CHFR and Paclitaxel Sensitivity of Ovarian Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Development of an Antibody for Studying CHFR Expression
2.2. CHFR Expression Varies in Clinical Ovarian Cancer
2.2.1. Clinical Characteristics
2.2.2. Relationship of CHFR Expression to Tumor Histology, Grade and Debulking Status
2.2.3. CHFR Expression, Overall Survival and Time to Progression in High Grade Serous Ovarian Cancer
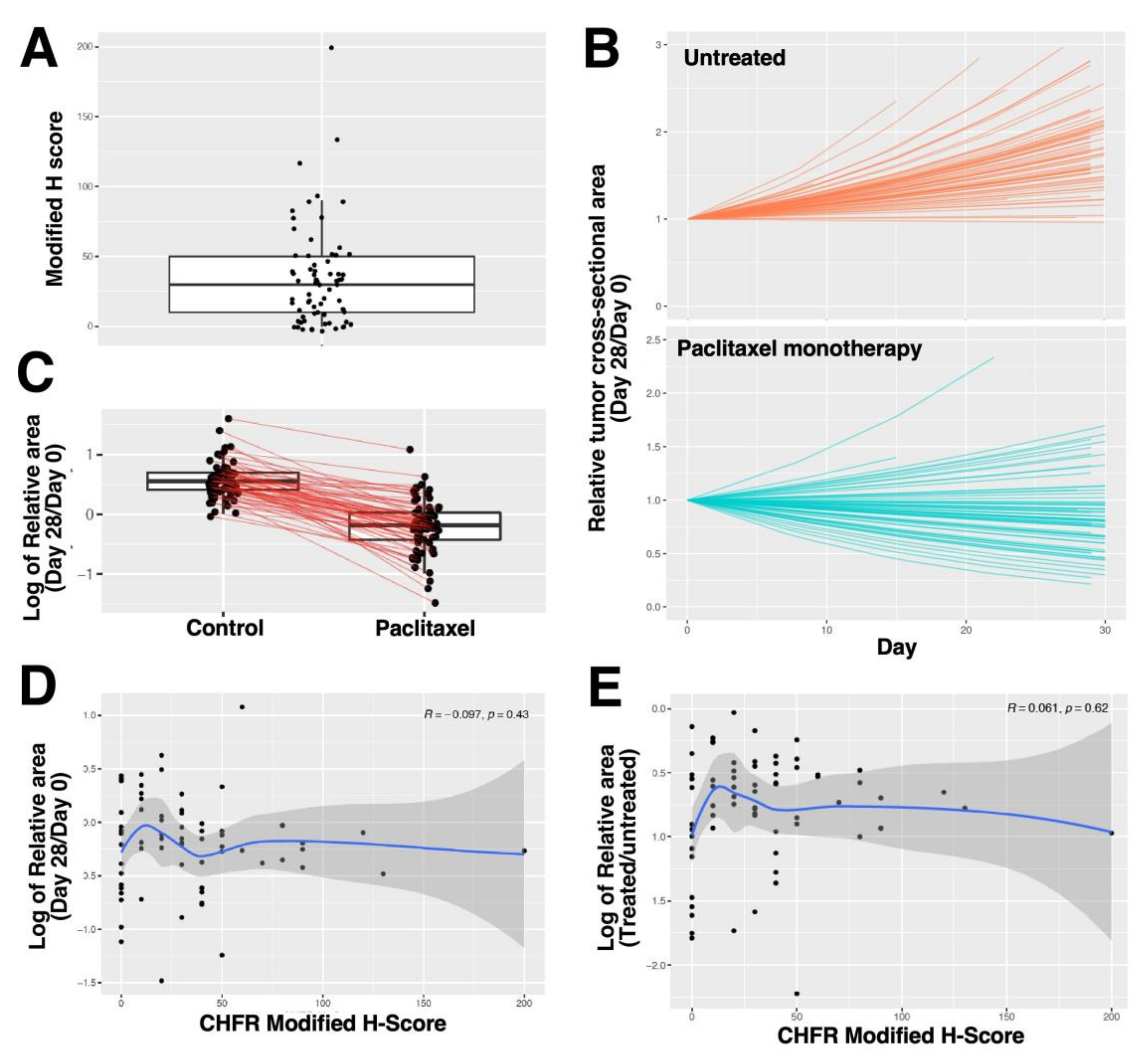
2.2.4. CHFR Expression and Response of Ovarian Cancer PDXs to Paclitaxel Monotherapy
3. Discussion
4. Methods
4.1. Reagents and Antibodies
4.2. Tissue Culture
4.3. Immunoblotting
4.4. Clinical Ovarian Cancer Specimens
4.5. Treatment of Ovarian Cancer PDXs with Paclitaxel Monotherapy
4.6. CHFR IHC and Determination of Modified H-Score
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mariatos, G.; Bothos, J.; Zacharatos, P.; Summers, M.K.; Scolnick, D.M.; Kittas, C.; Halazonetis, T.D.; Gorgoulis, V.G. Inactivating mutations targeting the chfr mitotic checkpoint gene in human lung cancer. Cancer Res. 2003, 63, 7185–7189. [Google Scholar]
- Corn, P.G.; Summers, M.K.; Fogt, F.; Virmani, A.K.; Gazdar, A.F.; Halazonetis, T.D.; El-Deiry, W.S. Frequent hypermethylation of the 5′ CpG island of the mitotic stress checkpoint gene Chfr in colorectal and non-small cell lung cancer. Carcinogenesis 2003, 24, 47–51. [Google Scholar] [CrossRef]
- Toyota, M.; Sasaki, Y.; Satoh, A.; Ogi, K.; Kikuchi, T.; Suzuki, H.; Mita, H.; Tanaka, N.; Itoh, F.; Issa, J.P.; et al. Epigenetic inactivation of CHFR in human tumors. Proc. Natl. Acad. Sci. USA 2003, 100, 7818–7823. [Google Scholar] [CrossRef]
- Privette, L.M.; Petty, E.M. CHFR: A Novel Mitotic Checkpoint Protein and Regulator of Tumorigenesis. Transl. Oncol. 2008, 1, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Derks, S.; Cleven, A.H.; Melotte, V.; Smits, K.M.; Brandes, J.C.; Azad, N.; van Criekinge, W.; de Bruine, A.P.; Herman, J.G.; van Engeland, M. Emerging evidence for CHFR as a cancer biomarker: From tumor biology to precision medicine. Cancer Metastasis Rev. 2014, 33, 161–171. [Google Scholar] [CrossRef]
- Ahel, I.; Ahel, D.; Matsusaka, T.; Clark, A.J.; Pines, J.; Boulton, S.J.; West, S.C. Poly(ADP-ribose)-binding zinc finger motifs in DNA repair/checkpoint proteins. Nature 2008, 451, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Oberoi, J.; Richards, M.W.; Crumpler, S.; Brown, N.; Blagg, J.; Bayliss, R. Structural basis of poly(ADP-ribose) recognition by the multizinc binding domain of checkpoint with forkhead-associated and RING Domains (CHFR). J. Biol. Chem. 2010, 285, 39348–39358. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, L.Y.; Yang, C.Y.; Wang, S.; Yu, X. The FHA and BRCT domains recognize ADP-ribosylation during DNA damage response. Genes Dev. 2013, 27, 1752–1768. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Chen, J.; Wong, J.; Fang, G. The checkpoint protein Chfr is a ligase that ubiquitinates Plk1 and inhibits Cdc2 at the G2 to M transition. J. Cell Biol. 2002, 156, 249–259. [Google Scholar] [CrossRef]
- Yu, X.; Minter-Dykhouse, K.; Malureanu, L.; Zhao, W.M.; Zhang, D.; Merkle, C.J.; Ward, I.M.; Saya, H.; Fang, G.; van Deursen, J.; et al. Chfr is required for tumor suppression and Aurora A regulation. Nat. Genet. 2005, 37, 401–406. [Google Scholar] [CrossRef]
- Burgess, A.; Labbe, J.C.; Vigneron, S.; Bonneaud, N.; Strub, J.M.; Van Dorsselaer, A.; Lorca, T.; Castro, A. Chfr interacts and colocalizes with TCTP to the mitotic spindle. Oncogene 2008, 27, 5554–5566. [Google Scholar] [CrossRef]
- Oh, Y.M.; Kwon, Y.E.; Kim, J.M.; Bae, S.J.; Lee, B.K.; Yoo, S.J.; Chung, C.H.; Deshaies, R.J.; Seol, J.H. Chfr is linked to tumour metastasis through the downregulation of HDAC1. Nat. Cell Biol. 2009, 11, 295–302. [Google Scholar] [CrossRef]
- Maddika, S.; Sy, S.M.; Chen, J. Functional interaction between Chfr and Kif22 controls genomic stability. J. Biol. Chem. 2009, 284, 12998–13003. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.A.; Toso, R.J.; Thrower, D.; Wilson, L. Mechanism of Mitotic Block and Inhibition of Cell Proliferation by Taxol at Low Concentrations. Proc. Natl. Acad. Sci. USA 1993, 90, 9552–9556. [Google Scholar] [CrossRef]
- Jordan, M.A.; Wendell, K.; Gardiner, S.; Derry, W.B.; Copp, H.; Wilson, L. Mitotic Block Induced in HeLa Cells by Low Concentrations of Paclitaxel (Taxol) Results in Abnormal Mitotic Exit and Apoptotic Cell Death. Cancer Res. 1996, 56, 816–825. [Google Scholar] [PubMed]
- Scolnick, D.M.; Halazonetis, T.D. Chfr defines a mitotic stress checkpoint that delays entry into metaphase. Nature 2000, 406, 430–435. [Google Scholar] [CrossRef]
- Summers, M.K.; Bothos, J.; Halazonetis, T.D. The CHFR mitotic checkpoint protein delays cell cycle progression by excluding Cyclin B1 from the nucleus. Oncogene 2005, 24, 2589–2598. [Google Scholar] [CrossRef]
- Mikhailov, A.; Rieder, C.L. Cell cycle: Stressed out of mitosis. Curr. Biol. 2002, 12, R331–R333. [Google Scholar] [CrossRef]
- Ogi, K.; Toyota, M.; Mita, H.; Satoh, A.; Kashima, L.; Sasaki, Y.; Suzuki, H.; Akino, K.; Nishikawa, N.; Noguchi, M.; et al. Small interfering RNA-induced CHFR silencing sensitizes oral squamous cell cancer cells to microtubule inhibitors. Cancer Biol. Ther. 2005, 4, 773–780. [Google Scholar] [CrossRef][Green Version]
- Blajeski, A.L.; Kottke, T.J.; Kaufmann, S.H. A multistep model for paclitaxel-induced apoptosis in human breast cancer cell lines. Exp. Cell Res. 2001, 270, 277–288. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Sudakin, V.; Bobiak, M.L.; Fisher, P.W.; Mattern, M.R.; Jablonski, S.A.; Hurle, M.R.; Zhu, Y.; Yen, T.J.; Zhou, B.B. Chfr regulates a mitotic stress pathway through its RING-finger domain with ubiquitin ligase activity. Cancer Res. 2002, 62, 1797–1801. [Google Scholar] [PubMed]
- Tanaka, M.; Chang, P.; Li, Y.; Li, D.; Overman, M.; Maru, D.M.; Sethi, S.; Phillips, J.; Bland, G.L.; Abbruzzese, J.L.; et al. Association of CHFR promoter methylation with disease recurrence in locally advanced colon cancer. Clin. Cancer Res. 2011, 17, 4531–4540. [Google Scholar] [CrossRef]
- Cleven, A.H.; Derks, S.; Draht, M.X.; Smits, K.M.; Melotte, V.; Van Neste, L.; Tournier, B.; Jooste, V.; Chapusot, C.; Weijenberg, M.P.; et al. CHFR promoter methylation indicates poor prognosis in stage II microsatellite stable colorectal cancer. Clin. Cancer Res. 2014, 20, 3261–3271. [Google Scholar] [CrossRef]
- Wang, M.; Shen, L.; Deng, D. Association between CHFR methylation and chemosensitivity of paclitaxel in advanced gastric cancer. Med. Oncol. 2014, 31, 907. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Lu, Y.; Herman, J.G.; Brock, M.V.; Zhao, P.; Guo, M. Predictive value of CHFR and MLH1 methylation in human gastric cancer. Gastric Cancer 2015, 18, 280–287. [Google Scholar] [CrossRef]
- Pillai, R.N.; Brodie, S.A.; Sica, G.L.; Shaojin, Y.; Li, G.; Nickleach, D.C.; Yuan, L.; Varma, V.A.; Bonta, D.; Herman, J.G.; et al. CHFR protein expression predicts outcomes to taxane-based first line therapy in metastatic NSCLC. Clin. Cancer Res. 2013, 19, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, Y.; Xu, C.; Xiao, L.; Shen, H.; Zhang, X.; Li, T.; Li, X. CHFR suppression by hypermethylation sensitizes endometrial cancer cells to paclitaxel. Int. J. Gynecol. Cancer 2011, 21, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Yang, Y.; Xu, C.; Shen, H. RNA interference targeting CHFR enhances taxol chemosensitivity in endometrial cancer cells. Oncol Rep. 2012, 28, 248–254. [Google Scholar] [CrossRef][Green Version]
- Banno, K.; Yanokura, M.; Kawaguchi, M.; Kuwabara, Y.; Akiyoshi, J.; Kobayashi, Y.; Iwata, T.; Hirasawa, A.; Fujii, T.; Susumu, N.; et al. Epigenetic inactivation of the CHFR gene in cervical cancer contributes to sensitivity to taxanes. Int. J. Oncol. 2007, 31, 713–720. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Cannistra, S.A.; Gershenson, D.M.; Recht, A. Ovarian Cancer, Fallopian Tube Carcinoma and Peritoneal Carcinoma. In Cancer: Principles & Practice of Oncology, 9th ed.; DeVita, V.T., Jr., Lawrence, T.S., Rosenberg, S.A., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011; pp. 1368–1391. [Google Scholar]
- Parmar, M.K.; Ledermann, J.A.; Colombo, N.; du Bois, A.; Delaloye, J.F.; Kristensen, G.B.; Wheeler, S.; Swart, A.M.; Qian, W.; Torri, V.; et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: The ICON4/AGO-OVAR-2.2 trial. Lancet 2003, 361, 2099–2106. [Google Scholar]
- Ludwig, A.H.; Bujko, M.; Bidzinski, M.; Kupryjanczyk, J. CHFR gene is neither mutated nor hypermethylated in ovarian cancer. Cancer Detect. Prev 2007, 31, 257–261. [Google Scholar] [CrossRef]
- Gao, Y.; Lou, G.; Zhang, G.M.; Sun, X.W.; Ma, Y.Y.; Yang, Y.M.; Liu, G. CHFR promoter hypermethylation and reduced CHFR mRNA expression in ovarian cancer. Int. J. Biol. Markers 2009, 24, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Feng, Y.; Wang, X.Y.; Zhang, Y.N.; Yuan, C.N.; Zhang, S.F.; Shen, Y.M.; Fu, Y.F.; Zhou, C.Y.; Li, X.; et al. The inhibition of UBC13 expression and blockage of the DNMT1-CHFR-Aurora A pathway contribute to paclitaxel resistance in ovarian cancer. Cell Death Dis. 2018, 9, 93. [Google Scholar] [CrossRef]
- Chatz, C.H.; Figg, W.D.; Chabner, B.A. Antimitotic Drugs. In Chemotherapy, Immunotherapy and Biotherapy: Principles and Practice, 6th ed.; Chabner, B.A., Longo, D.L., Eds.; Wolters Kluwer: Phildelphia, PA, USA, 2019; pp. 175–199. [Google Scholar]
- Yanokura, M.; Banno, K.; Kawaguchi, M.; Hirao, N.; Hirasawa, A.; Susumu, N.; Tsukazaki, K.; Aoki, D. Relationship of aberrant DNA hypermethylation of CHFR with sensitivity to taxanes in endometrial cancer. Oncol. Rep. 2007, 17, 41–48. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Y.; Wang, X.; Yu, X.; Wu, C.; Ding, S. Silencing of CHFR Sensitizes Gastric Carcinoma to PARP Inhibitor Treatment. Transl Oncol. 2020, 13, 113–121. [Google Scholar] [CrossRef]
- Patel, A.G.; De Lorenzo, S.B.; Flatten, K.S.; Poirier, G.G.; Kaufmann, S.H. Failure of iniparib to inhibit poly(ADP-Ribose) polymerase in vitro. Clin. Cancer Res. 2012, 18, 1655–1662. [Google Scholar] [CrossRef]
- Fu, Z.; Regan, K.; Zhang, L.; Muders, M.H.; Thibodeau, S.N.; French, A.; Wu, Y.; Kaufmann, S.H.; Lingle, W.L.; Chen, J.; et al. Deficiencies in CHFR and MLH1 synergistically enhance tumor susceptibility. J. Clin. Investig. 2009, 119, 2714–2724. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hackbarth, J.S.; Lee, S.-H.; Meng, X.W.; Vroman, B.T.; Kaufmann, S.H.; Karnitz, L.M. S-Peptide Epitope Tagging for Protein Purification, Expression Monitoring and Localization in Mammalian Cells. BioTechniques 2004, 37, 835–839. [Google Scholar]
- Patel, A.G.; Flatten, K.S.; Peterson, K.L.; Beito, T.G.; Schneider, P.A.; Perkins, A.L.; Harki, D.A.; Kaufmann, S.H. Immunodetection of Human Topoisomerase I-DNA Covalent Complexes. Nucleic Acids Res. 2016, 44, 2816–2826. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Ding, H.; Meng, X.W.; Peterson, K.L.; Schneider, P.A.; Karp, J.E.; Kaufmann, S.H. Constitutive BAK activation as a determinant of drug sensitivity in malignant lymphohematopoietic cells. Genes Dev. 2015, 29, 2140–2152. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kaufmann, S.H.; Svingen, P.A.; Gore, S.D.; Armstrong, D.K.; Cheng, Y.-C.; Rowinsky, E.K. Altered Formation of Topotecan-Stabilized Topoisomerase I-DNA Adducts in Human Leukemia Cells. Blood 1997, 89, 2098–2104. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.H. Reutilization of Immunoblots After Chemiluminescent Detection. Anal. Biochem. 2001, 296, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Markman, M.; Bundy, B.N.; Alberts, D.S.; Fowler, J.M.; Clark-Pearson, D.L.; Carson, L.F.; Wadler, S.; Sickel, J. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: An intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J. Clin. Oncol 2001, 19, 1001–1007. [Google Scholar]
- Ozols, R.F.; Bundy, B.N.; Greer, B.E.; Fowler, J.M.; Clarke-Pearson, D.; Burger, R.A.; Mannel, R.S.; DeGeest, K.; Hartenbach, E.M.; Baergen, R.; et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: A Gynecologic Oncology Group study. J. Clin. Oncol. 2003, 21, 3194–3200. [Google Scholar] [CrossRef] [PubMed]
- Weroha, S.J.; Becker, M.A.; Enderica-Gonzalez, S.; Harrington, S.C.; Oberg, A.L.; Maurer, M.J.; Perkins, S.E.; Alhilli, M.; Butler, K.A.; McKinstry, S.; et al. Tumorgrafts as in vivo surrogates for women with ovarian cancer. Clin. Cancer Res. 2014, 20, 1288–1297. [Google Scholar] [CrossRef]
- Wahner Hendrickson, A.E.; Hawthorne, K.M.; Goode, E.L.; Kalli, K.R.; Goergen, K.M.; Bakkum-Gamez, J.N.; Cliby, W.A.; Keeney, G.L.; Visscher, D.W.; Tarabishy, Y.; et al. Assessment of published models and prognostic variables in epithelial ovarian cancer at Mayo Clinic. Gynecol Oncol. 2015, 137, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Oberg, A.L.; Heinzen, E.P.; Hou, X.; Al Hilli, M.M.; Hurley, R.M.; Wahner Hendrickson, A.E.; Goergen, K.M.; Larson, M.C.; Becker, M.A.; Eckel-Passow, J.E.; et al. Statistical analysis of comparative tumor growth repeated measures experiments in the ovarian cancer patient derived xenograft (PDX) setting. Sci. Rep. 2021, 11, 8076. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]



| Pathology and Treatment | TMA Staining Overall (N = 417) | PDX Staining (N = 69) |
|---|---|---|
| Histology | ||
| High Grade Serous | 307 (73.6%) | 60 (87.0%) |
| Endometrioid | 49 (11.8%) | 3 (4.3%) |
| Clear Cell | 29 (7.0%) | 4 (5.8%) |
| Other | 32 (7.7%) | 2 (2.9%) |
| Stage | ||
| 1 | 66 (15.8%) | 2 (2.9%) |
| 2 | 32 (7.7%) | 2 (2.9%) |
| 3 | 257 (61.6%) | 49 (71.0%) |
| 4 | 62 (14.9%) | 16 (23.2%) |
| Grade | ||
| 1 | 21 (5.0%) | 2 (2.9%) |
| 2 | 35 (8.4%) | 3 (4.3%) |
| 3 | 361 (86.6%) | 64 (92.8%) |
| Debulking Status * | ||
| Optimal | 371(89.0%) | 64 (92.8%) |
| Sub-optimal | 46 (11.0%) | 3 (4.3%) |
| Unknown | - | 2 (2.9%) |
| Platinum/Taxane regimen | ||
| Yes | 322 (77.2%) | - |
| No | 12 (2.9%) | - |
| Unknown | 83 (19.9%) | - |
| Variable | Group | N | Median | IQR | p-Value 1 |
|---|---|---|---|---|---|
| Histology | Non-HGS | 110 | 192.5 | (160, 244) | 0.0048 |
| HGS | 307 | 220 | (180, 260) | ||
| Stage | 1 | 66 | 200 | (160, 240) | 0.053 |
| 2 | 32 | 187.5 | (137.5, 250) | ||
| 3 | 257 | 220 | (180, 260) | ||
| 4 | 62 | 210 | (170, 249) | ||
| Stage Grouped | Early (1 & 2) | 98 | 200 | (150, 244) | 0.016 |
| Advanced (3 & 4) | 319 | 220 | (180, 260) | ||
| Grade | 1 | 21 | 160 | (110, 180) | 5.4 × 10−5 |
| 2 | 35 | 210 | (175, 250) | ||
| 3 | 361 | 220 | (180, 260) | ||
| Grade Grouped | Low (1) | 21 | 160 | (110, 180) | 1.4 × 10−5 |
| High (2 & 3) | 396 | 220 | (180, 260) | ||
| Debulking Status Grouped | Optimal | 371 | 215 | (170, 260) | 0.49 |
| Sub-optimal | 46 | 210 | (160, 250) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wahner Hendrickson, A.E.; Visscher, D.W.; Hou, X.; Goergen, K.M.; Atkinson, H.J.; Beito, T.G.; Negron, V.; Lingle, W.L.; Bruzek, A.K.; Hurley, R.M.; et al. CHFR and Paclitaxel Sensitivity of Ovarian Cancer. Cancers 2021, 13, 6043. https://doi.org/10.3390/cancers13236043
Wahner Hendrickson AE, Visscher DW, Hou X, Goergen KM, Atkinson HJ, Beito TG, Negron V, Lingle WL, Bruzek AK, Hurley RM, et al. CHFR and Paclitaxel Sensitivity of Ovarian Cancer. Cancers. 2021; 13(23):6043. https://doi.org/10.3390/cancers13236043
Chicago/Turabian StyleWahner Hendrickson, Andrea E., Daniel W. Visscher, Xiaonan Hou, Krista M. Goergen, Hunter J. Atkinson, Thomas G. Beito, Vivian Negron, Wilma L. Lingle, Amy K. Bruzek, Rachel M. Hurley, and et al. 2021. "CHFR and Paclitaxel Sensitivity of Ovarian Cancer" Cancers 13, no. 23: 6043. https://doi.org/10.3390/cancers13236043
APA StyleWahner Hendrickson, A. E., Visscher, D. W., Hou, X., Goergen, K. M., Atkinson, H. J., Beito, T. G., Negron, V., Lingle, W. L., Bruzek, A. K., Hurley, R. M., Wagner, J. M., Flatten, K. S., Peterson, K. L., Schneider, P. A., Larson, M. C., Maurer, M. J., Kalli, K. R., Oberg, A. L., Weroha, S. J., & Kaufmann, S. H. (2021). CHFR and Paclitaxel Sensitivity of Ovarian Cancer. Cancers, 13(23), 6043. https://doi.org/10.3390/cancers13236043






