Carboplatin Dosing in Children Using Estimated Glomerular Filtration Rate: Equation Matters
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Treatment
2.2. Ethical Approval
2.3. Carboplatin Administration, Blood Sampling and Platinum Analysis
2.4. Renal Function
2.5. Pharmacokinetic and Statistical Analysis
- The percentage prediction error (%PE), defined as: which is a measure of bias;
- The absolute percentage prediction error (APE) , which is a measure of imprecision;
- Accuracy assessed by calculating the proportion of predicted AUC values within ± 30% of measured AUC (P30 accuracy), a commonly used accuracy measure in the evaluation of eGFR equations [20].
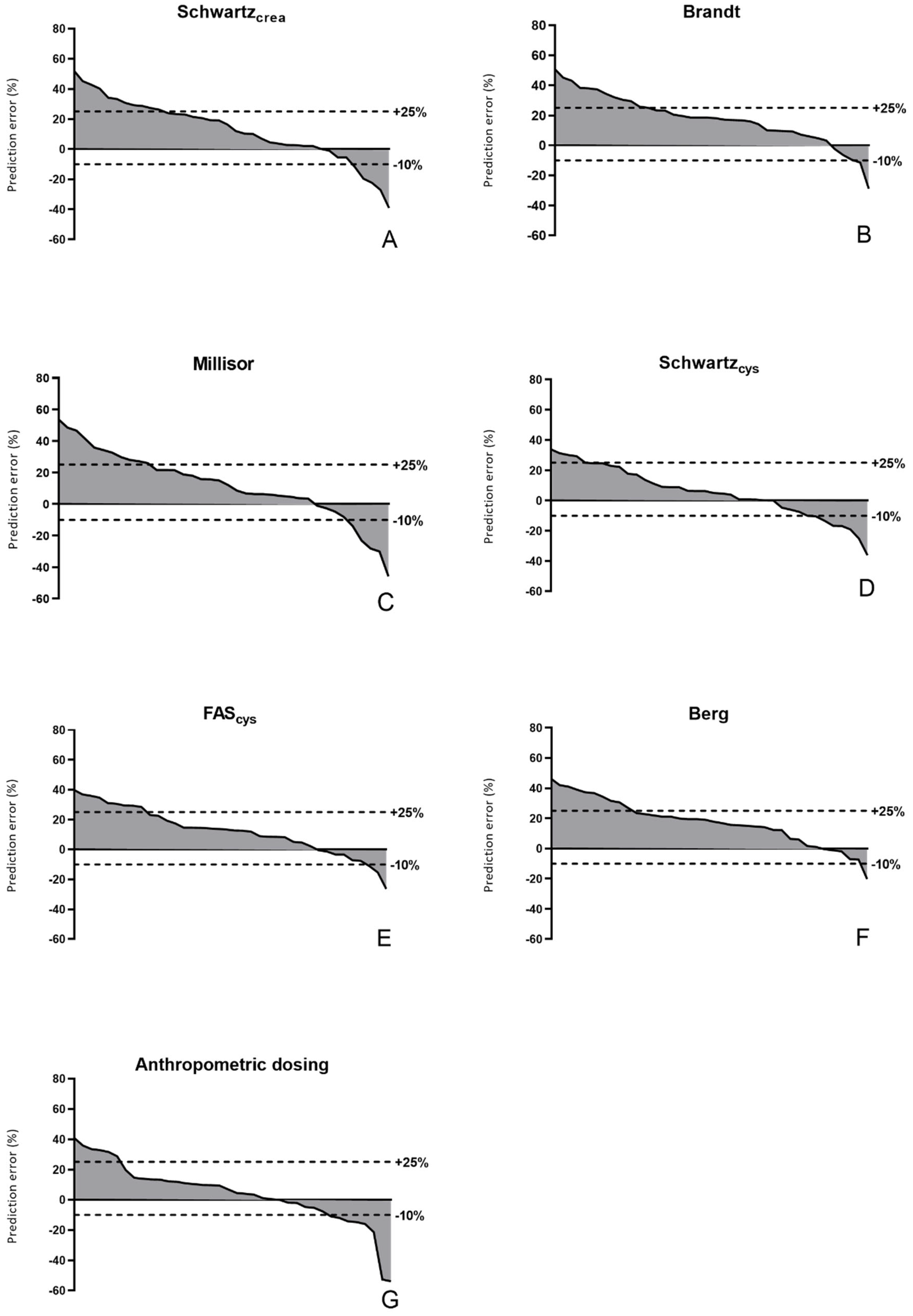
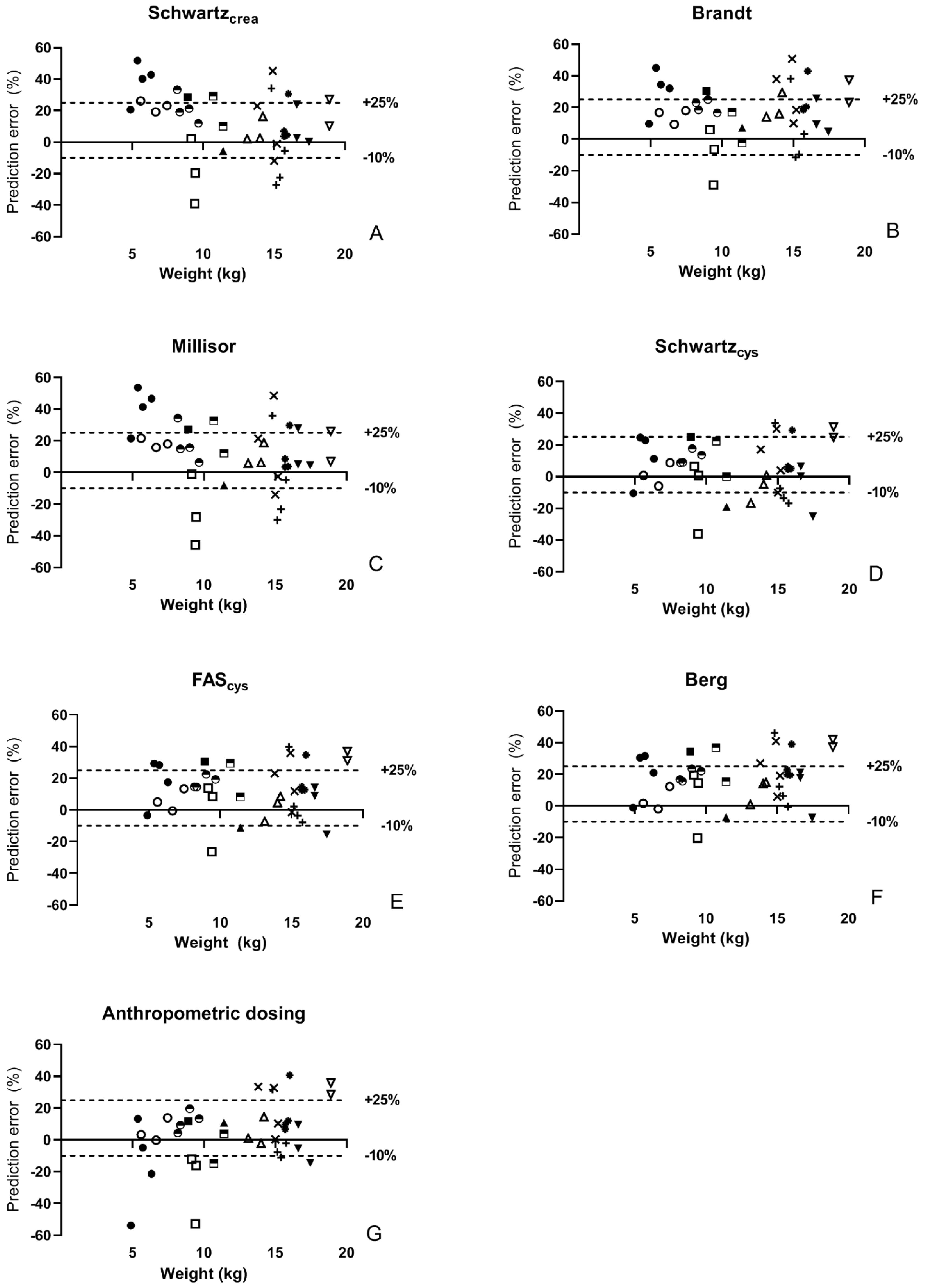
3. Results
3.1. Patients
3.2. Pharmacokinetics and Comparison of Equations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doz, F.; Pinkerton, R. What is the place of carboplatin in paediatric oncology? Eur. J. Cancer 1994, 30A, 194–201. [Google Scholar] [CrossRef]
- Geurtsen, M.L.; Kors, W.A.; Moll, A.C.; Smits, C. Long-term audiologic follow-up of carboplatin-treated children with retinoblastoma. Ophthalmic Genet. 2017, 38, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Go, R.S.; Adjei, A.A. Review of the comparative pharmacology and clinical activity of cisplatin and carboplatin. J. Clin. Oncol. 1999, 17, 409. [Google Scholar] [CrossRef]
- Calvert, A.H.; Newell, D.R.; A Gumbrell, L.; O’Reilly, S.; Burnell, M.; E Boxall, F.; Siddik, Z.H.; Judson, I.R.; E Gore, M.; Wiltshaw, E. Carboplatin dosage: Prospective evaluation of a simple formula based on renal function. J. Clin. Oncol. 1989, 7, 1748–1756. [Google Scholar] [CrossRef]
- Chatelut, E.; Boddy, A.V.; Peng, B.; Rubie, H.; Lavit, M.; Dezeuze, A.; Pearson, A.D.J.; Roché, H.; Robert, A.; Newell, D.R.; et al. Population pharmacokinetics of carboplatin in children. Clin. Pharmacol. Ther. 1996, 59, 436–443. [Google Scholar] [CrossRef]
- Newell, D.R.; Pearson, A.D.; Balmanno, K.; Price, L.; A Wyllie, R.; Keir, M.; Calvert, A.H.; Lewis, I.J.; Pinkerton, C.R.; Stevens, M.C. Carboplatin pharmacokinetics in children: The development of a pediatric dosing formula. The United Kingdom Children’s Cancer Study Group. J. Clin. Oncol. 1993, 11, 2314–2323. [Google Scholar] [CrossRef]
- Marina, N.M.; Rodman, J.H.; Murry, D.J.; Shema, S.J.; Bowmans, L.C.; Jones, D.P.; Furman, W.; Meyer, W.H.; Pratt, C.B. Phase I study of escalating targeted doses of carboplatin combined with ifosfamide and etoposide in treatment of newly diagnosed pediatric solid tumors. J. Natl. Cancer Inst. 1994, 86, 544–548. [Google Scholar] [CrossRef]
- Allen, S.; Wilson, M.W.; Watkins, A.; Billups, C.; Qaddoumi, I.; Haik, B.H.; Rodriguez-Galindo, C. Comparison of two methods for carboplatin dosing in children with retinoblastoma. Pediatr. Blood Cancer 2010, 55, 47–54. [Google Scholar] [CrossRef]
- Thomas, H.; Boddy, A.; English, M.W.; Hobson, R.; Imeson, J.; Lewis, I.; Morland, B.; Pearson, A.D.J.; Pinkerton, R.; Price, L.; et al. Prospective validation of renal function–based carboplatin dosing in children with cancer: A United Kingdom children’s cancer study group trial. J. Clin. Oncol. 2000, 18, 3614–3621. [Google Scholar] [CrossRef]
- Veal, G.J.; Errington, J.; Tilby, M.J.; Pearson, A.D.J.; Foot, A.B.M.; McDowell, H.; Ellershaw, C.; Pizer, B.; Nowell, G.M.; Pearson, D.G.; et al. Adaptive dosing and platinum–DNA adduct formation in children receiving high-dose carboplatin for the treatment of solid tumours. Br. J. Cancer 2007, 96, 725–731. [Google Scholar] [CrossRef]
- Newell, D.R.; Siddik, Z.H.; Gumbrell, L.A.; Boxall, F.E.; Gore, M.E.; Smith, I.E.; Calvert, A.H. Plasma free platinum pharma-cokinetics in patients treated with high dose carboplatin. Eur. J. Cancer Clin. Oncol. 1987, 23, 1399–1405. [Google Scholar] [CrossRef]
- Qaddoumi, I.; Bass, J.; Wu, J.; Billups, C.A.; Wozniak, A.W.; Merchant, T.E.; Haik, B.G.; Wilson, M.W.; Rodriguez-Galindo, C. Carboplatin-associated ototoxicity in children with retinoblastoma. J. Clin. Oncol. 2012, 30, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Jodrell, D.I.; Egorin, M.J.; Canetta, R.M.; Langenberg, P.; Goldbloom, E.P.; Burroughs, J.N.; Goodlow, J.L.; Tan, S.; Wiltshaw, E. Relationships between carboplatin exposure and tumor response and toxicity in patients with ovarian cancer. J. Clin. Oncol. 1992, 10, 520–528. [Google Scholar] [CrossRef]
- Schwartz, G.J.; Furth, S.L. Glomerular filtration rate measurement and estimation in chronic kidney disease. Pediatr. Nephrol. 2007, 22, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Millisor, V.E.; Roberts, J.K.; Sun, Y.; Tang, L.; Daryani, V.M.; Gregornik, D.; Cross, S.; Ward, D.; Pauley, J.L.; Molinelli, A.; et al. Derivation of new equations to estimate glomerular filtration rate in pediatric oncology patients. Pediatr. Nephrol. 2017, 32, 1575–1584. [Google Scholar] [CrossRef]
- Levin, A.; Stevens, P.E.; Bilous, R.W.; Coresh, J.; De Francisco, A.L.; De Jong, P.E.; Griffith, K.E.; Hemmelgarn, B.R.; Iseki, K.; Lamb, E.J.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Workgroup. Clinical Practice Guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2012, 3, 1–150. [Google Scholar]
- National Kidney Foundation. K/DOQI Clinical Practice Guidelines for chronic kidney disease: Evaluation, classification and stratification. Am. J. Kidney Dis. 2002, 39, S1–S266. [Google Scholar]
- Vera, N.G.; Villarreal, L.P.; Vargas, J.U. Carboplatin Dosing Accuracy by Estimation of Glomerular Filtration versus Creatinuria in Cancer Patients. Chemotherapy 2018, 63, 137–142. [Google Scholar] [CrossRef]
- Cathomas, R.; Klingbiel, D.; Geldart, T.; Mead, G.M.; Ellis, S.; Wheater, M.; Simmonds, P.; Nagaraj, N.; von Moos, R.; Fehr, M. Relevant risk of carboplatin underdosing in cancer patients with normal renal function using estimated GFR: Lessons from a stage I seminoma cohort. Ann. Oncol. 2014, 25, 1591–1597. [Google Scholar] [CrossRef]
- Den Bakker, E.; Gemke, R.J.B.J.; Bökenkamp, A. Endogenous markers for kidney function in children: A review. Crit. Rev. Clin. Lab. Sci. 2018, 55, 163–183. [Google Scholar] [CrossRef]
- Riccardi, R.; Riccardi, A.; Lasorella, A.; Di Rocco, C.; Carelli, G.; Tornesello, A.; Servidei, T.; Iavarone, A.; Mastrangelo, R. Clinical pharmacokinetics of carboplatin in children. Cancer Chemother. Pharmacol. 1994, 33, 477–483. [Google Scholar] [CrossRef]
- Ma, J.; Stoter, G.; Verweij, J.; Schellens, J.H.M. Comparison of ethanol plasma-protein precipitation with plasma ultrafiltration and trichloroacetic acid protein precipitation for the measurement of unbound platinum concentrations. Cancer Chemother. Pharmacol. 1996, 38, 391–394. [Google Scholar] [CrossRef]
- Verschraagen, M.; Boven, E.; Ruijter, R.; Born, K.; Berkhof, J.; Hausheer, F.H.; Vijgh, W.J.F. Pharmacokinetics and preliminary clinical data of the novel chemoprotectant BNP7787 and cisplatin and their metabolites. Clin. Pharmacol. Ther. 2003, 74, 157–169. [Google Scholar] [CrossRef]
- Verschraagen, M.; van der Born, K.; Zwiers, T.; van der Vijgh, W.J. Simultaneous determination of intact cisplatin and its metabolite monohydrated cisplatin in human plasma. J. Chromatogr. B 2002, 772, 273–281. [Google Scholar] [CrossRef]
- Giaccone, G.; González-Larriba, J.L.; van Oosterom, A.T.; Alfonso, R.; Smit, E.F.; Martens, M.; Peters, G.J.; van der Vijgh, W.J.F.; Smith, R.; Averbuch, S.; et al. Combination therapy with gefitinib, an epidermal growth factor receptor tyrosine kinase inhibitor, gemcitabine and cisplatin in patients with advanced solid tumors. Ann. Oncol. 2004, 15, 831–838. [Google Scholar] [CrossRef]
- Piéroni, L.; Delanaye, P.; Boutten, A.; Bargnoux, A.-S.; Rozet, E.; Delatour, V.; Carlier, M.-C.; Hanser, A.-M.; Cavalier, E.; Froissart, M.; et al. A multicentric evaluation of IDMS-traceable creatinine enzymatic assays. Clin. Chim. Acta 2011, 412, 2070–2075. [Google Scholar] [CrossRef]
- Eckfeldt, J.H.; Karger, A.; Miller, W.G.; Rynders, G.P.; Inker, L.A. Performance in measurement of serum Cystatin C by Laboratories participating in the College of American Pathologists 2014 CYS Survey. Arch. Pathol. Lab. Med. 2015, 139, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Leion, F.; Hegbrant, J.; den Bakker, E.; Jonsson, M.; Abrahamson, M.; Nyman, U.; Björk, J.; Lindström, V.; Larsson, A.; Bökenkamp, A.; et al. Estimating glomerular filtration rate (GFR) in children. The average between a cystatin C- and a creatinine-based equation improves estimation of GFR in both children and adults and enables diagnosing Shrunken Pore Syndrome. Scand. J. Clin. Lab. Investig. 2017, 77, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.J.; Schneider, M.F.; Maier, P.S.; Moxey-Mims, M.; Dharnidharka, V.R.; Warady, B.A.; Furth, S.L.; Muñoz, A. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int. 2012, 82, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.R.; Wong, C.; Jones, D.R.; Qualls, C.; McAfee, N.; Brewer, E.; Watkins, S.L. Glomerular filtration rate in children with solid tumors: Normative values and a new method for estimation. Pediatr. Hematol. Oncol. 2003, 20, 309–318. [Google Scholar] [CrossRef]
- Pottel, H.; Delanaye, P.; Schaeffner, E.; Dubourg, L.; Eriksen, B.O.; Melsom, T.; Lamb, E.J.; Rule, A.D.; Turner, S.T.; Glassock, R.J.; et al. Estimating glomerular filtration rate for the full age spectrum from serum creatinine and cystatin C. Nephrol. Dial. Transplant. 2017, 32, 497–507. [Google Scholar] [CrossRef]
- Berg, U.B.; Nyman, U.; Bäck, R.; Hansson, M.; Monemi, K.; Herthelius, M.; Björk, J. New standardized cystatin C and creatinine GFR equations in children validated with inulin clearance. Pediatr. Nephrol. 2015, 30, 1317–1326. [Google Scholar] [CrossRef]
- Björk, J.; Nyman, U.; Berg, U.; Delanaye, P.; Dubourg, L.; Goffin, K.; Grubb, A.; Hansson, M.; Littmann, K.; Åsling-Monemi, K.; et al. Validation of standardized creatinine and cystatin C GFR estimating equations in a large multicentre European cohort of children. Pediatr. Nephrol. 2019, 34, 1087–1098. [Google Scholar] [CrossRef]
- Ekhart, C.; Rodenhuis, S.; Schellens, J.H.M.; Beijnen, J.H.; Huitema, A.D.R. Carboplatin dosing in overweight and obese patients with normal renal function, does weight matter? Cancer Chemother. Pharmacol. 2009, 64, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Mosteller, R.D. Simplified calculation of body-surface area. N. Engl. J. Med. 1987, 317, 1098. [Google Scholar] [CrossRef]
- Filler, G.; Huang, S.-H.S.; Yasin, A. The usefulness of cystatin C and related formulae in pediatrics. Clin. Chem. Lab. Med. 2012, 50, 2081–2091. [Google Scholar] [CrossRef]
- Soveri, I.; Berg, U.B.; Björk, J.; Elinder, C.-G.; Grubb, A.; Mejare, I.; Sterner, G.; Bäck, S.-E. Measuring GFR: A systematic review. Am. J. Kidney Dis. 2014, 64, 411–424. [Google Scholar] [CrossRef] [PubMed]
- National Kidney Disease Education Program. CKD and Drug Dosing: Information for Providers. Available online: http://www.nkdep.nih.gov/resources/CKD-drug-dosing.shtml (accessed on 26 November 2021).
- Blufpand, H.N.; Westland, R.; van Wijk, J.A.; Roelandse-Koop, E.A.; Kaspers, G.J.; Bökenkamp, A. Height-independent estimation of glomerular filtration rate in children: An alternative to the Schwartz equation. J. Pediatr. 2013, 163, 1722–1727. [Google Scholar] [CrossRef] [PubMed]
- Veal, G.J.; Errington, J.; Hayden, J.; Hobin, D.; Murphy, D.; Dommett, R.M.; Tweddle, D.A.; Jenkinson, H.; Picton, S. Carboplatin therapeutic monitoring in preterm and full-term neonates. Eur. J. Cancer 2015, 51, 2022–2030. [Google Scholar] [CrossRef] [PubMed]
- Blufpand, H.N.; Tromp, J.; Abbink, F.C.; Stoffel-Wagner, B.; Bouman, A.A.; Meeteren, A.Y.S.-V.; van Wijk, J.A.; Kaspers, G.J.; Bökenkamp, A. Cystatin C more accurately detects mildly impaired renal function than creatinine in children receiving treatment for malignancy. Pediatr. Blood Cancer 2011, 57, 262–267. [Google Scholar] [CrossRef]
- Pottel, H.; Dubourg, L.; Schaeffner, E.; Eriksen, B.O.; Melsom, T.; Lamb, E.J.; Rule, A.D.; Turner, S.T.; Glassock, R.J.; De Souza, V.; et al. The diagnostic value of rescaled renal biomarkers serum creatinine and serum cystatin C and their relation with measured glomerular filtration rate. Clin. Chim. Acta 2017, 471, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Den Bakker, E.; Gemke, R.; Van Wijk, J.A.E.; Hubeek, I.; Stoffel-Wagner, B.; Bökenkamp, A. Combining GFR estimates from cystatin C and creatinine—what is the optimal mix? Pediatr. Nephrol. 2018, 33, 1553–1563. [Google Scholar] [CrossRef]
- Siddik, Z.H.; Newell, D.R.; Boxall, F.E.; Harrap, K.R. The comparative pharmacokinetics of carboplatin and cisplatin in mice and rats. Biochem. Pharmacol. 1987, 36, 1925–1932. [Google Scholar] [CrossRef]
- Wells, J.C.K.; Fewtrell, M.S.; Davies, P.S.W.; Williams, J.E.; Coward, W.A.; Cole, T.J. Prediction of total body water in infants and children. Arch. Dis. Child. 2005, 90, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Beumer, J.H.; Inker, L.A.; Levey, A.S. Improving carboplatin dosing based on estimated GFR. Am. J. Kidney Dis. 2018, 71, 163–165. [Google Scholar] [CrossRef]
- Janowitz, T.; Williams, E.H.; Marshall, A.; Ainsworth, N.; Thomas, P.; Sammut, S.J.; Shepherd, S.; White, J.; Mark, P.; Lynch, A.; et al. New model for estimating glomerular filtration rate in patients with cancer. J. Clin. Oncol. 2017, 35, 2798–2805. [Google Scholar] [CrossRef]
- Zorbas, Y.G.; Ichinose, M.N.; Sakagamis, M.B. Fluid electrolyte changes in physically healthy subjects during prolonged re-striction of motor activity and daily hyperhydration. Mater. Med. Pol. 1993, 25, 97–107. [Google Scholar]
- Dodgshun, A.J.; Quinlan, C.; Sullivan, M.J. Cystatin C based equation accurately estimates glomerular filtration rate in children with solid and central nervous system tumours: Enough evidence to change practice? Pediatr. Blood Cancer 2016, 63, 1535–1538. [Google Scholar] [CrossRef]
- Ekhart, C.; De Jonge, M.E.; Huitema, A.D.R.; Schellens, J.H.M.; Rodenhuis, S.; Beijnen, J.H. Flat Dosing of carboplatin is justified in adult patients with normal renal function. Clin. Cancer Res. 2006, 12, 6502–6508. [Google Scholar] [CrossRef]
- Hjorth, L.; Wiebe, T.; Karpman, D. Correct evaluation of renal glomerular filtration rate requires clearance assays. Pediatr. Nephrol. 2002, 17, 847–851. [Google Scholar] [CrossRef]
| Equations Based on Creatinine | |
| Equation 1 | |
| Equation 2 | |
| Equation 3 | |
| Equations Based on Cystatin C | |
| Equation 4 | |
| Equation 5 | |
| Equation 6 | |
| Number of Clearance Studies | Total 38 | <2 Years 18 | >2 Years 20 | p-Value |
|---|---|---|---|---|
| Age, years | 2.2 [0.5–3.3] | 0.5 [0.4–1.0] | 3.3 [3.0–4.0] | <0.001 |
| Body weight, kg | 13.5 [8.8–15.7] | 8.6 [6.2–9.5] | 15.6 [14.8–16.5] | <0.001 |
| BSA, m2 | 0.60 [0.41–0.66] | 0.40 [0.33–0.43] | 0.65 [0.63–0.67] | <0.001 |
| BMI, kg/m2 | 15.9 [15.1–18.6] | 17.9 [14.7–18.9] | 15.6 [15.1–16.4] | 0.11 |
| Creatinine, mg/dL a | 0.27 [0.22–0.32] | 0.25 [0.18–0.29] | 0.31 [0.24–0.33] | 0.008 |
| Cystatin C, mg/L | 0.80 [0.74–0.97] | 0.97 [0.86–1.09] | 0.75 [0.69–0.79] | <0.001 |
| eGFR-Schwartzcrea (mL/min/1.73 m2) | 104.3 [92.8–120.7] | 95.2 [82.6–116.7] | 108.9 [99.9–125.3] | <0.001 |
| eGFR-Schwartzcrea abs (mL/min) | 36.0 [21.7–43.0] | 21.7 [19.1–26.6] | 42.7 [39.3–44.9] | <0.001 |
| eGFR-Brandt (mL/min/1.73 m2) | 115.4 [97.9–128.7] | 97.8 [85.2–106.0] | 128.6 [123.4–138.9] | <0.001 |
| eGFR-Brandt abs (mL/min) | 43.5 [22.1–49.4] | 22.0 [18.6–24.0] | 49.2 [46.9–52.1] | <0.001 |
| eGFR-Millisor (mL/min/1.73 m2) | 103.4 [89.2–124.5] | 92.2 [76.9–119.3] | 109.2 [98.0–130.5] | <0.001 |
| eGFR-Millisor abs (mL/min) | 35.8 [21.7–43.3] | 21.3 [18.2–26.9] | 42.6 [37.8–46.4] | <0.001 |
| eGFR-Schwartzcys (mL/min/1.73 m2) | 99.7 [84.1–107.4] | 83.8 [75.3–94.2] | 106.6 [100.9–114.7] | <0.001 |
| eGFR-Schwartzcys abs (mL/min) | 33.6 [18.1–40.9] | 18.0 [13.8–24.4] | 40.6 [38.0–44.0] | <0.001 |
| eGFR-FAScys (mL/min/1.73 m2) | 109.4 [91.1–118.5] | 90.7 [80.8–102.9] | 117.6 [110.8–127.1] | <0.001 |
| eGFR-FAScys abs (mL/min) | 37.2 [19.6–45.2] | 19.4 [14.7–26.7] | 44.8 [41.7–48.7] | <0.001 |
| eGFR-Berg (mL/min/1.73 m2) | 118.5 [94.9–130.6] | 94.5 [82.1–110.1] | 129.3 [120.3–142.2] | <0.001 |
| eGFR-Berg abs (mL/min) | 41.3 [20.2–49.9] | 20.1 [14.8–28.5] | 49.2 [45.0–54.6] | <0.001 |
| Observed carboplatin clearance (mL/min/1.73 m2) | 104.3 [83.6–122.9] | 84.5 [75.3–106.2] | 116.7 [90.3–136.1] | 0.002 |
| Observed carboplatin clearance abs (mL/min) | 33.4 [18.8–45.4] | 18.7 [15.9–27.9] | 44.4 [37.3–49.3] | <0.001 |
| Observed carboplatin AUC (mg/mL.min) | 7.9 [7.0–8.6] | 7.7 [6.4–8.5] | 8.2 [7.3–10.7] | 0.09 |
| Bias (mg/mL.min) | %PE (%) | APE (%) | Accuracy (±30%) | Accuracy (−10 to 25%) | ||
|---|---|---|---|---|---|---|
| Schwartzcrea | Total N = 38 | 1.1 [0.1 to 2.5] | 14.2 [1.7 to 27.6] | 20.1 [5.5 to 28.8] | 78.9 | 57.9 |
| Brandt | 1.5 [0.6 to 2.4] | 18.3 [8.8 to 29.7] | 18.5 [9.7 to 29.7] | 76.3 | 63.2 | |
| Millisor | 1.0 [0.2 to 2.3] | 13.6 [2.3 to 27.4] | 18.4 [6.3 to 29.8] | 76.3 | 57.9 | |
| Schwartzcys | 0.4 [−0.5 to 1.6] | 5.7 [−6.3 to 18.9] | 10.9 [5.7 to 23.4] | 89.5 | 65.8 | |
| FAScys | 1.1 [0.1 to 2.0] | 13.1 [1.6 to 24.5] | 13.9 [8.1 to 26.9] | 84.2 | 68.4 | |
| Berg | 1.3 [0.4 to 2.3] | 18.5 [6.3 to 27.9] | 19.3 [11.0 to 27.9] | 76.3 | 71.1 | |
| Anthropometric dosing | 0.4 [−0.4 to 1.2] | 5.6 [−5.9 to 13.6] | 12.0 [5.2 to 20.1] | 81.6 | 55.3 | |
| Schwartzcrea | Infants N = 18 | 1.7 [0.6 to 2.5] | 21.0 [8.2 to 30.2] | 22.3 [17.4 to 34.8] | 72.2 | 50.0 |
| Brandt | 1.4 [0.5 to 2.0] | 17.0 [6.9 to 26.4] | 17.6 [8.9 to 29.2] | 77.8 | 66.7 | |
| Millisor | 1.3 [0.4 to 2.4] | 16.9 [4.5 to 33.0] | 21.6 [14.3 to 36.1] | 66.7 | 55.6 | |
| Schwartzcys | 0.7 [−0.1 to 1.5] | 8.8 [−1.5 to 18.9] | 10.9 [6.4 to 22.5] | 94.4 | 77.8 | |
| FAScys | 1.1 [0.3 to 1.9] | 14.1 [3.7 to 24.0] | 14.6 [8.4 to 26.9] | 94.4 | 66.7 | |
| Berg | 1.3 [0.1 to 2.2] | 16.3 [1.0 to 25.3] | 18.2 [11.0 to 25.3] | 77.8 | 72.2 | |
| Anthropometric dosing | 0.3 [−1.0 to 1.0] | 3.6 [−15.1 to 12.2] | 12.7 [4.7 to 17.0] | 88.9 | 55.6 | |
| Schwartzcrea | Older children N = 20 | 0.3 [−0.1 to 2.4] | 4.1 [−0.7 to 23.7] | 11.1 [3.0 to 26.3] | 85.0 | 65.0 |
| Brandt | 1.5 [0.7 to 3.7] | 19.0 [9.5 to 35.3] | 19.0 [10.4 to 35.3] | 75.0 | 60.0 | |
| Millisor | 0.4 [−0.1 to 2.4] | 6.1 [−1.1 to 24.8] | 11.3 [4.8 to 27.5] | 85.0 | 60.0 | |
| Schwartzcys | 0.4 [−0.7 to 2.4] | 4.3 [−9.4 to 22.9] | 11.7 [4.8 to 25.0] | 85.0 | 55.0 | |
| FAScys | 1.0 [−0.0 to 3.1] | 12.3 [−0.4 to 29.2] | 12.7 [7.3 to 29.2] | 75.0 | 70.0 | |
| Berg | 1.6 [0.5 to 3.7] | 19.3 [7.9 to 34.7] | 19.3 [8.6 to 34.7] | 75.0 | 70.0 | |
| Anthropometric dosing | 0.8 [−0.2 to 3.3] | 9.7 [−2.1 to 30.9] | 10.7 [5.7 to 30.9] | 75.0 | 55.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van de Velde, M.E.; den Bakker, E.; Blufpand, H.N.; Kaspers, G.L.; Abbink, F.C.H.; Kors, A.W.A.; Wilhelm, A.J.; Honeywell, R.J.; Peters, G.J.; Stoffel-Wagner, B.; et al. Carboplatin Dosing in Children Using Estimated Glomerular Filtration Rate: Equation Matters. Cancers 2021, 13, 5963. https://doi.org/10.3390/cancers13235963
van de Velde ME, den Bakker E, Blufpand HN, Kaspers GL, Abbink FCH, Kors AWA, Wilhelm AJ, Honeywell RJ, Peters GJ, Stoffel-Wagner B, et al. Carboplatin Dosing in Children Using Estimated Glomerular Filtration Rate: Equation Matters. Cancers. 2021; 13(23):5963. https://doi.org/10.3390/cancers13235963
Chicago/Turabian Stylevan de Velde, Mirjam E., Emil den Bakker, Hester N. Blufpand, Gertjan L. Kaspers, Floor C. H. Abbink, Arjenne W. A. Kors, Abraham J. Wilhelm, Richard J. Honeywell, Godefridus J. Peters, Birgit Stoffel-Wagner, and et al. 2021. "Carboplatin Dosing in Children Using Estimated Glomerular Filtration Rate: Equation Matters" Cancers 13, no. 23: 5963. https://doi.org/10.3390/cancers13235963
APA Stylevan de Velde, M. E., den Bakker, E., Blufpand, H. N., Kaspers, G. L., Abbink, F. C. H., Kors, A. W. A., Wilhelm, A. J., Honeywell, R. J., Peters, G. J., Stoffel-Wagner, B., Buffart, L. M., & Bökenkamp, A. (2021). Carboplatin Dosing in Children Using Estimated Glomerular Filtration Rate: Equation Matters. Cancers, 13(23), 5963. https://doi.org/10.3390/cancers13235963







