Novel Perspectives in Pseudomyxoma Peritonei Treatment
Abstract
Simple Summary
Abstract
1. Introduction
2. Standardization of PMP Treatment
3. Quality Assurance
4. New Surgical Approaches
4.1. Second-Look Strategy after Diagnosis of Appendiceal Mucinous Tumor
4.2. Surgery for Unresectable PMP
4.3. Pressurized Intraperitoneal Aerosolized Chemotherapy (PIPAC)
4.4. Intestinal Transplantation for End-Stage PMP
5. Systemic Treatments
5.1. Perioperative Systemic Chemotherapy
5.2. Palliative Systemic Chemotherapy
5.3. Anti-Angiogenic Treatment
6. Prognostic Significance of Pathological Markers
7. Novel Therapeutic Targets
7.1. Genomic and Transcriptomic Profile in PMP
7.2. Protein Expression in PMP
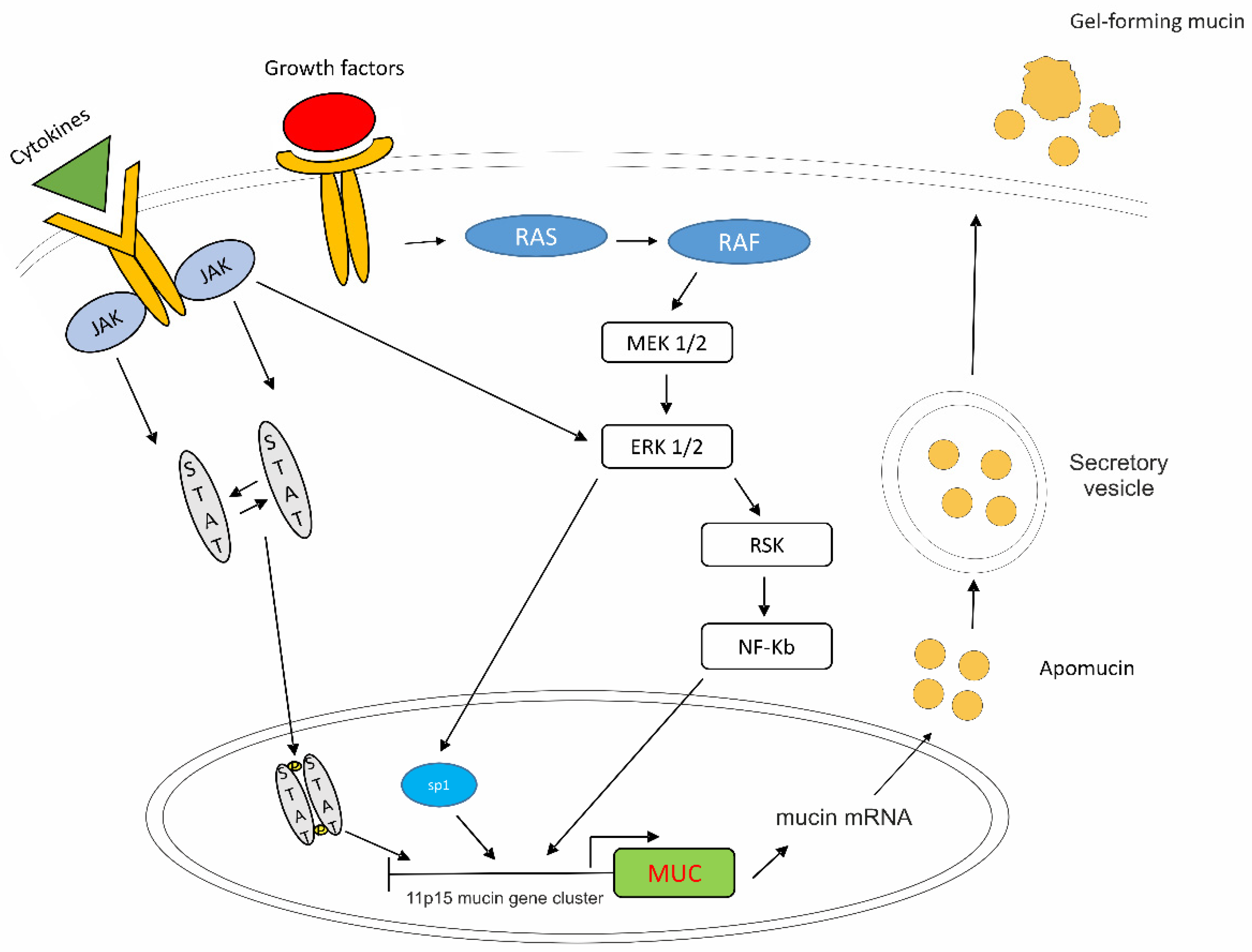
8. Mucin as a Therapeutic Target
8.1. Mucins in PMP
8.2. Mucolytic Agents in PMP
8.3. Targeting Expression of Mucin
9. Other Potential Therapeutic Strategies
9.1. JAK/STAT Pathway
9.2. The Dual Role of Anti-Inflammatory Drugs
9.3. Ubiquitin-Proteasome System (UPS)
9.4. EpCAM
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Werth, K. Klinische und anatomische Untersuchungen zur Lehre von den Bauchgeschwülsten und der Laparatomie. Arch. Gynäkol. 1884, 24, 100–118. [Google Scholar] [CrossRef]
- Miner, T.J.; Shia, J.; Jaques, D.P.; Klimstra, D.S.; Brennan, M.F.; Coit, D.G. Long-term survival following treatment of pseudomyxoma peritonei: An analysis of surgical therapy. Ann. Surg. 2005, 241, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol. 2006, 7, 69–76. [Google Scholar] [CrossRef]
- Kusamura, S.; Barretta, F.; Yonemura, Y.; Sugarbaker, P.H.; Moran, B.J.; Levine, E.A.; Goere, D.; Baratti, D.; Nizri, E.; Morris, D.L.; et al. The Role of Hyperthermic Intraperitoneal Chemotherapy in Pseudomyxoma Peritonei After Cytoreductive Surgery. JAMA Surg. 2021, 156, e206363. [Google Scholar] [CrossRef]
- Chua, T.C.; Al-Mohaimeed, K.; Liauw, W.; Morris, D.L. Pseudomyxoma peritonei: A need to establish evidence-based standard of care--is this the right trial? Ann. Surg. Oncol. 2009, 16, 2675–2677. [Google Scholar] [CrossRef]
- Sugarbaker, P.H.; Jablonski, K.A. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann. Surg. 1995, 221, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, J. Technology of Hyperthermic Intraperitoneal Chemotherapy in the United States, Europe, China, Japan, and Korea. Cancer J. 2009, 15, 249–254. [Google Scholar] [CrossRef]
- Foster, J.M.; Sleightholm, R.; Patel, A.; Shostrom, V.; Hall, B.; Neilsen, B.; Bartlett, D.; Smith, L. Morbidity and Mortality Rates Following Cytoreductive Surgery Combined With Hyperthermic Intraperitoneal Chemotherapy Compared With Other High-Risk Surgical Oncology Procedures. JAMA Netw. Open 2019, 2, e186847. [Google Scholar] [CrossRef]
- Chua, T.C.; Robertson, G.; Liauw, W.; Farrell, R.; Yan, T.D.; Morris, D.L. Intraoperative hyperthermic intraperitoneal chemotherapy after cytoreductive surgery in ovarian cancer peritoneal carcinomatosis: Systematic review of current results. J. Cancer Res. Clin. Oncol. 2009, 135, 1637–1645. [Google Scholar] [CrossRef]
- Govaerts, K.; Lurvink, R.J.; de Hingh, I.; van der Speeten, K.; Villeneuve, L.; Kusamura, S.; Kepenekian, V.; Deraco, M.; Glehen, O.; Moran, B.J.; et al. Appendiceal tumours and pseudomyxoma peritonei: Literature review with PSOGI/EURACAN clinical practice guidelines for diagnosis and treatment. Eur. J. Surg. Oncol. 2021, 47, 11–35. [Google Scholar] [CrossRef]
- Carr, N.J.; Cecil, T.D.; Mohamed, F.; Sobin, L.H.; Sugarbaker, P.H.; Gonzalez-Moreno, S.; Taflampas, P.; Chapman, S.; Moran, B.J.; Peritoneal Surface Oncology Group, I. A Consensus for Classification and Pathologic Reporting of Pseudomyxoma Peritonei and Associated Appendiceal Neoplasia: The Results of the Peritoneal Surface Oncology Group International (PSOGI) Modified Delphi Process. Am. J. Surg. Pathol. 2016, 40, 14–26. [Google Scholar] [CrossRef]
- Carr, N.J.; Sobin, L.H. Adenocarcinoma of the appendix. In WHO Classification of Tumours of the Digestive System, 4th ed.; Bosman, F.T., Carneiro, F., Hruban, R.H., Theise, N.D., Eds.; International Agency for Research on Cancer (IARC) Press: Lyon, France, 2010; Volume 3, pp. 122–125. [Google Scholar]
- Baratti, D.; Kusamura, S.; Milione, M.; Bruno, F.; Guaglio, M.; Deraco, M. Validation of the Recent PSOGI Pathological Classification of Pseudomyxoma Peritonei in a Single-Center Series of 265 Patients Treated by Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Ann. Surg. Oncol. 2018, 25, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Merkow, R.P.; Bilimoria, K.Y.; Ko, C.Y. Surgical quality measurement: An evolving science. JAMA Surg. 2013, 148, 586–587. [Google Scholar] [CrossRef]
- Alderson, D.; Cromwell, D. Publication of surgeon-specific outcomes. Br. J. Surg. 2014, 101, 1335–1337. [Google Scholar] [CrossRef] [PubMed]
- Kusamura, S.; Gonzalez-Moreno, S.; Nizri, E.; Baratti, D.; Guadagni, S.; Guaglio, M.; Battaglia, L.; Deraco, M. Learning Curve, Training Program, and Monitorization of Surgical Performance of Peritoneal Surface Malignancies Centers. Surg Oncol Clin. N Am. 2018, 27, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Kusamura, S.; Moran, B.J.; Sugarbaker, P.H.; Levine, E.A.; Elias, D.; Baratti, D.; Morris, D.L.; Sardi, A.; Glehen, O.; Deraco, M.; et al. Multicentre study of the learning curve and surgical performance of cytoreductive surgery with intraperitoneal chemotherapy for pseudomyxoma peritonei. Br. J. Surg. 2014, 101, 1758–1765. [Google Scholar] [CrossRef]
- Istl, A.C.; Gage, M.M.; Esquivel, J.; Ahuja, N.; Greer, J.B.; Johnston, F.M. Management of Low-Grade Appendiceal Mucinous Neoplasms (LAMN): An International Survey of Surgeons Performing CRS and HIPEC. Ann. Surg. Oncol. 2021, 28, 3831–3837. [Google Scholar] [CrossRef] [PubMed]
- Smeenk, R.M.; van Velthuysen, M.L.; Verwaal, V.J.; Zoetmulder, F.A. Appendiceal neoplasms and pseudomyxoma peritonei: A population based study. Eur. J. Surg. Oncol. 2008, 34, 196–201. [Google Scholar] [CrossRef]
- Furman, M.J.; Cahan, M.; Cohen, P.; Lambert, L.A. Increased risk of mucinous neoplasm of the appendix in adults undergoing interval appendectomy. JAMA Surg. 2013, 148, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Guaglio, M.; Sinukumar, S.; Kusamura, S.; Milione, M.; Pietrantonio, F.; Battaglia, L.; Guadagni, S.; Baratti, D.; Deraco, M. Clinical Surveillance After Macroscopically Complete Surgery for Low-Grade Appendiceal Mucinous Neoplasms (LAMN) with or Without Limited Peritoneal Spread: Long-Term Results in a Prospective Series. Ann. Surg. Oncol. 2018, 25, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Pai, R.K.; Beck, A.H.; Norton, J.A.; Longacre, T.A. Appendiceal mucinous neoplasms: Clinicopathologic study of 116 cases with analysis of factors predicting recurrence. Am. J. Surg. Pathol. 2009, 33, 1425–1439. [Google Scholar] [CrossRef] [PubMed]
- Yantiss, R.K.; Shia, J.; Klimstra, D.S.; Hahn, H.P.; Odze, R.D.; Misdraji, J. Prognostic significance of localized extra-appendiceal mucin deposition in appendiceal mucinous neoplasms. Am. J. Surg. Pathol. 2009, 33, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.M.; Sleightholm, R.L.; Wahlmeier, S.; Loggie, B.; Sharma, P.; Patel, A. Early identification of DPAM in at-risk low-grade appendiceal mucinous neoplasm patients: A new approach to surveillance for peritoneal metastasis. World J. Surg. Oncol. 2016, 14, 243. [Google Scholar] [CrossRef]
- Abudeeb, H.; Selvasekar, C.R.; O’Dwyer, S.T.; Chakrabarty, B.; Malcolmson, L.; Renehan, A.G.; Wilson, M.S.; Aziz, O. Laparoscopic cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for perforated low-grade appendiceal mucinous neoplasms. Surg. Endosc. 2020, 34, 5516–5521. [Google Scholar] [CrossRef] [PubMed]
- Arjona-Sanchez, A.; Aziz, O.; Passot, G.; Salti, G.; Esquivel, J.; van der Speeten, K.; Piso, P.; Nedelcut, D.S.; Sommariva, A.; Yonemura, Y.; et al. Laparoscopic cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for limited peritoneal metastasis. The PSOGI international collaborative registry. Eur. J. Surg. Oncol. 2021, 47, 1420–1426. [Google Scholar] [CrossRef]
- Andreasson, H.; Graf, W.; Nygren, P.; Glimelius, B.; Mahteme, H. Outcome differences between debulking surgery and cytoreductive surgery in patients with Pseudomyxoma peritonei. Eur. J. Surg. Oncol. 2012, 38, 962–968. [Google Scholar] [CrossRef]
- Benhaim, L.; Faron, M.; Gelli, M.; Sourrouille, I.; Honore, C.; Delhorme, J.B.; Elias, D.; Goere, D. Survival after complete cytoreductive surgery and HIPEC for extensive pseudomyxoma peritonei. Surg. Oncol. 2019, 29, 78–83. [Google Scholar] [CrossRef]
- Sgarbura, O.; Al Hosni, M.; Petruzziello, A.; Figueroa, R.; Khellaf, L.; Pissas, M.H.; Carrere, S.; Nougaret, S.; Bibeau, F.; Quenet, F. Complete pathologic response after two-stage cytoreductive surgery with HIPEC for bulky pseudomyxoma peritonei: Proof of concept. Int. J. Hyperth. 2020, 37, 585–591. [Google Scholar] [CrossRef]
- Solass, W.; Kerb, R.; Murdter, T.; Giger-Pabst, U.; Strumberg, D.; Tempfer, C.; Zieren, J.; Schwab, M.; Reymond, M.A. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: First evidence for efficacy. Ann. Surg. Oncol. 2014, 21, 553–559. [Google Scholar] [CrossRef]
- Alyami, M.; Hubner, M.; Grass, F.; Bakrin, N.; Villeneuve, L.; Laplace, N.; Passot, G.; Glehen, O.; Kepenekian, V. Pressurised intraperitoneal aerosol chemotherapy: Rationale, evidence, and potential indications. Lancet Oncol. 2019, 20, e368–e377. [Google Scholar] [CrossRef]
- Tempfer, C.B.; Solass, W.; Buerkle, B.; Reymond, M.A. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) with cisplatin and doxorubicin in a woman with pseudomyxoma peritonei: A case report. Gynecol. Oncol. Rep. 2014, 10, 32–35. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Robella, M.; Vaira, M.; de Simone, M. Safety and feasibility of pressurized intraperitoneal aerosol chemotherapy (PIPAC) associated with systemic chemotherapy: An innovative approach to treat peritoneal carcinomatosis. World J. Surg. Oncol. 2016, 14, 128. [Google Scholar] [CrossRef] [PubMed]
- Swain, D.; Mason, G.; Yates, A.; Burke, S.; Cecil, T.; Mohamed, F.; Dayal, S.; Tzivanakis, A.; Moran, B. Outcomes of home parenteral nutrition in 34 patients with intestinal failure from recurrent or progressive peritoneal malignancy of gastro-intestinal tract origin. Eur. J. Clin. Nutr. 2021, 75, 856–858. [Google Scholar] [CrossRef] [PubMed]
- Kahn, A.B.; Tulla, K.A.; Tzvetanov, I.G. Indications of Intestinal Transplantation. Gastroenterol. Clin. N. Am. 2019, 48, 575–583. [Google Scholar] [CrossRef]
- Reddy, S.C.T.; Allan, P.; Georgios, V.; Smith, A.; Holdaway, L.; Vokes, L.; Mohamad, F.; Moran, B.; Friend, P. Extending the Indications of Intestinal Transplantation—Modified multivisceral transplantation for end-stage pseudomyxoma peritoneii. Transplantation 2017, 101, S89. [Google Scholar] [CrossRef]
- Cecil, T.A.P.; Reddy, S.; Vrakas, G.; Giele, H.; Mohamed, F.; Vaidya, A.; Moran, B.; Friend, P. Cytoreductive surgery and multivisceral small bowel transplantation. Pleura Peritoneum 2018, 3, BC04. [Google Scholar]
- Munoz-Zuluaga, C.A.; King, M.C.; Ledakis, P.; Gushchin, V.; Sittig, M.; Nieroda, C.; Zambrano-Vera, K.; Sardi, A. Systemic chemotherapy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS/HIPEC) in patients with high-grade mucinous carcinoma peritonei of appendiceal origin. Eur. J. Surg. Oncol. 2019, 45, 1598–1606. [Google Scholar] [CrossRef]
- Asare, E.A.; Compton, C.C.; Hanna, N.N.; Kosinski, L.A.; Washington, M.K.; Kakar, S.; Weiser, M.R.; Overman, M.J. The impact of stage, grade, and mucinous histology on the efficacy of systemic chemotherapy in adenocarcinomas of the appendix: Analysis of the National Cancer Data Base. Cancer 2016, 122, 213–221. [Google Scholar] [CrossRef]
- Shapiro, J.F.; Chase, J.L.; Wolff, R.A.; Lambert, L.A.; Mansfield, P.F.; Overman, M.J.; Ohinata, A.; Liu, J.; Wang, X.; Eng, C. Modern systemic chemotherapy in surgically unresectable neoplasms of appendiceal origin: A single-institution experience. Cancer 2010, 116, 316–322. [Google Scholar] [CrossRef]
- Farquharson, A.L.; Pranesh, N.; Witham, G.; Swindell, R.; Taylor, M.B.; Renehan, A.G.; Rout, S.; Wilson, M.S.; O’Dwyer, S.T.; Saunders, M.P. A phase II study evaluating the use of concurrent mitomycin C and capecitabine in patients with advanced unresectable pseudomyxoma peritonei. Br. J. Cancer 2008, 99, 591–596. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Perrone, F.; Mennitto, A.; Gleeson, E.M.; Milione, M.; Tamborini, E.; Busico, A.; Settanni, G.; Berenato, R.; Caporale, M.; et al. Toward the molecular dissection of peritoneal pseudomyxoma. Ann. Oncol. 2016, 27, 2097–2103. [Google Scholar] [CrossRef]
- Raimondi, A.; Corallo, S.; Niger, M.; Antista, M.; Randon, G.; Morano, F.; Milione, M.; Kusamura, S.; Baratti, D.; Guaglio, M.; et al. Metronomic Capecitabine With Cyclophosphamide Regimen in Unresectable or Relapsed Pseudomyxoma Peritonei. Clin. Colorectal Cancer 2019, 18, e179–e190. [Google Scholar] [CrossRef]
- Hiraide, S.; Komine, K.; Sato, Y.; Ouchi, K.; Imai, H.; Saijo, K.; Takahashi, M.; Takahashi, S.; Shirota, H.; Takahashi, M.; et al. Efficacy of modified FOLFOX6 chemotherapy for patients with unresectable pseudomyxoma peritonei. Int. J. Clin. Oncol. 2020, 25, 774–781. [Google Scholar] [CrossRef]
- Overman, M.J.; McDermott, R.; Leach, J.L.; Lonardi, S.; Lenz, H.J.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.; Moss, R.A.; et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017, 18, 1182–1191. [Google Scholar] [CrossRef]
- Andersson, Y.; Fleten, K.G.; Abrahamsen, T.W.; Reed, W.; Davidson, B.; Flatmark, K. Anti-Angiogenic Treatment in Pseudomyxoma Peritonei-Still a Strong Preclinical Rationale. Cancers 2021, 13, 2819. [Google Scholar] [CrossRef]
- Dohan, A.; Lousquy, R.; Eveno, C.; Goere, D.; Broqueres-You, D.; Kaci, R.; Lehmann-Che, J.; Launay, J.M.; Soyer, P.; Bonnin, P.; et al. Orthotopic animal model of pseudomyxoma peritonei: An in vivo model to test anti-angiogenic drug effects. Am. J. Pathol. 2014, 184, 1920–1929. [Google Scholar] [CrossRef]
- Choe, J.H.; Overman, M.J.; Fournier, K.F.; Royal, R.E.; Ohinata, A.; Rafeeq, S.; Beaty, K.; Phillips, J.K.; Wolff, R.A.; Mansfield, P.F.; et al. Improved Survival with Anti-VEGF Therapy in the Treatment of Unresectable Appendiceal Epithelial Neoplasms. Ann. Surg. Oncol. 2015, 22, 2578–2584. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.; Gohda, Y.; Miyazaki, H.; Hayama, N.; Shimizu, S.; Igari, T.; Yano, H. A case of pseudomyxoma peritonei successfully treated with trifluridine/tipiracil (TAS-102) and bevacizumab after palliative debulking surgery. Chin. Clin. Oncol. 2021, 10, 29. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Berenato, R.; Maggi, C.; Caporale, M.; Milione, M.; Perrone, F.; Tamborini, E.; Baratti, D.; Kusamura, S.; Mariani, L.; et al. GNAS mutations as prognostic biomarker in patients with relapsed peritoneal pseudomyxoma receiving metronomic capecitabine and bevacizumab: A clinical and translational study. J. Transl. Med. 2016, 14, 125. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Foster, J.M.; Grotz, T.E.; Turaga, K.K.; Gatalica, Z.; Sharma, P.; Loggie, B.W. Analysis of immunohistochemical predictors of outcome in pseudomyxoma peritonei. J. Clin. Oncol. 2008, 26, 22067. [Google Scholar] [CrossRef]
- Shetty, S.; Thomas, P.; Ramanan, B.; Sharma, P.; Govindarajan, V.; Loggie, B. Kras mutations and p53 overexpression in pseudomyxoma peritonei: Association with phenotype and prognosis. J. Surg. Res. 2013, 180, 97–103. [Google Scholar] [CrossRef]
- Yan, F.; Lin, Y.; Zhou, Q.; Chang, H.; Li, Y. Pathological prognostic factors of pseudomyxoma peritonei: Comprehensive clinicopathological analysis of 155 cases. Hum. Pathol. 2020, 97, 9–18. [Google Scholar] [CrossRef]
- Yan, F.; Shi, F.; Li, X.; Chang, H.; Jin, M.; Li, Y. Prognostic significance of CEA, Ki67 and p53 in pseudomyxoma peritonei of appendiceal origin. J. Int. Med. Res. 2021, 49, 3000605211022297. [Google Scholar] [CrossRef] [PubMed]
- Arjona-Sanchez, A.; Martinez-Lopez, A.; Valenzuela-Molina, F.; Rufian-Andujar, B.; Rufian-Pena, S.; Casado-Adam, A.; Sanchez-Hidalgo, J.M.; Rodriguez-Ortiz, L.; Medina-Fernandez, F.J.; Diaz-Lopez, C.; et al. A Proposal for Modification of the PSOGI Classification According to the Ki-67 Proliferation Index in Pseudomyxoma Peritonei. Ann. Surg. Oncol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kuracha, M.R.; Thomas, P.; Loggie, B.W.; Govindarajan, V. Bilateral blockade of MEK- and PI3K-mediated pathways downstream of mutant KRAS as a treatment approach for peritoneal mucinous malignancies. PLoS ONE 2017, 12, e0179510. [Google Scholar] [CrossRef]
- Lin, Y.L.; Ma, R.; Li, Y. The biological basis and function of GNAS mutation in pseudomyxoma peritonei: A review. J. Cancer Res. Clin. Oncol. 2020, 146, 2179–2188. [Google Scholar] [CrossRef] [PubMed]
- Ang, C.; Stollman, A.; Zhu, H.; Sarpel, U.; Scarborough, B.; Sahni, G.; Millis, S.Z. Clinical Benefit from Trametinib in a Patient with Appendiceal Adenocarcinoma with a GNAS R201H Mutation. Case Rep. Oncol. 2017, 10, 548–552. [Google Scholar] [CrossRef]
- Davison, J.M.; Choudry, H.A.; Pingpank, J.F.; Ahrendt, S.A.; Holtzman, M.P.; Zureikat, A.H.; Zeh, H.J.; Ramalingam, L.; Zhu, B.; Nikiforova, M.; et al. Clinicopathologic and molecular analysis of disseminated appendiceal mucinous neoplasms: Identification of factors predicting survival and proposed criteria for a three-tiered assessment of tumor grade. Mod. Pathol. 2014, 27, 1521–1539. [Google Scholar] [CrossRef]
- Shimizu, T.; Tolcher, A.W.; Papadopoulos, K.P.; Beeram, M.; Rasco, D.W.; Smith, L.S.; Gunn, S.; Smetzer, L.; Mays, T.A.; Kaiser, B.; et al. The clinical effect of the dual-targeting strategy involving PI3K/AKT/mTOR and RAS/MEK/ERK pathways in patients with advanced cancer. Clin. Cancer Res. 2012, 18, 2316–2325. [Google Scholar] [CrossRef]
- Temraz, S.; Mukherji, D.; Shamseddine, A. Dual Inhibition of MEK and PI3K Pathway in KRAS and BRAF Mutated Colorectal Cancers. Int. J. Mol. Sci. 2015, 16, 22976–22988. [Google Scholar] [CrossRef]
- Canon, J.; Rex, K.; Saiki, A.Y.; Mohr, C.; Cooke, K.; Bagal, D.; Gaida, K.; Holt, T.; Knutson, C.G.; Koppada, N.; et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature 2019, 575, 217–223. [Google Scholar] [CrossRef]
- Hallin, J.; Engstrom, L.D.; Hargis, L.; Calinisan, A.; Aranda, R.; Briere, D.M.; Sudhakar, N.; Bowcut, V.; Baer, B.R.; Ballard, J.A.; et al. The KRAS(G12C) Inhibitor MRTX849 Provides Insight toward Therapeutic Susceptibility of KRAS-Mutant Cancers in Mouse Models and Patients. Cancer Discov. 2020, 10, 54–71. [Google Scholar] [CrossRef]
- Roberts, D.L.; O’Dwyer, S.T.; Stern, P.L.; Renehan, A.G. Global gene expression in pseudomyxoma peritonei, with parallel development of two immortalized cell lines. Oncotarget 2015, 6, 10786–10800. [Google Scholar] [CrossRef]
- Levine, E.A.; Blazer, D.G., 3rd; Kim, M.K.; Shen, P.; Stewart, J.H.t.; Guy, C.; Hsu, D.S. Gene expression profiling of peritoneal metastases from appendiceal and colon cancer demonstrates unique biologic signatures and predicts patient outcomes. J. Am. Coll. Surg. 2012, 214, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Levine, E.A.; Votanopoulos, K.I.; Qasem, S.A.; Philip, J.; Cummins, K.A.; Chou, J.W.; Ruiz, J.; D’Agostino, R.; Shen, P.; Miller, L.D. Prognostic Molecular Subtypes of Low-Grade Cancer of the Appendix. J. Am. Coll. Surg. 2016, 222, 493–503. [Google Scholar] [CrossRef]
- Gleeson, E.M.; Feldman, R.; Mapow, B.L.; Mackovick, L.T.; Ward, K.M.; Morano, W.F.; Rubin, R.R.; Bowne, W.B. Appendix-derived Pseudomyxoma Peritonei (PMP): Molecular Profiling Toward Treatment of a Rare Malignancy. Am. J. Clin. Oncol. 2018, 41, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, R.; Xiu, J.; Johnston, C.; Goldberg, R.M.; Philip, P.A.; Seeber, A.; Naseem, M.; Lo, J.H.; Arai, H.; Battaglin, F.; et al. Molecular Profiling of Appendiceal Adenocarcinoma and Comparison with Right-sided and Left-sided Colorectal Cancer. Clin. Cancer Res. 2019, 25, 3096–3103. [Google Scholar] [CrossRef] [PubMed]
- Borazanci, E.; Millis, S.Z.; Kimbrough, J.; Doll, N.; Von Hoff, D.; Ramanathan, R.K. Potential actionable targets in appendiceal cancer detected by immunohistochemistry, fluorescent in situ hybridization, and mutational analysis. J. Gastrointest. Oncol. 2017, 8, 164–172. [Google Scholar] [CrossRef]
- Kufe, D.W. Mucins in cancer: Function, prognosis and therapy. Nat. Rev. Cancer 2009, 9, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Dhanisha, S.S.; Guruvayoorappan, C.; Drishya, S.; Abeesh, P. Mucins: Structural diversity, biosynthesis, its role in pathogenesis and as possible therapeutic targets. Crit. Rev. Oncol. Hematol. 2018, 122, 98–122. [Google Scholar] [CrossRef]
- Pillai, K.; Akhter, J.; Mekkawy, A.; Chua, T.C.; Morris, D.L. Physical and chemical characteristics of mucin secreted by pseudomyxoma peritonei (PMP). Int. J. Med. Sci. 2017, 14, 18–28. [Google Scholar] [CrossRef][Green Version]
- Pillai, K.; Akhter, J.; Chua, T.C.; Morris, D.L. A formulation for in situ lysis of mucin secreted in pseudomyxoma peritonei. Int. J. Cancer 2014, 134, 478–486. [Google Scholar] [CrossRef]
- Amini, A.; Masoumi-Moghaddam, S.; Ehteda, A.; Liauw, W.; Morris, D.L. Depletion of mucin in mucin-producing human gastrointestinal carcinoma: Results from in vitro and in vivo studies with bromelain and N-acetylcysteine. Oncotarget 2015, 6, 33329–33344. [Google Scholar] [CrossRef]
- Samuni, Y.; Goldstein, S.; Dean, O.M.; Berk, M. The chemistry and biological activities of N-acetylcysteine. Biochim. Biophys. Acta 2013, 1830, 4117–4129. [Google Scholar] [CrossRef]
- Pavan, R.; Jain, S.; Shraddha; Kumar, A. Properties and therapeutic application of bromelain: A review. Biotechnol. Res. Int. 2012, 2012, 976203. [Google Scholar] [CrossRef] [PubMed]
- Valle, S.J.; Akhter, J.; Mekkawy, A.H.; Lodh, S.; Pillai, K.; Badar, S.; Glenn, D.; Power, M.; Liauw, W.; Morris, D.L. A novel treatment of bromelain and acetylcysteine (BromAc) in patients with peritoneal mucinous tumours: A phase I first in man study. Eur. J. Surg. Oncol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Pigny, P.; Guyonnet-Duperat, V.; Hill, A.S.; Pratt, W.S.; Galiegue-Zouitina, S.; d’Hooge, M.C.; Laine, A.; Van-Seuningen, I.; Degand, P.; Gum, J.R.; et al. Human mucin genes assigned to 11p15.5: Identification and organization of a cluster of genes. Genomics 1996, 38, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Van Seuningen, I.; Perrais, M.; Pigny, P.; Porchet, N.; Aubert, J.P. Sequence of the 5′-flanking region and promoter activity of the human mucin gene MUC5B in different phenotypes of colon cancer cells. Biochem. J. 2000, 348 Pt. 3, 675–686. [Google Scholar] [CrossRef]
- Van Seuningen, I.; Pigny, P.; Perrais, M.; Porchet, N.; Aubert, J.P. Transcriptional regulation of the 11p15 mucin genes. Towards new biological tools in human therapy, in inflammatory diseases and cancer? Front. Biosci. 2001, 6, D1216–D1234. [Google Scholar] [CrossRef]
- Vincent, A.; Perrais, M.; Desseyn, J.L.; Aubert, J.P.; Pigny, P.; van Seuningen, I. Epigenetic regulation (DNA methylation, histone modifications) of the 11p15 mucin genes (MUC2, MUC5AC, MUC5B, MUC6) in epithelial cancer cells. Oncogene 2007, 26, 6566–6576. [Google Scholar] [CrossRef]
- Li, J.D.; Feng, W.; Gallup, M.; Kim, J.H.; Gum, J.; Kim, Y.; Basbaum, C. Activation of NF-kappaB via a Src-dependent Ras-MAPK-pp90rsk pathway is required for Pseudomonas aeruginosa-induced mucin overproduction in epithelial cells. Proc. Natl. Acad. Sci. USA 1998, 95, 5718–5723. [Google Scholar] [CrossRef]
- Zhou, X.; Tu, J.; Li, Q.; Kolosov, V.P.; Perelman, J.M. Hypoxia induces mucin expression and secretion in human bronchial epithelial cells. Transl. Res. 2012, 160, 419–427. [Google Scholar] [CrossRef]
- Dilly, A.K.; Song, X.; Zeh, H.J.; Guo, Z.S.; Lee, Y.J.; Bartlett, D.L.; Choudry, H.A. Mitogen-activated protein kinase inhibition reduces mucin 2 production and mucinous tumor growth. Transl. Res. 2015, 166, 344–354. [Google Scholar] [CrossRef]
- Perrais, M.; Pigny, P.; Copin, M.C.; Aubert, J.P.; van Seuningen, I. Induction of MUC2 and MUC5AC mucins by factors of the epidermal growth factor (EGF) family is mediated by EGF receptor/Ras/Raf/extracellular signal-regulated kinase cascade and Sp1. J. Biol. Chem. 2002, 277, 32258–32267. [Google Scholar] [CrossRef]
- Alakus, H.; Babicky, M.L.; Ghosh, P.; Yost, S.; Jepsen, K.; Dai, Y.; Arias, A.; Samuels, M.L.; Mose, E.S.; Schwab, R.B.; et al. Genome-wide mutational landscape of mucinous carcinomatosis peritonei of appendiceal origin. Genome Med. 2014, 6, 43. [Google Scholar] [CrossRef]
- Choudry, H.A.; Mavanur, A.; O’Malley, M.E.; Zeh, H.J.; Guo, Z.; Bartlett, D.L. Chronic anti-inflammatory drug therapy inhibits gel-forming mucin production in a murine xenograft model of human pseudomyxoma peritonei. Ann. Surg. Oncol. 2012, 19, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Fremin, C.; Meloche, S. From basic research to clinical development of MEK1/2 inhibitors for cancer therapy. J. Hematol. Oncol. 2010, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Bibi, R.; Pranesh, N.; Saunders, M.P.; Wilson, M.S.; O’Dwyer, S.T.; Stern, P.L.; Renehan, A.G. A specific cadherin phenotype may characterise the disseminating yet non-metastatic behaviour of pseudomyxoma peritonei. Br. J. Cancer 2006, 95, 1258–1264. [Google Scholar] [CrossRef]
- Chakraborty, S.; Kubatzky, K.F.; Mitra, D.K. An Update on Interleukin-9: From Its Cellular Source and Signal Transduction to Its Role in Immunopathogenesis. Int. J. Mol. Sci. 2019, 20, 2113. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.X.; Leonard, W.J. The Common Cytokine Receptor gamma Chain Family of Cytokines. Cold Spring Harb. Perspect. Biol. 2018, 10, a028449. [Google Scholar] [CrossRef]
- Lv, X.; Wang, X. The role of interleukin-9 in lymphoma. Leuk. Lymphoma 2013, 54, 1367–1372. [Google Scholar] [CrossRef]
- Hu, B.; Qiu-Lan, H.; Lei, R.E.; Shi, C.; Jiang, H.X.; Qin, S.Y. Interleukin-9 Promotes Pancreatic Cancer Cells Proliferation and Migration via the miR-200a/Beta-Catenin Axis. Biomed. Res. Int. 2017, 2017, 2831056. [Google Scholar] [CrossRef]
- Ma, J.; Tong, H.F.; Lin, J.H.; Chen, F.N.; Wu, C.X.; Cao, C.Z.; Wu, J.; Hu, S.Q. miR-208b-5p inhibits invasion of non-small cell lung cancer through the STAT3 pathway by targeting interleukin-9. Oncol. Lett. 2020, 20, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Nalleweg, N.; Chiriac, M.T.; Podstawa, E.; Lehmann, C.; Rau, T.T.; Atreya, R.; Krauss, E.; Hundorfean, G.; Fichtner-Feigl, S.; Hartmann, A.; et al. IL-9 and its receptor are predominantly involved in the pathogenesis of UC. Gut 2015, 64, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.; Bartolome, R.A.; Mendes, M.; Barderas, R.; Fernandez-Acenero, M.J.; Pelaez-Garcia, A.; Pena, C.; Lopez-Lucendo, M.; Villar-Vazquez, R.; de Herreros, A.G.; et al. Proteome profiling of cancer-associated fibroblasts identifies novel proinflammatory signatures and prognostic markers for colorectal cancer. Clin. Cancer Res. 2013, 19, 6006–6019. [Google Scholar] [CrossRef]
- Qureshy, Z.; Johnson, D.E.; Grandis, J.R. Targeting the JAK/STAT pathway in solid tumors. J. Cancer Metastasis Treat. 2020, 6, 2384–2393. [Google Scholar] [CrossRef]
- Adams, J. The proteasome: A suitable antineoplastic target. Nat. Rev. Cancer 2004, 4, 349–360. [Google Scholar] [CrossRef]
- Wu, K.L.; van Wieringen, W.; Vellenga, E.; Zweegman, S.; Lokhorst, H.M.; Sonneveld, P. Analysis of the efficacy and toxicity of bortezomib for treatment of relapsed or refractory multiple myeloma in community practice. Haematologica 2005, 90, 996–997. [Google Scholar]
- Dispenzieri, A.; Jacobus, S.; Vesole, D.H.; Callandar, N.; Fonseca, R.; Greipp, P.R. Primary therapy with single agent bortezomib as induction, maintenance and re-induction in patients with high-risk myeloma: Results of the ECOG E2A02 trial. Leukemia 2010, 24, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Aghajanian, C.; Blessing, J.A.; Darcy, K.M.; Reid, G.; DeGeest, K.; Rubin, S.C.; Mannel, R.S.; Rotmensch, J.; Schilder, R.J.; Riordan, W.; et al. A phase II evaluation of bortezomib in the treatment of recurrent platinum-sensitive ovarian or primary peritoneal cancer: A Gynecologic Oncology Group study. Gynecol. Oncol. 2009, 115, 215–220. [Google Scholar] [CrossRef]
- Song, X.; Dilly, A.K.; Choudry, H.A.; Bartlett, D.L.; Kwon, Y.T.; Lee, Y.J. Hypoxia Promotes Synergy between Mitomycin C and Bortezomib through a Coordinated Process of Bcl-xL Phosphorylation and Mitochondrial Translocation of p53. Mol. Cancer Res. 2015, 13, 1533–1543. [Google Scholar] [CrossRef]
- Haglund, C.; Mohanty, C.; Fryknäs, M.; D’Arcy, P.; Larsson, R.; Linder, S.; Rickardson, L. Identification of an inhibitor of the ubiquitin–proteasome system that induces accumulation of polyubiquitinated proteins in the absence of blocking of proteasome function. MedChemComm 2014, 5, 376–385. [Google Scholar] [CrossRef]
- Huang, L.; Yang, Y.; Yang, F.; Liu, S.; Zhu, Z.; Lei, Z.; Guo, J. Functions of EpCAM in physiological processes and diseases (Review). Int. J. Mol. Med. 2018, 42, 1771–1785. [Google Scholar] [CrossRef] [PubMed]
- Went, P.; Vasei, M.; Bubendorf, L.; Terracciano, L.; Tornillo, L.; Riede, U.; Kononen, J.; Simon, R.; Sauter, G.; Baeuerle, P.A. Frequent high-level expression of the immunotherapeutic target Ep-CAM in colon, stomach, prostate and lung cancers. Br. J. Cancer 2006, 94, 128–135. [Google Scholar] [CrossRef]
- Engebraaten, O.; Sivam, G.; Juell, S.; Fodstad, O. Systemic immunotoxin treatment inhibits formation of human breast cancer metastasis and tumor growth in nude rats. Int. J. Cancer 2000, 88, 970–976. [Google Scholar] [CrossRef]
- Andersson, Y.; Engebraaten, O.; Fodstad, O. Synergistic anti-cancer effects of immunotoxin and cyclosporin in vitro and in vivo. Br. J. Cancer 2009, 101, 1307–1315. [Google Scholar] [CrossRef]
- Antignani, A.; Fitzgerald, D. Immunotoxins: The role of the toxin. Toxins 2013, 5, 1486–1502. [Google Scholar] [CrossRef] [PubMed]
- Flatmark, K.; Guldvik, I.J.; Svensson, H.; Fleten, K.G.; Florenes, V.A.; Reed, W.; Giercksky, K.E.; Fodstad, O.; Andersson, Y. Immunotoxin targeting EpCAM effectively inhibits peritoneal tumor growth in experimental models of mucinous peritoneal surface malignancies. Int. J. Cancer 2013, 133, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Froysnes, I.S.; Andersson, Y.; Larsen, S.G.; Davidson, B.; Oien, J.T.; Olsen, K.H.; Giercksky, K.E.; Julsrud, L.; Fodstad, O.; Dueland, S.; et al. Novel Treatment with Intraperitoneal MOC31PE Immunotoxin in Colorectal Peritoneal Metastasis: Results From the ImmunoPeCa Phase 1 Trial. Ann. Surg. Oncol. 2017, 24, 1916–1922. [Google Scholar] [CrossRef]
- Thorgersen, E.B.; Asvall, J.; Froysnes, I.S.; Schjalm, C.; Larsen, S.G.; Dueland, S.; Andersson, Y.; Fodstad, O.; Mollnes, T.E.; Flatmark, K. Increased Local Inflammatory Response to MOC31PE Immunotoxin After Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Ann. Surg. Oncol. 2021, 28, 5252–5262. [Google Scholar] [CrossRef]
- Froysnes, I.S.; Andersson, Y.; Larsen, S.G.; Davidson, B.; Oien, J.T.; Julsrud, L.; Fodstad, O.; Dueland, S.; Flatmark, K. ImmunoPeCa trial: Long-term outcome following intraperitoneal MOC31PE immunotoxin treatment in colorectal peritoneal metastasis. Eur. J. Surg. Oncol. 2021, 47, 134–138. [Google Scholar] [CrossRef] [PubMed]

| Drugs | Carrier Solution | Duration (min) |
|---|---|---|
| Oxaliplatin-Based | ||
| Oxaliplatin (360–460 mg/m2) i.p. + 5FU (400 mg/m2) and LV (20 mg/m2) i.v. | 5% dextrose solution, 2 L/m2 | 30 |
| Oxaliplatin (200 mg/m2) | 5% dextrose solution, 3 L | 120 |
| MMC-Based | ||
| MMC (35 mg/m2) or 40 mg (Fixed Dose) | 1.5% dextrose peritoneal dialysis solution, 3 L | 90 |
| MMC (10 mg/m2) | Sodium chloride solution, 0.9% 1 L | 60 |
| MMC (3.3 mg/L/m2) + Cisplatin (25 mg/m2/L) | Sodium chloride solution, 2.5 L/m2 | 60 |
| Treatment | Recommendations |
|---|---|
| CRS-HIPEC | All patients with a confirmed diagnosis of PMP should be treated in a referral center for CRS followed by HIPEC. |
| Palliative Surgery ± HIPEC | A debulking surgery with or without HIPEC provides disease control in high-risk patients or unresectable disease (primary or recurrent). |
| Systemic Chemotherapy | Adjuvant systemic chemotherapy should be considered in high-grade/signet ring PMP. In unresectable patients, palliative chemotherapy is effective in a minority of cases. |
| PIPAC | As palliative option within clinical studies. |
| Mucolytic Agents | As palliative option within clinical studies. |
| Small Bowel Transplant | In very selected patients with end-stage disease within clinical studies. |
| Regimen | N. of Patients | Median PFS (months) | Median OS (months) | RR (%) | DCR (%) |
|---|---|---|---|---|---|
| Capecitabine/5FU [40] | 54 | 7.6 | 56 | 24 | 56 |
| Capecitabine + MMC [41] | 40 | nr | 84 | 8 | 42 |
| Capecitabine + Bevacizumab [42] | 15 | 8.2 | 91 a | 20 | 87 |
| Capecitabine + Ciclophosphamide [43] | 23 | 9.5 | 73.7 a | 4 | 87 |
| FOLFOX6 [44] | 8 | 8.0 | 26 | 20 | 65 |
| Analyzed Molecular Factors | N. of Patients | Association with Histological Grade | Association with Survival | Reference |
|---|---|---|---|---|
| COX-2, HER-2, EGFR, MUC2, Ki67 | 65 | EGFR, Ki67 | Ki67 | [51] |
| p53/KRAS Mutations a | 194/64 | p53 | p53 | [52] |
| dMMR, MUC b, Ki67, p53 | 155 | - | - | [53] |
| CEA, Ki67 and p53 | 141 | Ki67, p53 | Ki67, p53 | [54] |
| Ki67, p53 | 117 | Ki67 | Ki67 | [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sommariva, A.; Tonello, M.; Rigotto, G.; Lazzari, N.; Pilati, P.; Calabrò, M.L. Novel Perspectives in Pseudomyxoma Peritonei Treatment. Cancers 2021, 13, 5965. https://doi.org/10.3390/cancers13235965
Sommariva A, Tonello M, Rigotto G, Lazzari N, Pilati P, Calabrò ML. Novel Perspectives in Pseudomyxoma Peritonei Treatment. Cancers. 2021; 13(23):5965. https://doi.org/10.3390/cancers13235965
Chicago/Turabian StyleSommariva, Antonio, Marco Tonello, Giulia Rigotto, Nayana Lazzari, Pierluigi Pilati, and Maria Luisa Calabrò. 2021. "Novel Perspectives in Pseudomyxoma Peritonei Treatment" Cancers 13, no. 23: 5965. https://doi.org/10.3390/cancers13235965
APA StyleSommariva, A., Tonello, M., Rigotto, G., Lazzari, N., Pilati, P., & Calabrò, M. L. (2021). Novel Perspectives in Pseudomyxoma Peritonei Treatment. Cancers, 13(23), 5965. https://doi.org/10.3390/cancers13235965







