.; Meurer, M.; MacKenzie, K.; Kavallaris, M.; et al. Effective Management of Advanced Angiosarcoma by the Synergistic Combination of Propranolol and Vinblastine-based Metronomic Chemotherapy: A Bench to Bedside Study. EBioMedicine 2016, 6, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Kaira, K.; Okubo, Y.; Utsumi, D.; Yasuda, M.; Asao, T.; Nishiyama, M.; Takahashi, K.; Ishikawa, O. Positive PD-L1 Expression Predicts Worse Outcome in Cutaneous Angiosarcoma. J. Glob. Oncol. 2017, 3, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Toulmonde, M.; Penel, N.; Adam, J.; Chevreau, C.; Blay, J.-Y.; Le Cesne, A.; Bompas, E.; Piperno-Neumann, S.; Cousin, S.; Grellety, T.; et al. Use of PD-1 Targeting, Macrophage Infiltration, and IDO Pathway Activation in Sarcomas. JAMA Oncol. 2018, 4, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Woodall, C.E.; Scoggins, C.R.; Lewis, A.M.; Mcmasters, K.M.; Martin, R.C. Hepatic Malignant Epithelioid Hemangioendothelioma: A Case Report and Review of the Literature. Am. Surg. 2008, 74, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.W.; Enzinger, F.M. Epithelioid hemangioendothelioma a vascular tumor often mistaken for a carcinoma. Cancer 1982, 50, 970–981. [Google Scholar] [CrossRef]
- Ellis, G.L.; Kratochvil, F.J. Epithelioid hemangioendothelioma of the head and neck: A clinicopathologic report of twelve cases. Oral Surg. Oral Med. Oral Pathol. 1986, 61, 61–68. [Google Scholar] [CrossRef]
- Bollinger, B.K.; Laskin, W.B.; Knight, C.B. Epithelioid hemangioendothelioma with multiple site involvement. Literature review and observations. Cancer 1994, 73, 610–615. [Google Scholar] [CrossRef]
- Ebo, C.M.; Boever, J.A.; Adriaens, P.A.; Roels, H. Hemangioendothelioma of the gingiva. Histopathologic and therapeutic considerations. J. Clin. Periodontol. 1986, 13, 11–18. [Google Scholar] [CrossRef]
- Moran, W.J.; Dobleman, T.J.; Bostwick, D.G. Epithelioid hemangioendothelioma (histiocytoid hemangioma) of the palate. Laryngoscope 1987, 97, 1299–1302. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, V.C.; Marcucci, G.; Sesso, A.; de Araújo, N.S. Epithelioid hemangioendothelioma of the gingiva: Case report and ultrastructural study. Oral Surg. Oral Med. Oral Pathol. 1987, 63, 472–477. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, Y.C. Unusual extrahepatic metastasis to the soft tissue of the left cervical neck area from hepatic epithelioid hemangioendothelioma. Hepatology 2011, 54, 1480–1481. [Google Scholar] [CrossRef]
- Boscaino, A.; Errico, M.E.; Orabona, P.; Tornillo, L.; Staibano, S.; Donofrio, V.; de Rosa, G. Epithelioid Hemangioendothelioma of the Larynx. Tumori J. 1999, 85, 515–518. [Google Scholar] [CrossRef]
- Fletcher, C.D.M. International Academy of P (2013) WHO classification of tumours of soft tissue and bone. In World Health Or-ganization Classification of Tumours, 4th ed.; IARC Press: Lyon, France, 1997. [Google Scholar]
- Sardaro, A.; Bardoscia, L.; Petruzzelli, M.F.; Portaluri, M. Epithelioid hemangioendothelioma: An overview and update on a rare vascular tumor. Oncol. Rev. 2014, 8, 259. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.; Massad, M.; Pollak, C.; Rubin, C.; Yeh, J.; Wang, J.; Edelman, G.; Yeh, J.; Prasad, S.; Weinberg, G. Clinical Patterns and Outcome in Epithelioid Hemangioendothelioma With or Without Pulmonary Involvement. Chest 2011, 140, 1312–1318. [Google Scholar] [CrossRef]
- Errani, C.; Zhang, L.; Sung, Y.S.; Hajdu, M.; Singer, S.; Maki, R.G.; Healey, J.H.; Antonescu, C.R. A novel WWTR1-CAMTA1 gene fusion is a consistent abnormality in epithelioid hemangioendothelioma of different anatomic sites. Genes Chromosom. Cancer 2011, 50, 644–653. [Google Scholar] [CrossRef]
- Seligson, N.D.; Awasthi, A.; Millis, S.Z.; Turpin, B.K.; Meyer, C.F.; Grand’Maison, A.; Liebner, D.A.; Hays, J.L.; Chen, J.L. Common Secondary Genomic Variants Associated With Advanced Epithelioid Hemangioendothelioma. JAMA Netw. Open 2019, 2, e1912416. [Google Scholar] [CrossRef]
- Antonescu, C.R.; Le Loarer, F.; Mosquera, J.-M.; Sboner, A.; Zhang, L.; Chen, C.-L.; Chen, H.-W.; Pathan, N.; Krausz, T.; Dickson, B.; et al. NovelYAP1-TFE3fusion defines a distinct subset of epithelioid hemangioendothelioma. Genes Chromosom. Cancer 2013, 52, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Mascarelli, P.E.; Iredell, J.R.; Maggi, R.; Weinberg, G.; Breitschwerdt, E.B. Bartonella Species Bacteremia in Two Patients with Epithelioid Hemangioendothelioma. J. Clin. Microbiol. 2011, 49, 4006–4012. [Google Scholar] [CrossRef]
- Brahmbhatt, A.N.; Skalski, K.A.; Bhatt, A. Vascular lesions of the head and neck: An update on classification and imaging review. Insights Imaging 2020, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mehrabi, A.; Kashfi, A.; Fonouni, H.; Schemmer, P.; Schmied, B.M.; Weitz, J.; Friess, H.; Buchler, M.W.; Schmidt, J. Primary malignant hepatic epithelioid hemangioendothelioma. Cancer 2006, 107, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Deyrup, A.T.; Tighiouart, M.; Montag, A.G.; Weiss, S.W. Epithelioid Hemangioendothelioma of Soft Tissue: A Proposal for Risk Stratification Based on 49 Cases. Am. J. Surg. Pathol. 2008, 32, 924–927. [Google Scholar] [CrossRef] [PubMed]
- Stacchiotti, S.; Miah, A.; Frezza, A.; Messiou, C.; Morosi, C.; Caraceni, A.; Antonescu, C.; Bajpai, J.; Baldini, E.; Bauer, S.; et al. Epithelioid hemangioendothelioma, an ultra-rare cancer: A consensus paper from the community of experts. ESMO Open 2021, 6, 100170. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, G.; Hu, P.; Pang, L.; Gu, T.; Yu, H.; Luo, R.; Yang, X.; Shi, H. Imaging characteristics and prognostic values of hepatic epithelioid hemangioendothelioma on 18F-FDG PET/CT. Clin. Exp. Med. 2020, 20, 557–567. [Google Scholar] [CrossRef]
- Dong, A.; Dong, H.; Wang, Y.; Gong, J.; Lu, J.; Zuo, C. MRI and FDG PET/CT Findings of Hepatic Epithelioid Hemangioendothelioma. Clin. Nucl. Med. 2013, 38, e66–e73. [Google Scholar] [CrossRef]
- Siddiqui, M.; Evans, H.L.; Ro, J.Y.; Ayala, A.G. Epithelioid haemangioendothelioma of the thyroid gland: A case report and review of literature. Histopathology 1998, 32, 473–476. [Google Scholar] [CrossRef]
- Hassan, I.; Barth, P.; Celik, I.; Hoffmann, S.; Langer, P.; Ramaswamy, A.; Wagner, H.-J.; Rothmund, M.; Zielke, A. An Authentic Malignant Epithelioid Hemangioendothelioma of the Thyroid: A Case Report and Review of the Literature. Thyroid 2005, 15, 1377–1381. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, J.; Ordonez, N.G.; Luna, M.A.; Williams, M.D.; Weber, R.S.; El-Naggar, A.K. Epithelioid Hemangioendothelioma of the Head and Neck: Role of Podoplanin in the Differential Diagnosis. Head Neck Pathol. 2007, 2, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.S.Y.; Chiu, T.W.; Wong, G.K.C.; Zhu, X.L.; Kwok, M.W.T.; Ho, C.M.; Burd, A.D.R. Epithelioid haemangioendothelioma of the anterior skull base: What is the optimal treatment? Hong Kong Med. J. 2009, 15, 308–310. [Google Scholar] [PubMed]
- Patnayak, R.; Jena, A.; Reddy, M.K.; Chowhan, A.K.; Rao, L.C.; Rukhamangadha, N. Epithelioid Hemangioendothelioma of Nasal Cavity. J. Lab. Physicians 2010, 2, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Al-Faky, Y.H.; Al Malki, S.; Raddaoui, E. Hemangioendothelioma of the eyelid can mimic chalazion. Oman J. Ophthalmol. 2011, 4, 142–143. [Google Scholar] [CrossRef]
- Ma, S.-R.; Li, K.-C.; Xu, Y.-Q.; Wang, Y.-M.; Ma, W.-L.; Li, Q. Primary epithelioid hemangioendothelioma in the clival region: A case report and literature review. Neuropathology 2010, 31, 519–522. [Google Scholar] [CrossRef]
- Banerjee, S.; Chakravarty, S.; Sarkar, S.; Bera, S.P. A Rare Case of A Giant Hemangioendothelioma of Neck. Bengal J. Otolaryngol. Head Neck Surg. 2015, 23, 120–122. [Google Scholar] [CrossRef]
- Drazin, D.; Gandhi, R.; Slodkowska, E.; Boulos, A.S. Epithelioid Hemangioendothelioma of the Mastoid: Resection for Recurrence and Adjuvant Radiation with 8-Year Followup. Case Rep. Surg. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Ali, S.; Odell, E.; Whaites, E.; Robinson, P.; Challacombe, S. Epithelioid Haemangioendothelioma of the mandibular gingiva: Case report and literature review. Int. J. Surg. Case Rep. 2015, 14, 194–198. [Google Scholar] [CrossRef][Green Version]
- Shah, A.A.; Ohori, N.P.; Yip, L.; Coyne, C.; Antonescu, C.R.; Seethala, R.R. Epithelioid Hemangioendothelioma: A Rare Primary Thyroid Tumor with Confirmation of WWTR1 and CAMTA1 Rearrangements. Endocr. Pathol. 2016, 27, 147–152. [Google Scholar] [CrossRef]
- Hanege, F.M.; Uzun, L.; Yavuz, C.; Ozkanli, S.; Kurtgoz, S. Epithelioid hemangioendothelioma of the nasal septum. B ENT 2016, 12, 155–157. [Google Scholar]
- Jain, S.; Sancheti, S.; Singh, J.N.; Malik, A.; Devi, K.T. Epithelioid hemangioendothelioma of hypopharynx: A rare presentation. Indian J. Dent. 2016, 7, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Ogita, S.; Nomura, K.; Ogawa, T.; Watanabe, M.; Higashi, K.; Katori, Y.; Tominaga, T. Nasal cavity epithelioid hemangioendothelioma invading the anterior skull base. Surg. Neurol. Int. 2016, 7, 53. [Google Scholar] [CrossRef]
- Salgarelli, A.C.; Bellini, P.; Maccio, L.; Setti, G. Epithelioid hemangioendothelioma of the mandibular gingiva: A rare case of metastasis 4 years after radical excision and literature review. J. Oral Maxillofac. Pathol. JOMFP 2016, 20, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Brill, J.B.; Schwartz, I.E.; Prescher, L.M.; Pratt, T.C. A Case of an Epithelioid Hemangioendothelioma Arising from the Innominate Vein Mimicking Cervical Metastatic Lymphadenopathy. Case Rep. Surg. 2016, 2016, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Duzer, S.; Akyigit, A.; Arslan Solmaz, O.; Sakallioglu, O.; Kilicarslan, A.; Polat, C. An Epithelioid Hemangioendothelioma of the Head and Neck. J. Craniofac. Surg. 2017, 28, e638–e640. [Google Scholar] [CrossRef] [PubMed]
- Koide, Y.; Sasaki, E.; Masago, K.; Fujita, S.; Beppu, S.; Nishikawa, D.; Suzuki, H.; Hasegawa, Y.; Yatabe, Y.; Hanai, N. Epithelioid hemangioendothelioma of the parotid gland: A case report. Int. Cancer Conf. J. 2019, 8, 39–42. [Google Scholar] [CrossRef]
- Ennouhi, M.; Guerrouani, A.; Moussaoui, A. Epithelioid hemangioendothelioma, an uncommon tumor of the eyelid: A case report. J. Stomatol. Oral Maxillofac. Surg. 2018, 119, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Jamshidian-Tehrani, M.; Eshraghi, B.; Zarei, M.; Nozarian, Z.; Rafizadeh, S.M.; Ghadimi, H. Successful Total Resection of an Orbital Epithelioid Hemangioendothelioma with the Aid of Endovascular Embolization. Ocul. Oncol. Pathol. 2018, 5, 50–53. [Google Scholar] [CrossRef]
- Suarez-Zamora, D.A.; Rodriguez-Urrego, P.A.; Hakim-Tawil, J.A.; Palau-Lazaro, M.A. Epithelioid hemangioendothelioma of the parotid gland: A case report in an unusual location with a review of the literature. Rev. Esp. Patol. 2019, 52, 260–264. [Google Scholar] [CrossRef]
- Komatsu, Y.; Miyamoto, I.; Ohashi, Y.; Katagiri, K.; Saito, D.; Obara, M.; Takeda, Y.; Shiga, K.; Yamada, H. Primary epithelioid angiosarcoma originating from the mandibular gingiva: A case report of an extremely rare oral lesion. World J. Surg. Oncol. 2020, 18, 1–6. [Google Scholar] [CrossRef]
- Lui, J.T.; Kang, A.T.; DiFrancesco, L.M.; Warshawski, S.J.; Randall, D.R. Epithelioid Hemangioendothelioma Presenting as Unilateral Vocal Fold Paralysis: A Case Report and Literature Review. Ear Nose Throat J. 2021, 100, S433–S435. [Google Scholar] [CrossRef] [PubMed]
- Cirkin, D.S.; Secinti, I.E.; Dogan, E.; Ozler, G.S. Epithelioid hemangioendothelioma in the tongue: A rare case report. Turk. J. Pathol. 2021, 1. [Google Scholar] [CrossRef] [PubMed]
- Mevio, N.; Pilollo, F.; Achena, A.; Roncoroni, L.; Ormellese, G.; Placentino, A.; Dragonetti, A.G. 3D endoscopic endonasal craniectomy for intenstinal type adeno-carcinoma (ITAC) of the nasal cavity. Am. J. Otolaryngol. 2021, 42, 103061. [Google Scholar] [CrossRef] [PubMed]
- Meccariello, G.; Maniaci, A.; Bianchi, G.; Cammaroto, G.; Iannella, G.; Catalano, A.; Sgarzani, R.; de Vito, A.; Capaccio, P.; Pelucchi, S.; et al. Neck dissection and trans oral robotic surgery for oropharyngeal squamous cell carcinoma. Auris Nasus Larynx 2021, 3. [Google Scholar] [CrossRef]
- Viet, C.T.; Dierks, E.J.; Cheng, A.C.; Patel, A.A.; Chang, S.-C.; Couey, M.A.; Watters, A.L.; Hoang, T.; Xiao, H.D.; Crittenden, M.R.; et al. Transoral robotic surgery and neck dissection for HPV-positive oropharyngeal carcinoma: Importance of nodal count in survival. Oral Oncol. 2020, 109, 104770. [Google Scholar] [CrossRef] [PubMed]
- Sybert, D.R.; Steffee, A.D.; Keppler, L.; Biscup, R.S.; Enker, P. Seven-Year Follow-up of Vertebral Excision and Reconstruction for Malignant Hemangioendothelioma of Bone. Spine 1995, 20, 841–844. [Google Scholar] [CrossRef]
- Kitaichi, M.; Nagai, S.; Nishimura, K.; Itoh, H.; Asamoto, H.; Izumi, T.; Dail, D. Pulmonary epithelioid haemangioendothelioma in 21 patients, including three with partial spontaneous regression. Eur. Respir. J. 1998, 12, 89–96. [Google Scholar] [CrossRef]
- Frezza, A.M.; Ravi, V.; Vullo, S.L.; Vincenzi, B.; Tolomeo, F.; Chen, T.W.; Teterycz, P.; Baldi, G.G.; Italiano, A.; Penel, N.; et al. Systemic therapies in advanced epithelioid haemangioendothelioma: A retrospective international case series from the World Sarcoma Network and a review of literature. Cancer Med. 2021, 10, 2645–2659. [Google Scholar] [CrossRef]
- Stacchiotti, S.; Simeone, N.; Vullo, S.L.; Baldi, G.G.; Brunello, A.; Vincenzi, B.; Palassini, E.; Dagrada, G.; Collini, P.; Morosi, C.; et al. Activity of sirolimus in patients with progressive epithelioid hemangioendothelioma: A case-series analysis within the Italian Rare Cancer Network. Cancer 2021, 127, 569–576. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, H.; Jiang, H.; Chen, E.; Zhang, J.; Xie, X. Apatinib for the treatment of pulmonary epithelioid hemangioendothelioma. Medicine 2017, 96, e8507. [Google Scholar] [CrossRef]
- Kobayashi, N.; Shimamura, T.; Tokuhisa, M.; Goto, A.; Ichikawa, Y. Sorafenib Monotherapy in a Patient with Unresectable Hepatic Epithelioid Hemangioendothelioma. Case Rep. Oncol. 2016, 9, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Semenisty, V.; Naroditsky, I.; Keidar, Z.; Bar-Sela, G. Pazopanib for metastatic pulmonary epithelioid hemangioendothelioma—A suitable treatment option: Case report and review of anti-angiogenic treatment options. BMC Cancer 2015, 15, 402. [Google Scholar] [CrossRef]
- Mascarenhas, R.C.; Sanghvi, A.N.; Friedlander, L.; Geyer, S.J.; Beasley, H.S.; van Thiel, D.H. Thalidomide Inhibits the Growth and Progression of Hepatic Epithelioid Hemangioendothelioma. Oncology 2004, 67, 471–475. [Google Scholar] [CrossRef]
- Kassam, A.; Mandel, K. Metastatic Hepatic Epithelioid Hemangioendothelioma in a Teenage Girl. J. Pediatr. Hematol. 2008, 30, 550–552. [Google Scholar] [CrossRef]
- Moon, S.-B.; Kwon, H.-J.; Park, K.-W.; Yun, W.-J.; Jung, S.-E. Clinical Experience with Infantile Hepatic Hemangioendothelioma. World J. Surg. 2009, 33, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Radzikowska, E.; Szczepulska-Wójcik, E.; Chabowski, M.; Oniszh, K.; Langfort, R.; Roszkowski, K. Pulmonary epithelioid haeman-gioendothelioma--interferon 2-alpha treatment—Case report. Adv. Respir. Med. 2008, 76, 281–285. [Google Scholar]

| Benign | Locally Aggressive or Borderline | Malignant |
|---|---|---|
| Infantile hemangioma/Hemangioma of infancy Congenital hemangioma Tufted angioma Spindle-cell hemangioma Epithelioid hemangioma Pyogenic granuloma | Kaposiform hemangioendothelioma Retiform hemangioendothelioma Papillary intralymphatic angioendothelioma Composite hemangioendothelioma Pseudomyogenic hemangioendothelioma Polymorphous hemangioendothelioma Hemangioendothelioma not otherwise specified Kaposi sarcoma | Angiosarcoma Epithelioid hemangioendothelioma |
| Others: Hobnail hemangioma Microvenular hemangioma Anastomosing hemangioma Glomeruloid hemangioma Papillary hemangioma Intravascular papillary endothelial hyperplasia Cutaneous epithelioid angiomatous nodule Acquired elastotic hemangioma Littoral cell hemangioma of the spleen | ||
| Related lesions: Eccrine angiomatous hamartoma Reactive angioendotheliomatosis Bacillary angiomatosis |
| Author, Year [Reference] | Treatment | Number of Patients | ORR | Median PFS (Months) | Median OS (Months) |
|---|---|---|---|---|---|
| Fata 1999 [24] | paclitaxel | 9 | 89% | 5 | NR |
| Butt 2002 [25] | doxorubicin | 33 | 33% | NR | NR |
| Nagano 2002 [26] | docetaxel | 39 | 67% | 9.5 | NR |
| Fury 2005 [27] | Doxorubicin paclitaxel | 30 41 | NR NR | 3.7–5.4 4 | NR NR |
| Schlemmer 2008 [16] | paclitaxel | 32 | 63% | 7.6 | NR |
| Ryan 2008 [28] | sorafenib | 9 | 11% | 4.7 | 13.5 |
| George 2009 [29] | sunitinib | 2 | 0% | NR | NR |
| Maki 2009 [30] | sorafenib | 37 | 14% | 3.8 | 14.9 |
| Penel 2012 [31] | paclitaxel doxorubicin different chemotherapies | 47 70 16 | 45.5 30.9 12.3 | 5.6 3.9 3.2 | 13.1 11 9.7 |
| Stacchiotti 2012 [32] | gemcitabine | 25 | 64 | 7 | 17 |
| Ray-Coquard 2012 [33] | sorafenib | 41 | 14.6 | 2 | 9.7 |
| Italiano 2012 [34] | paclitaxel doxorubicin | 75 42 | 53 29 | 5.8 3 | 10.3 5.5 |
| Agulnik 2013 [35] | bevacizumab | 23 | 9 | 3 | 11 |
| Young 2014 [36] | anthracycline based | 108 | 25 | 4.9 | NR |
| DÁngelo 2015 [37] | anthracycline-based taxane-based other agents | 74 74 30 | 25–33 31 NR | 3.4 3.6 3.0 | 12 11.6 17.8 |
| Ray-Coquard 2015 [38] | paclitaxel paclitaxel+ bevacizumab | 26 24 | 45.8 28.5 | 6.6 6.6 | 19.5 15.9 |
| Kollar 2017 [39] | pazopanib | 40 | 20 | 3 | 9.9 |
| Lebellec 2018 [40] | paclitaxel paclitaxel+ bevacizumab | 18 24 | NR NR | 5.5 5.7 | NR NR |
| Agulnik 2021 [41] | regorafenib | 23 | 17.4 | 5.5 | NR |
| Wagner 2021 [42] | nivolumab+ ipilimumab | 16 | 25 | NR (6 months PFS: 38%) | NR |
| Author Year | Gender | Age | Localization | Clinical Presentation | Metastasis at Diagnosis | Initial Therapy | Recurrence | Therapy at Recurrence | Follow-Up |
|---|---|---|---|---|---|---|---|---|---|
| Siddiqui 1998 [72] | f | 44 | thyroid | local neck discomfort, gradual increase in size of mass, weakness, hoarseness | no | resection | no | no | 2 years: NED |
| Hassan 2005 [73] | f | 73 | thyroid | mass, hoarseness, dysphagia, weight loss | no | resection | local recurrence at 9 months | palliative surgery, 2 months of subcutaneous interferon-alpha therapy | died 13 months after diagnosis |
| Naqvi 2008 [74] | m | 4 | nasal cavity | NR | no | resection | local recurrence after 3 and 5 years | resection (2×) | 10 years: NED |
| f | 17 | gingiva | NR | no | resection, radio- and chemotherapy | local recurrence after 4 years; local recurrence+ lymph node metastases after 5 years. | resection NR | NR | |
| f | 66 | gingiva | NR | lymph node metastasis | resection lymph node dissection | no | NA | 10 months: NED | |
| m | 71 | tongue | NR | no | resection | NR | NR | NR | |
| Wong 2009 [75] | m | 50 | forehead | skin lesion, visual disturbance | no | resection | NR | NR | NR |
| Patnayak 2010 [76] | m | 40 | nasal cavity | swelling, intermittent epistaxis | no | resection | NR | NR | 9 months: NED |
| Al-Faky 2011 [77] | f | 27 | eyelid | mass | no | resection | no | NA | 2 years: NED |
| Banerjee 2013 [78] | f | 30 | neck | huge neck swelling | no | resection | NR | NR | 6 months: NED |
| Drazin 2013 [79] | m | 62 | mastoid | dizziness, nausea | no | resection | local recurrence after 15 months | resection, radiotherapy 59.4 Gy | 8 years: NED |
| Ma 2013 [80] | f | 58 | clivus | headache, visual detoriation | no | resection | NR | NR | NR |
| Ali 2015 [81] | f | 23 | gingiva | swelling | no | resection | local recurrence after 7 years | resection | 16 years: NED |
| Shah 2016 [82] | f | 35 | thyroid | local neck discomfort, sore throat, hoarseness, dysphagia, weight loss | no | resection, neck dissection | residual disease | radio-chemotherapy | NR |
| Hanege 2016 [83] | m | 62 | nasal septum | epistaxis, congestion | no | resection | no | NA | 3 years: NED |
| Sancheti 2016 [84] | f | 25 | hypopharynx | dysphagia, difficulties in breathing | no | resection | no | NA | 1 year: NED |
| Ogita 2016 [85] | f | 27 | nasal cavity | epistaxis, pain | no | resection | no | NA | 2 years: NED |
| Salgarelli 2016 [86] | m | 33 | mandibular gingiva | lesion | no | resection | 3 neck lymph node metastases 4 years later | resection | NR |
| Brill 2016 [87] | f | 39 | mediastinum | mass | no | resection | no | NA | 1 year: NED |
| Duzer 2017 [88] | f | 26 | neck | lump in the neck | no | resection, postoperative radiotherapy | NR | NR | NR |
| Koide 2018 [89] | f | 70 | parotid gland | swelling, pain | no | resection, neck dissection, adjuvant radiation 60 Gy | distant metastases (right lung, lumbar spine, liver) 5 months after surgery | no | died 13 months after diagnosis |
| Ennouhi 2018 [90] | NR | NR | eyelid | mass | no | resection | no | NA | 5 years: NED |
| Jamshidian-Tehrani 2019 [91] | m | 30 | orbit | proptosis, hypoglobus | no | resection | NR | NR | NR |
| Suarez-Zamora 2019 [92] | f | 62 | parotid gland | painless mass | no | resection | no | NA | NR |
| Komatsu 2020 [93] | m | 66 | gingiva | gingival swelling | no | resection, paclitaxel | no | NA | 1 year: NED |
| Lui 2021 [94] | male | 52 | vocal fold | dysphonia | no | radiation therapy (5000 cGy) | multiple pulmonary metastases at one year | NR | NR (metastases at one year) |
| Cirkin 2021 [95] | m | 55 | tongue | lumps in the tongue, pain | no | resection | NR | NR | NR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiegand, S.; Dietz, A.; Wichmann, G. Malignant Vascular Tumors of the Head and Neck—Which Type of Therapy Works Best? Cancers 2021, 13, 6201. https://doi.org/10.3390/cancers13246201
Wiegand S, Dietz A, Wichmann G. Malignant Vascular Tumors of the Head and Neck—Which Type of Therapy Works Best? Cancers. 2021; 13(24):6201. https://doi.org/10.3390/cancers13246201
Chicago/Turabian StyleWiegand, Susanne, Andreas Dietz, and Gunnar Wichmann. 2021. "Malignant Vascular Tumors of the Head and Neck—Which Type of Therapy Works Best?" Cancers 13, no. 24: 6201. https://doi.org/10.3390/cancers13246201
APA StyleWiegand, S., Dietz, A., & Wichmann, G. (2021). Malignant Vascular Tumors of the Head and Neck—Which Type of Therapy Works Best? Cancers, 13(24), 6201. https://doi.org/10.3390/cancers13246201






