Molecular and Cellular Mechanisms of Perineural Invasion in Oral Squamous Cell Carcinoma: Potential Targets for Therapeutic Intervention
Abstract
:Simple Summary
Abstract
1. Introduction
2. Clinical Overview of Oral Squamous Cell Carcinoma
2.1. Clinical Presentation
2.2. Diagnosis
2.3. Staging
2.4. Treatment Overview
2.5. Treatment Considerations
3. Definitions for Perineural Invasion (PNI)
4. Clinical Impact of Perineural Invasion in Oral Cavity Squamous Cell Carcinoma
4.1. Prevalence of Perineural Invasion
4.2. Impact of Perineural Invasion on Locoregional Control and Survival
4.3. Surgical Resection and Elective Neck Dissection for Perineural Invasion
4.4. Adjuvant Radiotherapy or Chemoradiation for Perineural Invasion
5. Potential Mechanisms of Perineural Invasion
5.1. Cellular Observations in Perineural Invasion
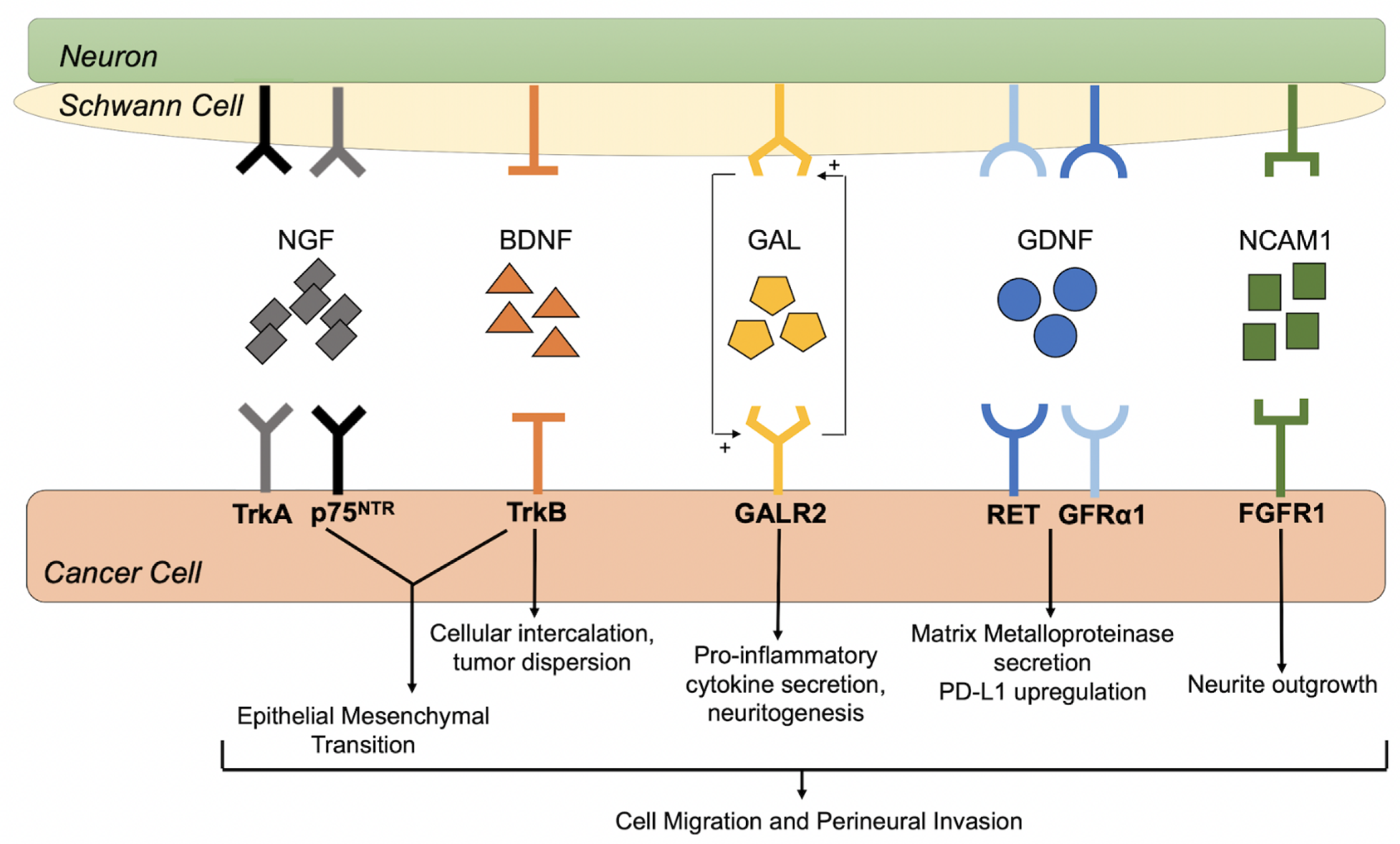
5.2. Nerve Growth Factor and Tropomyosine Receptor Kinase A
5.3. Brain Derived Neurotrophic Factor and Tropomyosine Receptor Kinase B
5.4. Glial Cell-Derived Neurotrophic Factor and Receptors
5.5. Galanin and Galanin Receptors
5.6. Neural Cell Adhesion Molecule 1 and Fibroblast Growth Factor Receptor 1
5.7. Alternative Pathways Influencing PNI and Tumor Progression
6. Potential Therapeutic Interventions for Perineural Invasion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AJCC | American Joint Committee on Cancer |
| AP-1 | Activator protein-1 |
| BDNF | Brain-derived neurotrophic factor |
| CI | Confidence interval |
| cN | Clinical nodal |
| CT | Computed tomography |
| DNA | Deoxyribonucleic acid |
| DOI | Depth of invasion |
| EGFR | Epidermal growth factor receptors |
| EMT | Epithelial to mesenchymal transition |
| ENE | Extracapsular nodal extension |
| ERK | Extracellular-regulated protein kinase |
| FDA | Food and Drug Administration |
| FGFR | Fibroblast growth factor receptor |
| GALR | Galanin receptor |
| GDNF | Glial cell-derived neurotrophic factor |
| GFAP | Glial fibrillary acidic protein |
| GFRalpha1 | GDNF family receptor-alpha 1 |
| HPV | Human papilloma virus |
| HR | Hazard ratio |
| IMRT | Intensity-modulated radiation therapy |
| LOH | Loss of heterozygosity |
| M | Metastasis |
| MAPK | Mitogen-activated protein kinase |
| MMP | Matrix metalloproteinase |
| MR | Magnetic resonance |
| mRNA | Messenger ribonucleic acid |
| NCAM | Neural cell adhesion molecule |
| NFATC | Nuclear factor of activated T cells |
| NGF | Nerve growth factor |
| OR | Odds ratio |
| p75NTR | p75 neurotrophin receptors |
| PD-L1 | Programed cell death -ligand 1 |
| pN | Pathological nodal |
| PNI | Perineural invasion |
| RET | REarranged during Transfection |
| SCC | Squamous cell carcinoma |
| shRNA | Short hairpin ribonucleic acid |
| T | Tumor |
| TNF | Tumor necrosis factor |
| TrkA | Tropomyosin receptor kinase A |
| TrkB | Tropomyosin receptor kinase B |
References
- National Cancer Institute (US). SEER Cancer Stat Facts: Oral Cavity and Pharynx Cancer. Available online: https://seer.cancer.gov/statfacts/html/oralcav.html (accessed on 3 September 2021).
- Chi, A.C.; Day, T.A.; Neville, B.W. Oral cavity and oropharyngeal squamous cell carcinoma-an update. CA Cancer J. Clin. 2015, 65, 401–421. [Google Scholar] [CrossRef]
- American Cancer Society. Cancer Facts & Figures 2021; American Cancer Society: Atlanta, GA, USA, 2021. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.L.; Foster, C.S.; A Dinsdale, E.; Pittam, M.R. Perineural spread by squamous carcinomas of the head and neck: A morphological study using antiaxonal and antimyelin monoclonal antibodies. J. Clin. Pathol. 1983, 36, 269–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soo, K.-C.; Carter, R.L.; O’Brien, C.J.; Barr, L.; Bliss, J.M.; Shaw, H.J. Prognostic implications of perineural spread in squamous carcinomas of the head and neck. Laryngoscope 1986, 96, 1145–1148. [Google Scholar] [CrossRef]
- Kurtz, K.A.; Hoffman, H.T.; Zimmerman, M.B.; Robinson, R.A. Perineural and Vascular Invasion in Oral Cavity Squamous Carcinoma: Increased Incidence on Re-review of Slides and by Using Immunohistochemical Enhancement. Arch. Pathol. Lab. Med. 2005, 129, 354–359. [Google Scholar] [CrossRef]
- Alkhadar, H.; Macluskey, M.; White, S.; Ellis, I. Perineural invasion in oral squamous cell carcinoma: Incidence, prognostic impact and molecular insight. J. Oral Pathol. Med. 2020, 49, 994–1003. [Google Scholar] [CrossRef]
- Wei, P.-Y.; Li, W.-Y.; Tai, S.-K. Discrete Perineural Invasion Focus Number in Quantification for T1-T2 Oral Squamous Cell Carcinoma. Otolaryngol. Head Neck Surg. 2019, 160, 635–641. [Google Scholar] [CrossRef]
- Schmitd, L.; Scanlon, C.; D’Silva, N. Perineural Invasion in Head and Neck Cancer. J. Dent. Res. 2018, 97, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.; Barnes, L.; Mazariegos, J.; Taylor, F.; Johnson, J.; Bs, R.L.W. Prognostic factors in mobile tongue and floor of mouth carcinoma. Cancer 1989, 64, 1195–1202. [Google Scholar] [CrossRef]
- Aivazian, K.; Ebrahimi, A.; Low, T.-H.H.; Gao, K.; Clifford, A.; Shannon, K.; Clark, J.R.; Gupta, R. Perineural invasion in oral squamous cell carcinoma: Quantitative subcategorisation of perineural invasion and prognostication. J. Surg. Oncol. 2015, 111, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Tai, S.-K.; Li, W.-Y.; Yang, M.-H.; Chang, S.-Y.; Chu, P.-Y.; Tsai, T.-L.; Wang, Y.-F.; Chang, P.M.-H. Treatment for T1-2 Oral Squamous Cell Carcinoma with or Without Perineural Invasion: Neck Dissection and Postoperative Adjuvant Therapy. Ann. Surg. Oncol. 2012, 19, 1995–2002. [Google Scholar] [CrossRef]
- Fagan, J.J.; Collins, B.; Barnes, L.; D’Amico, F.; Myers, E.N.; Johnson, J.T. Perineural Invasion in Squamous Cell Carcinoma of the Head and Neck. Arch. Otolaryngol. Head Neck Surg. 1998, 124, 637–640. [Google Scholar] [CrossRef] [Green Version]
- Kim, R.Y.; Helman, J.I.; Braun, T.M.; Ward, B.B. Increased Presence of Perineural Invasion in the Tongue and Floor of the Mouth: Could It Represent a More Aggressive Oral Squamous Cell Carcinoma, or Do Larger Aggressive Tumors Cause Perineural Invasion? J. Oral Maxillofac. Surg. 2019, 77, 852–858. [Google Scholar] [CrossRef]
- Binmadi, N.O.; Basile, J.R. Perineural invasion in oral squamous cell carcinoma: A discussion of significance and review of the literature. Oral Oncol. 2011, 47, 1005–1010. [Google Scholar] [CrossRef]
- Azam, S.H.; Pecot, C.V. Cancer’s got nerve: Schwann cells drive perineural invasion. J. Clin. Investig. 2016, 126, 1242–1244. [Google Scholar] [CrossRef] [Green Version]
- Deborde, S.; Wong, R.J. How Schwann cells facilitate cancer progression in nerves. Cell. Mol. Life Sci. 2017, 74, 4405–4420. [Google Scholar] [CrossRef]
- Roger, E.; Martel, S.; Bertrand-Chapel, A.; Depollier, A.; Chuvin, N.; Pommier, R.; Yacoub, K.; Caligaris, C.; Cardot, V.; Chauvet, V.; et al. Schwann cells support oncogenic potential of pancreatic cancer cells through TGFβ signaling. Cell Death Dis. 2019, 10, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T.; Fan, Q.; Wang, Y.; Cui, Y.; Wang, Z.; Yang, L.; Sun, X.; Wang, Y. Schwann Cell-Derived CCL2 Promotes the Perineural Invasion of Cervical Cancer. Front. Oncol. 2020, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Ein, L.; Bracho, O.; Mei, C.; Patel, J.; Boyle, T.; Monje, P.; Fernandez-Valle, C.; Bas, E.; Thomas, G.; Weed, D.; et al. Inhibition of tropomyosine receptor kinase B on the migration of human Schwann cell and dispersion of oral tongue squamous cell carcinoma in vitro. Head Neck 2019, 41, 4069–4075. [Google Scholar] [CrossRef]
- Ein, L.; Mei, C.; Bracho, O.; Bas, E.; Monje, P.; Weed, D.; Sargi, Z.; Thomas, G.; Dinh, C. Modulation of BDNF–TRKB Interactions on Schwann Cell-induced Oral Squamous Cell Carcinoma Dispersion In Vitro. Anticancer. Res. 2019, 39, 5933–5942. [Google Scholar] [CrossRef] [PubMed]
- The American Cancer Society. What Are Oral Cavity and Oropharyngeal Cancers? Available online: https://www.cancer.org/cancer/oral-cavity-and-oropharyngeal-cancer/about/what-is-oral-cavity-cancer.html#references (accessed on 30 September 2021).
- PDQ Adult Treatment Editorial Board. Lip and Oral Cavity Cancer Treatment (Adult) (PDQ(R)): Health Professional Version. In PDQ Cancer Information Summaries; National Cancer Institute: Bethesda, MD USA, 2002. [Google Scholar]
- The American Cancer Society Medical and Editorial Content Team. Signs and Symptoms of Oral Cavity and Oropharyngeal Cancer. Available online: https://www.cancer.org/cancer/oral-cavp (accessed on 9 October 2021).
- Massey, B.T. Physiology of oral cavity, pharynx, and upper esophageal sphincter. GI Motil. Online 2006. [Google Scholar] [CrossRef]
- Bakst, R.L.; Glastonbury, C.M.; Parvathaneni, U.; Katabi, N.; Hu, K.S.; Yom, S. Perineural Invasion and Perineural Tumor Spread in Head and Neck Cancer. Int. J. Radiat. Oncol. 2019, 103, 1109–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mello, F.W.; Melo, G.; Pasetto, J.J.; Silva, C.A.B.; Warnakulasuriya, S.; Rivero, E. The synergistic effect of tobacco and alcohol consumption on oral squamous cell carcinoma: A systematic review and meta-analysis. Clin. Oral Investig. 2019, 23, 2849–2859. [Google Scholar] [CrossRef]
- Petti, S.; Masood, M.; Scully, C. The Magnitude of Tobacco Smoking-Betel Quid Chewing-Alcohol Drinking Interaction Effect on Oral Cancer in South-East Asia. A Meta-Analysis of Observational Studies. PLoS ONE 2013, 8, e78999. [Google Scholar] [CrossRef]
- The American Cancer Society Medical and Editorial Content Team. Tests for Oral Cavity and Oropharyngeal Cancers. Available online: https://www.cancer.org/cancer/oral-cavity-and-oropharyngeal-cancer/detection-diagnosis-staging/how-diagnosed.html (accessed on 30 September 2021).
- Maymone, M.B.; Greer, R.O.; Burdine, L.K.; Dao-Cheng, A.; Venkatesh, S.; Sahitya, P.C.; Maymone, A.C.; Kesecker, J.; Vashi, N.A. Benign oral mucosal lesions: Clinical and pathological findings. J. Am. Acad. Dermatol. 2019, 81, 43–56. [Google Scholar] [CrossRef]
- Gonsalves, W.C.; Chi, A.C.; Neville, B.W. Common Oral Lesions: Part I. Superficial Mucosal Lesions. Am. Fam. Phys. 2007, 75, 501–506. [Google Scholar]
- Trotta, B.M.; Pease, C.S.; Rasamny, J.J.; Raghavan, P.; Mukherjee, S. Oral Cavity and Oropharyngeal Squamous Cell Cancer: Key Imaging Findings for Staging and Treatment Planning. Radiographics 2011, 31, 339–354. [Google Scholar] [CrossRef]
- Pałasz, P.; Adamski, Ł.; Górska-Chrząstek, M.; Starzyńska, A.; Studniarek, M. Contemporary Diagnostic Imaging of Oral Squamous Cell Carcinoma–A Review of Literature. Pol. J. Radiol. 2017, 82, 193–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Lazor, J.W.; Assadsangabi, R.; Shah, J. An Imager’s Guide to Perineural Tumor Spread in Head and Neck Cancers: Radiologic Footprints on 18F-FDG PET, with CT and MRI Correlates. J. Nucl. Med. 2019, 60, 304–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, V.-H. Imaging of perineural spread in head and neck tumours. Cancer Imaging 2010, 10, S92–S98. [Google Scholar] [CrossRef] [Green Version]
- Machiels, J.-P.; Leemans, C.R.; Golusinski, W.; Grau, C.; Licitra, L.; Gregoire, V. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS–ESMO–ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1462–1475. [Google Scholar] [CrossRef] [PubMed]
- Koo, K.; Harris, R.; Wiesenfeld, D.; Iseli, T.A. A role for panendoscopy? Second primary tumour in early stage squamous cell carcinoma of the oral tongue. J. Laryngol. Otol. 2015, 129, S27–S31. [Google Scholar] [CrossRef]
- Rodriguez-Bruno, K.; Ali, M.J.; Wang, S.J. Role of panendoscopy to identify synchronous second primary malignancies in patients with oral cavity and oropharyngeal squamous cell carcinoma. Head Neck 2011, 33, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.B.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; American College of Surgeons: Chicago, IL, USA, 2017. [Google Scholar]
- Ebrahimi, A.; Gil, Z.; Amit, M.; Yen, T.-C.; Liao, C.-T.; Chaturvedi, P.; Agarwal, J.P.; Kowalski, L.P.; Kreppel, M.; Cernea, C.R.; et al. Primary Tumor Staging for Oral Cancer and a Proposed Modification Incorporating Depth of Invasion: An international multicenter retrospective study. JAMA Otolaryngol. Neck Surg. 2014, 140, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, D.K.; Patel, S.G.; Shah, J.P. Changes in the 8th Edition of the American Joint Committee on Cancer (AJCC) Staging of Head and Neck Cancer: Rationale and Implications. Curr. Oncol. Rep. 2019, 21, 1–7. [Google Scholar] [CrossRef]
- Wreesmann, V.B.; Katabi, N.; Ba, F.L.P.; Montero, P.H.; Ma, J.C.M.; Gönen, M.; Carlson, D.L.; Ganly, I.; Shah, J.P.; A Ghossein, R.; et al. Influence of extracapsular nodal spread extent on prognosis of oral squamous cell carcinoma. Head Neck 2016, 38, E1192–E1199. [Google Scholar] [CrossRef]
- Laske, R.D.; Scholz, I.; Ikenberg, K.; Meerwein, C.; Vital, D.G.; Studer, G.; Rössle, M.; Huber, G.F. Perineural Invasion in Squamous Cell Carcinoma of the Oral Cavity: Histology, Tumor Stage, and Outcome. Laryngoscope Investig. Otolaryngol. 2016, 1, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Zanoni, D.K.; Montero, P.H.; Migliacci, J.C.; Shah, J.P.; Wong, R.J.; Ganly, I.; Patel, S.G. Survival outcomes after treatment of cancer of the oral cavity (1985–2015). Oral Oncol. 2019, 90, 115–121. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Head and Neck Cancers (Version 3.2021). Available online: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (accessed on 23 October 2021).
- Chinn, S.; Myers, J.N. Oral Cavity Carcinoma: Current Management, Controversies, and Future Directions. J. Clin. Oncol. 2015, 33, 3269–3276. [Google Scholar] [CrossRef] [Green Version]
- Kuhnt, T.; Stang, A.; Wienke, A.; Vordermark, D.; Schweyen, R.; Hey, J. Potential risk factors for jaw osteoradionecrosis after radiotherapy for head and neck cancer. Radiat. Oncol. 2016, 11, 101. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, R.J.; Burtness, B.; Husain, Z.A.; Judson, B.L.; Bhatia, A.; Sasaki, C.T.; Yarbrough, W.G.; Mehra, S. Treatment guidelines and patterns of care in oral cavity squamous cell carcinoma: Primary surgical resection vs. nonsurgical treatment. Oral Oncol. 2017, 71, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Koyfman, S.A.; Ismaila, N.; Crook, D.; D’Cruz, A.; Rodriguez, C.P.; Sher, D.J.; Silbermins, D.; Sturgis, E.M.; Tsue, T.T.; Weiss, J.; et al. Management of the Neck in Squamous Cell Carcinoma of the Oral Cavity and Oropharynx: ASCO Clinical Practice Guideline. J. Clin. Oncol. 2019, 37, 1753–1774. [Google Scholar] [CrossRef]
- Szturz, P.; Vermorken, J.B. Management of recurrent and metastatic oral cavity cancer: Raising the bar a step higher. Oral Oncol. 2020, 101, 104492. [Google Scholar] [CrossRef]
- Shanti, R.M.; O’Malley, B.W. Surgical Management of Oral Cancer. Dent. Clin. N. Am. 2018, 62, 77–86. [Google Scholar] [CrossRef]
- Rigby, M.H.; Taylor, S.M. Soft tissue reconstruction of the oral cavity: A review of current options. Curr. Opin. Otolaryngol. Head Neck Surg. 2013, 21, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Patel, U.A.; Hartig, G.K.; Hanasono, M.M.; Lin, D.T.; Richmon, J.D. Locoregional Flaps for Oral Cavity Reconstruction: A Review of Modern Options. Otolaryngol. Neck Surg. 2017, 157, 201–209. [Google Scholar] [CrossRef]
- Haughey, B.H.; Fredrickson, J.M.; Lerrick, A.J.; Sclaroff, A.; Gay, W.D. Fibular and Iliac Crest Osteomuscular Free Flap Reconstruction of the Oral Cavity. Laryngoscope 1994, 104, 1305–1313. [Google Scholar] [CrossRef]
- Bachaud, J.-M.; Cohen-Jonathan, E.; Alzieu, C.; David, J.-M.; Serrano, E.; Daly-Schveitzer, N. Combined postoperative radiotherapy and Weekly Cisplatin infusion for locally advanced head and neck carcinoma: Final report of a randomized trial. Int. J. Radiat. Oncol. 1996, 36, 999–1004. [Google Scholar] [CrossRef]
- Bernier, J.; Cooper, J.S.; Pajak, T.F.; Van Glabbeke, M.; Bourhis, J.; Forastiere, A.; Ozsahin, E.M.; Jacobs, J.R.; Jassem, J.; Ang, K.-K.; et al. Defining risk levels in locally advanced head and neck cancers: A comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck 2005, 27, 843–850. [Google Scholar] [CrossRef]
- Cooper, J.S.; Pajak, T.F.; Forastiere, A.A.; Jacobs, J.; Campbell, B.; Saxman, S.B.; Kish, J.A.; Kim, H.E.; Cmelak, A.J.; Rotman, M.; et al. Postoperative Concurrent Radiotherapy and Chemotherapy for High-Risk Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2004, 350, 1937–1944. [Google Scholar] [CrossRef] [Green Version]
- Kalyankrishna, S.; Grandis, J.R. Epidermal Growth Factor Receptor Biology in Head and Neck Cancer. J. Clin. Oncol. 2006, 24, 2666–2672. [Google Scholar] [CrossRef]
- Grandis, J.R.; Melhem, M.F.; Gooding, W.E.; Day, R.S.; Holst, V.A.; Wagener, M.M.; Drenning, S.D.; Tweardy, D.J. Levels of TGF-α and EGFR Protein in Head and Neck Squamous Cell Carcinoma and Patient Survival. J. Natl. Cancer Inst. 1998, 90, 824–832. [Google Scholar] [CrossRef] [Green Version]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus Cetuximab for Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermorken, J.B.; Trigo, J.; Hitt, R.; Koralewski, P.; Diaz-Rubio, E.; Rolland, F.; Knecht, R.; Amellal, N.; Schueler, A.; Baselga, J. Open-Label, Uncontrolled, Multicenter Phase II Study to Evaluate the Efficacy and Toxicity of Cetuximab As a Single Agent in Patients with Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck Who Failed to Respond to Platinum-Based Therapy. J. Clin. Oncol. 2007, 25, 2171–2177. [Google Scholar] [CrossRef] [PubMed]
- Bauml, J.M.; Vinnakota, R.; Park, Y.A.; Bates, S.E.; Fojo, T.; Aggarwal, C.; Di Stefano, J.; Knepley, C.; Limaye, S.; Mamtani, R.; et al. Cisplatin versus cetuximab with definitive concurrent radiotherapy for head and neck squamous cell carcinoma: An analysis of Veterans Health Affairs data. Cancer 2019, 125, 406–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stokes, W.A.; Sumner, W.A.; Breggren, K.L.; Rathbun, J.T.; Raben, D.; McDermott, J.D.; Gan, G.; Karam, S.D. A comparison of concurrent cisplatin versus cetuximab with radiotherapy in locally-advanced head and neck cancer: A bi-institutional analysis. Rep. Pract. Oncol. Radiother. 2017, 22, 389–395. [Google Scholar] [CrossRef]
- Burtness, B.; Harrington, K.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, A.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Kitamura, N.; Sento, S.; Yoshizawa, Y.; Sasabe, E.; Kudo, Y.; Yamamoto, T. Current Trends and Future Prospects of Molecular Targeted Therapy in Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2020, 22, 240. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Soulières, D.; Le Tourneau, C.; Dinis, J.; Licitra, L.; Ahn, M.-J.; Soria, A.; Machiels, J.-P.; Mach, N.; Mehra, R.; et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet 2019, 393, 156–167. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.-W.; Tao, L.-Y.; Jiang, Y.-S.; Yang, J.-Y.; Huo, Y.-M.; Liu, D.-J.; Li, J.; Fu, X.-L.; He, R.; Lin, C.; et al. Perineural invasion reprograms the immune microenvironment through cholinergic signaling in pancreatic ductal adenocarcinoma. Cancer Res. 2020, 80, 1991–2003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peltonen, S.; Alanne, M.; Peltonen, J. Barriers of the peripheral nerve. Tissue Barriers 2013, 1, e24956. [Google Scholar] [CrossRef] [Green Version]
- Batsakis, J.G. Nerves and neurotropic carcinomas. Ann. Otol. Rhinol. Laryngol. 1985, 94, 426–427. [Google Scholar]
- Liebig, C.; Ayala, G.; Wilks, J.A.; Berger, D.H.; Albo, D. Perineural invasion in cancer: A review of the literature. Cancer 2009, 115, 3379–3391. [Google Scholar] [CrossRef]
- Chi, A.C.; Katabi, N.; Chen, H.-S.; Cheng, Y.-S.L. Interobserver Variation among Pathologists in Evaluating Perineural Invasion for Oral Squamous Cell Carcinoma. Head Neck Pathol. 2016, 10, 451–464. [Google Scholar] [CrossRef] [Green Version]
- Yan, F.; Cheng, Y.-S.L.; Katabi, N.; Nguyen, S.A.; Chen, H.-S.; Morgan, P.; Zhang, K.; Chi, A.C. Interobserver Variation in Evaluating Perineural Invasion for Oral Squamous Cell Carcinoma: Phase 2 Survey Study. Head Neck Pathol. 2021, 15, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Varsha, B.K.; Radhika, M.B.; Makarla, S.; Kuriakose, M.A.; Kiran, G.S.; Padmalatha, G.V. Perineural invasion in oral squamous cell carcinoma: Case series and review of literature. J. Oral Maxillofac. Pathol. 2015, 19, 335–341. [Google Scholar] [CrossRef]
- Dunn, M.; Morgan, M.B.; Beer, T. Perineural Invasion: Identification, Significance, and a Standardized Definition. Dermatol. Surg. 2009, 35, 214–221. [Google Scholar] [CrossRef]
- Keerthi, R.; Dutta, A.; Agarwal, S.; Kani, V.; Khatua, A. Perineural Invasion of Oral Squamous Cell Carcinoma: A New Hurdle for Surgeons. J. Maxillofac. Oral Surg. 2018, 17, 59–63. [Google Scholar] [CrossRef]
- Conte, C.C.; Ergin, M.; Ricci, A.; Deckers, P.J. Clinical and pathologic prognostic variables in oropharyngeal squamous cell carcinoma. Am. J. Surg. 1989, 157, 582–584. [Google Scholar] [CrossRef]
- Lydiatt, W.M.; Patel, S.G.; O’Sullivan, B.; Brandwein, M.S.; Ridge, J.A.; Migliacci, J.C.; Loomis, A.M.; Shah, J.P. Head and neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 122–137. [Google Scholar] [CrossRef]
- Carrillo, J.; Carrillo, L.C.; Cano, A.; Ramirez–Ortega, M.C.; Chanona, J.G.; Avilés, A.; Herrera-Goepfert, R.; Corona-Rivera, J.; Ochoa-Carrillo, F.J.; Oñate-Ocaña, L.F. Retrospective cohort study of prognostic factors in patients with oral cavity and oropharyngeal squamous cell carcinoma. Head Neck 2016, 38, 536–541. [Google Scholar] [CrossRef]
- Low, T.-H.; Gao, K.; Gupta, R.; Clifford, A.; Elliott, M.; Ch’Ng, S.; Milross, C.; Clark, J.R. Factors predicting poor outcomes in T1N0 oral squamous cell carcinoma: Indicators for treatment intensification. ANZ J. Surg. 2016, 86, 366–371. [Google Scholar] [CrossRef]
- Subramaniam, N.; Ms, S.M.; Balasubramanian, D.; Low, T.-H.; Vidhyadharan, S.; Clark, J.R.; Thankappan, K.; Iyer, S. Adverse pathologic features in T1/2 oral squamous cell carcinoma classified by the American Joint Committee on Cancer eighth edition and implications for treatment. Head Neck 2018, 40, 2123–2128. [Google Scholar] [CrossRef]
- Lee, L.; De Paz, D.; Lin, C.; Fan, K.; Wang, H.; Hsieh, C.; Lee, L.; Yen, T.; Liao, C.; Yeh, C.; et al. Prognostic impact of extratumoral perineural invasion in patients with oral cavity squamous cell carcinoma. Cancer Med. 2019, 8, 6185–6194. [Google Scholar] [CrossRef] [PubMed]
- Caponio, V.C.A.; Troiano, G.; Togni, L.; Zhurakivska, K.; Santarelli, A.; Laino, L.; Rubini, C.; Muzio, L.L.; Mascitti, M. Pattern and localization of perineural invasion predict poor survival in oral tongue carcinoma. Oral Dis. 2021, in press. [Google Scholar] [CrossRef]
- Chatzistefanou, I.; Lubek, J.; Markou, K.; Ord, R.A. The role of neck dissection and postoperative adjuvant radiotherapy in cN0 patients with PNI-positive squamous cell carcinoma of the oral cavity. Oral Oncol. 2014, 50, 753–758. [Google Scholar] [CrossRef]
- Nguyen, E.; McKenzie, J.; Clarke, R.; Lou, S.; Singh, T. The Indications for Elective Neck Dissection in T1N0M0 Oral Cavity Squamous Cell Carcinoma. J. Oral Maxillofac. Surg. 2021, 79, 1779–1793. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Cheng, A.; Alzahrani, S.; Li, B.; Han, Z.; Ward, B.B. Elective Neck Dissection in T1N0M0 Oral Squamous Cell Carcinoma: When Is It Necessary? J. Oral Maxillofac. Surg. 2020, 78, 2306–2315. [Google Scholar] [CrossRef]
- Liao, C.-T.; Chang, J.T.-C.; Wang, H.-M.; Ng, S.-H.; Hsueh, C.; Lee, L.-Y.; Lin, C.-H.; Chen, I.-H.; Huang, S.-F.; Cheng, A.-J.; et al. Does Adjuvant Radiation Therapy Improve Outcomes In pT1-3N0 Oral Cavity Cancer with Tumor-Free Margins and Perineural Invasion? Int. J. Radiat. Oncol. 2008, 71, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.; Mair, M.; Singhvi, H.; Mishra, A.; Nair, S.V.; Agrawal, J.; Chaturvedi, P. Perineural invasion: Independent prognostic factor in oral cancer that warrants adjuvant treatment. Head Neck 2018, 40, 1780–1787. [Google Scholar] [CrossRef]
- Rajappa, S.; Ram, D.; Shukla, H.; Mandal, G.; Venkatasubramaniyan, M.; Dubey, A.; Agarwal, M.; Kumar, R.; Dewan, A. Oncological benefits of postoperative radiotherapy in node-negative early stage cancer of the oral cavity with isolated perineural invasion. Br. J. Oral Maxillofac. Surg. 2019, 57, 454–459. [Google Scholar] [CrossRef]
- Chinn, S.; Spector, M.E.; Bellile, E.L.; McHugh, J.B.; Gernon, T.J.; Bradford, C.R.; Wolf, G.T.; Eisbruch, A.; Chepeha, D.B. Impact of Perineural Invasion in the Pathologically N0 Neck in Oral Cavity Squamous Cell Carcinoma. Otolaryngol. Neck Surg. 2013, 149, 893–899. [Google Scholar] [CrossRef] [Green Version]
- Fan, K.-H.; Chen, Y.-C.; Lin, C.-Y.; Kang, C.-J.; Lee, L.-Y.; Huang, S.-F.; Liao, C.-T.; Ng, S.-H.; Wang, H.-M.; Chang, J.T.-C. Postoperative radiotherapy with or without concurrent chemotherapy for oral squamous cell carcinoma in patients with three or more minor risk factors: A propensity score matching analysis. Radiat. Oncol. 2017, 12, 184. [Google Scholar] [CrossRef] [Green Version]
- Babar, A.; Woody, N.; Ghanem, A.; Tsai, J.; Dunlap, N.; Schymick, M.; Liu, H.; Burkey, B.; Lamarre, E.; Ku, J.; et al. Outcomes of Post-Operative Treatment with Concurrent Chemoradiotherapy (CRT) in High-Risk Resected Oral Cavity Squamous Cell Carcinoma (OCSCC): A Multi-Institutional Collaboration. Curr. Oncol. 2021, 28, 2409–2419. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, C.S.; Banerjee, R.; Inglehart, R.C.; Liu, M.; Russo, N.; Hariharan, A.; Van Tubergen, E.A.; Corson, S.L.; Asangani, I.; Mistretta, C.M.; et al. Galanin modulates the neural niche to favour perineural invasion in head and neck cancer. Nat. Commun. 2015, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Amit, M.; Takahashi, H.; Dragomir, M.P.; Lindemann, A.; Gleber-Netto, F.O.; Pickering, C.R.; Anfossi, S.; Osman, A.A.; Cai, Y.; Wang, R.; et al. Loss of p53 drives neuron reprogramming in head and neck cancer. Nature 2020, 578, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Kolokythas, A.; Cox, D.P.; Dekker, N.; Schmidt, B.L. Nerve Growth Factor and Tyrosine Kinase A Receptor in Oral Squamous Cell Carcinoma: Is There an Association with Perineural Invasion? J. Oral Maxillofac. Surg. 2010, 68, 1290–1295. [Google Scholar] [CrossRef]
- Pascual, G.; Domínguez, D.; Elosúa-Bayes, M.; Beckedorff, F.; Laudanna, C.; Bigas, C.; Douillet, D.; Greco, C.; Symeonidi, A.; Hernández, I.; et al. Dietary palmitic acid promotes a prometastatic memory via Schwann cells. Nature 2021, 599, 485–490. [Google Scholar] [CrossRef]
- Fujii-Nishimura, Y.; Yamazaki, K.; Masugi, Y.; Douguchi, J.; Kurebayashi, Y.; Kubota, N.; Ojima, H.; Kitago, M.; Shinoda, M.; Hashiguchi, A.; et al. Mesenchymal-epithelial transition of pancreatic cancer cells at perineural invasion sites is induced by Schwann cells. Pathol. Int. 2018, 68, 214–223. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.-H.; Zhang, B.-Y.; Zhou, B.; Zhu, C.-Z.; Sun, L.-Q.; Feng, Y.-J. Perineural invasion of cancer: A complex crosstalk between cells and molecules in the perineural niche. Am. J. Cancer Res. 2019, 9, 1–21. [Google Scholar]
- Amit, M.; Na’Ara, S.; Gil, Z. Mechanisms of cancer dissemination along nerves. Nat. Rev. Cancer 2016, 16, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Magnon, C.; Hall, S.J.; Lin, J.; Xue, X.; Gerber, L.; Freedland, S.J.; Frenette, P.S. Autonomic Nerve Development Contributes to Prostate Cancer Progression. Science 2013, 341, 143. [Google Scholar] [CrossRef] [Green Version]
- Zahalka, A.H.; Arnal-Estapé, A.; Maryanovich, M.; Nakahara, F.; Cruz, C.D.; Finley, L.W.S.; Frenette, P.S. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science 2017, 358, 321–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in Neuronal Development and Function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [Green Version]
- Demir, I.E.; Tieftrunk, E.; Schorn, S.; Friess, H.; Ceyhan, G.O. Nerve growth factor & TrkA as novel therapeutic targets in cancer. Biochim. Biophys. Acta 2016, 1866, 37–50. [Google Scholar] [CrossRef]
- Ma, J.; Jiang, Y.; Jiang, Y.; Sun, Y.; Zhao, X. Expression of nerve growth factor and tyrosine kinase receptor A and correlation with perineural invasion in pancreatic cancer. J. Gastroenterol. Hepatol. 2008, 23, 1852–1859. [Google Scholar] [CrossRef] [PubMed]
- Djakiew, D.; Pflug, B.R.; Delsite, R.; Onoda, M.; Lynch, J.H.; Arand, G.; Thompson, E.W. Chemotaxis and chemokinesis of human prostate tumor cell lines in response to human prostate stromal cell secretory proteins containing a nerve growth factor-like protein. Cancer Res. 1993, 53, 1416–1420. [Google Scholar] [PubMed]
- Bapat, A.A.; Munoz, R.M.; Von Hoff, D.D.; Han, H. Blocking Nerve Growth Factor Signaling Reduces the Neural Invasion Potential of Pancreatic Cancer Cells. PLoS ONE 2016, 11, e0165586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saloman, J.L.; Singhi, A.D.; Hartman, D.J.; Normolle, D.P.; Albers, K.M.; Davis, B.M. Systemic Depletion of Nerve Growth Factor Inhibits Disease Progression in a Genetically Engineered Model of Pancreatic Ductal Adenocarcinoma. Pancreas 2018, 47, 856–863. [Google Scholar] [CrossRef]
- Shen, W.-R.; Wang, Y.-P.; Chang, J.Y.-F.; Yu, S.-Y.; Chen, H.-M.; Chiang, C.-P. Perineural invasion and expression of nerve growth factor can predict the progression and prognosis of oral tongue squamous cell carcinoma. J. Oral Pathol. Med. 2014, 43, 258–264. [Google Scholar] [CrossRef]
- Lin, C.; Ren, Z.; Yang, X.; Yang, R.; Chen, Y.; Liu, Z.; Dai, Z.; Zhang, Y.; He, Y.; Zhang, C.; et al. Nerve growth factor (NGF)-TrkA axis in head and neck squamous cell carcinoma triggers EMT and confers resistance to the EGFR inhibitor erlotinib. Cancer Lett. 2020, 472, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.-H.; Lui, M.-T.; Tu, H.-F.; Wu, C.-H.; Lo, W.-L.; Yang, C.-C.; Chang, K.-W.; Kao, S.-Y. Oral carcinoma with perineural invasion has higher nerve growth factor expression and worse prognosis. Oral Dis. 2013, 20, 268–274. [Google Scholar] [CrossRef]
- Alkhadar, H.; Macluskey, M.; White, S.; Ellis, I. Nerve growth factor-induced migration in oral and salivary gland tumour cells utilises the PI3K/Akt signalling pathway: Is there a link to perineural invasion? J. Oral Pathol. Med. 2020, 49, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Grille, S.J.; Bellacosa, A.; Upson, J.; Klein-Szanto, A.J.; Van Roy, F.; Lee-Kwon, W.; Donowitz, M.; Tsichlis, P.N.; LaRue, L. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 2003, 63, 2172–2178. [Google Scholar]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, A.; Moya-Alvarado, G.; Gonzalez-Billaut, C.; Bronfman, F.C. Cellular and molecular mechanisms regulating neuronal growth by brain-derived neurotrophic factor. Cytoskeleton 2016, 73, 612–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, S.; Wang, W.; Hu, Z.; Shan, C.; Wang, L.; Wu, B.; Yang, Z.; Yang, X.; Lei, D. BDNF mediated TrkB activation contributes to the EMT progression and the poor prognosis in human salivary adenoid cystic carcinoma. Oral Oncol. 2015, 51, 64–70. [Google Scholar] [CrossRef]
- Kowalski, P.J.; Paulino, A.F. Perineural invasion in adenoid cystic carcinoma: Its causation/promotion by brain-derived neurotrophic factor. Hum. Pathol. 2002, 33, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Okugawa, Y.; Tanaka, K.; Inoue, Y.; Kawamura, M.; Kawamoto, A.; Hiro, J.; Saigusa, S.; Toiyama, Y.; Ohi, M.; Uchida, K.; et al. Brain-derived neurotrophic factor/tropomyosin-related kinase B pathway in gastric cancer. Br. J. Cancer 2013, 108, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Albini, A. Tumor and endothelial cell invasion of basement membranes. The matrigel chemoinvasion assay as a tool for dissecting molecular mechanisms. Pathol. Oncol. Res. 1998, 4, 230–241. [Google Scholar] [CrossRef]
- Miknyoczki, S.J.; Lang, D.; Huang, L.; Klein-Szanto, A.J.; Dionne, C.A.; Ruggeri, B.A. Neurotrophins and Trk receptors in human pancreatic ductal adenocarcinoma: Expression patterns and effects onIn vitro invasive behavior. Int. J. Cancer 1999, 81, 417–427. [Google Scholar] [CrossRef]
- Ketterer, K.; Rao, S.; Friess, H.; Weiss, J.; Büchler, M.W.; Korc, M. Reverse transcription-PCR analysis of laser-captured cells points to potential paracrine and autocrine actions of neurotrophins in pancreatic cancer. Clin. Cancer Res. 2003, 9, 5127–5136. [Google Scholar] [PubMed]
- Sclabas, G.M.; Fujioka, S.; Schmidt, C.; Li, Z.; A I Frederick, W.; Yang, W.; Yokoi, K.; Evans, D.B.; Abbruzzese, J.L.; Hess, K.R.; et al. Overexpression of tropomysin-related kinase B in metastatic human pancreatic cancer cells. Clin. Cancer Res. 2005, 11, 440–449. [Google Scholar]
- Yilmaz, T.; Jiffar, T.; de la Garza, G.; Lin, H.; MacIntyre, T.; Brown, J.L.; Myers, J.N.; Kupferman, M.E. Therapeutic targeting of Trk supresses tumor proliferation and enhances cisplatin activity in HNSCC. Cancer Biol. Ther. 2010, 10, 644–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; A Werner, J.; Mandic, R. Implications of tropomyosin-related kinase B (TrkB) in head and neck cancer. Anticancer Res. 2007, 27, 3121–3126. [Google Scholar] [PubMed]
- E Kupferman, M.; Jiffar, T.; El-Naggar, A.; Yilmaz, T.; Zhou, G.; Xie, T.; Feng, L.; Wang, J.; Holsinger, F.C.; Yu, D.; et al. TrkB induces EMT and has a key role in invasion of head and neck squamous cell carcinoma. Oncogene 2010, 29, 2047–2059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudás, J.; Bitsche, M.; Schartinger, V.; Falkeis, C.; Sprinzl, G.M.; Riechelmann, H. Fibroblasts produce brain-derived neurotrophic factor and induce mesenchymal transition of oral tumor cells. Oral Oncol. 2011, 47, 98–103. [Google Scholar] [CrossRef] [Green Version]
- Jing, S.; Wen, D.; Yu, Y.; Holst, P.L.; Luo, Y.; Fang, M.; Tamir, R.; Antonio, L.; Hu, Z.; Cupples, R.; et al. GDNF–Induced Activation of the Ret Protein Tyrosine Kinase Is Mediated by GDNFR-α, a Novel Receptor for GDNF. Cell 1996, 85, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- He, S.; Chen, C.-H.; Chernichenko, N.; Bakst, R.L.; Barajas, F.; Deborde, S.; Allen, P.J.; Vakiani, E.; Yu, Z.; Wong, R.J. GFR 1 released by nerves enhances cancer cell perineural invasion through GDNF-RET signaling. Proc. Natl. Acad. Sci. USA 2014, 111, E2008–E2017. [Google Scholar] [CrossRef] [Green Version]
- Gil, Z.; Cavel, O.; Kelly, K.; Brader, P.; Rein, A.; Gao, S.P.; Carlson, D.L.; Shah, J.; Fong, Y.; Wong, R.J. Paracrine Regulation of Pancreatic Cancer Cell Invasion by Peripheral Nerves. J. Natl. Cancer Inst. 2010, 102, 107–118. [Google Scholar] [CrossRef]
- Ban, K.; Feng, S.; Shao, L.; Ittmann, M. RET Signaling in Prostate Cancer. Clin. Cancer Res. 2017, 23, 4885–4896. [Google Scholar] [CrossRef] [Green Version]
- Chuang, J.-Y.; Tsai, C.-F.; Chang, S.-W.; Chiang, I.-P.; Huang, S.-M.; Lin, H.-Y.; Yeh, W.-L.; Lu, D.-Y. Glial cell line-derived neurotrophic factor induces cell migration in human oral squamous cell carcinoma. Oral Oncol. 2013, 49, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Cao, W.; Ren, Z.; Tang, Y.; Zhang, C.; Yang, R.; Chen, Y.; Liu, Z.; Peng, C.; Wang, L.; et al. GDNF secreted by nerves enhances PD-L1 expression via JAK2-STAT1 signaling activation in HNSCC. Oncoimmunology 2017, 6, e1353860. [Google Scholar] [CrossRef] [PubMed]
- Elliott-Hunt, C.R.; Pope, R.J.P.; Vanderplank, P.; Wynick, D. Activation of the galanin receptor 2 (GalR2) protects the hippocampus from neuronal damage. J. Neurochem. 2007, 100, 780–789. [Google Scholar] [CrossRef] [Green Version]
- Berger, A.; Lang, R.; Moritz, K.; Santic, R.; Hermann, A.; Sperl, W.; Kofler, B. Galanin Receptor Subtype GalR2 Mediates Apoptosis in SH-SY5Y Neuroblastoma Cells. Endocrinology 2004, 145, 500–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilaberte, Y.; Vera, J.; Coscojuela, C.; Roca, M.; Parrado, C.; González, S. Expression of Galanin in Melanocytic Tumors. Actas Dermosifiliogr. 2007, 98, 24–34. [Google Scholar] [CrossRef]
- Kim, K.Y.; Kee, M.K.; Chong, S.A.; Nam, M.J. Galanin Is Up-Regulated in Colon Adenocarcinoma. Cancer Epidemiol. Biomark. Prev. 2007, 16, 2373–2378. [Google Scholar] [CrossRef] [Green Version]
- Rauch, I.; Kofler, B. The Galanin System in Cancer. Exp. Suppl. 2010, 102, 223–241. [Google Scholar] [CrossRef]
- Berger, A.; Santic, R.; Hauser-Kronberger, C.; Schilling, F.H.; Kogner, P.; Ratschek, M.; Gamper, A.; Jones, N.; Sperl, W.; Kofler, B. Galanin and galanin receptors in human cancers. Neuropeptides 2005, 39, 353–359. [Google Scholar] [CrossRef]
- Xia, C.-Y.; Yuan, C.-X.; Yuan, C.-G. Galanin inhibits the proliferation of glial olfactory ensheathing cells. Neuropeptides 2005, 39, 453–459. [Google Scholar] [CrossRef]
- Pearlstein, R.P.; Benninger, M.S.; Carey, T.E.; Zarbo, R.J.; Torres, F.X.; Rybicki, B.A.; Van Dyke, D.L. Loss of 18q predicts poor survival of patients with squamous cell carcinoma of the head and neck. Genes Chromosom. Cancer 1998, 21, 333–339. [Google Scholar] [CrossRef]
- Takebayashi, S.; Ogawa, T.; Jung, K.Y.; Muallem, A.; Mineta, H.; Fisher, S.G.; Grenman, R.; Carey, T. Identification of new minimally lost regions on 18q in head and neck squamous cell carcinoma. Cancer Res. 2000, 60, 3397–3403. [Google Scholar]
- Jacoby, A.S.; Webb, G.C.; Liu, M.L.; Kofler, B.; Hort, Y.J.; Fathi, Z.; Bottema, C.; Shine, J.; Iismaa, T.P. Structural Organization of the Mouse and Human GALR1 Galanin Receptor Genes (GalnrandGALNR) and Chromosomal Localization of the Mouse Gene. Genomics 1997, 45, 496–508. [Google Scholar] [CrossRef]
- Henson, B.S.; Neubig, R.R.; Jang, I.; Ogawa, T.; Zhang, Z.; Carey, T.; D’Silva, N.J. Galanin Receptor 1 Has Anti-proliferative Effects in Oral Squamous Cell Carcinoma. J. Biol. Chem. 2005, 280, 22564–22571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, R.; Henson, B.S.; Russo, N.; Tsodikov, A.; D’Silva, N.J. Rap1 mediates galanin receptor 2-induced proliferation and survival in squamous cell carcinoma. Cell. Signal. 2011, 23, 1110–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanazawa, T.; Iwashita, T.; Kommareddi, P.; Nair, T.; Misawa, K.; Misawa, Y.; Ueda, Y.; Tono, T.; E Carey, T. Galanin and galanin receptor type 1 suppress proliferation in squamous carcinoma cells: Activation of the extracellular signal regulated kinase pathway and induction of cyclin-dependent kinase inhibitors. Oncogene 2007, 26, 5762–5771. [Google Scholar] [CrossRef] [Green Version]
- Misawa, K.; Kanazawa, T.; Misawa, Y.; Uehara, T.; Imai, A.; Takahashi, G.; Takebayashi, S.; Cole, A.; E Carey, T.; Mineta, H. Galanin Has Tumor Suppressor Activity and Is Frequently Inactivated by Aberrant Promoter Methylation in Head and Neck Cancer. Transl. Oncol. 2013, 6, 338–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanazawa, T.; Kommareddi, P.K.; Iwashita, T.; Kumar, B.; Misawa, K.; Misawa, Y.; Jang, I.; Nair, T.S.; Iino, Y.; Carey, T. Galanin Receptor Subtype 2 Suppresses Cell Proliferation and Induces Apoptosis in p53 Mutant Head and Neck Cancer Cells. Clin. Cancer Res. 2009, 15, 2222–2230. [Google Scholar] [CrossRef] [Green Version]
- Williams, E.J.; Furness, J.; Walsh, F.S.; Doherty, P. Activation of the FGF receptor underlies neurite outgrowth stimulated by L1, N-CAM, and N-cadherin. Neuron 1994, 13, 583–594. [Google Scholar] [CrossRef]
- Hinsby, A.M.; Berezin, V.; Bock, E. Molecular mechanisms of NCAM function. Front. Biosci. 2004, 9, 2227–2244. [Google Scholar] [CrossRef]
- Brugière, C.; El Bouchtaoui, M.; Leboeuf, C.; Gapihan, G.; El Far, R.A.; Sy, M.; Lepage, A.L.; Ratajczak, P.; Janin, A.; Verneuil, L. Perineural Invasion in Human Cutaneous Squamous Cell Carcinoma Is Linked to Neurotrophins, Epithelial-Mesenchymal Transition, and NCAM1. J. Investig. Dermatol. 2018, 138, 2063–2066. [Google Scholar] [CrossRef] [Green Version]
- Deborde, S.; Omelchenko, T.; Lyubchik, A.; Zhou, Y.; He, S.; McNamara, W.F.; Chernichenko, N.; Lee, S.-Y.; Barajas, F.; Chen, C.-H.; et al. Schwann cells induce cancer cell dispersion and invasion. J. Clin. Investig. 2016, 126, 1538–1554. [Google Scholar] [CrossRef] [Green Version]
- Li, R. Neural cell adhesion molecule is upregulated in nerves with prostate cancer invasion. Hum. Pathol. 2003, 34, 457–461. [Google Scholar] [CrossRef]
- Stierli, S.; Imperatore, V.; Lloyd, A.C. Schwann cell plasticity-roles in tissue homeostasis, regeneration, and disease. Glia 2019, 67, 2203–2215. [Google Scholar] [CrossRef]
- Vural, E.; Hutcheson, J.; Korourian, S.; Kechelava, S.; Hanna, E. Correlation of Neural Cell Adhesion Molecules with Perineural Spread of Squamous Cell Carcinoma of the Head and Neck. Otolaryngol. Head Neck Surg. 2000, 122, 717–720. [Google Scholar] [CrossRef]
- McLaughlin, R.B.; Montone, K.T.; Wall, S.J.; Chalian, A.A.; Weinstein, G.S.; Roberts, S.A.; Wolf, P.F.; Weber, R.S. Nerve cell adhesion molecule expression in squamous cell carcinoma of the head and neck: A predictor of propensity toward perineural spread. Laryngoscope 1999, 109, 821–826. [Google Scholar] [CrossRef]
- Solares, C.A.; Brown, I.; Boyle, G.M.; Parsons, P.G.; Panizza, B. Neural cell adhesion molecule expression: No correlation with perineural invasion in cutaneous squamous cell carcinoma of the head and neck. Head Neck 2009, 31, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Banh, R.S.; Biancur, D.E.; Yamamoto, K.; Sohn, A.S.; Walters, B.; Kuljanin, M.; Gikandi, A.; Wang, H.; Mancias, J.D.; Schneider, R.J.; et al. Neurons Release Serine to Support mRNA Translation in Pancreatic Cancer. Cell 2020, 183, 1202–1218. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Michael, I.P.; Zhang, P.; Saghafinia, S.; Knott, G.; Jiao, W.; McCabe, B.D.; Galván, J.A.; Robinson, H.P.C.; Zlobec, I.; et al. Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature 2019, 573, 526–531. [Google Scholar] [CrossRef]
- Mauffrey, P.; Tchitchek, N.; Barroca, V.; Bemelmans, A.-P.; Firlej, V.; Allory, Y.; Roméo, P.-H.; Magnon, C. Progenitors from the central nervous system drive neurogenesis in cancer. Nature 2019, 569, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Bakst, R.L.; Lee, N.; He, S.; Chernichenko, N.; Chen, C.-H.; Linkov, G.; Le, H.C.; Koutcher, J.; Vakiani, E.; Wong, R.J. Radiation Impairs Perineural Invasion by Modulating the Nerve Microenvironment. PLoS ONE 2012, 7, e39925. [Google Scholar] [CrossRef] [Green Version]
- Meldolesi, J. Neurotrophin Trk Receptors: New Targets for Cancer Therapy. Rev. Physiol. Biochem. Pharmacol. 2018, 174, 67–79. [Google Scholar] [CrossRef]
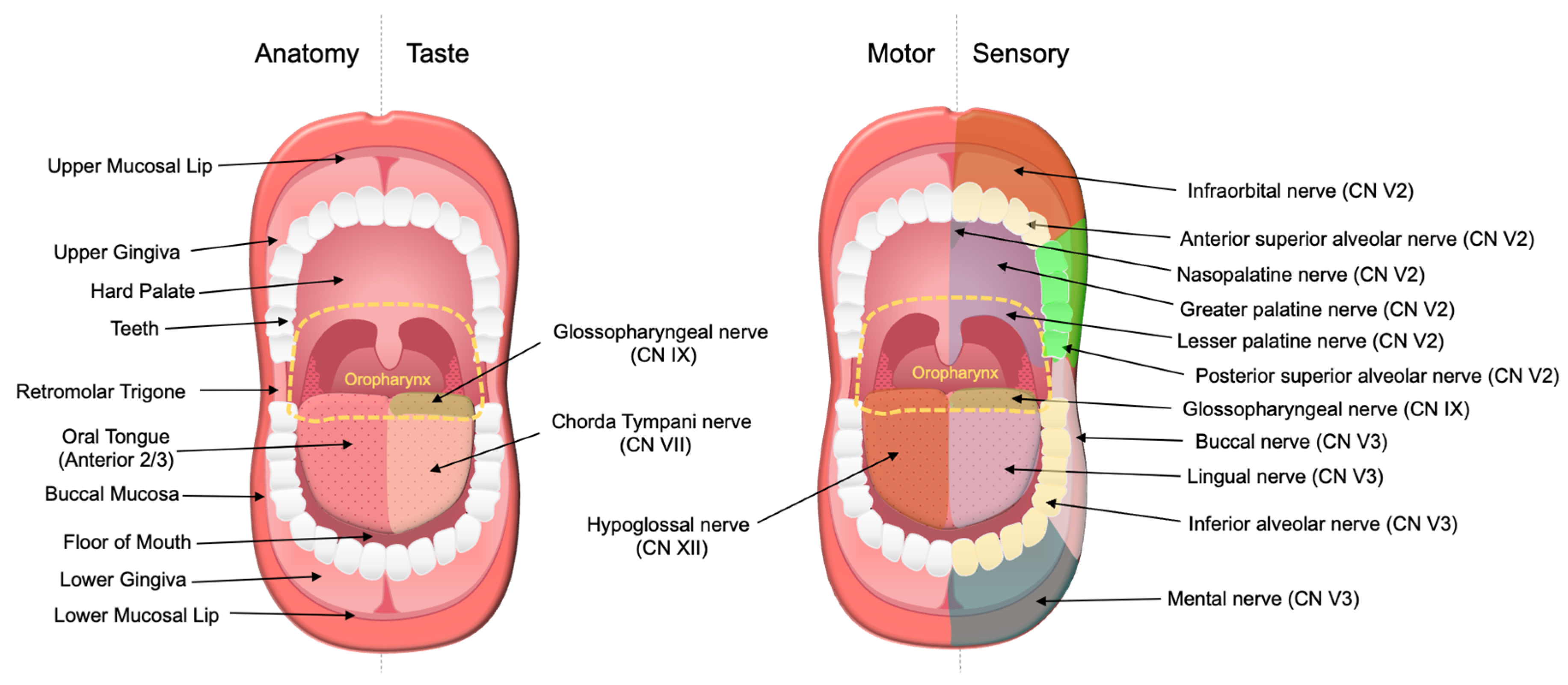
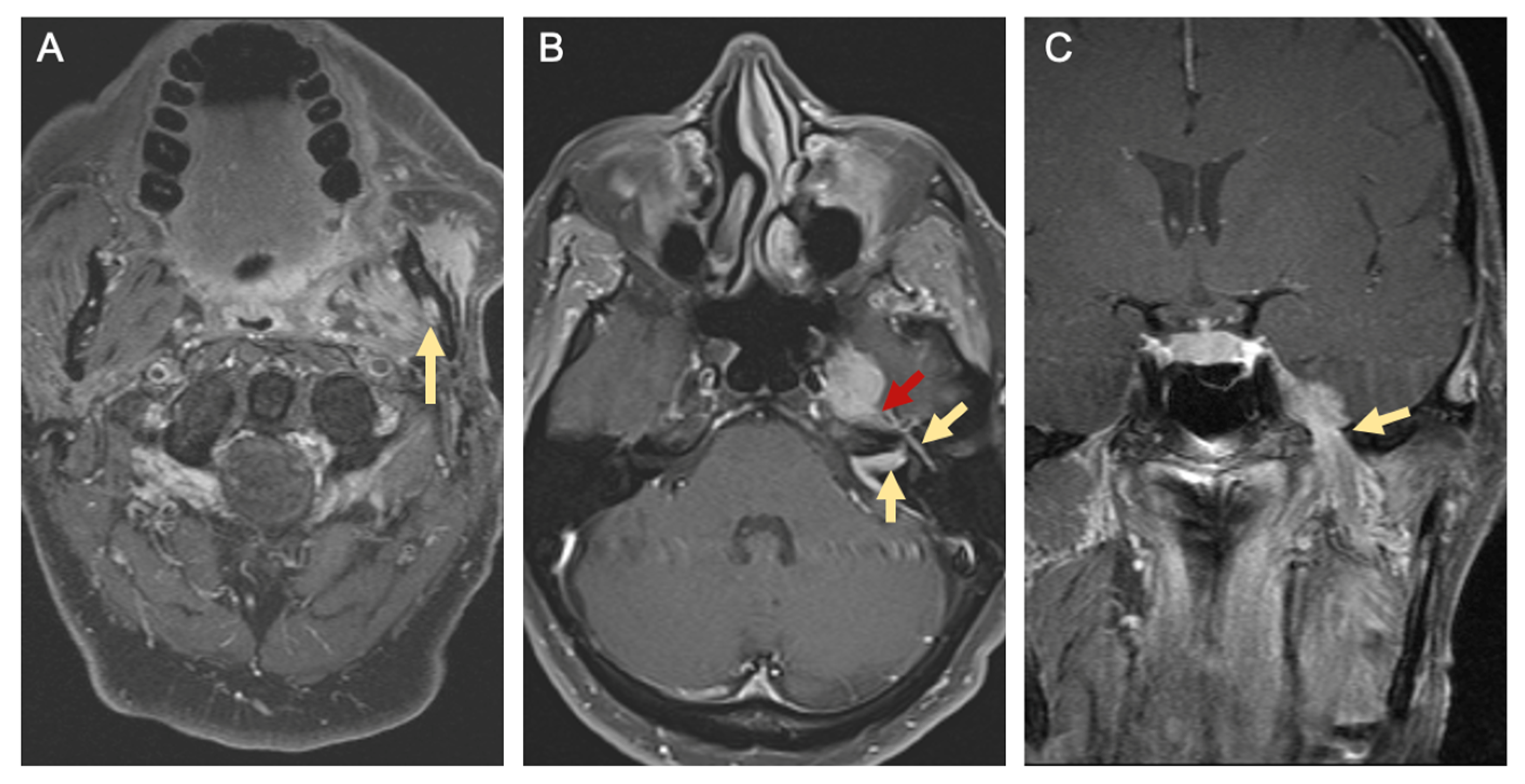
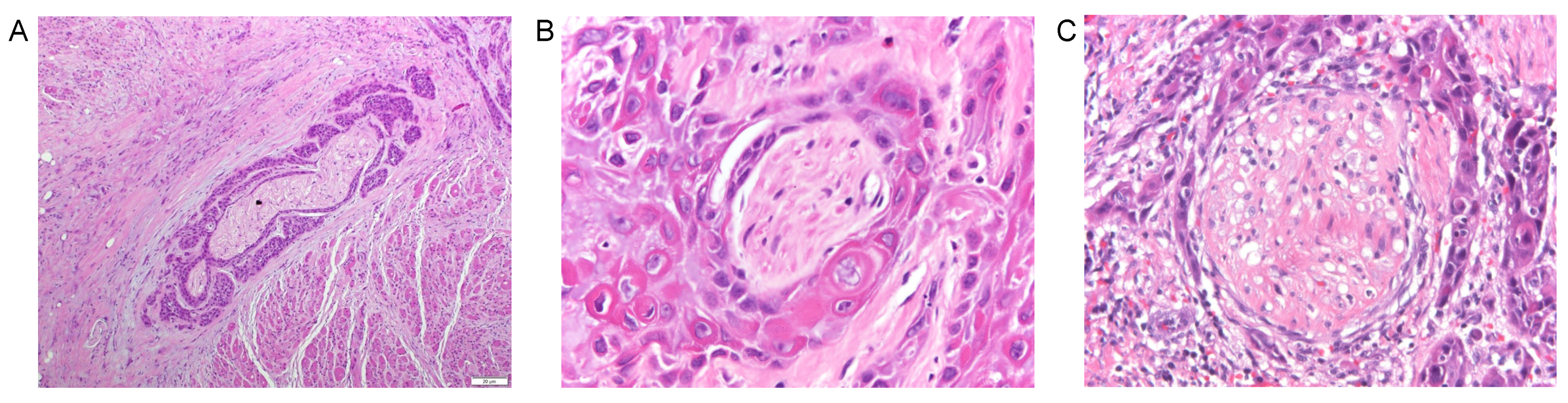
| Primary Tumor (T) | |||
| TX | Primary tumor cannot be assessed | ||
| Tis | Carcinoma in situ | ||
| T1 | Tumor ≤ 2 cm and DOI ≤ 5 mm | ||
| T2 | Tumor ≤ 2 cm, DOI > 5 mm and ≤10 mm; or Tumor > 2 cm but ≤4 cm and DOI ≤ 10 mm | ||
| T3 | Tumor > 4 cm; or any tumor with DOI > 10 mm but ≤20 mm | ||
| T4a | Moderately Advanced Local Disease: Tumor invades adjacent structures only (e.g., through cortical bone of the mandible or maxilla, or involves the maxillary sinus or skin of the face); or Extensive tumor with bilateral tongue involvement; and/or DOI > 20 mm | ||
| T4b | Very advanced local disease: Tumor invades masticator space, pterygoid plates, or skull base and/or encases the internal carotid artery | ||
| Clinical Assessment of Regional Lymph Nodes (cN) | |||
| NX | Regional lymph nodes cannot be assessed | ||
| N0 | No regional lymph node metastasis | ||
| N1 | Metastasis in a single ipsilateral lymph node, ≤3 cm and ENE− | ||
| N2a | Metastasis in a single ipsilateral node, >3 cm and ≤6 cm and ENE− | ||
| N2b | Metastases in multiple ipsilateral nodes, ≤6 cm and ENE– | ||
| N2c | Metastases in bilateral or contralateral lymph nodes, ≤6 cm and ENE− | ||
| N3a | Metastasis in a lymph node > 6 cm and ENE− | ||
| N3b | Metastasis in any lymph node(s) with ENE+ clinically; or Metastasis in a single ipsilateral node, >3 cm and ENE+; or Metastasis to multiple ipsilateral, contralateral, or bilateral nodes and ENE+ | ||
| Pathologic Assessment of Regional Lymph Nodes (pN) | |||
| NX | Regional lymph nodes cannot be assessed | ||
| N0 | No regional lymph node metastasis | ||
| N1 | Metastasis in a single ipsilateral lymph node, ≤3 cm and ENE− | ||
| N2a | Metastasis in a single ipsilateral node, ≤3 cm and ENE+; or Metastasis in a single ipsilateral node > 3 cm and ≤6 cm and ENE− | ||
| N2b | Metastases in multiple ipsilateral nodes, ≤6 cm and ENE– | ||
| N2c | Metastases in bilateral or contralateral lymph nodes, ≤6 cm and ENE− | ||
| N3a | Metastasis in a lymph node > 6 cm and ENE− | ||
| N3b | Metastasis in a single ipsilateral node > 3 cm with ENE+; or Metastasis to multiple ipsilateral, contralateral, or bilateral nodes, any with ENE+; or Metastasis to a single contralateral node of any size and ENE+ | ||
| Distant Metastasis (M) | |||
| M0 | No distant metastases | ||
| M1 | Distant metastases | ||
| AJCC Prognostic Stage Groups | |||
| Stage 0 | Tis | N0 | M0 |
| Stage I | T1 | N0 | M0 |
| Stage II | T2 | N0 | M0 |
| Stage III | T3 | N0 | M0 |
| T1–T3 | N1 | M0 | |
| Stage IVa | T4a | N0 | M0 |
| T4a | N1 | M0 | |
| T1–T4a | N2 | M0 | |
| Stage IVb | Any T | N3 | M0 |
| T4b | Any N | M0 | |
| Stage IVc | Any T | Any N | M1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Misztal, C.I.; Green, C.; Mei, C.; Bhatia, R.; Velez Torres, J.M.; Kamrava, B.; Moon, S.; Nicolli, E.; Weed, D.; Sargi, Z.; et al. Molecular and Cellular Mechanisms of Perineural Invasion in Oral Squamous Cell Carcinoma: Potential Targets for Therapeutic Intervention. Cancers 2021, 13, 6011. https://doi.org/10.3390/cancers13236011
Misztal CI, Green C, Mei C, Bhatia R, Velez Torres JM, Kamrava B, Moon S, Nicolli E, Weed D, Sargi Z, et al. Molecular and Cellular Mechanisms of Perineural Invasion in Oral Squamous Cell Carcinoma: Potential Targets for Therapeutic Intervention. Cancers. 2021; 13(23):6011. https://doi.org/10.3390/cancers13236011
Chicago/Turabian StyleMisztal, Carly I., Carlos Green, Christine Mei, Rita Bhatia, Jaylou M. Velez Torres, Brandon Kamrava, Seo Moon, Elizabeth Nicolli, Donald Weed, Zoukaa Sargi, and et al. 2021. "Molecular and Cellular Mechanisms of Perineural Invasion in Oral Squamous Cell Carcinoma: Potential Targets for Therapeutic Intervention" Cancers 13, no. 23: 6011. https://doi.org/10.3390/cancers13236011
APA StyleMisztal, C. I., Green, C., Mei, C., Bhatia, R., Velez Torres, J. M., Kamrava, B., Moon, S., Nicolli, E., Weed, D., Sargi, Z., & Dinh, C. T. (2021). Molecular and Cellular Mechanisms of Perineural Invasion in Oral Squamous Cell Carcinoma: Potential Targets for Therapeutic Intervention. Cancers, 13(23), 6011. https://doi.org/10.3390/cancers13236011






