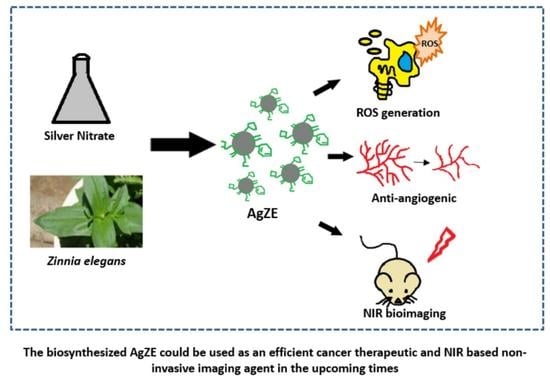
Biosynthesized Silver Nanoparticles for Cancer Therapy and In Vivo Bioimaging
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Cell Lines
2.1.2. Preparation of Ethanolic Zinnia elegans Plant Leaf Extract
2.1.3. Synthesis of Silver Nanoparticle Using Ethanolic ZE Plant Leaf Extract
2.2. Characterization
2.3. In Vitro Experiments
2.3.1. Cell Culture Experiments
2.3.2. Cell Viability Assay Using MTT Reagent
2.3.3. ICPOES for Determination of Silver
2.3.4. Scratch Assay
2.3.5. Transwell Migration Assay
2.3.6. Cell Cycle Assay
2.3.7. Cellular Apoptosis Assay
2.3.8. Reactive Oxygen Species (ROS) Assay
2.3.9. Western Blot Analysis
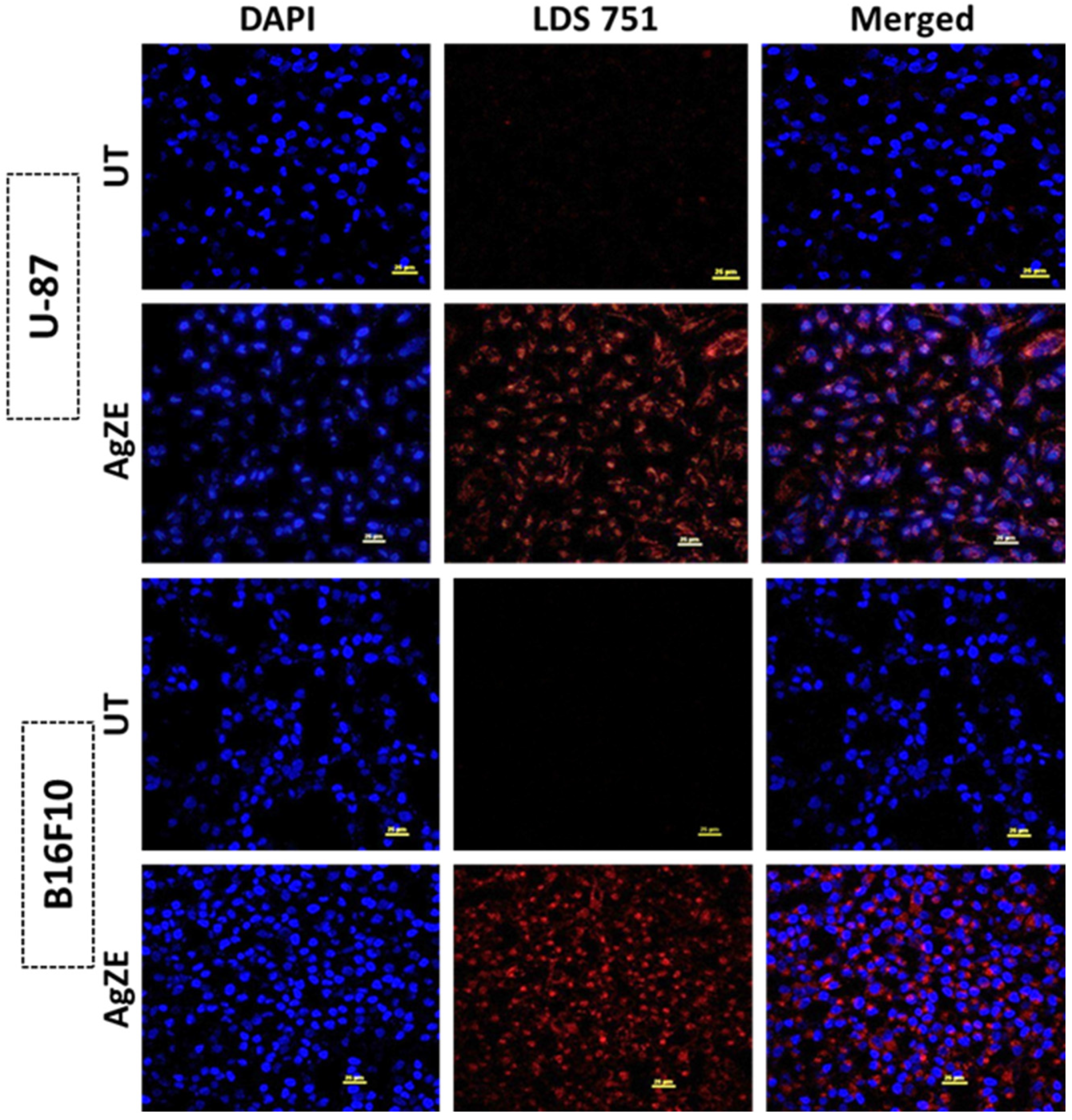
2.3.10. Cellular Imaging by Confocal Microscopy
2.4. In Vivo Assays
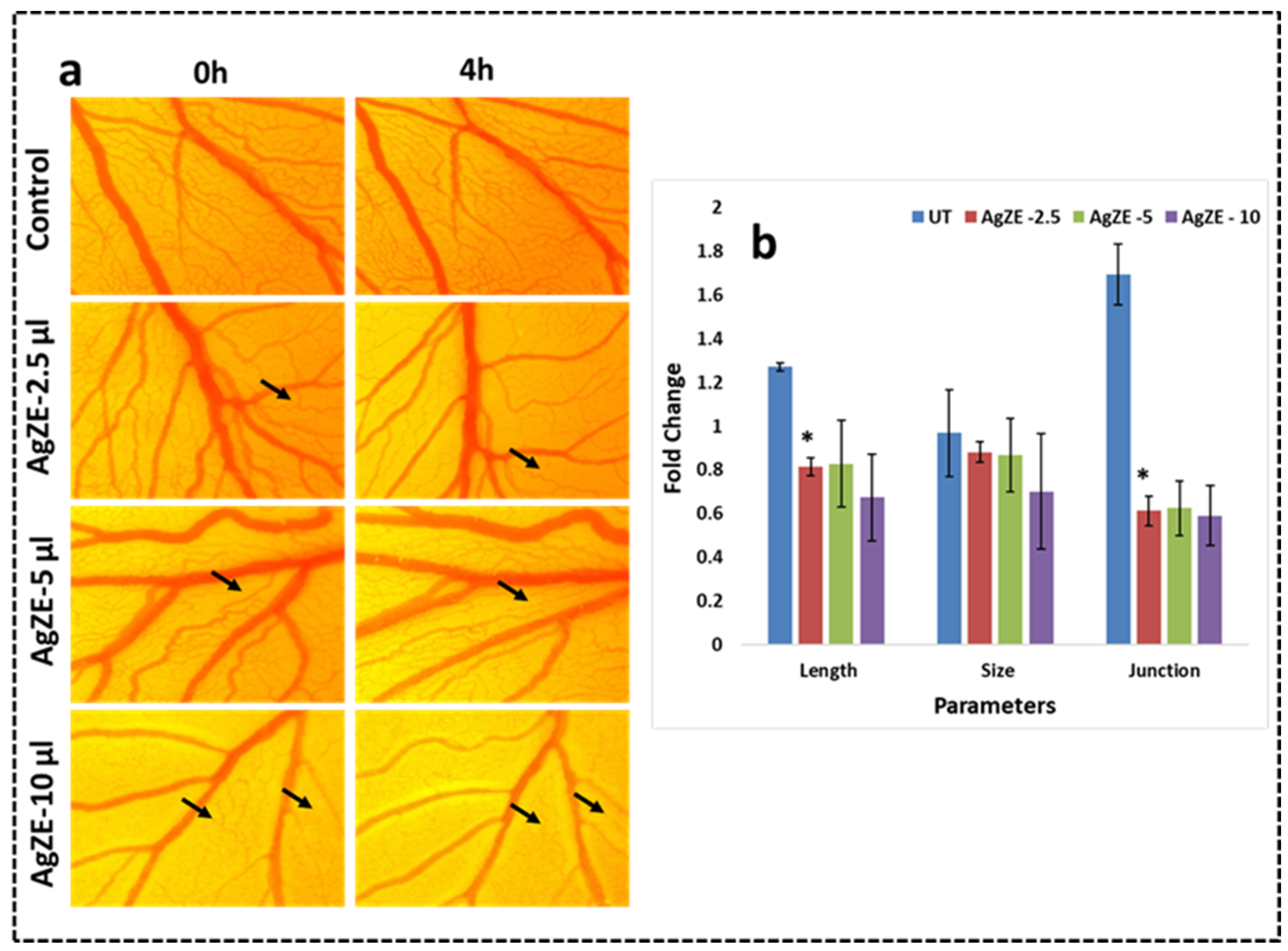
2.4.1. Chorioallantoic Membrane (CAM) Assay
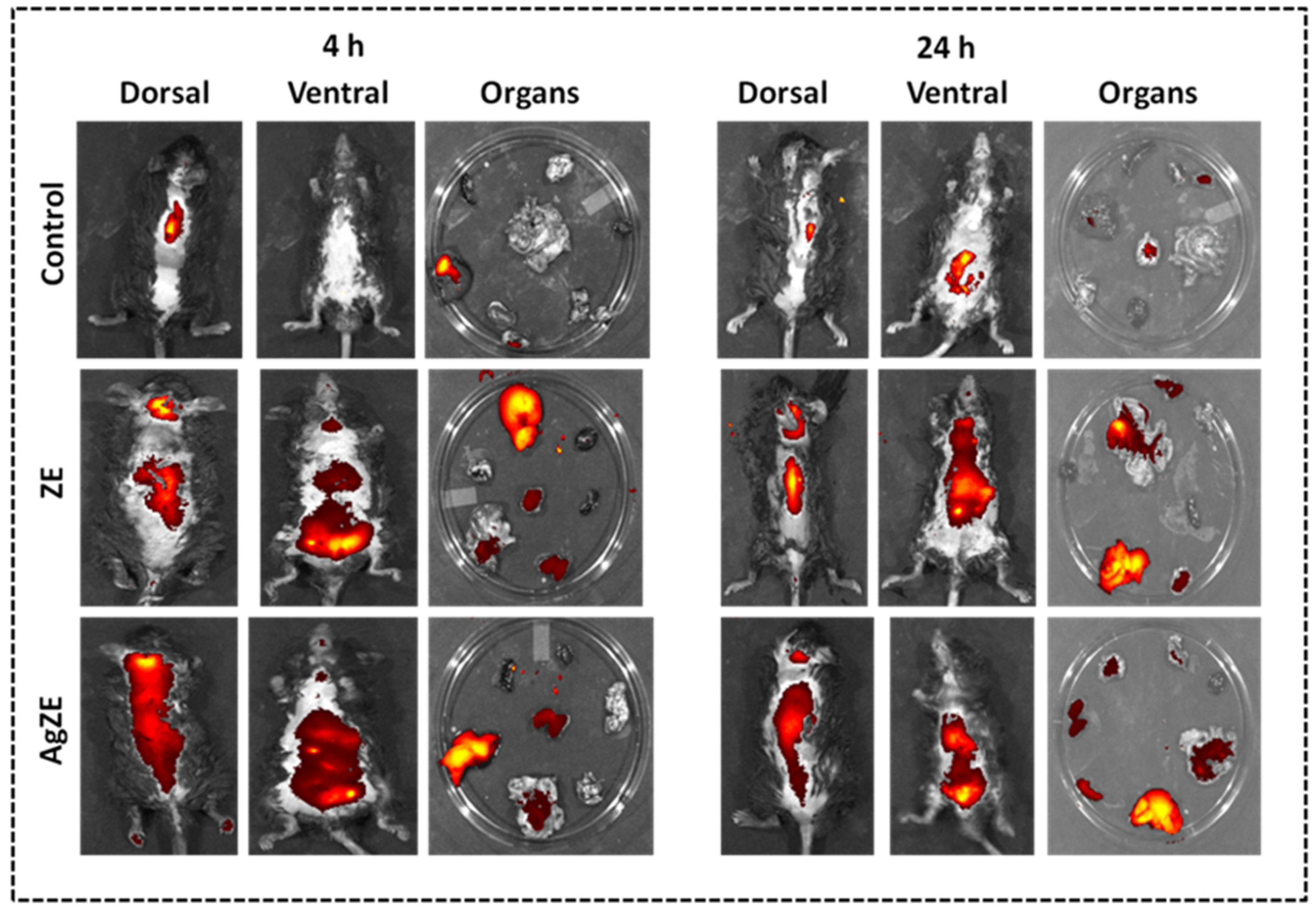
2.4.2. Non-Invasive Imaging In Vivo
2.4.3. Biodistribution Study in Tumor Model
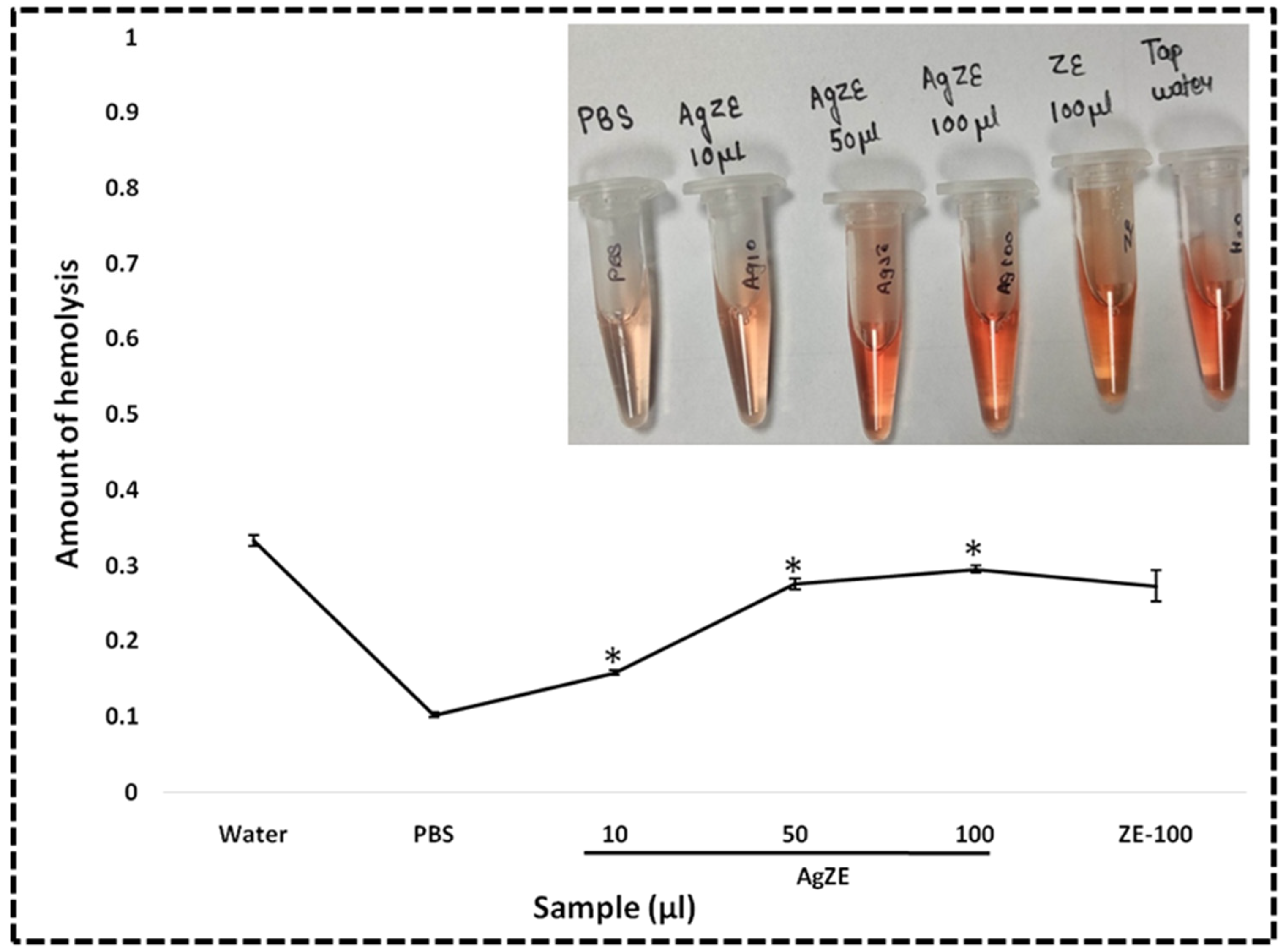
2.4.4. Hemolysis Assay
2.4.5. Statistical Analysis
3. Results
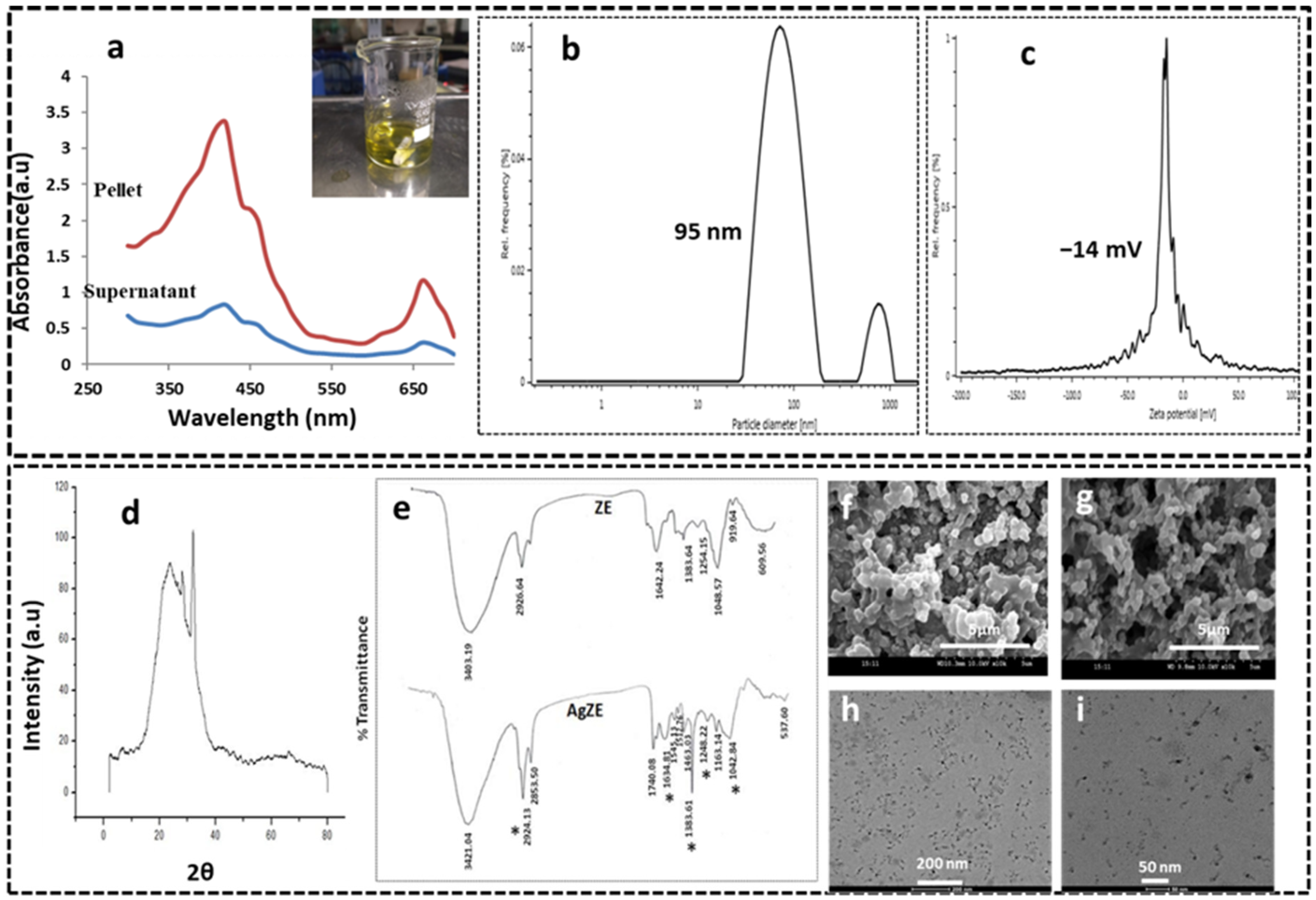
3.1. Synthesis and Characterization of AgZE
3.2. UV-Visible Spectroscopy
3.3. Dynamic Light Scattering (DLS) Analysis
3.4. X-ray Diffraction (XRD) Analysis
3.5. Fourier Transformed Infrared Spectroscopy (FTIR)
3.6. Scanning Electron Microscope (SEM)
3.7. Transmission Electron Microscope (TEM)
3.8. Fluorescence Measurements
3.9. Inductively Coupled Plasma Optical Emission Spectrometry (ICPOES) Analysis
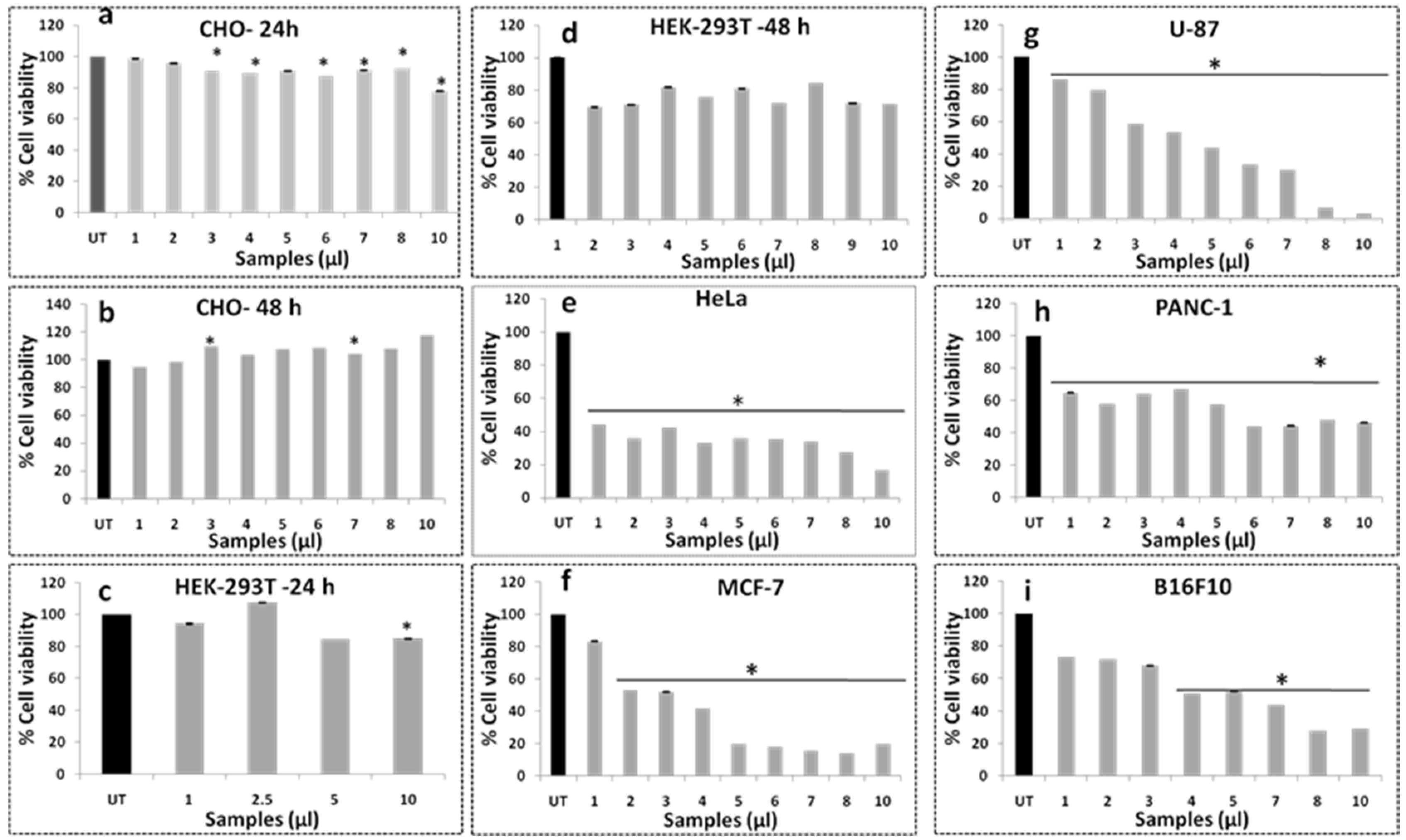
3.10. Cell Viability Assay
3.11. Studies on Anticancer Activity of AgZE on Glioblastoma Cells
3.12. Cell Uptake Study
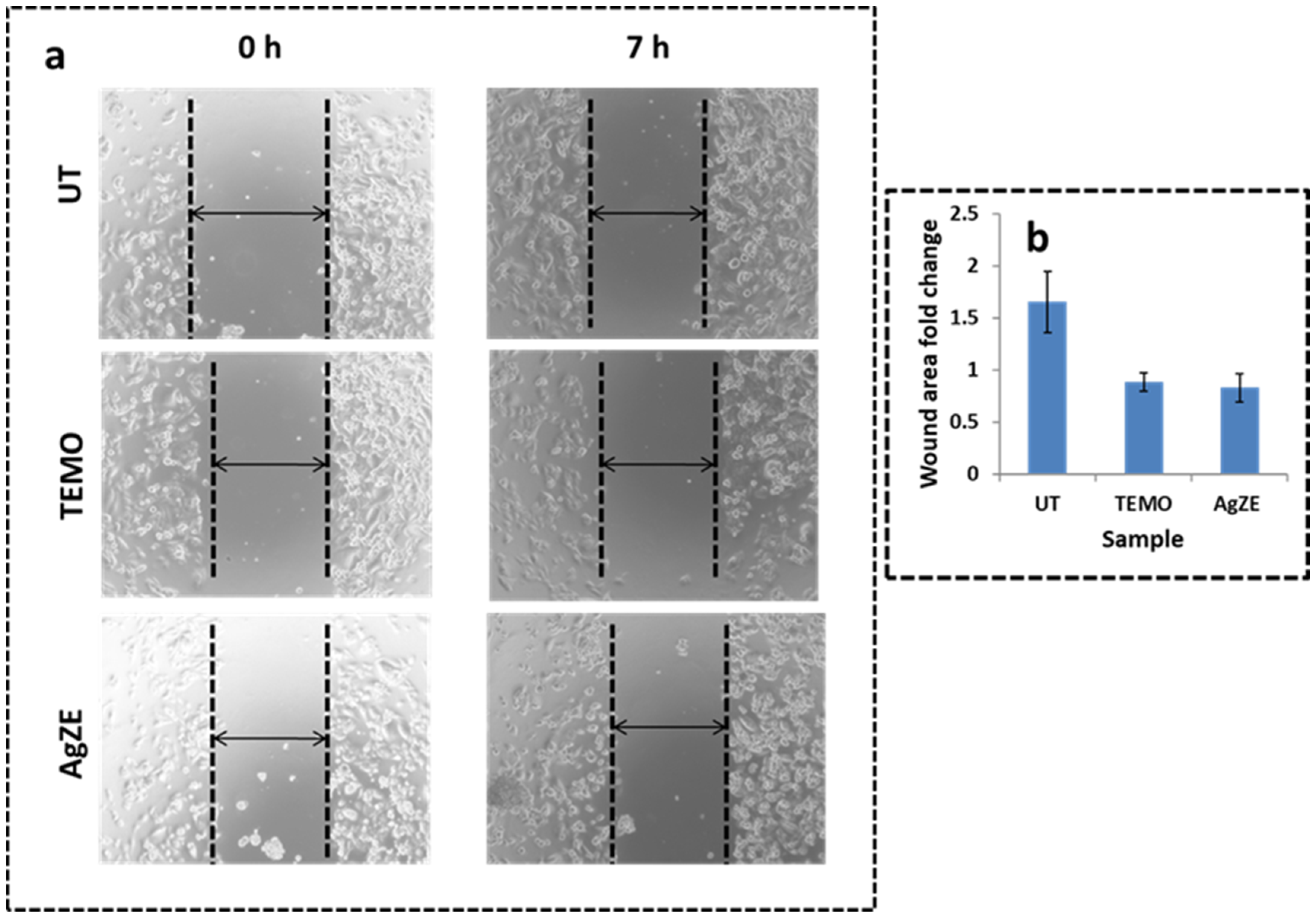
3.13. Scratch Assay
3.14. Transwell Migration Assay
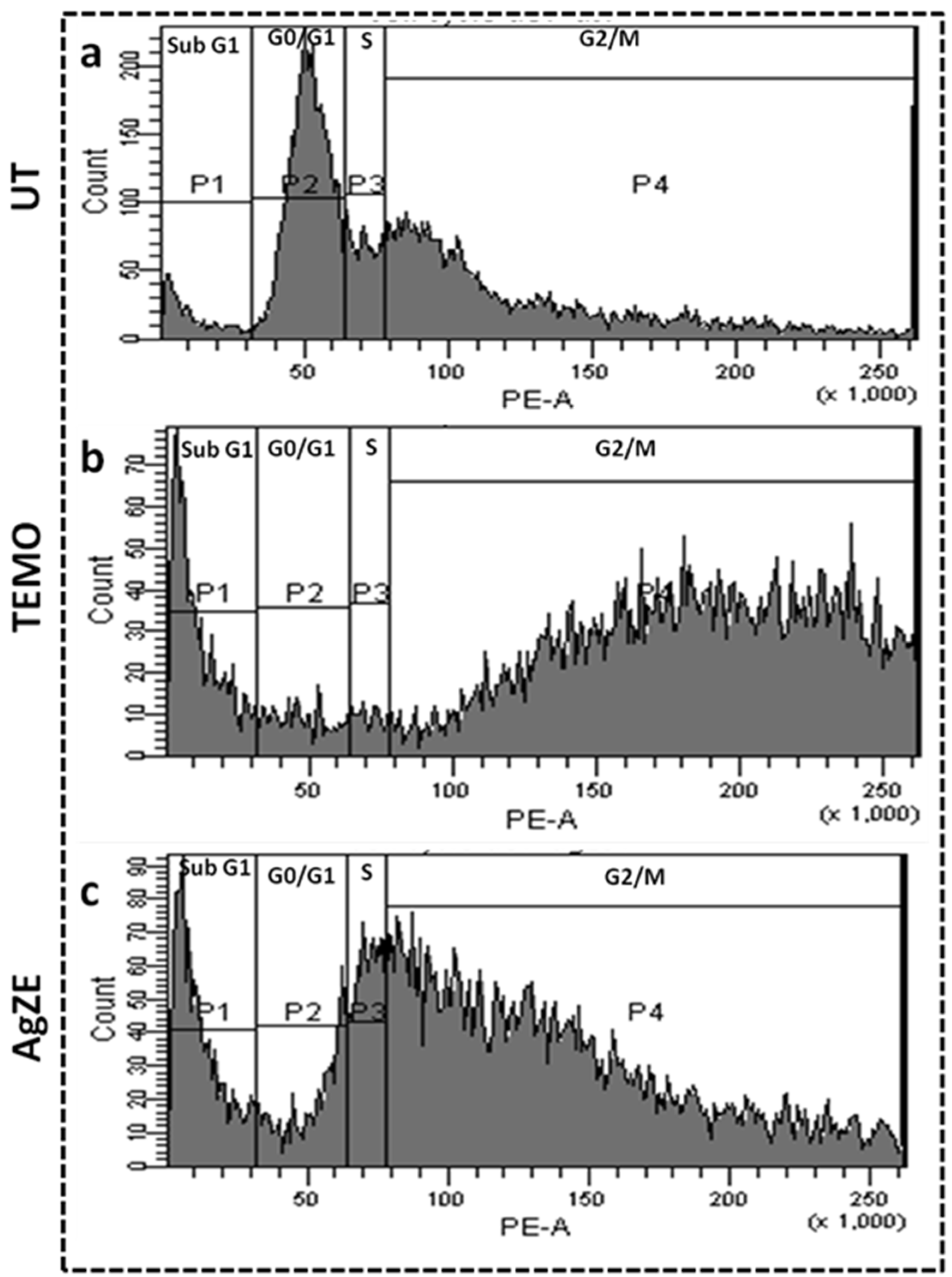
3.15. Cell Cycle Assay
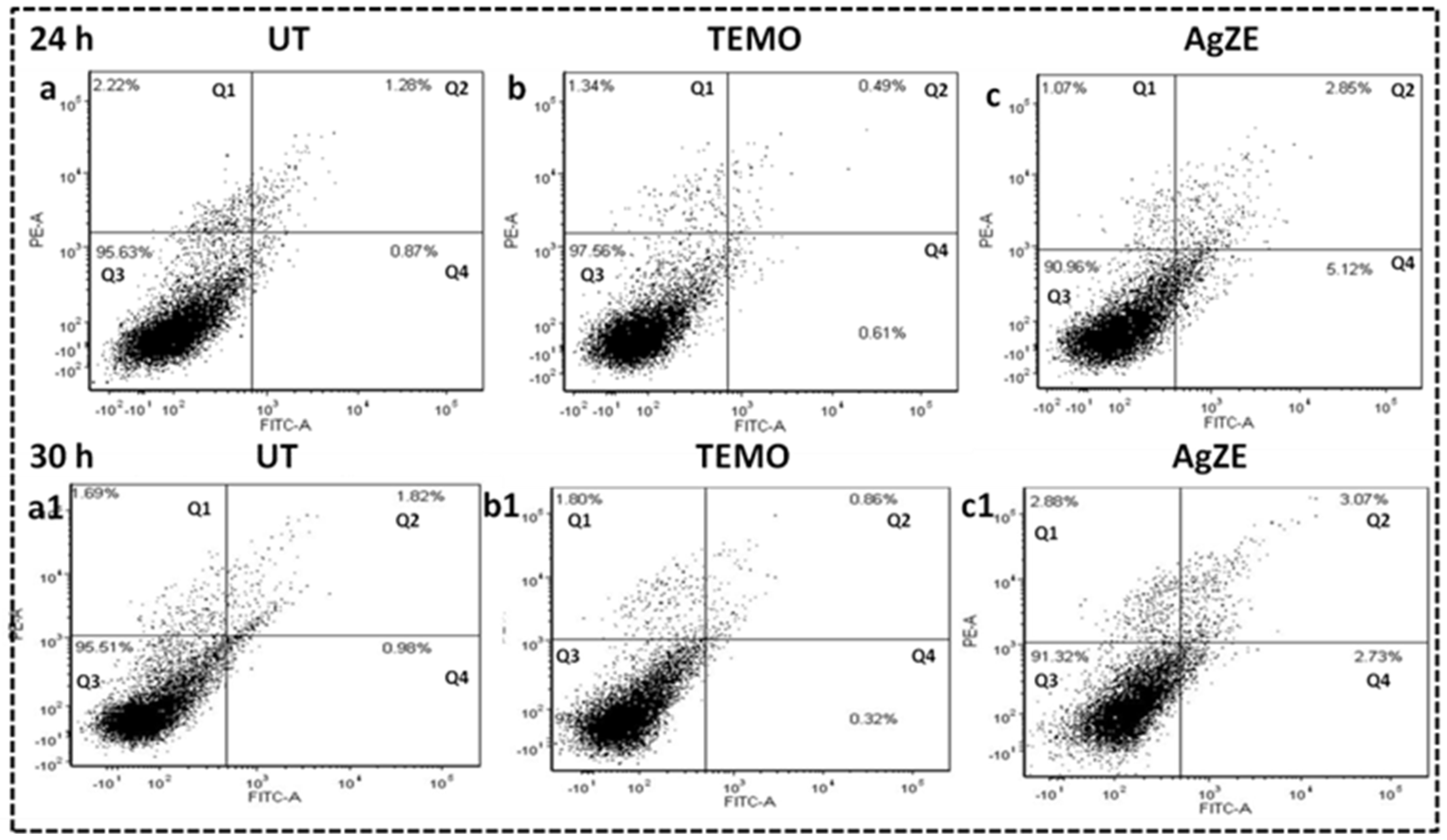
3.16. Cellular Apoptosis Assay
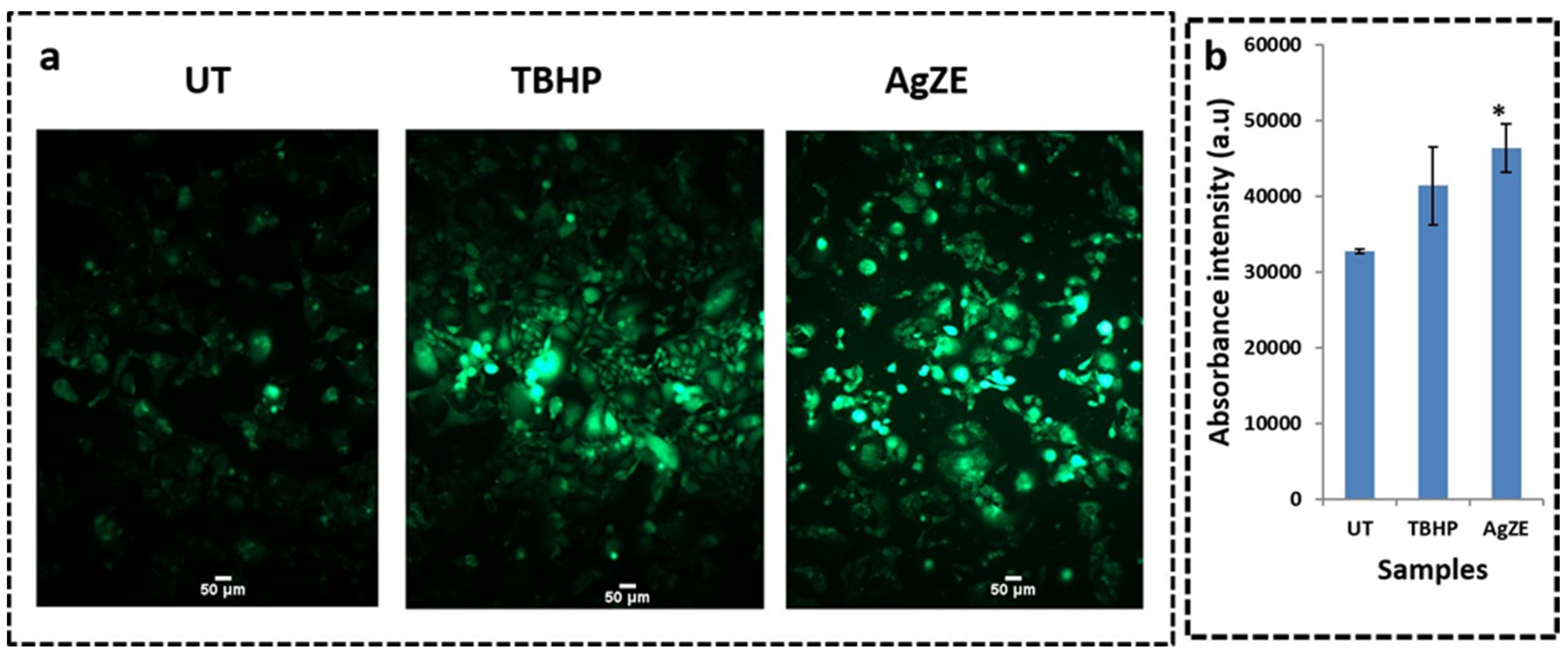
3.17. Reactive Oxygen Species (ROS) Determination
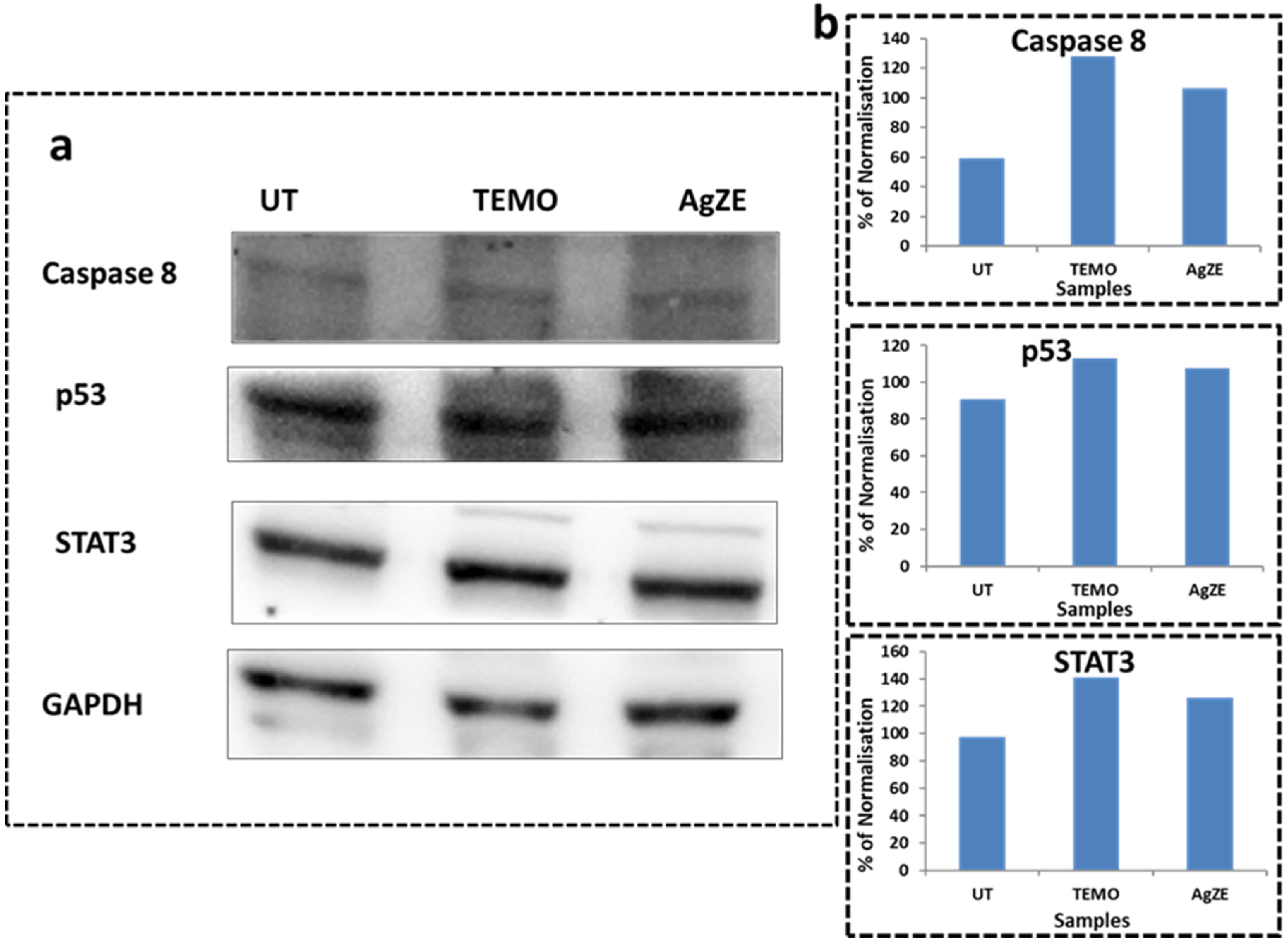
3.18. Western Blot Analysis
3.19. Cellular Imaging by Confocal Microscopy
3.20. Chorioallantoic Membrane (CAM) Assay
3.21. Non-Invasive Imaging of AgZE In Vivo
3.22. Biodistribution Analysis by ICPOES
3.23. Pilot Study for Biodistribution Analysis in Tumor Model
3.24. Hemolysis Assay
4. Mechanism behind Anticancer Activity of AgZE
5. Discussion
6. Preclinical Approach and Future Perspective
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Zhang, Y.-B.; Pan, X.-F.; Chen, J.; Cao, A.; Zhang, Y.-G.; Xia, L.; Wang, J.; Li, H.; Liu, G.; Pan, A. Combined lifestyle factors, incident cancer, and cancer mortality: A systematic review and meta-analysis of prospective cohort studies. Br. J. Cancer 2020, 122, 1085–1093. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Haque, S.; Patra, C.R. Biologically synthesized gold nanoparticles as a near-infrared-based bioimaging agent. Nanomedicine 2021, 16, 613–616. [Google Scholar] [CrossRef]
- Pannerselvam, B.; Thiyagarajan, D.; Pazhani, A.; Thangavelu, K.P.; Kim, H.J.; Rangarajulu, S.K. Copperpod Plant Synthesized AgNPs Enhance Cytotoxic and Apoptotic Effect in Cancer Cell Lines. Processes 2021, 9, 888. [Google Scholar] [CrossRef]
- Ratan, Z.A.; Haidere, M.F.; Nurunnabi, M.; Shahriar, S.M.; Ahammad, A.J.S.; Shim, Y.Y.; Reaney, M.J.T.; Cho, J.Y. Green Chemistry Synthesis of Silver Nanoparticles and Their Potential Anticancer Effects. Cancers 2020, 12, 855. [Google Scholar] [CrossRef] [Green Version]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Khorrami, S.; Zarrabi, A.; Khaleghi, M.; Danaei, M.; Mozafari, M.R. Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int. J. Nanomed. 2018, 13, 8013–8024. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Kotcherlakota, R.; Haque, S.; Bhattacharya, D.; Kumar, J.M.; Chakravarty, S.; Patra, C.R. Improved delivery of doxorubicin using rationally designed PEGylated platinum nanoparticles for the treatment of melanoma. Mater. Sci. Eng. C 2020, 108, 110375. [Google Scholar] [CrossRef]
- Burdușel, A.C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Chowdhury, D.; Kotcherlakota, R.; Patra, S. Potential Theranostics Application of Bio-Synthesized Silver Nanoparticles (4-in-1 System). Theranostics 2014, 4, 316–335. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Sushma, V.; Patra, S.; Barui, A.K.; Bhadra, M.P.; Sreedhar, B.; Patra, C.R. Green chemistry approach for the synthesis and stabilization of biocompatible gold nanoparticles and their potential applications in cancer therapy. Nanotechnology 2012, 23, 455103. [Google Scholar] [CrossRef]
- Nethi, S.K.; Mukherjee, S.; Veeriah, V.; Barui, A.K.; Chatterjee, S.; Patra, C.R. Bioconjugated gold nanoparticles accelerate the growth of new blood vessels through redox signaling. Chem. Commun. 2014, 50, 14367–14370. [Google Scholar] [CrossRef]
- Limame, R.; Wouters, A.; Pauwels, B.; Fransen, E.; Peeters, M.; Lardon, F.; De Wever, O.; Pauwels, P. Comparative Analysis of Dynamic Cell Viability, Migration and Invasion Assessments by Novel Real-Time Technology and Classic Endpoint Assays. PLoS ONE 2012, 7, e46536. [Google Scholar] [CrossRef]
- Gurunathan, S.; Jeong, J.-K.; Han, J.W.; Zhang, X.-F.; Park, J.H.; Kim, J.-H. Multidimensional effects of biologically synthesized silver nanoparticles in Helicobacter pylori, Helicobacter felis, and human lung (L132) and lung carcinoma A549 cells. Nanoscale Res. Lett. 2015, 10, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Baharara, J.; Namvar, F.; Mousavi, M.; Ramezani, T.; Mohamad, R. Anti-Angiogenesis Effect of Biogenic Silver Nanoparticles Synthesized Using Saliva officinalis on Chick Chorioalantoic Membrane (CAM). Molecules 2014, 19, 13498–13508. [Google Scholar] [CrossRef] [Green Version]
- Siddique, S.; Chow, J.C. Application of nanomaterials in biomedical imaging and cancer therapy. Nanomaterials 2020, 10, 1700. [Google Scholar] [CrossRef]
- Ding, F.; Zhan, Y.; Lu, X.; Sun, Y. Recent advances in near-infrared II fluorophores for multifunctional biomedical imaging. Chem. Sci. 2018, 9, 4370–4380. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Park, S.; Yoon, J.; Shin, I. Recent progress in the development of near-infrared fluorescent probes for bioimaging applications. Chem. Soc. Rev. 2014, 43, 16–29. [Google Scholar] [CrossRef]
- Zhao, J.; Zhong, D.; Zhou, S. NIR-I-to-NIR-II fluorescent nanomaterials for biomedical imaging and cancer therapy. J. Mater. Chem. B 2017, 6, 349–365. [Google Scholar] [CrossRef]
- Cao, J.; Zhu, B.; Zheng, K.; He, S.; Meng, L.; Song, J.; Yang, H. Recent Progress in NIR-II Contrast Agent for Biological Imaging. Front. Bioeng. Biotechnol. 2020, 7, 487. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y. Carbon Nano-Allotrope/Magnetic Nanoparticle Hybrid Nanomaterials as T2 Contrast Agents for Magnetic Resonance Imaging Applications. J. Funct. Biomater. 2018, 9, 16. [Google Scholar] [CrossRef]
- Liu, J.M.; Chen, J.-T.; Yan, X.-P. Near Infrared Fluorescent Trypsin Stabilized Gold Nanoclusters as Surface Plasmon Enhanced Energy Transfer Biosensor and in vivo Cancer Imaging Bioprobe. Anal. Chem. 2013, 85, 3238–3245. [Google Scholar] [CrossRef]
- Roy, S.; Baral, A.; Bhattacharjee, R.; Jana, B.; Datta, A.; Ghosh, S.; Banerjee, A. Preparation of multi-coloured different sized fluorescent gold clusters from blue to NIR, structural analysis of the blue emitting Au7cluster, and cell-imaging by the NIR gold cluster. Nanoscale 2015, 7, 1912–1920. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, Y.; Hou, S.; Upputuri, P.K.; Wu, D.; Li, J.; Wang, P.; Zhen, X.; Pramanik, M.; Pu, K.; et al. Compact Plasmonic Blackbody for Cancer Theranosis in the Near-Infrared II Window. ACS Nano 2018, 12, 2643–2651. [Google Scholar] [CrossRef]
- Suganya, K.U.; Govindaraju, K.; Vani, C.V.; Kirubagaran, R.; Kumar, T.A.; Tamilselvan, S.; Veeramani, V.; Kumar, V.G. Nanoscale Chlorophyll-Liposome Composite (NCLC) Fluorescent Probe for In vivo Bio-imaging. J. Clust. Sci. 2017, 28, 2969–2977. [Google Scholar] [CrossRef]
- Kotcherlakota, R.; Nimushakavi, S.; Roy, A.; Yadavalli, H.C.; Mukherjee, S.; Haque, S.; Patra, C.R. Biosynthesized Gold Nanoparticles: In vivo Study of Near-Infrared Fluorescence (NIR)-Based Bio-imaging and Cell Labeling Applications. ACS Biomater. Sci. Eng. 2019, 5, 5439–5452. [Google Scholar] [CrossRef]
- Mohamed, A.H.; Ahmed, F.A.; Ahmed, O.K. Hepatoprotective and Antioxidant Activity of Zinnia elegans Leaves Ethanolic Extract. Int. J. Sci. Eng. Res. 2015, 6, 154–161. [Google Scholar]
- Gomaa, A.A.-R.; Samy, M.N.; Desoukey, S.Y.; Kamel, M. A comprehensive review of phytoconstituents and biological activities of genus Zinnia. J. Adv. Biomed. Pharm. Sci. 2018, 2, 29–37. [Google Scholar] [CrossRef]
- Das, S.; Roy, A.; Barui, A.K.; Alabbasi, M.M.A.; Kuncha, M.; Sistla, R.; Sreedhar, B.; Patra, C.R. Anti-angiogenic vanadium pentoxide nanoparticles for the treatment of melanoma and their in vivo toxicity study. Nanoscale 2020, 12, 7604–7621. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Kotcherlakota, R.; Haque, S.; Das, S.; Nuthi, S.; Bhattacharya, D.; Madhusudana, K.; Chakravarty, S.; Sistla, R.; Patra, C.R. Silver Prussian Blue Analogue Nanoparticles: Rationally Designed Advanced Nanomedicine for Multifunctional Biomedical Applications. ACS Biomater. Sci. Eng. 2020, 6, 690–704. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Zhou, F.; Wu, Z.; Zhang, F.; Niu, K.; Kang, Y.; Liu, X.; Wang, Q.; Wang, Y.; Wang, Q. Inhibition of cancer cell migration with CuS@ mSiO(2)-PEG nanoparticles by repressing MMP-2/MMP-9 expression. Int. J. Nanomed. 2017, 13, 103–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovács, D.; Igaz, N.; Marton, A.; Rónavári, A.; Bélteky, P.; Bodai, L.; Spengler, G.; Tiszlavicz, L.; Rázga, Z.; Hegyi, P.; et al. Core-shell nanoparticles suppress metastasis and modify the tumour-supportive activity of cancer-associated fibroblasts. J. Nanobiotechnol. 2020, 18, 18–20. [Google Scholar] [CrossRef]
- Ghanbari, M.Z.; Rastegari, P.M.; Mohammadi, M.h.; Mansouri, K. Cancer cells change their glucose metabolism to overcome increased ROS: One step from cancer cell to cancer stem cell? Biomed. Pharmacother. 2019, 112, 108690. [Google Scholar] [CrossRef]
- Souza, C.; Mônico, D.A.; Tedesco, A.C. Implications of dichlorofluoresceinphotoinstability for detection of UVA-induced oxidative stress in fibroblasts and keratinocyte cells. Photochem. Photobiol. Sci. 2020, 19, 40–48. [Google Scholar] [CrossRef]
- Mukherjee, S.; Vinothkumar, B.; Prashanthi, S.; Bangal, P.R.; Sreedhar, B.; Patra, C.R. Potential therapeutic and diagnostic applications of one-step in situ biosynthesized gold nanoconjugates (2-in-1 system) in cancer treatment. RSC Adv. 2013, 3, 2318–2329. [Google Scholar] [CrossRef]
- Londoño, R.S.M.; Jeronimo, C.R.; Millán, M.B.M.; Rivera, M.E.M.; Rodriguez, G.M.E. Effect of the Nano Crystal Size on the X-ray Diffraction Patterns of Biogenic Hydroxyapatite from Human, Bovine, and Porcine Bones. Sci. Rep. 2019, 9, 5915. [Google Scholar] [CrossRef]
- Satapute, P.; Patil, B.; Kaliwal, B. Biofabrication of Silver Nanoparticle Synthesized by Erigeron canadensis L. leaf Extract *Corresponding Author. Int. J. Sci. Res. Rev. 2017, 6, 49–58. [Google Scholar]
- Asharani, P.V.; Low, K.M.G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Raja, G.; Jang, Y.-K.; Suh, J.-S.; Kim, H.-S.; Ahn, S.H.; Kim, T.-J. Microcellular Environmental Regulation of Silver Nanoparticles in Cancer Therapy: A Critical Review. Cancers 2020, 12, 664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vu, B.T.; Shahin, S.A.; Croissant, J.; Fatieiev, Y.; Matsumoto, K.; Doan, T.L.-H.; Yik, T.; Simargi, S.; Contreras, A.; Ratliff, L.; et al. Chick chorioallantoic membrane assay as an in vivo model to study the effect of nanoparticle-based anticancer drugs in ovarian cancer. Sci. Rep. 2018, 8, 8524. [Google Scholar] [CrossRef]
- Rodrigo, M.A.M.; Casar, B.; Michalkova, H.; Jimenez, A.M.; Heger, Z.; Adam, V. Extending the Applicability of In Ovo and Ex Ovo Chicken Chorioallantoic Membrane Assays to Study Cytostatic Activity in Neuroblastoma Cells. Front. Oncol. 2021, 11, 707366. [Google Scholar] [CrossRef] [PubMed]
- Kalishwaralal, K.; Banumathi, E.; Pandian, S.R.K.; Deepak, V.; Muniyandi, J.; Eom, S.H.; Gurunathan, S. Silver nanoparticles inhibit VEGF induced cell proliferation and migration in bovine retinal endothelial cells. Colloids Surf. B Biointerfaces 2009, 73, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.C.; Madeira, V.M.; Almeida, L.M.; Custódio, J.B. Hemolysis of human erythrocytes induced by tamoxifen is related to disruption of membrane structure. Biochim. Biophys. Acta (BBA) Biomembr. 2000, 1464, 49–61. [Google Scholar] [CrossRef] [Green Version]
- Haley, K.M.; Russell, T.B.; Boshkov, L.; Leger, R.M.; Garratty, G.; Recht, M.; Nazemi, K.J. Fatal carboplatin-induced immune hemolytic anemia in a child with a brain tumor. J. Blood Med. 2014, 5, 55–58. [Google Scholar] [CrossRef] [Green Version]
- Lu, D.; Liu, Q.; Zhang, T.; Cai, Y.; Yin, Y.; Jiang, G. Stable silver isotope fractionation in the natural transformation process of silver nanoparticles. Nat. Nanotechnol. 2016, 11, 682–686. [Google Scholar] [CrossRef]
- Patra, C.R.; Bhattacharya, R.; Wang, E.; Katarya, A.; Lau, J.S.; Dutta, S.; Muders, M.H.; Wang, S.; Buhrow, S.A.; Safgren, S.L.; et al. Targeted Delivery of Gemcitabine to Pancreatic Adenocarcinoma Using Cetuximab as a Targeting Agent. Cancer Res. 2008, 68, 1970–1978. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.H.; Israelsen, W.J.; Lee, D.; Vionnie, W.; Jeanson, N.T.; Clish, C.B.; Cantley, L.C.; Vander Heiden, M.G.; Scadden, D.T. Cell-State-Specific Metabolic Dependency in Hematopoiesis and Leukemogenesis. Cell 2014, 158, 1309–1323. [Google Scholar] [CrossRef] [Green Version]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 1252–1276. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Agarwal, S.; Seth, B.; Yadav, A.; Nair, S.; Bhatnagar, P.; Karmakar, M.; Kumari, M.; Chauhan, L.K.S.; Patel, D.K.; et al. Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer’s disease model via canonical Wnt/β-catenin pathway. ACS Nano 2014, 8, 76–103. [Google Scholar] [CrossRef]
- Velasquez, J.T.; Watts, M.E.; Todorovic, M.; Nazareth, L.; Pastrana, E.; Diaz, N.J.; Lim, F.; Ekberg, J.A.; Quinn, R.J.; John, J.A.S. Low-Dose Curcumin Stimulates Proliferation, Migration and Phagocytic Activity of Olfactory Ensheathing Cells. PLoS ONE 2014, 9, e111787. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Patra, C.R. Biologically synthesized metal nanoparticles: Recent advancement and future perspectives in cancer theranostics. Future Sci. 2017, 3, FSO203. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-C.; Lin, M.-L.; Meng, M.; Chen, S.-S. Galangin Induces p53-independent S-phase Arrest and Apoptosis in Human Nasopharyngeal Carcinoma Cells Through Inhibiting PI3K−AKT Signaling Pathway. Anticancer Res. 2018, 38, 1377–1389. [Google Scholar] [CrossRef]
- Salehi, S.; Shandiz, S.A.S.; Ghanbar, F.; Darvish, M.R.; Ardestani, M.S.; Mirzaie, A.; Jafari, M. Phytosynthesis of silver nanoparticles using Artemisia marschalliana Sprengel aerial part extract and assessment of their antioxidant, anticancer, and antibacterial properties. Int. J. Nanomed. 2016, 11, 1835–1846. [Google Scholar]
- Quan, J.-H.; Gao, F.F.; Ismail, H.A.H.A.; Yuk, J.-M.; Cha, G.-H.; Chu, J.-Q.; Lee, Y.-H. Silver Nanoparticle-Induced Apoptosis in ARPE-19 Cells Is Inhibited by Toxoplasma gondii Pre-Infection Through Suppression of NOX4-Dependent ROS Generation. Int. J. Nanomed. 2020, 15, 3695–3716. [Google Scholar] [CrossRef]
- Liu, J.; Uematsu, H.; Tsuchida, N.; Ikeda, M.A. Essential role of caspase-8 in p53/p73-dependent apoptosis induced by etoposide in head and neck carcinoma cells. Mol. Cancer 2011, 10, 95. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.-S.; Lee, K.-S.; Kim, J.-H.; Kim, C.-K.; Lee, G.; Choe, J.; Won, M.-H.; Kim, T.-H.; Jeoung, D.; Lee, H.; et al. The caspase-8/Bid/cytochrome c axis links signals from death receptors to mitochondrial reactive oxygen species production. Free Radic. Biol. Med. 2017, 112, 567–577. [Google Scholar] [CrossRef]
- Chinen, A.B.; Guan, C.M.; Ferrer, J.R.; Barnaby, S.N.; Merkel, T.J.; Mirkin, C.A. Nanoparticle Probes for the Detection of Cancer Biomarkers, Cells, and Tissues by Fluorescence. Chem. Rev. 2015, 115, 10530–10574. [Google Scholar] [CrossRef] [Green Version]
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haque, S.; Norbert, C.C.; Acharyya, R.; Mukherjee, S.; Kathirvel, M.; Patra, C.R. Biosynthesized Silver Nanoparticles for Cancer Therapy and In Vivo Bioimaging. Cancers 2021, 13, 6114. https://doi.org/10.3390/cancers13236114
Haque S, Norbert CC, Acharyya R, Mukherjee S, Kathirvel M, Patra CR. Biosynthesized Silver Nanoparticles for Cancer Therapy and In Vivo Bioimaging. Cancers. 2021; 13(23):6114. https://doi.org/10.3390/cancers13236114
Chicago/Turabian StyleHaque, Shagufta, Caroline Celine Norbert, Rajarshi Acharyya, Sudip Mukherjee, Muralidharan Kathirvel, and Chitta Ranjan Patra. 2021. "Biosynthesized Silver Nanoparticles for Cancer Therapy and In Vivo Bioimaging" Cancers 13, no. 23: 6114. https://doi.org/10.3390/cancers13236114
APA StyleHaque, S., Norbert, C. C., Acharyya, R., Mukherjee, S., Kathirvel, M., & Patra, C. R. (2021). Biosynthesized Silver Nanoparticles for Cancer Therapy and In Vivo Bioimaging. Cancers, 13(23), 6114. https://doi.org/10.3390/cancers13236114