NS-11021 Modulates Cancer-Associated Processes Independently of BK Channels in Melanoma and Pancreatic Duct Adenocarcinoma Cell Lines
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Chemicals
2.3. Patch Clamp Recording
2.4. Ca2+ Imaging with FURA-2-AM
2.5. Cell Viability Assay
2.6. Wound Healing Assay
2.7. Cell Proliferation Assay
2.8. Statistics
3. Results
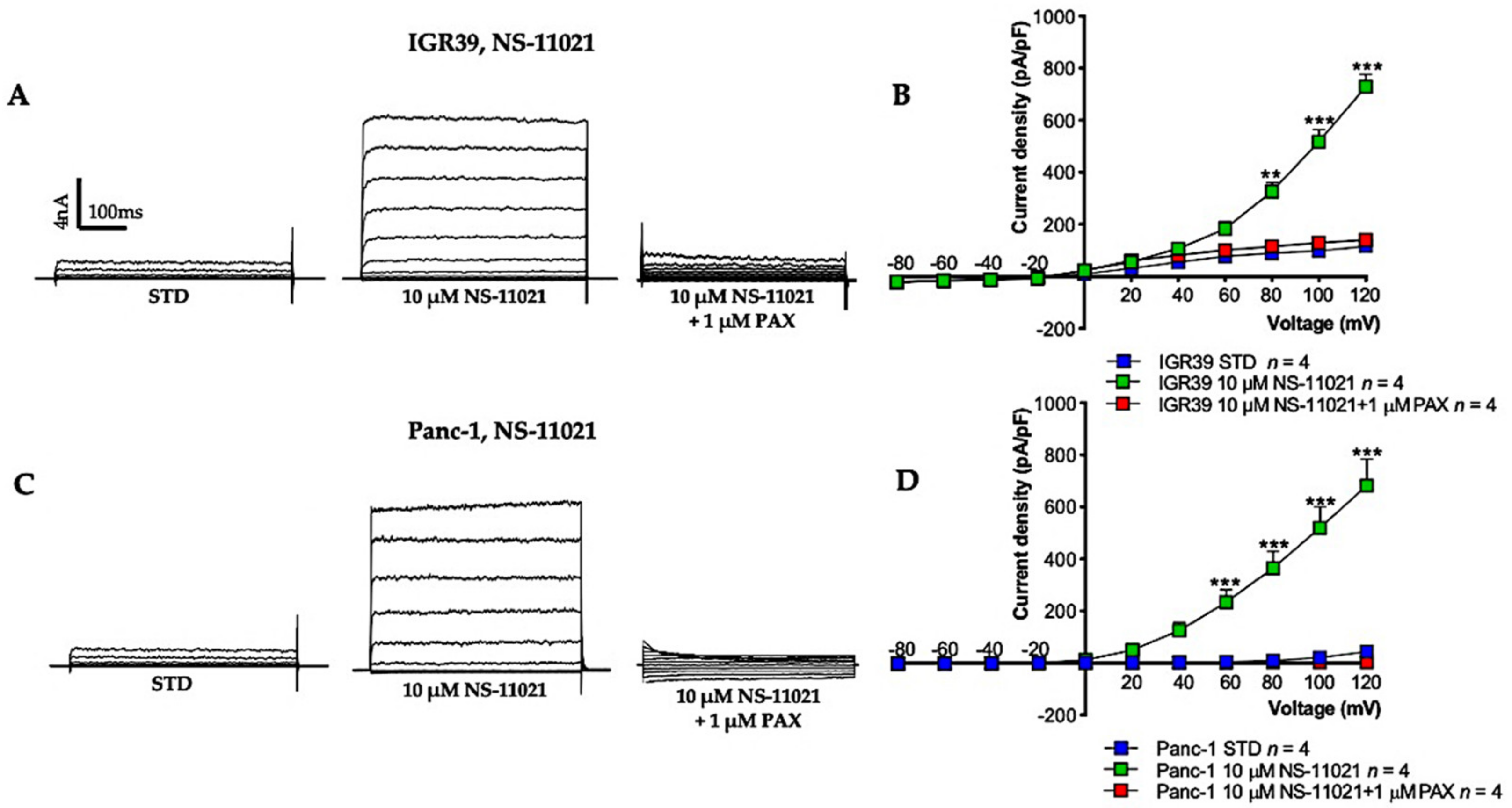
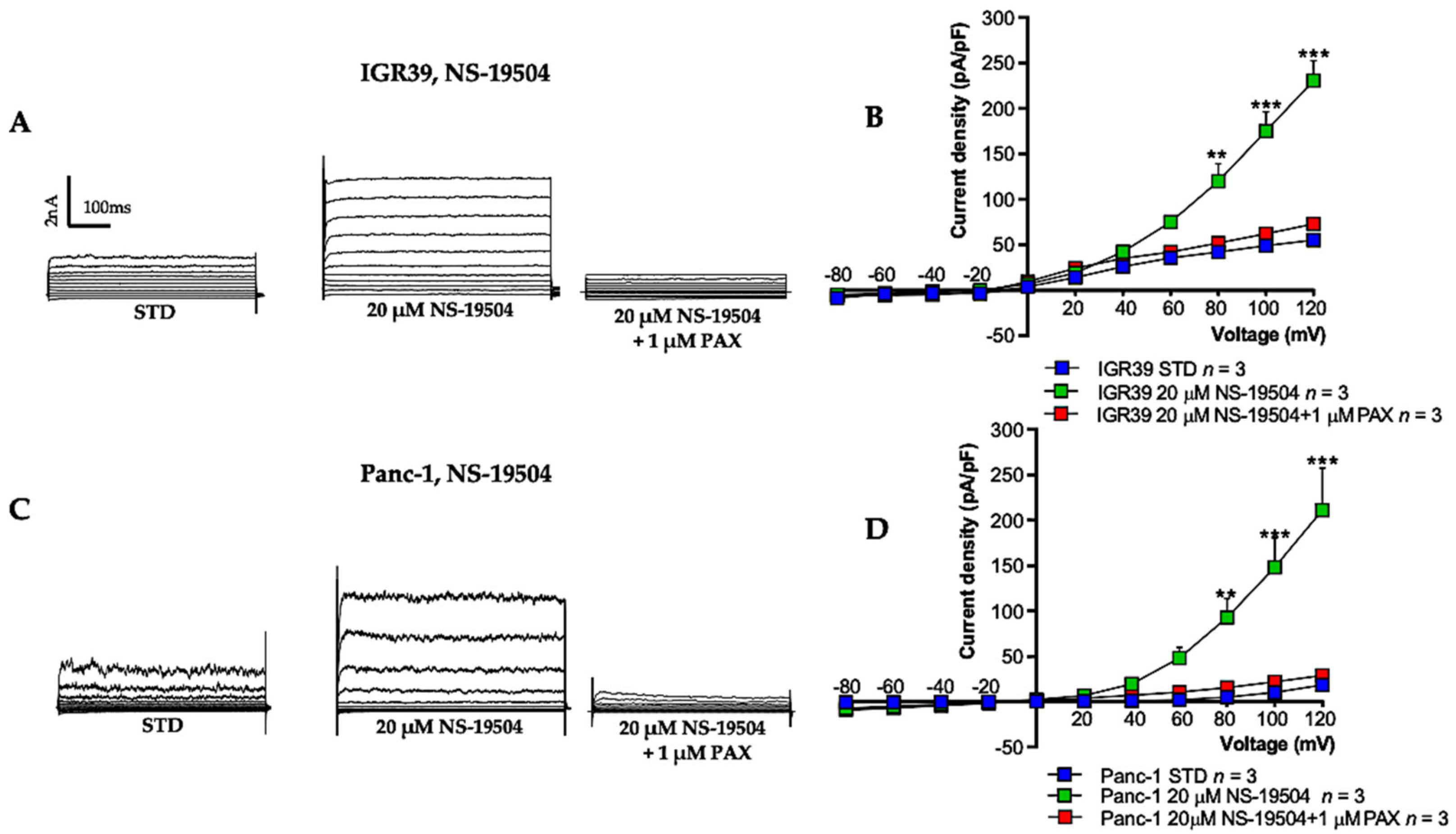
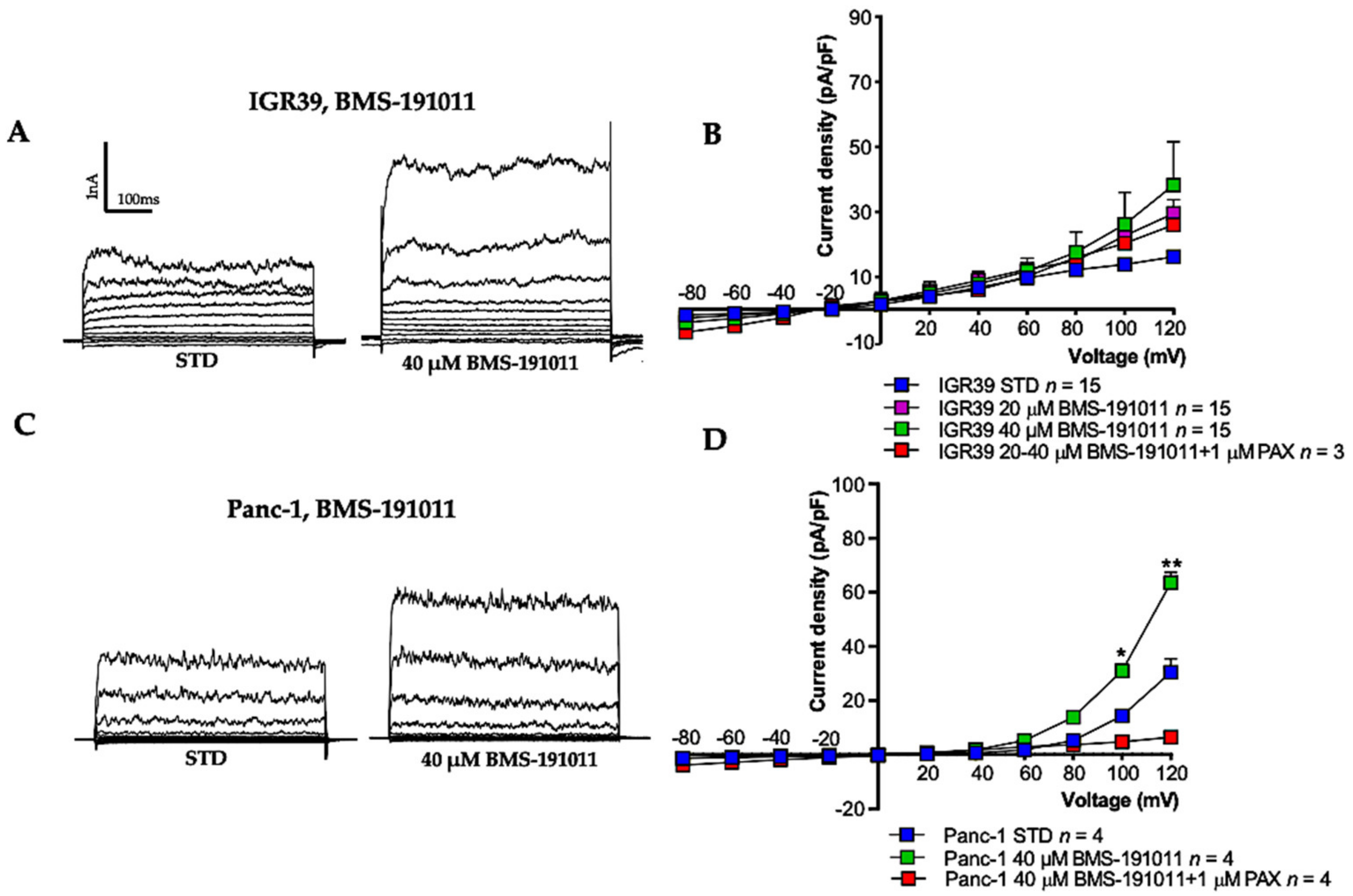
3.1. BK Channel Activators: NS-11021 and NS-19504
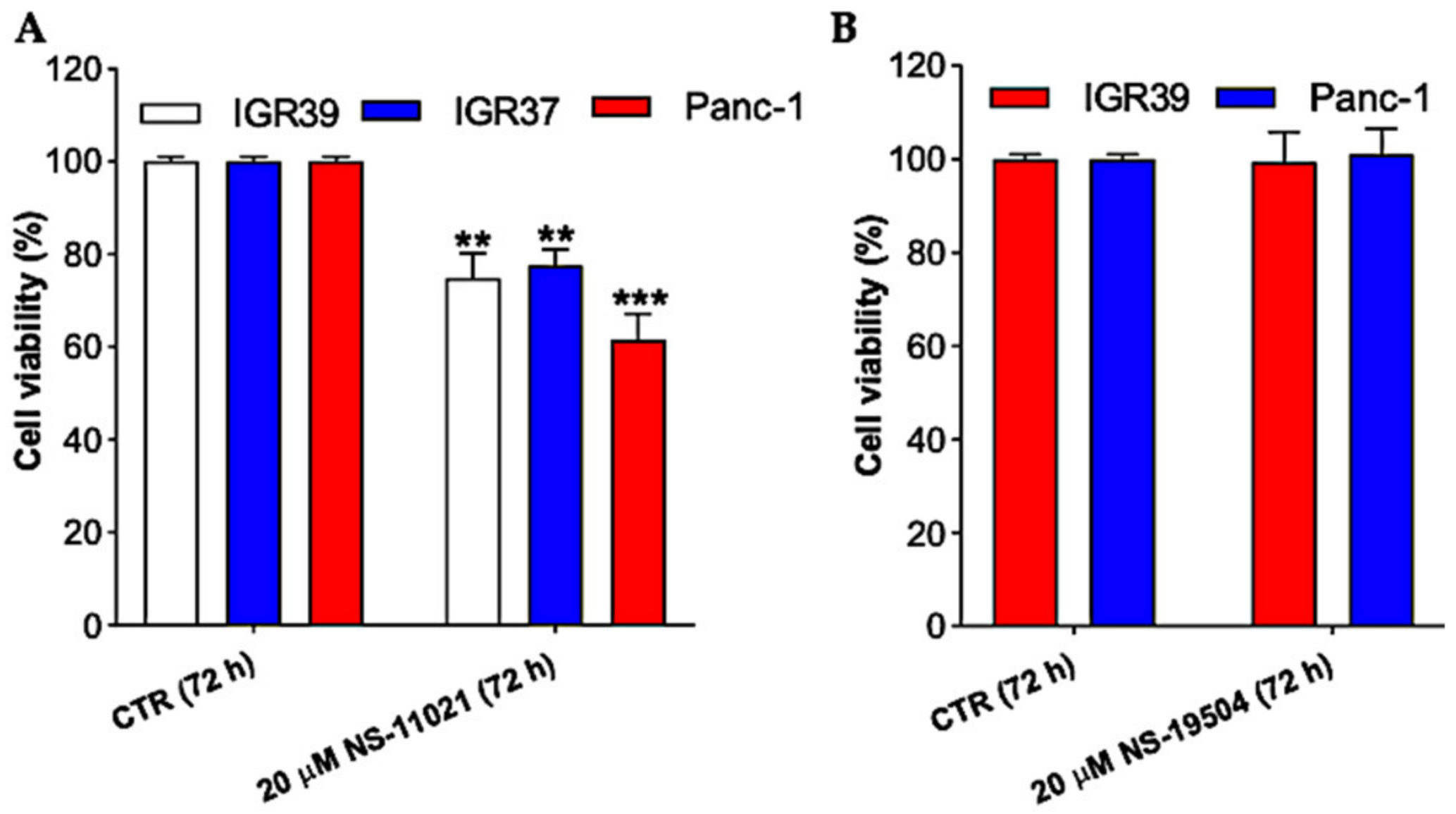
3.2. BK Channel Activation and Cell Viability in IGR39 and Panc-1 Cells
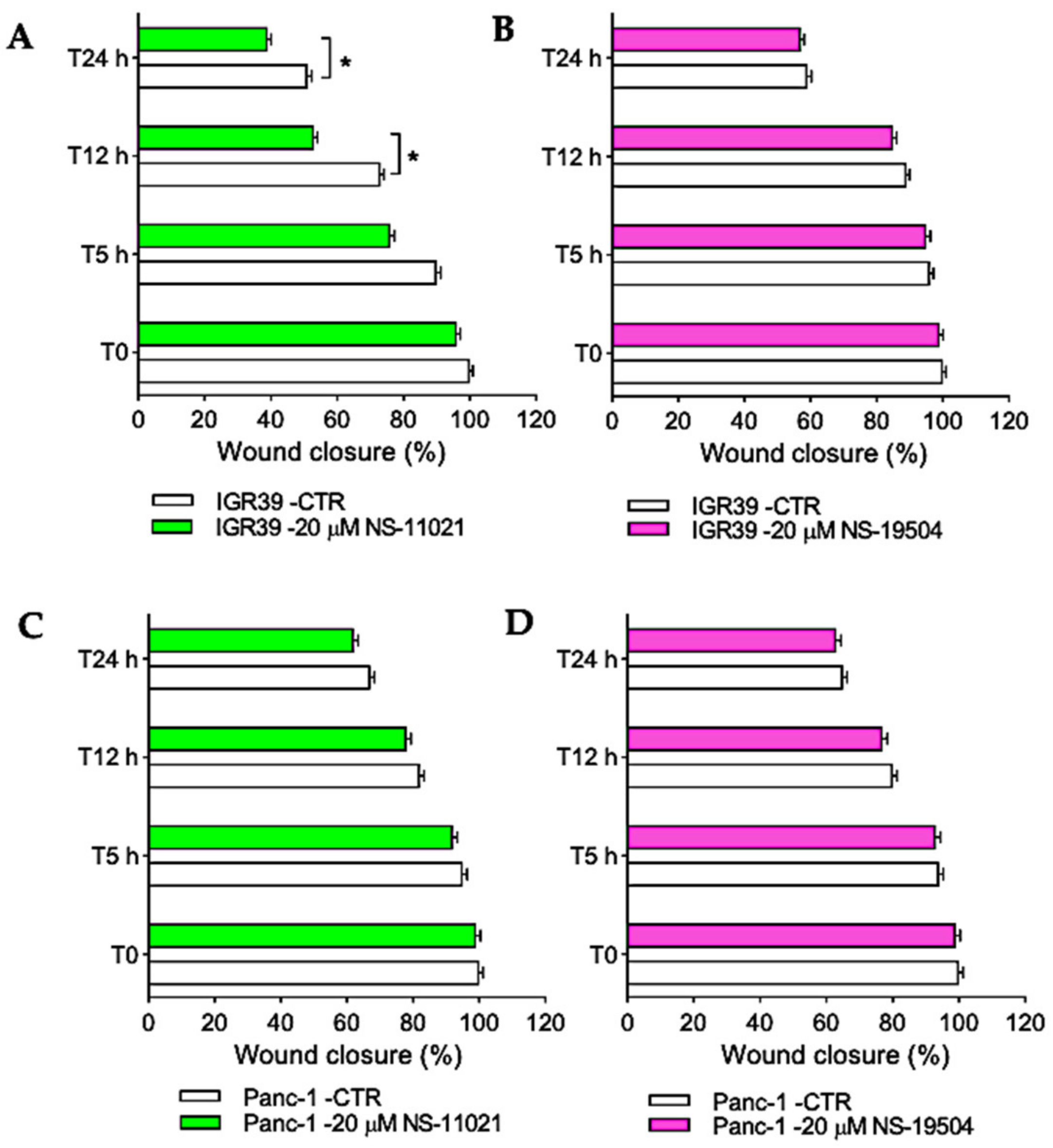
3.3. Effect of BK Channel Activators on Migration in IGR39 and Panc-1 Cells
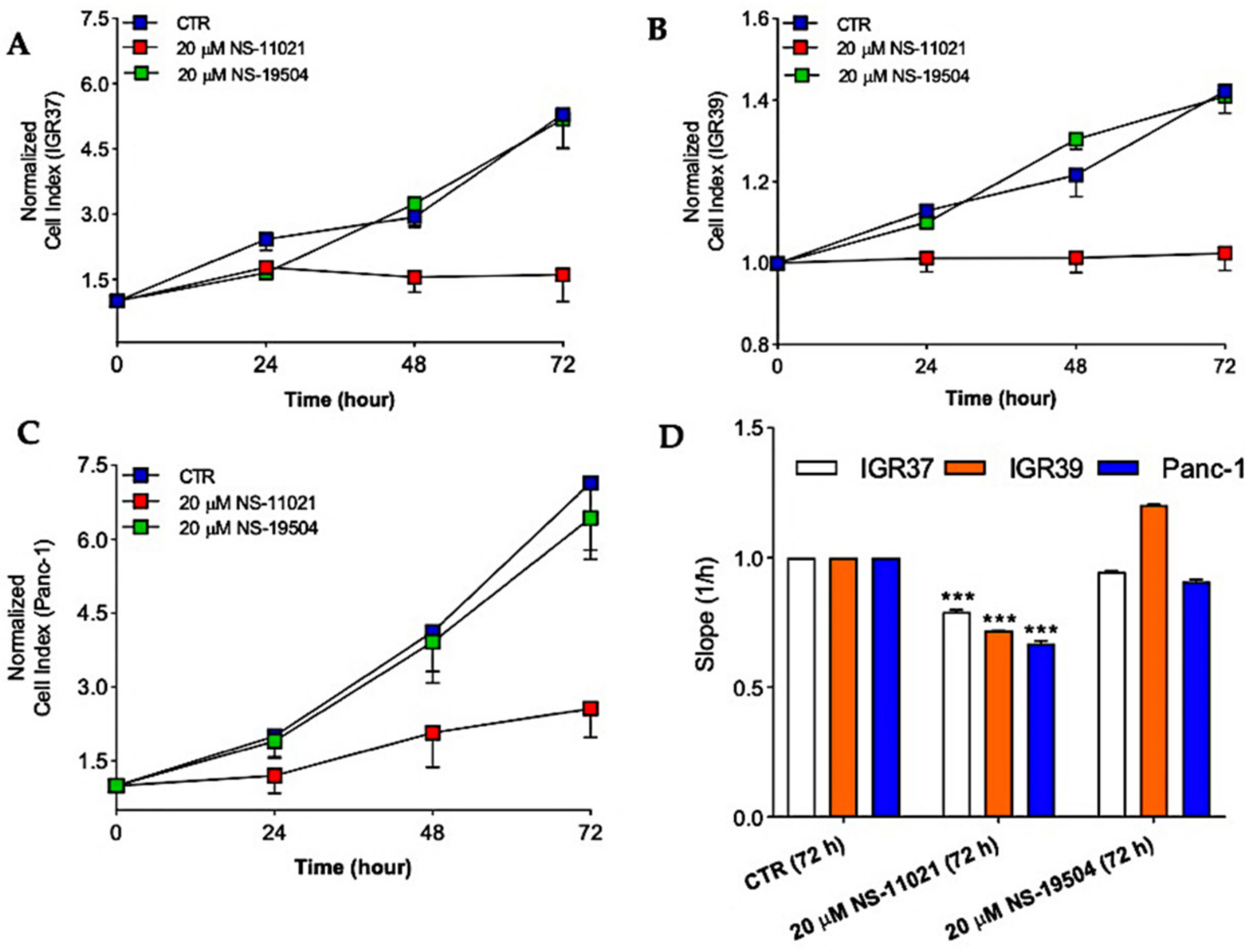
3.4. BK Channel Openers and Cell Proliferation in IGR39 and Panc-1 Cells
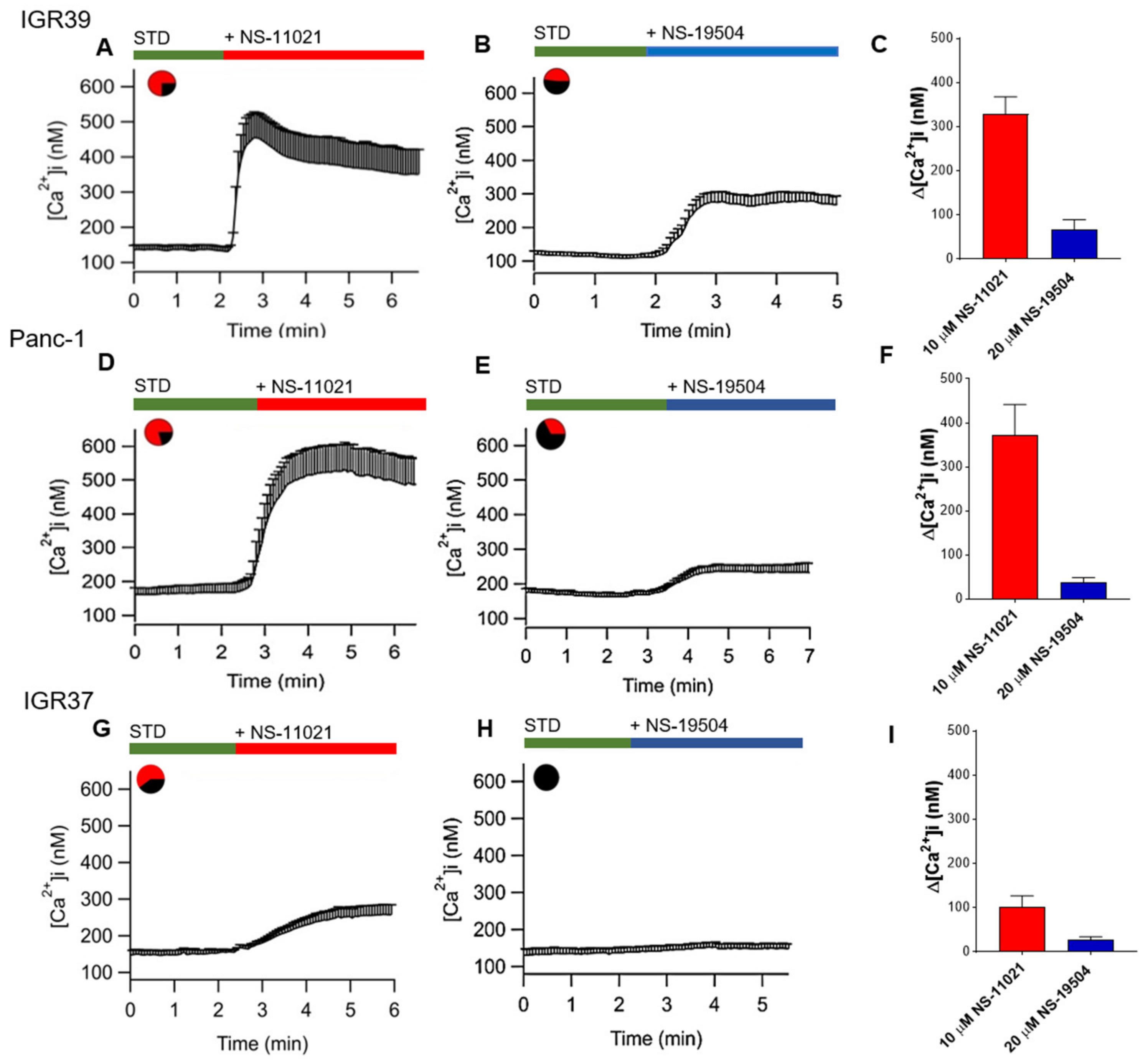
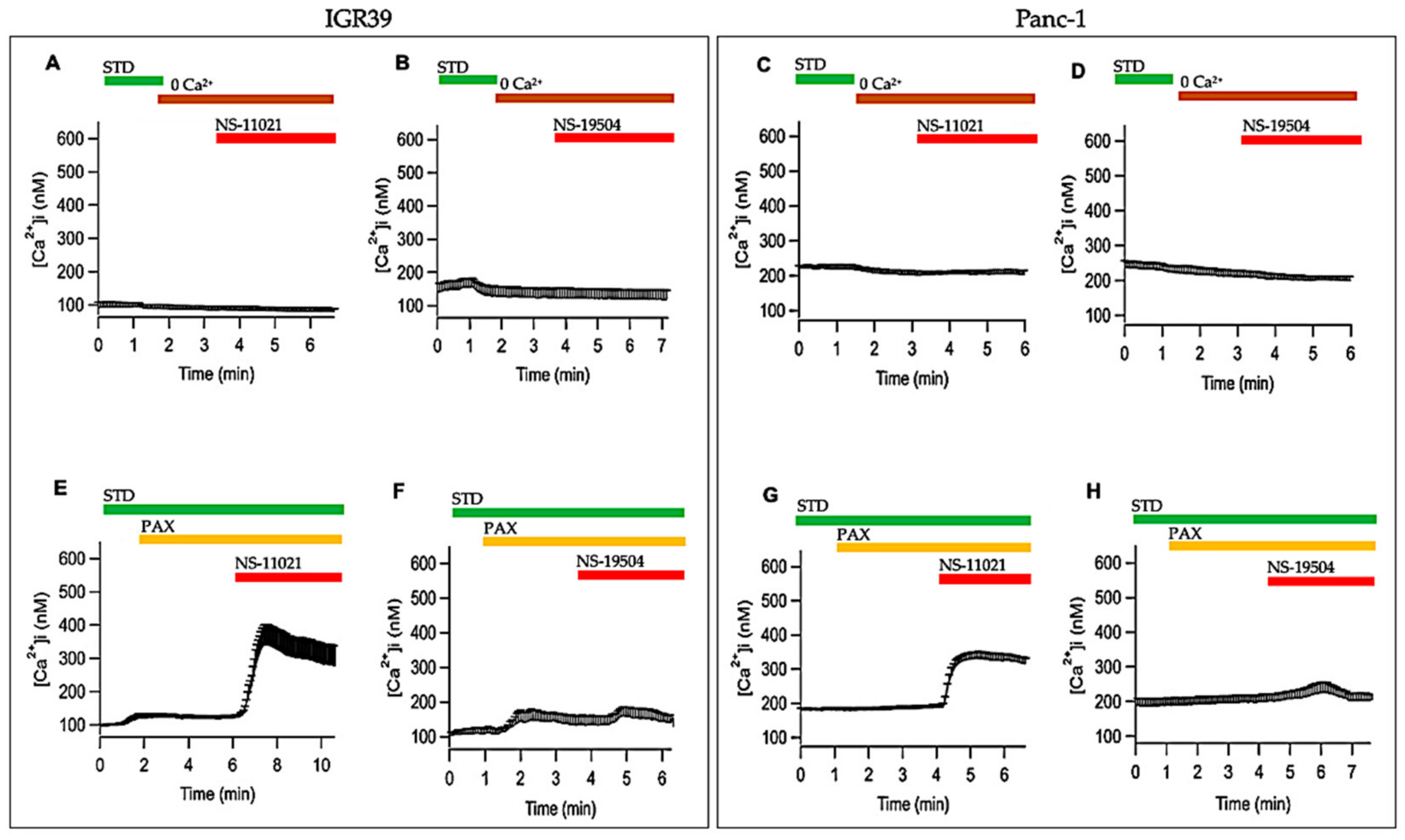
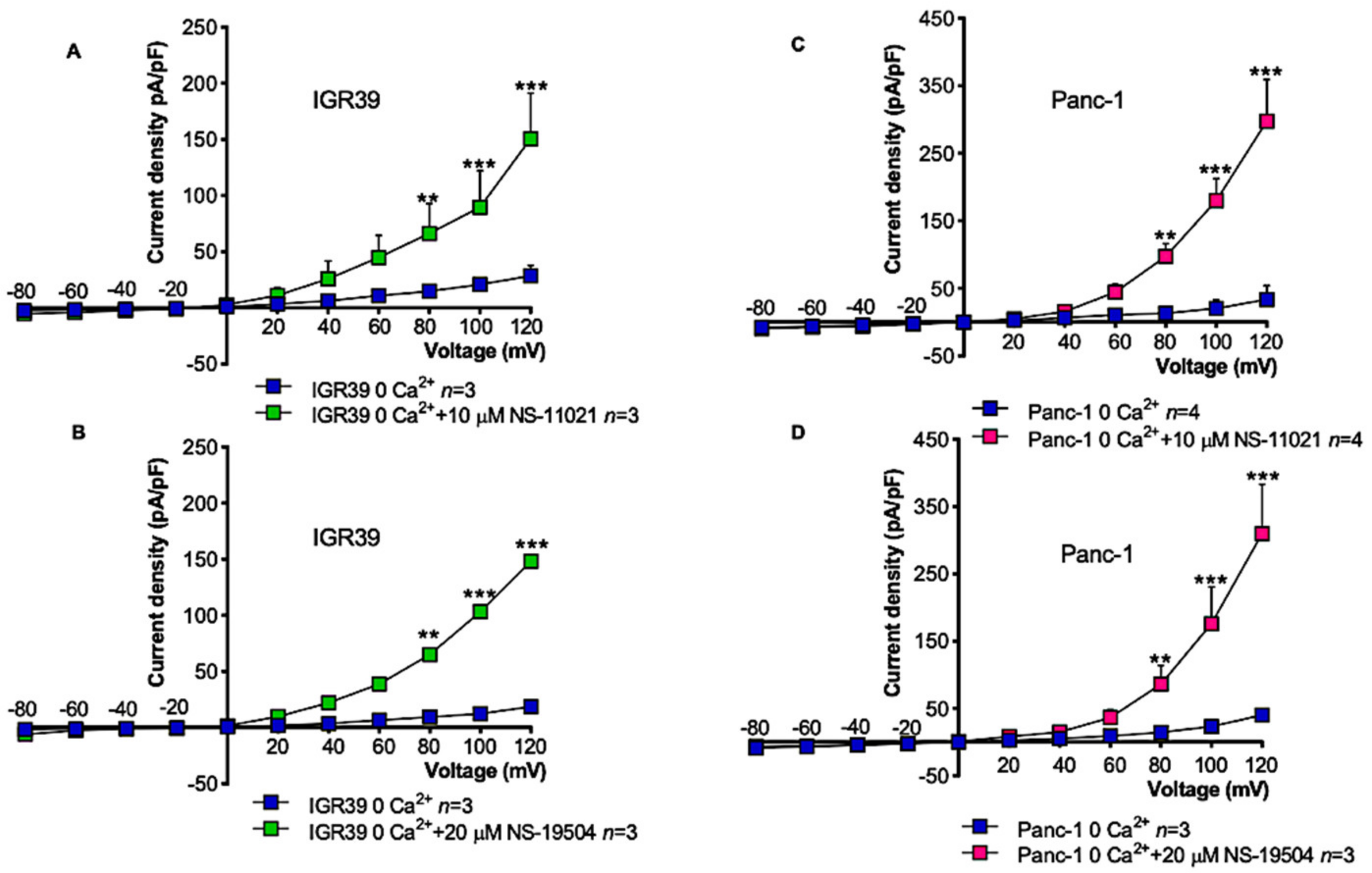
3.5. Measurement of Intracellular Ca2+ Levels in IGR39 and Panc-1 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ge, L.; Hoa, N.T.; Wilson, Z.; Arismendi-Morillo, G.; Kong, X.T.; Tajhya, R.B.; Beeton, C.; Jadus, M.R. Big Potassium (BK) ion channels in biology, disease and possible targets for cancer immunotherapy. Int. Immunopharmacol. 2014, 22, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Latorre, R.; Brauchi, S. Large conductance Ca2+-activated K+ (BK) channel: Activation by Ca2+ and voltage. Biol. Res. 2006, 39, 385–401. [Google Scholar] [CrossRef] [PubMed]
- Ponnalagu, D.; Singh, H. Anion Channels of Mitochondria. Handb. Exp. Pharmacol. 2017, 240, 71–101. [Google Scholar] [CrossRef] [PubMed]
- Latorre, R.; Castillo, K.; Carrasquel-Ursulaez, W.; Sepulveda, R.V.; Gonzalez-Nilo, F.; Gonzalez, C.; Alvarez, O. Molecular Determinants of BK Channel Functional Diversity and Functioning. Physiol. Rev. 2017, 97, 39–87. [Google Scholar] [CrossRef] [PubMed]
- Cui, J. The action of a BK channel opener. J. Gen. Physiol. 2020, 152, e202012571. [Google Scholar] [CrossRef]
- Schickling, B.M.; England, S.K.; Aykin-Burns, N.; Norian, L.A.; Leslie, K.K.; Frieden-Korovkina, V.P. BKCa channel inhibitor modulates the tumorigenic ability of hormone-independent breast cancer cells via the Wnt pathway. Oncol. Rep. 2015, 33, 533–538. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, X.B.; Wulfsen, I.; Utku, E.; Sausbier, U.; Sausbier, M.; Wieland, T.; Ruth, P.; Korth, M. Dual role of protein kinase C on BK channel regulation. Proc. Natl. Acad. Sci. USA 2010, 107, 8005–8010. [Google Scholar] [CrossRef] [PubMed]
- Nardi, A.; Olesen, S.P. BK channel modulators: A comprehensive overview. Curr. Med. Chem. 2008, 15, 1126–1146. [Google Scholar] [CrossRef]
- Chen, Q.; Tao, J.; Hei, H.; Li, F.; Wang, Y.; Peng, W.; Zhang, X. Up-Regulatory Effects of Curcumin on Large Conductance Ca2+-Activated K+ Channels. PLoS ONE 2015, 10, e0144800. [Google Scholar] [CrossRef][Green Version]
- Geng, Y.; Magleby, K.L. Single-channel kinetics of BK (Slo1) channels. Front. Physiol. 2014, 5, 532. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qu, C.; Han, C.; Chen, M.M.; An, L.J.; Zou, W. Potassium channels and their role in glioma: A mini review. Mol. Membr. Biol. 2019, 35, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Abdul Kadir, L.; Stacey, M.; Barrett-Jolley, R. Emerging Roles of the Membrane Potential: Action Beyond the Action Potential. Front. Physiol. 2018, 9, 1661. [Google Scholar] [CrossRef] [PubMed]
- Sizemore, G.; McLaughlin, S.; Newman, M.; Brundage, K.; Ammer, A.; Martin, K.; Pugacheva, E.; Coad, J.; Mattes, M.D.; Yu, H.G. Opening large-conductance potassium channels selectively induced cell death of triple-negative breast cancer. BMC Cancer 2020, 20, 595. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.G.; Liu, W.C.; Dong, S.; Du, C.; Wang, X.J.; Li, J.S.; Xie, X.P.; Wu, L.; Ma, D.C.; Yu, Z.B.; et al. Activation of BK(Ca) channels in zoledronic acid-induced apoptosis of MDA-MB-231 breast cancer cells. PLoS ONE 2012, 7, e37451. [Google Scholar] [CrossRef]
- Khaitan, D.; Sankpal, U.T.; Weksler, B.; Meister, E.A.; Romero, I.A.; Couraud, P.O.; Ningaraj, N.S. Role of KCNMA1 gene in breast cancer invasion and metastasis to brain. BMC Cancer 2009, 9, 258. [Google Scholar] [CrossRef]
- Brandalise, F.; Ratto, D.; Leone, R.; Olivero, F.; Roda, E.; Locatelli, C.A.; Grazia Bottone, M.; Rossi, P. Deeper and Deeper on the Role of BK and Kir4.1 Channels in Glioblastoma Invasiveness: A Novel Summative Mechanism? Front. Neurosci. 2020, 14, 595664. [Google Scholar] [CrossRef] [PubMed]
- Goda, A.A.; Siddique, A.B.; Mohyeldin, M.; Ayoub, N.M.; El Sayed, K.A. The Maxi-K (BK) Channel Antagonist Penitrem A as a Novel Breast Cancer-Targeted Therapeutic. Mar. Drugs 2018, 16, 157. [Google Scholar] [CrossRef] [PubMed]
- Noda, S.; Chikazawa, K.; Suzuki, Y.; Imaizumi, Y.; Yamamura, H. Involvement of the gamma1 subunit of the large-conductance Ca(2+)-activated K(+) channel in the proliferation of human somatostatinoma cells. Biochem. Biophys. Res. Commun. 2020, 525, 1032–1037. [Google Scholar] [CrossRef]
- Li, N.; Liu, L.; Li, G.; Xia, M.; Du, C.; Zheng, Z. The role of BKCa in endometrial cancer HEC-1-B cell proliferation and migration. Gene 2018, 655, 42–47. [Google Scholar] [CrossRef]
- Nausch, B.; Rode, F.; Jorgensen, S.; Nardi, A.; Korsgaard, M.P.; Hougaard, C.; Bonev, A.D.; Brown, W.D.; Dyhring, T.; Strobaek, D.; et al. NS19504: A novel BK channel activator with relaxing effect on bladder smooth muscle spontaneous phasic contractions. J. Pharmacol. Exp. Ther. 2014, 350, 520–530. [Google Scholar] [CrossRef]
- Bentzen, B.H.; Olesen, S.P.; Ronn, L.C.; Grunnet, M. BK channel activators and their therapeutic perspectives. Front. Physiol. 2014, 5, 389. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.L.; Jo, H.; Wang, X.Y.; Ji, T.T.; Lin, H.X.; Park, C.S.; Cui, Y.M. Synthesis and BK channel-opening activity of 2-amino-1,3-thiazole derivatives. Bioorg. Med. Chem. Lett. 2021, 43, 128083. [Google Scholar] [CrossRef]
- Mughal, A.; Sun, C.; O’Rourke, S.T. Apelin Reduces Nitric Oxide-Induced Relaxation of Cerebral Arteries by Inhibiting Activation of Large-Conductance, Calcium-Activated K Channels. J. Cardiovasc. Pharmacol. 2018, 71, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Shannan, B.; Perego, M.; Somasundaram, R.; Herlyn, M. Heterogeneity in Melanoma. In Melanoma; Cancer Treatment and Research; Springer: Cham, Switzerland, 2016; Volume 167, pp. 1–15. [Google Scholar] [CrossRef]
- Cros, J.; Raffenne, J.; Couvelard, A.; Pote, N. Tumor Heterogeneity in Pancreatic Adenocarcinoma. Pathobiology 2018, 85, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, S.; Barbieri, R.; Pusch, M.; Gavazzo, P. Gain of function of sporadic/familial hemiplegic migraine-causing SCN1A mutations: Use of an optimized cDNA. Cephalalgia 2019, 39, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [CrossRef]
- McCauley, J.; Zivanovic, A.; Skropeta, D. Bioassays for anticancer activities. Methods Mol. Biol. 2013, 1055, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Li, N.; Tong, X.; Zeng, J.; He, S.; Gai, T.; Bai, Y.; Liu, L.; Lu, K.; Shen, J.; et al. Ara-c induces cell cycle G1/S arrest by inducing upregulation of the INK4 family gene or directly inhibiting the formation of the cell cycle-dependent complex CDK4/cyclin D1. Cell Cycle 2019, 18, 2293–2306. [Google Scholar] [CrossRef] [PubMed]
- Ferrera, L.; Barbieri, R.; Picco, C.; Zuccolini, P.; Remigante, A.; Bertelli, S.; Fumagalli, M.R.; Zifarelli, G.; La Porta, C.A.M.; Gavazzo, P.; et al. TRPM2 Oxidation Activates Two Distinct Potassium Channels in Melanoma Cells through Intracellular Calcium Increase. Int. J. Mol. Sci. 2021, 22, 8359. [Google Scholar] [CrossRef]
- Zuccolini, P.; Ferrera, L.; Remigante, A.; Picco, C.; Barbieri, R.; Bertelli, S.; Moran, O.; Gavazzo, P.; Pusch, M. The VRAC blocker DCPIB directly gates the BK channels and increases intracellular Ca2+ in melanoma and Pancreatic Duct Adenocarcinoma (PDAC) cell lines. Brit. J. Pharmacol. 2021. under review. [Google Scholar]
- Zhou, Y.; Lingle, C.J. Paxilline inhibits BK channels by an almost exclusively closed-channel block mechanism. J. Gen. Physiol. 2014, 144, 415–440. [Google Scholar] [CrossRef]
- Bentzen, B.H.; Nardi, A.; Calloe, K.; Madsen, L.S.; Olesen, S.P.; Grunnet, M. The small molecule NS11021 is a potent and specific activator of Ca2+-activated big-conductance K+ channels. Mol. Pharmacol. 2007, 72, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Suzuki, S.; Sakamoto, K.; Nakahara, T.; Ishii, K. BMS-191011, an opener of large-conductance Ca2+-activated potassium channels, dilates rat retinal arterioles in vivo. Biol. Pharm. Bull. 2011, 34, 150–152. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rockman, M.E.; Vouga, A.G.; Rothberg, B.S. Molecular mechanism of BK channel activation by the smooth muscle relaxant NS11021. J. Gen. Physiol. 2020, 152, e201912506. [Google Scholar] [CrossRef]
- Budelli, G.; Geng, Y.; Butler, A.; Magleby, K.L.; Salkoff, L. Properties of Slo1 K+ channels with and without the gating ring. Proc. Natl. Acad. Sci. USA 2013, 110, 16657–16662. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chen, T.; Suo, Q.; Shi, R.; Khan, H.; Ma, Y.; Tang, Y.; Yang, G.Y.; Zhang, Z. BK Channel-Mediated Microglial Phagocytosis Alleviates Neurological Deficit After Ischemic Stroke. Front. Cell. Neurosci. 2021, 15, 683769. [Google Scholar] [CrossRef] [PubMed]
- Oeggerli, M.; Tian, Y.; Ruiz, C.; Wijker, B.; Sauter, G.; Obermann, E.; Guth, U.; Zlobec, I.; Sausbier, M.; Kunzelmann, K.; et al. Role of KCNMA1 in breast cancer. PLoS ONE 2012, 7, e41664. [Google Scholar] [CrossRef] [PubMed]
- Kraft, R.; Krause, P.; Jung, S.; Basrai, D.; Liebmann, L.; Bolz, J.; Patt, S. BK channel openers inhibit migration of human glioma cells. Pflugers Arch. 2003, 446, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, H.; Ohi, Y.; Muraki, K.; Watanabe, M.; Imaizumi, Y. BK channel activation by NS-1619 is partially mediated by intracellular Ca2+ release in smooth muscle cells of porcine coronary artery. Br. J. Pharmacol. 2001, 132, 828–834. [Google Scholar] [CrossRef]
- Qiu, Z.; Dubin, A.E.; Mathur, J.; Tu, B.; Reddy, K.; Miraglia, L.J.; Reinhardt, J.; Orth, A.P.; Patapoutian, A. SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell 2014, 157, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Xi, L.; Wang, H.; Huang, X.; Ma, X.; Han, Z.; Wu, P.; Ma, X.; Lu, Y.; Wang, G.; et al. The potassium ion channel opener NS1619 inhibits proliferation and induces apoptosis in A2780 ovarian cancer cells. Biochem. Biophys. Res. Commun. 2008, 375, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Bloch, M.; Ousingsawat, J.; Simon, R.; Schraml, P.; Gasser, T.C.; Mihatsch, M.J.; Kunzelmann, K.; Bubendorf, L. KCNMA1 gene amplification promotes tumor cell proliferation in human prostate cancer. Oncogene 2007, 26, 2525–2534. [Google Scholar] [CrossRef]
- Rosa, P.; Catacuzzeno, L.; Sforna, L.; Mangino, G.; Carlomagno, S.; Mincione, G.; Petrozza, V.; Ragona, G.; Franciolini, F.; Calogero, A. BK channels blockage inhibits hypoxia-induced migration and chemoresistance to cisplatin in human glioblastoma cells. J. Cell Physiol. 2018, 233, 6866–6877. [Google Scholar] [CrossRef] [PubMed]
- Cuddapah, V.A.; Sontheimer, H. Ion channels and transporters [corrected] in cancer. 2. Ion channels and the control of cancer cell migration. Am. J. Physiol. Cell Physiol. 2011, 301, C541–C549. [Google Scholar] [CrossRef] [PubMed]
- Pardo, L.A.; Stuhmer, W. The roles of K(+) channels in cancer. Nat. Rev. Cancer 2014, 14, 39–48. [Google Scholar] [CrossRef] [PubMed]
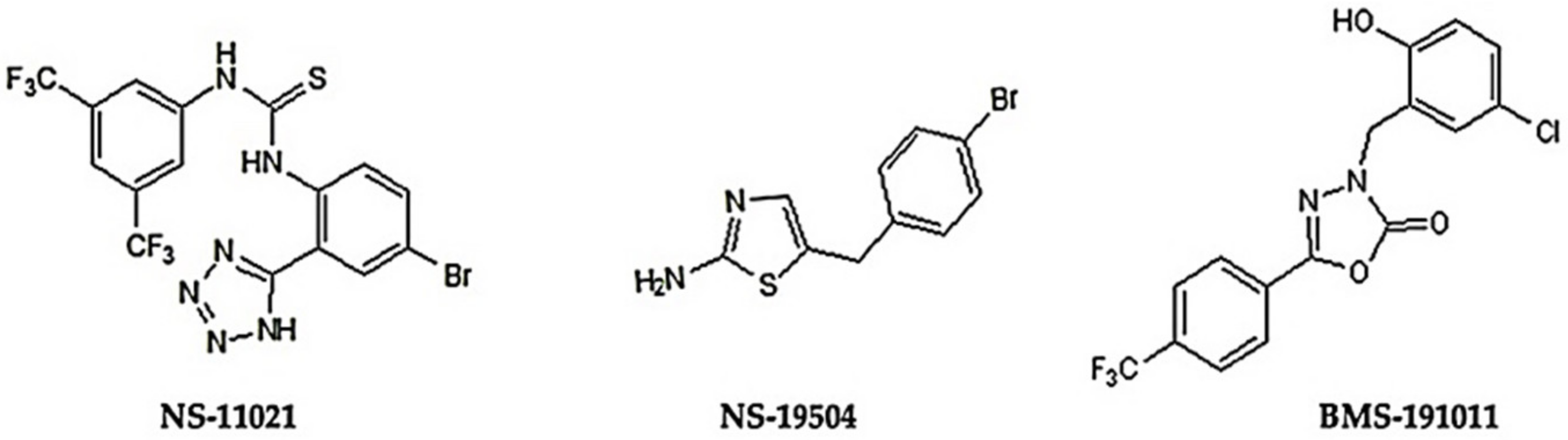
| Variable | NaCl | KCl | CaCl2 | MgCl2 | HEPES | EGTA |
|---|---|---|---|---|---|---|
| STD | 145 mM | 5 mM | 2 mM | 1 mM | 10 mM | - |
| STD 0 Ca2+ | 145 mM | 5 mM | - | 1 mM | 10 mM | 3 mM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Remigante, A.; Zuccolini, P.; Barbieri, R.; Ferrera, L.; Morabito, R.; Gavazzo, P.; Pusch, M.; Picco, C. NS-11021 Modulates Cancer-Associated Processes Independently of BK Channels in Melanoma and Pancreatic Duct Adenocarcinoma Cell Lines. Cancers 2021, 13, 6144. https://doi.org/10.3390/cancers13236144
Remigante A, Zuccolini P, Barbieri R, Ferrera L, Morabito R, Gavazzo P, Pusch M, Picco C. NS-11021 Modulates Cancer-Associated Processes Independently of BK Channels in Melanoma and Pancreatic Duct Adenocarcinoma Cell Lines. Cancers. 2021; 13(23):6144. https://doi.org/10.3390/cancers13236144
Chicago/Turabian StyleRemigante, Alessia, Paolo Zuccolini, Raffaella Barbieri, Loretta Ferrera, Rossana Morabito, Paola Gavazzo, Michael Pusch, and Cristiana Picco. 2021. "NS-11021 Modulates Cancer-Associated Processes Independently of BK Channels in Melanoma and Pancreatic Duct Adenocarcinoma Cell Lines" Cancers 13, no. 23: 6144. https://doi.org/10.3390/cancers13236144
APA StyleRemigante, A., Zuccolini, P., Barbieri, R., Ferrera, L., Morabito, R., Gavazzo, P., Pusch, M., & Picco, C. (2021). NS-11021 Modulates Cancer-Associated Processes Independently of BK Channels in Melanoma and Pancreatic Duct Adenocarcinoma Cell Lines. Cancers, 13(23), 6144. https://doi.org/10.3390/cancers13236144








