Adjusted Comparison of Outcomes between Patients from CARTITUDE-1 versus Multiple Myeloma Patients with Prior Exposure to PI, Imid and Anti-CD-38 from a German Registry
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.1.1. Patients Treated with Ciltacabtagene Autoleucel in CARTITUDE-1
2.1.2. Patients Receiving Treatments from Real World Clinical Practice in Therapie Monitor
2.2. Analysis Populations and Design
2.3. Baseline Characteristics for Population Alignment
2.4. Endpoints
2.5. Statistical Methods
2.6. Role of the Sponsor
3. Results
3.1. Patient Population
3.2. Treatment Regimens Received in the Real-World Clinical Practice Cohort
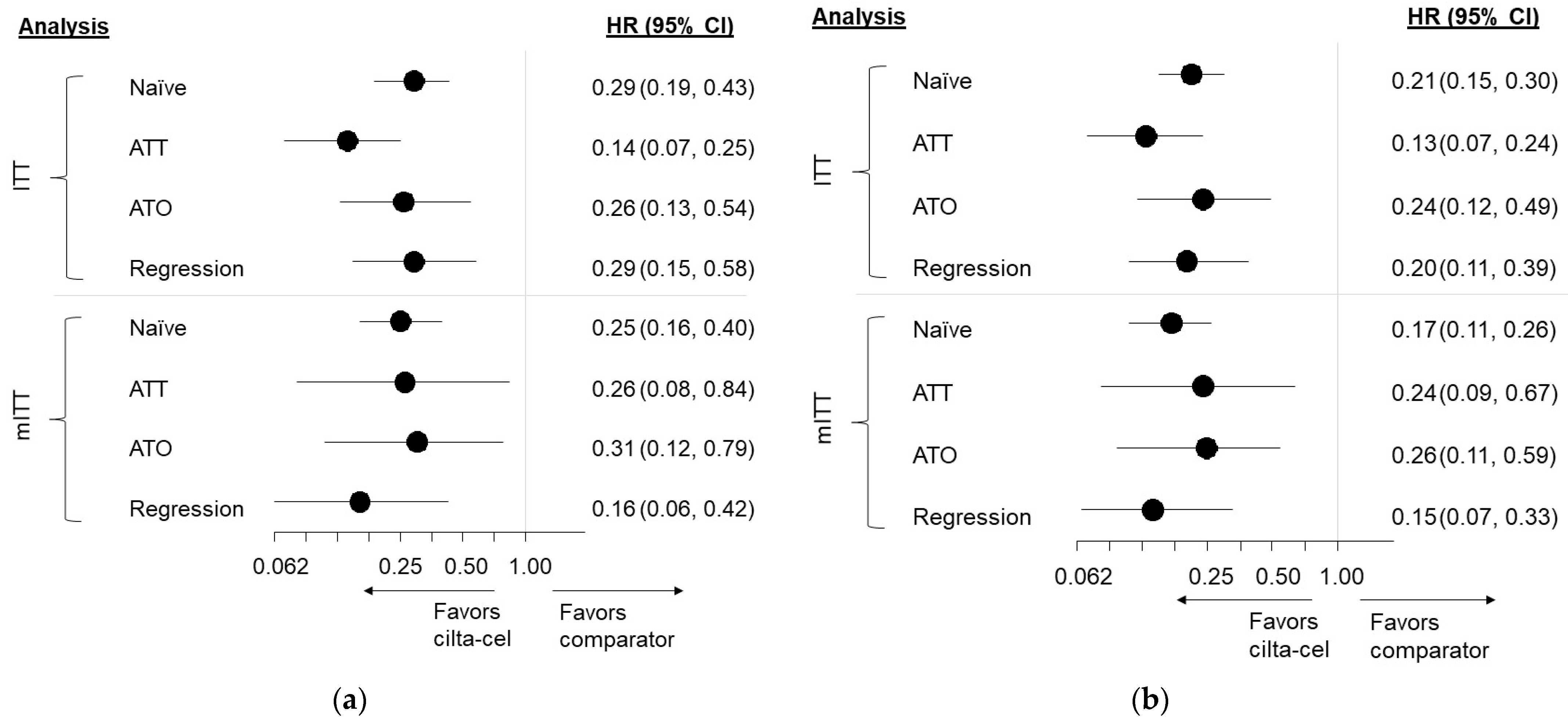
3.3. Findings, ITT Analyses
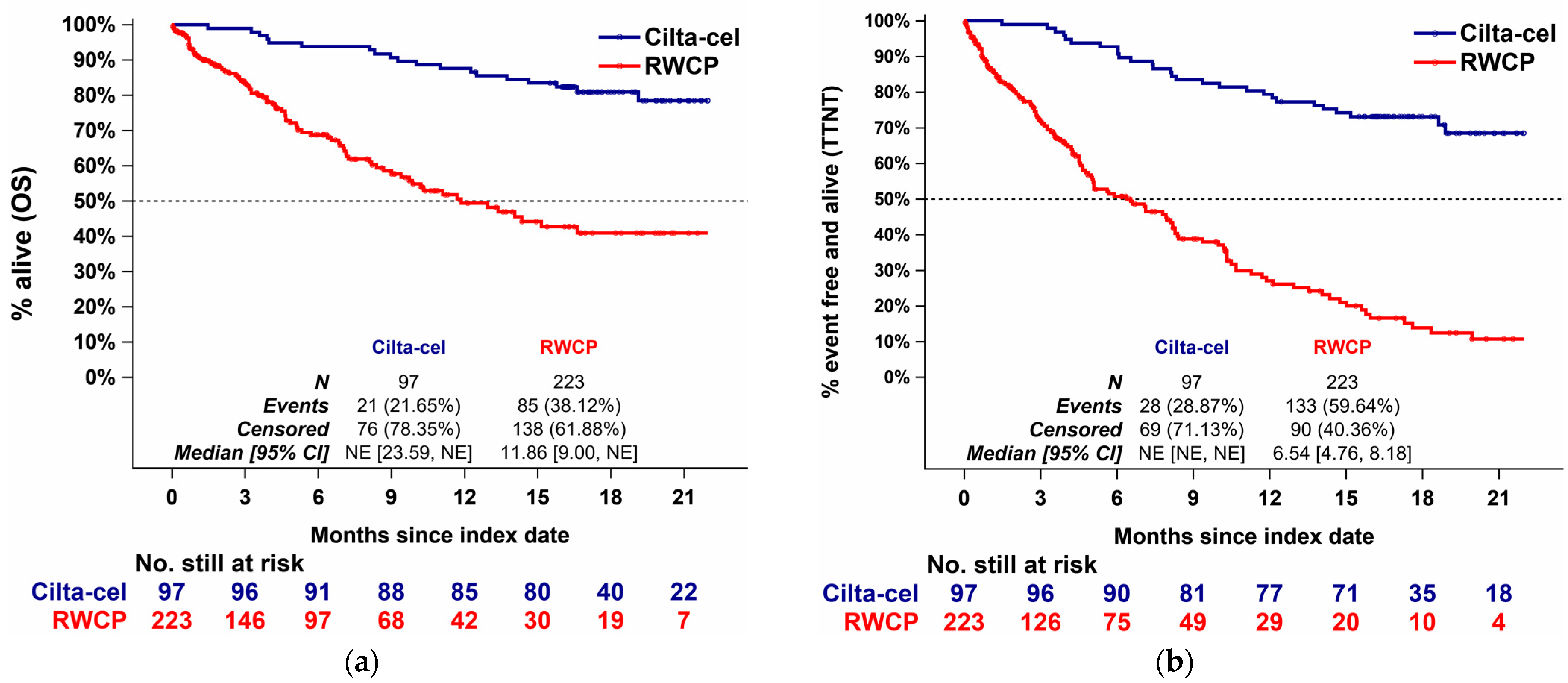
3.3.1. Comparison Results, Overall Survival
3.3.2. Comparison Results, Time to Next Treatment
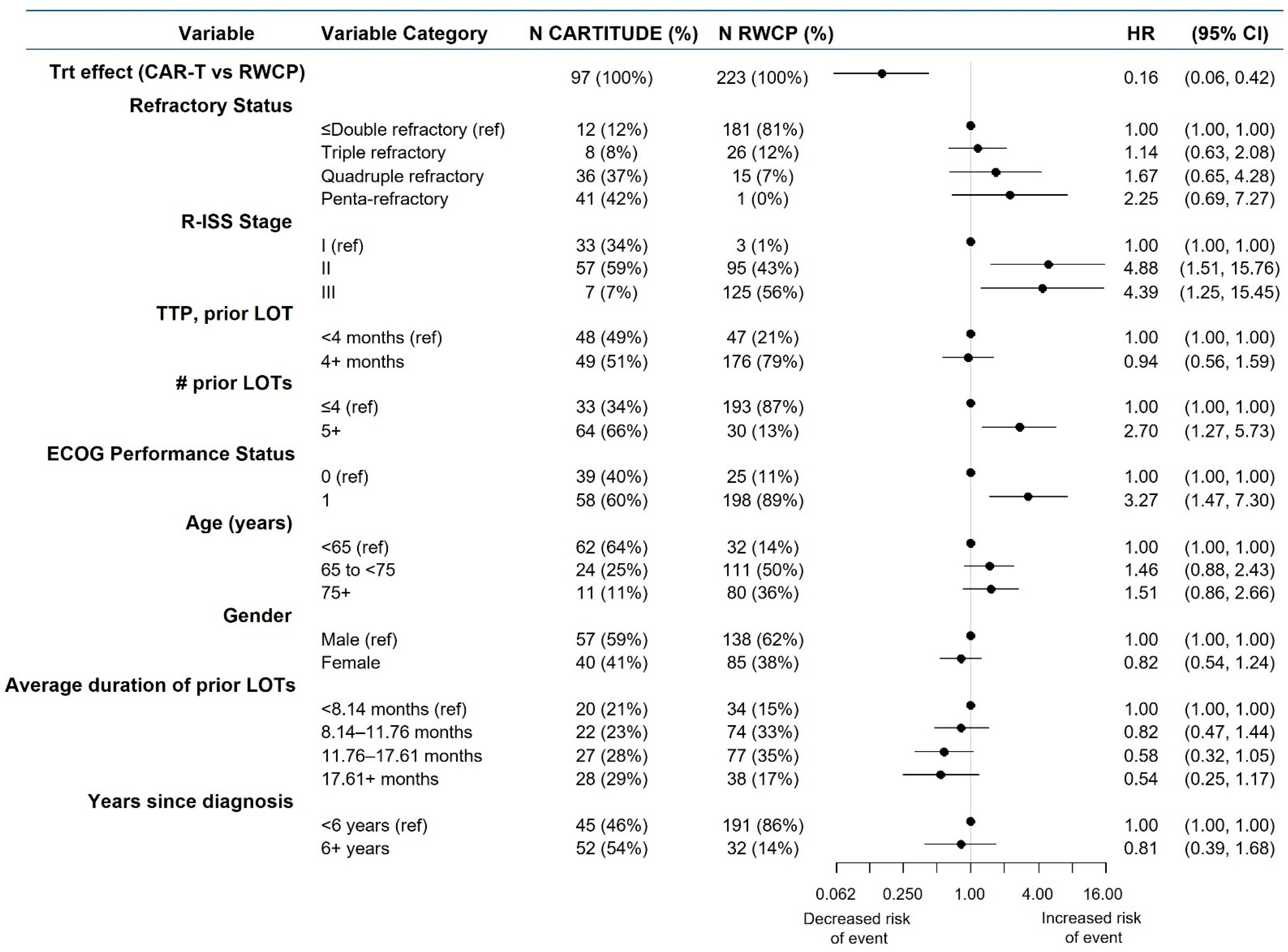
3.4. Findings, mITT Analyses
3.4.1. Comparison Results, Overall Survival
3.4.2. Comparison Results, Time to Next Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kyle, R.A.; Rajkumar, S.V. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia 2009, 23, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Raab, M.S.; Cavo, M.; Delforge, M.; Driessen, C.; Fink, L.; Flinois, A.; Gonzalez-McQuire, S.; Safaei, R.; Karlin, L.; Mateos, M.V.; et al. Multiple myeloma: Practice patterns across Europe. Br. J. Haematol. 2016, 175, 66–76. [Google Scholar] [CrossRef] [Green Version]
- Benyamini, N.; Adir, S.; Gatt, M.; Coen, Y.; Avivi, I.; Trestman, S.; Shvetz, O.; Tadmor, T.; Dally, N.; Cober, Y.; et al. Real-Life Data on the Outcome of Daratumomab-Refractory Myeloma Patients: Multi-Center Experience. Blood 2018, 132, 3259. [Google Scholar] [CrossRef]
- Haefliger, B.; Diels, J.; Ghilotti, F.; Sliwka, H.; Potamianou, A.; Bacon, T.; Kellermann, L. Baseline characteristics and survival outcomes of patients with tri exposed multiple myeloma in a German registry. In Proceedings of the EHA2021—European Hematology Association Conference, Virtual Meeting, 9–17 June 2021. [Google Scholar]
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer Oxf. Engl. 2013, 49, 1374–1403. [Google Scholar] [CrossRef] [Green Version]
- Usmani, S.Z.; Nahi, H.; Plesner, T.; Weiss, B.M.; Bahlis, N.J.; Belch, A.; Voorhees, P.; Laubach, J.; van de Donk, N.; Ahmadi, T.; et al. Daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma: Final resultsfrom the phase 2 GEN501 and SIRIUS trials. Lancet Haematol. 2020, 7, e447–e455. [Google Scholar] [CrossRef]
- Miguel, J.S.; Weisel, K.; Moreau, P.; Lacy, M.; Song, K.; Delforge, M.; Karlin, L.; Goldschmidt, H.; Banos, A.; Oriol, A.; et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): A randomised, open-label, phase 3 trial. Lancet Oncol. 2013, 14, 1055–1066. [Google Scholar] [CrossRef] [Green Version]
- San-Miguel, J.F.; Hungria, V.T.M.; Yoon, S.-S.; Beksac, M.; Dimopoulos, M.A.; Elghandour, A.; Jedrzejczak, W.; Gunther, A.; Nakorn, T.; Siritanaratkul, N.; et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: A multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014, 15, 1195–1206. [Google Scholar] [CrossRef]
- Orlowski, R.Z.; Nagler, A.; Sonneveld, P.; Bladé, J.; Hajek, R.; Spencer, A.; San Miguel, J.; Robak, T.; Dmoszynska, A.; Horvath, N.; et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: Combination therapy improves time to progression. J. Clin. Oncol. 2007, 25, 3892–3901. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Moreau, P.; Palumbo, A.; Joshua, D.; Pour, L.; Hájek, R.; Facon, T.; Ludwig, H.; Oriol, A.; Goldschmidt, H.; et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): A randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016, 17, 27–38. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Chen, C.; Spencer, A.; Niesvizky, R.; Attal, M.; Stadtmauer, E.A.; Petrucci, M.; Yu, Z.; Olsesnyckyj, M.; Zeldis, J.; et al. Long-term follow-up on overall survival from the MM-009 and MM-010 phase III trials of lenalidomide plus dexamethasone in patients with relapsed or refractory multiple myeloma. Leukemia 2009, 23, 2147–2152. [Google Scholar] [CrossRef] [Green Version]
- Dimopoulos, M.A.; Moreau, P.; Terpos, E.; Mateos, M.V.; Zweegman, S.; Cook, G.; Delforge, M.; Hajek, R.; Schjesvold, F.; Cavo, M.; et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 309–322. [Google Scholar] [CrossRef]
- Gandhi, U.H.; Cornell, R.F.; Lakshman, A.; Gahvari, Z.J.; McGehee, E.; Jagosky, M.H.; Gupta, R.; Varnado, W.; Fiala, M.; Chhabra, S.; et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia 2019, 33, 2266–2275. [Google Scholar] [CrossRef] [PubMed]
- Robak, P.; Drozdz, I.; Szemraj, J.; Robak, T. Drug resistance in multiple myeloma. Cancer Treat. Rev. 2018, 70, 199–208. [Google Scholar] [CrossRef]
- Kumar, V.; Ailawadhi, M.; Dutta, N.; Abdulazeez, M.; Aggarwal, C.S.; Quintero, G.; Baksh, M.; Roy, V.; Sher, T.; Alegria, V.; et al. Trends in Early Mortality from Multiple Myeloma: A Population-Based Analysis. Clin. Lymphoma Myeloma Leuk. 2021, 21, e449–e455. [Google Scholar] [CrossRef] [PubMed]
- Majzner, R.G.; Mackall, C.L. Clinical lessons learned from the first leg of the CAR T cell journey. Nat. Med. 2019, 25, 1341–1355. [Google Scholar] [CrossRef]
- Madduri, D.; Berdeja, J.G.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; O’Donnell, E.; et al. CARTITUDE-1: Phase 1b/2 Study of Ciltacabtagene Autoleucel, a B-Cell Maturation Antigen–Directed Chimeric Antigen Receptor T Cell Therapy, in Relapsed/Refractory Multiple Myeloma. Blood 2020, 136, 22–25. [Google Scholar] [CrossRef]
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet 2021, 398, 314–324. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. A Study of JNJ-68284528, a Chimeric Antigen Receptor T Cell (CAR-T) Therapy Directed against B-Cell Maturation Antigen (BCMA) in Participants with Relapsed or Refractory Multiple Myeloma (CARTITUDE-1). 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT03548207 (accessed on 15 June 2021).
- Usmani, S.; Berdeja, J.; Madduri, D.; Jakubowiak, A.; Agha, M.; Cohen, D.; Hari, P.; Yeh, T.M.; Olyslager, Y.; Banerjee, A.; et al. Ciltacabtagene autoleucel, a B-cell maturation antigen (BCMA)-directed chimeric antigen receptor T-cell (CAR-T) therapy, in relapsed/refractory multiple myeloma (R/R MM): Updated results from CARTITUDE-1. In Proceedings of the American Society of Clinical Oncology (ASCO) Annual Meeting, Virtual Meeting, 4–8 June 2021. [Google Scholar]
- Merz, M.; Patel, V.; Kutikova, L.; Lebioda, A.; Schoel, M.; Kellermann, L.; Goldschmidt, H. Treatment Patterns in Patients (pts) with Refractory/Relapsed Multiple Myeloma (RRMM) in Germany between 2016 and 2018. Blood 2019, 134, 4734. [Google Scholar] [CrossRef]
- Backenroth, D. How to choose a time zero for patients in external control arms. Pharm. Stat. 2021, 20, 783–792. [Google Scholar] [CrossRef]
- Van der Laan, M.; Petersen, M.; Joffe, M. History-adjusted marginal structural models and statically-optimal dynamic treatment regimens. Int. J. Biostat. 2005, 1, 4. [Google Scholar] [CrossRef]
- Petersen, M.L.; Deeks, S.G.; Martin, J.N.; van der Laan, M.J. History-adjusted marginal structural models for estimating time-varying effect modification. Am. J. Epidemiol. 2007, 166, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Hernán, M.A.; Robins, J.M. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. Am. J. Epidemiol. 2016, 183, 758–764. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Dalton, J. A unified approach to measuring the effect size between two groups using SAS®. In Proceedings of the SAS Global Forum, Orlando, FL, USA, 22–25 April 2012. [Google Scholar]
- Li, F.; Morgan, K.; Zaslavsky, A. Balancing Covariates via Propensity Score Weighting. J. Am. Stat. Assoc. 2018, 113, 390–400. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Thomas, L.E.; Li, F. Addressing Extreme Propensity Scores via the Overlap Weights. Am. J. Epidemiol. 2019, 188, 250–257. [Google Scholar] [CrossRef] [Green Version]
- Grambsch, P.; Therneau, T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994, 81, 515–526. [Google Scholar] [CrossRef]
- Mehra, M.; Vogel, M.; Valluri, S.; Nair, S.; Schecter, J.; Slowik, R.; Jagannath, S.; Weisel, K.; Usmani, S. Patient characteristics, treatment patterns and outcomes in patients with triple class refractory multiple myeloma. In Proceedings of the EHA25 VIRTUAL: 25th EHA Annual Congress: “Unfolding the future!”, Virtual Meeting, 11–21 June 2020. [Google Scholar]
- Merz, M.; Vande Broek, I.; Perez, M.; Kolb, B.; Symeonidis, A.; Nikolousis, E.; Zomas, A.; Gonzalez, F.; Kellermann, L.; Goldschmidt, H. Evolving Treatment Trends in Relapsed/Refractory Multiple Myeloma in Europe from 2016 to 2018: Analysis of a Multi-National Survey. Blood 2019, 134, 3115. [Google Scholar] [CrossRef]
- Merz, M.; Kellermann, L.; Poenisch, W.; Tischler, H.-J.; Kohnke, J.; Knauf, W.; Goldschmidt, H. Diagnosis and treatment of multiple myeloma in Germany: Analysis of a nationwide multi-institutional survey. Ann. Hematol. 2017, 96, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Lin, Y.; Martin, T.; Chhabra, S.; Usmani, S.; Jagannath, S.; Callandar, N.S.; Berdeja, J.G.; Kang, Y.; Vij, R.; et al. Cilta-cel versus conventional treatment in patients with relapse/refractory multiple myeloma. J. Clin. Oncol. 2021, 39, 8030. [Google Scholar] [CrossRef]
- Weisel, K.; Martin, T.; Krishnan, A.; Jagannath, S.; Qi, K.; Londhe, A.; Mehra, M.; Nair, S.; Diels, J.; Vogel, M.; et al. Comparison of ciltacabtagene autoleucel (cilta-cel) in CARTITUDE-1 versus standard of care in triple-class exposed multiple myeloma patients in clinical trials of daratumumab. In Proceedings of the EHA2021—European Hematology Association Conference, Virtual Meeting, 9–17 June 2021; p. 060921. [Google Scholar]
- Martin, T.; Krishnan, A.; Yong, K.; Weisel, K.; Mehra, M.; Nair, S.; Qi, K.; Londhe, A.; Diels, J.; Crivera, C.; et al. Comparison of outcomes with ciltacabtagene autoleucel (cilta-cel) in CARTITUDE-1 versus real-world standard of care (RW SOC) for patients with triple-class exposed relapsed/refractory multiple myeloma. J. Clin. Oncol. 2021, 39, 8045. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merz, M.; Goldschmidt, H.; Hari, P.; Agha, M.; Diels, J.; Ghilotti, F.; Perualila, N.J.; Cabrieto, J.; Haefliger, B.; Sliwka, H.; et al. Adjusted Comparison of Outcomes between Patients from CARTITUDE-1 versus Multiple Myeloma Patients with Prior Exposure to PI, Imid and Anti-CD-38 from a German Registry. Cancers 2021, 13, 5996. https://doi.org/10.3390/cancers13235996
Merz M, Goldschmidt H, Hari P, Agha M, Diels J, Ghilotti F, Perualila NJ, Cabrieto J, Haefliger B, Sliwka H, et al. Adjusted Comparison of Outcomes between Patients from CARTITUDE-1 versus Multiple Myeloma Patients with Prior Exposure to PI, Imid and Anti-CD-38 from a German Registry. Cancers. 2021; 13(23):5996. https://doi.org/10.3390/cancers13235996
Chicago/Turabian StyleMerz, Maximilian, Hartmut Goldschmidt, Parameswaran Hari, Mounzer Agha, Joris Diels, Francesca Ghilotti, Nolen J. Perualila, Jedelyn Cabrieto, Benjamin Haefliger, Henrik Sliwka, and et al. 2021. "Adjusted Comparison of Outcomes between Patients from CARTITUDE-1 versus Multiple Myeloma Patients with Prior Exposure to PI, Imid and Anti-CD-38 from a German Registry" Cancers 13, no. 23: 5996. https://doi.org/10.3390/cancers13235996
APA StyleMerz, M., Goldschmidt, H., Hari, P., Agha, M., Diels, J., Ghilotti, F., Perualila, N. J., Cabrieto, J., Haefliger, B., Sliwka, H., Schecter, J. M., Jackson, C. C., Olyslager, Y., Akram, M., Nesheiwat, T., Kellermann, L., & Jagannath, S. (2021). Adjusted Comparison of Outcomes between Patients from CARTITUDE-1 versus Multiple Myeloma Patients with Prior Exposure to PI, Imid and Anti-CD-38 from a German Registry. Cancers, 13(23), 5996. https://doi.org/10.3390/cancers13235996





