Primary Skull Base Chondrosarcomas: A Systematic Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Study Selection
2.3. Data Extraction
2.4. Data Synthesis and Quality Assessment
2.5. Statistical Analysis
3. Results
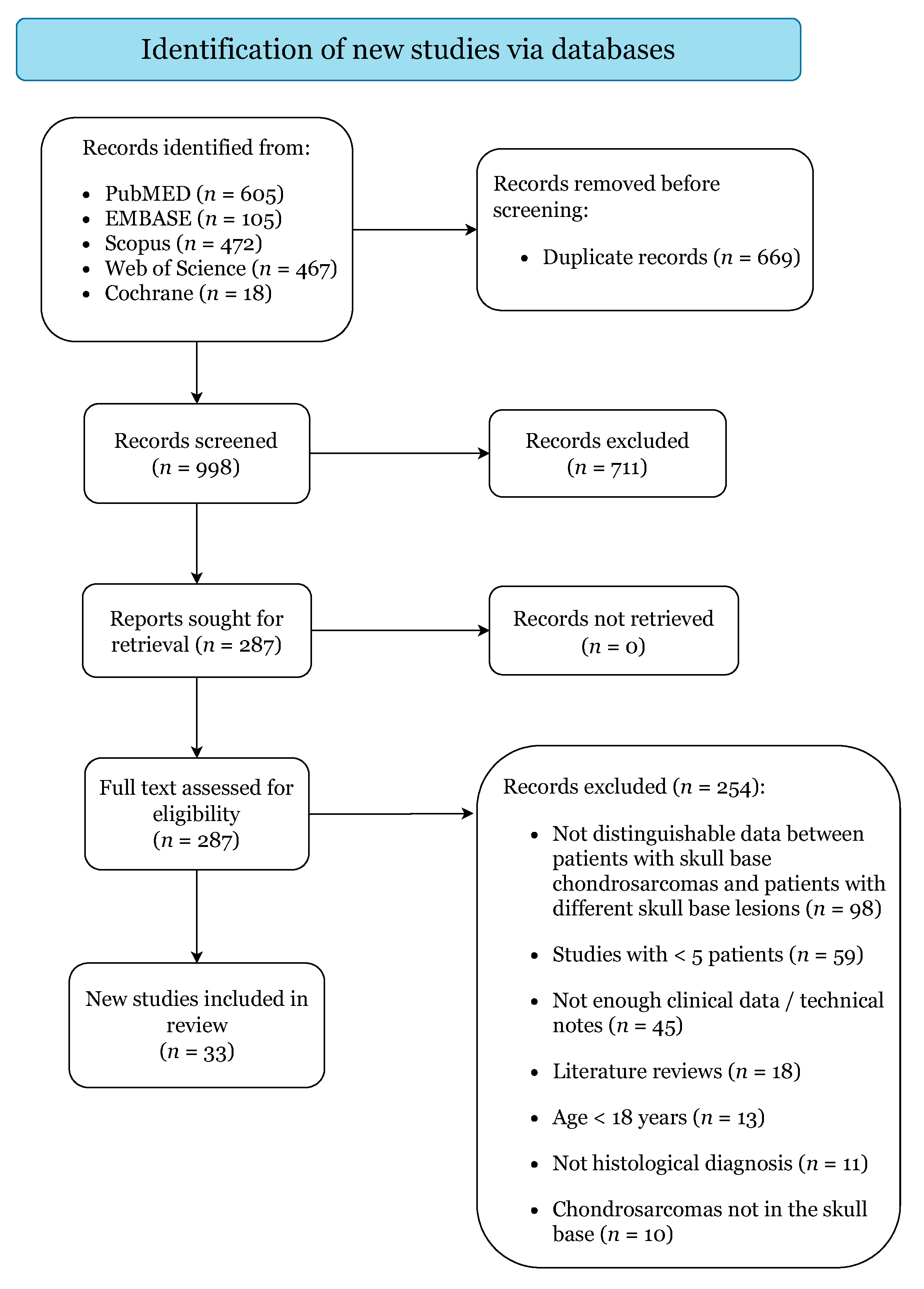
3.1. Study Selection
3.2. Demographics and Clinical Characteristics
3.3. Management Strategies
3.4. Treatment Outcomes, Complications, and Survival
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cianfriglia, F.; Pompili, A.; Occhipinti, E. Intracranial malignant cartilaginous tumours. Report of two cases and review of literature. Acta Neurochir. 1978, 45, 163–175. [Google Scholar] [CrossRef]
- Richardson, M.S. Pathology of skull base tumors. Otolaryngol. Clin. N. Am. 2001, 34, 1025–1042. [Google Scholar] [CrossRef]
- Noël, G.; Feuvret, L.; Calugaru, V.; Hadadi, K.; Baillet, F.; Mazeron, J.; Habrand, J. Chondrosarcomas of the base of the skull in Ollier’s disease or Maffucci’s syndrome Three Case Reports and Review of the Literature. Acta Oncol. 2004, 43, 705–710. [Google Scholar] [CrossRef]
- Tzortzidis, F.; Elahi, F.; Wright, D.C.; Temkin, N.; Natarajan, S.K.; Sekhar, L.N. Patient Outcome at Long-term Follow-up after Aggressive Microsurgical Resection of Cranial Base Chondrosarcomas. Neurosurgery 2006, 58, 1090–1098. [Google Scholar] [CrossRef]
- Bloch, O.G.; Jian, B.J.; Yang, I.; Han, S.J.; Aranda, D.; Ahn, B.J.; Parsa, A.T. A systematic review of intracranial chondrosarcoma and survival. J. Clin. Neurosci. 2009, 16, 1547–1551. [Google Scholar] [CrossRef] [Green Version]
- Amer, K.M.; Munn, M.; Congiusta, D.; Abraham, J.A.; Basu Mallick, A. Survival and Prognosis of Chondrosarcoma Subtypes: SEER Database Analysis. J. Orthop. Res. 2020, 38, 311–319. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board (Ed.) Soft Tissue and Bone Tumours; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Kremenevski, N.; Schlaffer, S.-M.; Coras, R.; Kinfe, T.M.; Graillon, T.; Buchfelder, M. Skull Base Chordomas and Chondrosarcomas. Neuroendocrinology 2020, 110, 836–847. [Google Scholar] [CrossRef]
- Yeom, K.W.; Lober, R.M.; Mobley, B.C.; Harsh, G.; Vogel, H.; Allagio, R.; Pearson, M.; Edwards, M.S.B.; Fischbein, N.J. Diffusion-Weighted MRI: Distinction of Skull Base Chordoma from Chondrosarcoma. Am. J. Neuroradiol. 2013, 34, 1056–1061. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Wang, K.; Ma, X.; Liu, Z.; Wang, S.; Du, J.; Tian, K.; Zhou, X.; Wei, W.; Sun, K.; et al. Radiomic analysis of multiparametric magnetic resonance imaging for differentiating skull base chordoma and chondrosarcoma. Eur. J. Radiol. 2019, 118, 81–87. [Google Scholar] [CrossRef]
- Patel, S.; Nunna, R.S.; Ryoo, J.S.; Ansari, D.; Chaudhry, N.S.; Mehta, A.I. Outcomes and Patterns of Care in Adult Skull Base Chondrosarcoma Patients in the United States. World Neurosurg. 2021, 150, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, L.N.; Pranatartiharan, R.; Chanda, A.; Wright, D.C. Chordomas and chondrosarcomas of the skull base: Results and complications of surgical management. Neurosurg. Focus 2001, 10, 1–4. [Google Scholar] [CrossRef]
- Kano, H.; Sheehan, J.; Sneed, P.K.; McBride, H.L.; Young, B.; Duma, C.; Mathieu, D.; Seymour, Z.; McDermott, M.W.; Kondziolka, D.; et al. Skull base chondrosarcoma radiosurgery: Report of the North American Gamma Knife Consortium. J. Neurosurg. 2015, 123, 1268–1275. [Google Scholar] [CrossRef]
- Lu, V.M.; O’Connor, K.P.; Mahajan, A.; Carlson, M.L.; Van Gompel, J.J. Carbon ion radiotherapy for skull base chordomas and chondrosarcomas: A systematic review and meta-analysis of local control, survival, and toxicity outcomes. J. Neurooncol. 2020, 147, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Holtzman, A.L.; Rotondo, R.L.; Rutenberg, M.S.; Indelicato, D.J.; Mercado, C.E.; Rao, D.; Tavanaiepour, D.; Morris, C.G.; Louis, D.; Flampouri, S.; et al. Proton therapy for skull-base chondrosarcoma, a single-institution outcomes study. J. Neurooncol. 2019, 142, 557–563. [Google Scholar] [CrossRef]
- Hasegawa, H.; Shin, M.; Kondo, K.; Hanakita, S.; Mukasa, A.; Kin, T.; Saito, N. Role of endoscopic transnasal surgery for skull base chondrosarcoma: A retrospective analysis of 19 cases at a single institution. J. Neurosurg. 2018, 128, 1438–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, H.; Vakharia, K.; Graffeo, C.S.; Carlson, M.L.; Pollock, B.E.; Brown, P.D.; Perry, A.; Van Gompel, J.J.; Driscoll, C.L.W.; Link, M.J. Long-term outcomes of grade I/II skull base chondrosarcoma: An insight into the role of surgery and upfront radiotherapy. J. Neurooncol. 2021, 153, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Jeremy, H.; Iain, C.; Glasziou, P.; Greenhalgh, T.; Heneghan, C.; Liberati, A.; Moschetti, I.; Phillips, B.; Thornton, H. Explanation of the 2011 Oxford Centre for Evidence-Based Medicine (OCEBM) Levels of Evidence (Background Document). Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence (accessed on 7 June 2021).
- Joanna Briggs Institute Checklist for Case Series. Available online: https://jbi.global/critical-appraisal-tools (accessed on 7 June 2021).
- Watters, M.W.R.; Brookes, G.B. Chondrosarcoma of the temporal bone. Clin. Otolaryngol. 1995, 20, 53–58. [Google Scholar] [CrossRef]
- Korten, A.G.G.C.; Ter Berg, H.J.W.; Spincemaille, G.H.; van der Laan, R.T.; Van de Wel, A.M. Intracranial chondrosarcoma: Review of the literature and report of 15 cases. J. Neurol. Neurosurg. Psychiatry 1998, 65, 88–92. [Google Scholar] [CrossRef]
- Crockard, H.A.; Cheeseman, A.; Steel, T.; Revesz, T.; Holton, J.L.; Plowman, N.; Singh, A.; Crossman, J. A multidisciplinary team approach to skull base chondrosarcomas. J. Neurosurg. 2001, 95, 184–189. [Google Scholar] [CrossRef]
- Oghalai, J.S.; Buxbaum, J.L.; Jackler, R.K.; McDermott, M.W. Skull Base Chondrosarcoma Originating from the Petroclival Junction. Otol. Neurotol. 2005, 26, 1052–1060. [Google Scholar] [CrossRef]
- Brackmann, D.E.; Teufert, K.B. Chondrosarcoma of the Skull Base. Otol. Neurotol. 2006, 27, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Wanebo, J.E.; Bristol, R.E.; Porter, R.R.; Coons, S.W.; Spetzler, R.F. Management of Cranial Base Chondrosarcomas. Neurosurgery 2006, 58, 249–255. [Google Scholar] [CrossRef]
- Schulz-Ertner, D.; Nikoghosyan, A.; Hof, H.; Didinger, B.; Combs, S.E.; Jäkel, O.; Karger, C.P.; Edler, L.; Debus, J. Carbon ion radiotherapy of skull base chondrosarcomas. Int. J. Radiat. Oncol. 2007, 67, 171–177. [Google Scholar] [CrossRef]
- Samii, A.; Gerganov, V.; Herold, C.; Gharabaghi, A.; Hayashi, N.; Samii, M. Surgical treatment of skull base chondrosarcomas. Neurosurg. Rev. 2009, 32, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Sanna, M.; Bacciu, A.; Pasanisi, E.; Piazza, P.; Fois, P.; Falcioni, M. Chondrosarcomas of the Jugular Foramen. Laryngoscope 2008, 118, 1719–1728. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Veeravagu, A.; Feroze, A.H.; Lee, M.; Harsh, G.R.; Soltys, S.G.; Gibbs, I.C.; Adler, J.R.; Chang, S.D. CyberKnife radiosurgery for the management of skull base and spinal chondrosarcomas. J. Neurooncol. 2013, 114, 209–218. [Google Scholar] [CrossRef]
- Sbaihat, A.; Bacciu, A.; Pasanisi, E.; Sanna, M. Skull Base Chondrosarcomas: Surgical Treatment and Results. Ann. Otol. Rhinol. Laryngol. 2013, 122, 763–770. [Google Scholar] [CrossRef]
- Mesquita Filho, P.M.; Ditzel Filho, L.F.S.; Prevedello, D.M.; Martinez, C.A.N.; Fiore, M.E.; Dolci, R.L.; Otto, B.A.; Carrau, R.L. Endoscopic endonasal surgical management of chondrosarcomas with cerebellopontine angle extension. Neurosurg. Focus 2014, 37, E13. [Google Scholar] [CrossRef] [Green Version]
- Uhl, M.; Mattke, M.; Welzel, T.; Oelmann, J.; Habl, G.; Jensen, A.D.; Ellerbrock, M.; Haberer, T.; Herfarth, K.K.; Debus, J. High control rate in patients with chondrosarcoma of the skull base after carbon ion therapy: First report of long-term results. Cancer 2014, 120, 1579–1585. [Google Scholar] [CrossRef] [Green Version]
- Weber, D.C.; Badiyan, S.; Malyapa, R.; Albertini, F.; Bolsi, A.; Lomax, A.J.; Schneider, R. Long-term outcomes and prognostic factors of skull-base chondrosarcoma patients treated with pencil-beam scanning proton therapy at the Paul Scherrer Institute. Neuro. Oncol. 2016, 18, 236–243. [Google Scholar] [CrossRef] [Green Version]
- Carlson, M.L.; O’Connell, B.P.; Breen, J.T.; Wick, C.C.; Driscoll, C.L.; Haynes, D.S.; Thompson, R.C.; Isaacson, B.; Gidley, P.W.; Kutz, J.W.; et al. Petroclival Chondrosarcoma. Otol. Neurotol. 2016, 37, 940–950. [Google Scholar] [CrossRef]
- Feuvret, L.; Bracci, S.; Calugaru, V.; Bolle, S.; Mammar, H.; De Marzi, L.; Bresson, D.; Habrand, J.-L.; Mazeron, J.-J.; Dendale, R.; et al. Efficacy and Safety of Adjuvant Proton Therapy Combined With Surgery for Chondrosarcoma of the Skull Base: A Retrospective, Population-Based Study. Int. J. Radiat. Oncol. 2016, 95, 312–321. [Google Scholar] [CrossRef]
- Shin, M.; Kondo, K.; Hanakita, S.; Hasegawa, H.; Yoshino, M.; Teranishi, Y.; Kin, T.; Saito, N. Endoscopic transsphenoidal anterior petrosal approach for locally aggressive tumors involving the internal auditory canal, jugular fossa, and cavernous sinus. J. Neurosurg. 2017, 126, 212–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raza, S.M.; Gidley, P.W.; Meis, J.M.; Grosshans, D.R.; Bell, D.; DeMonte, F. Multimodality Treatment of Skull Base Chondrosarcomas: The Role of Histology Specific Treatment Protocols. Neurosurgery 2017, 81, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Weng, J.-C.; Zhang, G.-J.; Hao, S.-Y.; Tang, J.; Zhang, L.-W.; Wang, L.; Wu, Z.; Jia, W.; Zhang, J.-T. Proposed Treatment Paradigm for Intracranial Chondrosarcomas Based on Multidisciplinary Coordination. World Neurosurg. 2018, 109, e517–e530. [Google Scholar] [CrossRef]
- Simon, F.; Feuvret, L.; Bresson, D.; Guichard, J.-P.; El Zein, S.; Bernat, A.-L.; Labidi, M.; Calugaru, V.; Froelich, S.; Herman, P.; et al. Surgery and protontherapy in Grade I and II skull base chondrosarcoma: A comparative retrospective study. PLoS ONE 2018, 13, e0208786. [Google Scholar] [CrossRef]
- Weber, D.C.; Murray, F.; Combescure, C.; Calugaru, V.; Alapetite, C.; Albertini, F.; Bolle, S.; Goudjil, F.; Pica, A.; Walser, M.; et al. Long term outcome of skull-base chondrosarcoma patients treated with high-dose proton therapy with or without conventional radiation therapy. Radiother. Oncol. 2018, 129, 520–526. [Google Scholar] [CrossRef]
- Zhang, K.; Qu, P.; Zhang, E.; Dai, C.; Shu, Y.; Chen, B. Primary temporal bone chondrosarcoma: Experience with 10 cases. Acta Otolaryngol. 2019, 139, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Venteicher, A.S.; McDowell, M.M.; Goldschmidt, E.; Wang, E.W.; Snyderman, C.H.; Gardner, P.A. A preoperative risk classifier that predicts tumor progression in patients with cranial base chondrosarcomas. J. Neurosurg. 2020, 134, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.N.; Sekhar, L.N.; Schramm, V.L.; Janecka, I.P. Chordoma and chondrosarcoma of the cranial base. Neurosurgery 1989, 931. [Google Scholar] [CrossRef]
- Bourgouin, P.M.; Tampieri, D.; Robitaille, Y.; Robert, F.; Bergeron, D.; del Carpio, R.; Melanson, D.; Ethier, R. Low-Grade Myxoid Chondrosarcoma of the Base of the Skull. J. Comput. Assist. Tomogr. 1992, 16, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Sasaki, T.; Takakura, K.; Ishida, T. Chondrosarcoma of the Skull Base: Report of Six Cases. Skull Base 1992, 2, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Urie, M.M.; Fullerton, B.; Tatsuzaki, H.; Birnbaum, S.; Suit, H.D.; Convery, K.; Skates, S.; Goitein, M. A dose response analysis of injury to cranial nerves and/or nuclei following proton beam radiation therapy. Int. J. Radiat. Oncol. 1992, 23, 27–39. [Google Scholar] [CrossRef]
- Stapleton, S.R.; Wilkins, P.R.; Archer, D.J.; Uttley, D. Chondrosarcoma of the Skull Base. Neurosurgery 1993, 32, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Chin, O.Y.; Dubal, P.M.; Sheikh, A.B.; Unsal, A.A.; Park, R.C.W.; Baredes, S.; Eloy, J.A. Laryngeal chondrosarcoma: A systematic review of 592 cases. Laryngoscope 2017, 127, 430–439. [Google Scholar] [CrossRef]
- Nunna, R.S.; Patel, S.; Patil, S.N.; Ansari, D.; Burch, T.G.; Mehta, A.I.; Chapman, J.; Oskouian, R.J. Incidence, Management, and Outcomes of Adult Patients with Spinal Chondrosarcoma in the United States. World Neurosurg. 2021, 149, e316–e328. [Google Scholar] [CrossRef]
- Pennington, Z.; Ehresman, J.; Pittman, P.D.; Ahmed, A.K.; Lubelski, D.; McCarthy, E.F.; Goodwin, C.R.; Sciubba, D.M. Chondrosarcoma of the spine: A narrative review. Spine J. 2021. [Google Scholar] [CrossRef]
- Khan, M.N.; Husain, Q.; Kanumuri, V.V.; Boghani, Z.; Patel, C.R.; Liu, J.K.; Eloy, J.A. Management of sinonasal chondrosarcoma: A systematic review of 161 patients. Int. Forum Allergy Rhinol. 2013, 3, 670–677. [Google Scholar] [CrossRef]
- Stanbouly, D.; Litman, E.; Vasilyeva, D.; Philipone, E. Mesenchymal Chondrosarcoma in the Maxilla: A Case Report and Literature Review. J. Oral Maxillofac. Surg. 2021, 79, 1706–1711. [Google Scholar] [CrossRef] [PubMed]
- Pansuriya, T.C.; Kroon, H.M.; Bovée, J.V.M.G. Enchondromatosis: Insights on the different subtypes. Int. J. Clin. Exp. Pathol. 2010, 3, 557–569. [Google Scholar]
- Herget, G.W.; Strohm, P.; Rottenburger, C.; Kontny, U.; Krauß, T.; Bohm, J.; Sudkamp, N.; Uhl, M. Insights into Enchondroma, Enchondromatosis and the risk of secondary Chondrosarcoma. Review of the literature with an emphasis on the clinical behaviour, radiology, malignant transformation and the follow up. Neoplasma 2014, 61, 365–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edem, I.; DeMonte, F.; Raza, S.M. Advances in the management of primary bone sarcomas of the skull base. J. Neurooncol. 2020, 150, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Bin Alamer, O.; Haider, A.S.; Haider, M.; Sagoo, N.S.; Robertson, F.C.; Arrey, E.N.; Aoun, S.G.; Yu, K.; Cohen-Gadol, A.A.; El Ahmadieh, T.Y. Primary and radiation induced skull base osteosarcoma: A systematic review of clinical features and treatment outcomes. J. Neurooncol. 2021, 153, 183–202. [Google Scholar] [CrossRef]
- Oot, R.F.; Melville, G.E.; New, P.F.; Austin-Seymour, M.; Munzenrider, J.; Pile-Spellman, J.; Spagnoli, M.; Shoukimas, G.M.; Momose, K.J.; Carroll, R. The role of MR and CT in evaluating clival chordomas and chondrosarcomas. Am. J. Roentgenol. 1988, 151, 567–575. [Google Scholar] [CrossRef] [Green Version]
- Umana, G.E.; Pucci, R.; Palmisciano, P.; Cassoni, A.; Ricciardi, L.; Tomasi, S.O.; Strigari, L.; Scalia, G.; Valentini, V. Cerebrospinal Fluid Leaks Following Anterior Skull Base Trauma: A Systematic Review of the Literature. World Neurosurg. 2021. [Google Scholar] [CrossRef]
- Umana, G.E.; Scalia, G.; Graziano, F.; Maugeri, R.; Alberio, N.; Barone, F.; Crea, A.; Fagone, S.; Giammalva, G.R.; Brunasso, L.; et al. Navigated Transcranial Magnetic Stimulation Motor Mapping Usefulness in the Surgical Management of Patients Affected by Brain Tumors in Eloquent Areas: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12. [Google Scholar] [CrossRef]
- Giammalva, G.R.; Musso, S.; Salvaggio, G.; Pino, M.A.; Gerardi, R.M.; Umana, G.E.; Midiri, M.; Iacopino, D.G.; Maugeri, R. Coplanar Indirect-Navigated Intraoperative Ultrasound: Matching Un-navigated Probes With Neuronavigation During Neurosurgical Procedures. How We Do It. Oper. Neurosurg. 2021, 21, 485–490. [Google Scholar] [CrossRef]
- Zając, A.E.; Kopeć, S.; Szostakowski, B.; Spałek, M.J.; Fiedorowicz, M.; Bylina, E.; Filipowicz, P.; Szumera-Ciećkiewicz, A.; Tysarowski, A.; Czarnecka, A.M.; et al. Chondrosarcoma-from Molecular Pathology to Novel Therapies. Cancers 2021, 13, 2390. [Google Scholar] [CrossRef]
- Holliday, E.B.; Frank, S.J. Proton Radiation Therapy for Head and Neck Cancer: A Review of the Clinical Experience to Date. Int. J. Radiat. Oncol. 2014, 89, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Lesueur, P.; Calugaru, V.; Nauraye, C.; Stefan, D.; Cao, K.; Emery, E.; Reznik, Y.; Habrand, J.L.; Tessonnier, T.; Chaikh, A.; et al. Proton therapy for treatment of intracranial benign tumors in adults: A systematic review. Cancer Treat. Rev. 2019, 72, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Palmisciano, P.; Haider, A.S.; Nwagwu, C.D.; Wahood, W.; Aoun, S.G.; Abdullah, K.G.; El Ahmadieh, T.Y. Bevacizumab vs. laser interstitial thermal therapy in cerebral radiation necrosis from brain metastases: A systematic review and meta-analysis. J. Neurooncol. 2021, 154, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Traylor, J.I.; Pernik, M.N.; Plitt, A.R.; Lim, M.; Garzon-Muvdi, T. Immunotherapy for Chordoma and Chondrosarcoma: Current Evidence. Cancers 2021, 13, 2408. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, I.J.; Lin, C.-Y.; Kuo, S.-J.; Su, C.-M.; Tang, C.-H. An update on current and future treatment options for chondrosarcoma. Expert Rev. Anticancer Ther. 2019, 19, 773–786. [Google Scholar] [CrossRef]
| Characteristics | Value |
|---|---|
| Cohort size (no.) | 1307 |
| Demographics | |
| Median age, range (years) | 42.5, 18–85 |
| Gender (female) | 603 (53%) |
| Syndromes | No. (%) |
| Ollier’s | 12 (0.9%) |
| Maffucci’s disease | 3 (0.2%) |
| Laterality (n = 55) | No. (%) |
| Right | 29 (52.7%) |
| Left | 10 (18.2%) |
| Midline | 4 (7.3%) |
| Bilateral | 12 (21.8%) |
| Locations (n = 859) | No. (%) |
| Petrous Bone | 325 (37.8%) |
| Clivus | 202 (23.5%) |
| Petroclival synchondrosis | 174 (20.2%) |
| Sphenoid Bone | 103 (12%) |
| Supra/Parasellar region | 64 (7.4%) |
| Others | 188 (21.9%) |
| Extra-Axial Compression/Invasion (n = 630) | No. (%) |
| Brainstem | 313 (49.7%) |
| Cavernous Sinus | 267 (42.4%) |
| Optic Apparatus | 242 (38.4%) |
| Temporal Lobe Compression | 139 (22.1%) |
| Sphenoid Sinus | 51 (8.1%) |
| Internal Carotid Artery | 49 (7.7%) |
| Others | 68(10.8%) |
| Tumor Size (n = 681) | |
| Median, range (cm3) | 24.5, 0.9–88.4 |
| Characteristics | Value |
|---|---|
| Presenting Symptoms (n = 811) | No. (%) |
| Median duration, range (months) | 16, 0.1–312 |
| Diplopia | 237 (29.2%) |
| Headache | 177 (21.8%) |
| Vision Impairment | 64 (7.9%) |
| Hearing Loss | 41 (5.1%) |
| Hypopituitarism | 29 (3.6%) |
| Motor Deficits | 29 (3.6%) |
| Vertigo | 29 (3.6%) |
| Others | 69 (8.5%) |
| No Symptoms | 51 (6.3%) |
| Cranial Nerve Neuropathies (n = 810) | No. (%) |
| I | 3 (0.4%) |
| II | 28 (3.5%) |
| III | 101 (12.5%) |
| IV | 31 (3.8%) |
| V | 157 (19.4%) |
| VI | 256 (31.6%) |
| VII | 77 (9.5%) |
| VIII | 75 (9.3%) |
| IX | 47 (5.8%) |
| X | 60 (7.4%) |
| XI | 29 (3.6%) |
| XII | 59 (7.3%) |
| Multiple | 164 (20.2%) |
| Histopathological Types (n = 579) | No. (%) |
| Conventional | 501 (86.5%) |
| Myxoid | 44 (7.6%) |
| Mesenchymal | 32 (5.5%) |
| Undifferentiated | 2 (0.3%) |
| WHO Grade (n = 928) | No. (%) |
| I/Low | 556 (59.9%) |
| II/Intermediate | 349 (37.6%) |
| III/High | 23 (2.5%) |
| Characteristics | Value |
|---|---|
| Surgery (n = 1307) | No. (%) |
| Biopsy | 87 (6.6%) |
| Surgical Resection | 1220 (93.3%) |
| Open | 1092 (89.5%) |
| Endoscopic | 111 (9.1%) |
| Combined (Open + Endo) | 17 (1.4%) |
| Extent of Surgical Resection (n = 776) | No. (%) |
| Gross Total Resection (100%) | 293 (37.8%) |
| Subtotal Resection (80–99%) | 355 (45.7%) |
| Partial Resection (<80%) | 128 (16.5%) |
| Surgical Approach (n = 521) | No. (%) |
| Endonasal Transsphenoidal | 111 (21.3%) |
| Frontotemporal Orbitozygomatic | 92 (17.6%) |
| Pterional | 62 (11.9%) |
| Infra-Temporal | 35 (6.7%) |
| Sub-Temporal | 32 (6.1%) |
| Trans-Petrosal | 32 (6.1%) |
| Retro-Sigmoid | 30 (5.8%) |
| Sub-Temporal + Infra-Temporal | 28 (5.4%) |
| Sub-Frontal/Bi-Frontal | 19 (3.6%) |
| Fronto-Temporal | 14 (2.7%) |
| Others | 61 (11.7%) |
| Conventional Photon-based Radiotherapy (n = 1307) | 421 (32.2%) |
| Median dose (Gy), range | 55, 6.5–70 |
| External Beam Radiation Therapy | 249 (59.1%) |
| Gamma Knife | 82 (19.5%) |
| Intensity Modulated Radiation Therapy | 40 (9.5%) |
| Linear Accelerator (LINAC) | 34 (8.1%) |
| Cyber Knife | 16 (3.8%) |
| Proton-based Radiotherapy (n = 1307) | 654 (50%) |
| Median dose (GyE), range | 70, 12–76 |
| Carbon-based Radiotherapy (n = 1307) | 133 (10.2%) |
| Median dose (GyE), range | 60, 57–69 |
| Characteristics | Value |
|---|---|
| Post-Surgical Complications (n = 555) | No. (%) |
| Cerebrospinal Fluid Leak | 36 (6.5%) |
| Transient | 88 (15.9%) |
| Cranial Nerve Neuropathies | 58 (10.5%) |
| Meningitis/Brain Abscess | 16 (2.9%) |
| Aphasia | 4 (0.7%) |
| Hearing Impairment | 4 (0.7%) |
| Others | 6 (1.1%) |
| Persistent | 59 (10.6%) |
| Cranial Nerve Neuropathies | 37 (6.7%) |
| Intracerebral Hemorrhage | 8 (1.4%) |
| Ischemic Stroke | 4 (0.7%) |
| Hearing Loss | 2 (0.4%) |
| Pulmonary Embolism | 2 (0.4%) |
| Sepsis | 2 (0.4%) |
| Others | 4 (0.7%) |
| Severe Post-Radiotherapy Complications (n = 818) | No. (%) |
| Total | 251 (30.7%) |
| Hypopituitarism | 126 (15.4%) |
| Hearing Loss | 58 (7.1%) |
| Radiation Necrosis | 30 (3.7%) |
| Cranial Nerve Neuropathies | 14 (1.7%) |
| Osteoradionecrosis | 7 (0.9%) |
| ntracranial Hemorrhage | 4 (0.5%) |
| Memory Loss | 4 (0.5%) |
| Seizure | 4 (0.5%) |
| Brainstem Gliomas | 2 (0.2%) |
| Locoregional Brain Edema | 2 (0.2%) |
| Otitis Media | 2 (0.2%) |
| Convexity Meningioma | 1 (0.1%) |
| Mucositis | 1 (0.1%) |
| Supratentorial Glioblastoma | 1 (0.1%) |
| Symptom Improvement (n = 316) | 138 (46.7%) |
| Radiological Response (n = 247) | No. (%) |
| Reduced tumor volumes | 67 (27.1%) |
| Stable tumor volumes | 144 (58.3%) |
| ncreased tumor volumes | 36 (14.6%) |
| Recurrence (n = 1307) | No. (%) |
| Local Recurrence | 211 (16.1%) |
| Distant Metastases | 7 (0.5%) |
| Treatment for Local Recurrence (n = 211) | No. (%) |
| Surgical resection alone | 33 (15.6%) |
| Radiotherapy alone | 29 (13.7%) |
| Surgical resection + Radiotherapy | 17 (8.1%) |
| Stereotactic radiosurgery alone | 11 (52%) |
| Proton therapy alone | 4 (1.9%) |
| Surgical resection + Stereotactic radiosurgery | 3 (1.4%) |
| Radiotherapy + Carbon therapy | 1 (0.5%) |
| Radiotherapy + Stereotactic radiosurgery | 1 (0.5%) |
| No treatment | 25 (11.8%) |
| Not reported | 87 (41.2%) |
| Outcome (months) | Median, range |
| Follow-up (n = 1307) | 67, 0.1–376 |
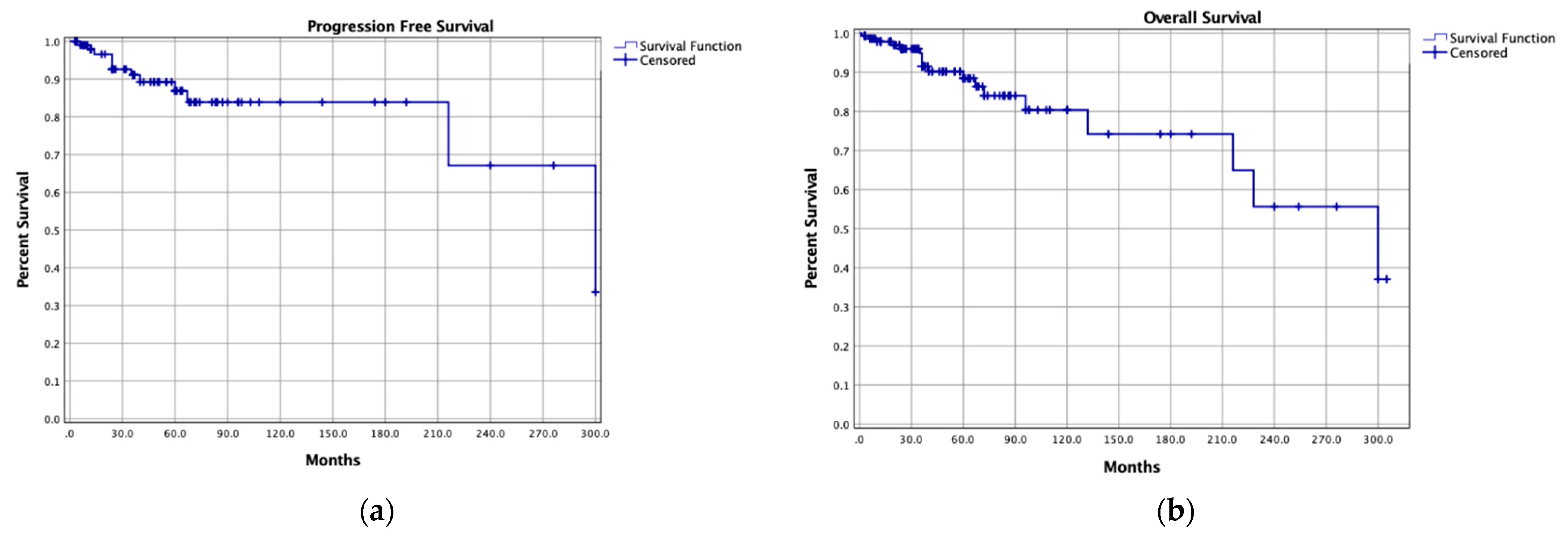
| Progression Free Survival (n = 159) | 36, 0.1–300 |
| 5-year rate | 84.3% |
| 10-year rate | 67.4% |
| Overall Survival (n = 233) | 67, 0.1–376 |
| 5-year rate | 94% |
| 10-year rate | 84% |
| Status (n = 1192) | No. (%) |
| Alive | 1058 (88.8%) |
| Dead | 134 (11.2%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmisciano, P.; Haider, A.S.; Sabahi, M.; Nwagwu, C.D.; Bin Alamer, O.; Scalia, G.; Umana, G.E.; Cohen-Gadol, A.A.; El Ahmadieh, T.Y.; Yu, K.; et al. Primary Skull Base Chondrosarcomas: A Systematic Review. Cancers 2021, 13, 5960. https://doi.org/10.3390/cancers13235960
Palmisciano P, Haider AS, Sabahi M, Nwagwu CD, Bin Alamer O, Scalia G, Umana GE, Cohen-Gadol AA, El Ahmadieh TY, Yu K, et al. Primary Skull Base Chondrosarcomas: A Systematic Review. Cancers. 2021; 13(23):5960. https://doi.org/10.3390/cancers13235960
Chicago/Turabian StylePalmisciano, Paolo, Ali S. Haider, Mohammadmahdi Sabahi, Chibueze D. Nwagwu, Othman Bin Alamer, Gianluca Scalia, Giuseppe E. Umana, Aaron A. Cohen-Gadol, Tarek Y. El Ahmadieh, Kenny Yu, and et al. 2021. "Primary Skull Base Chondrosarcomas: A Systematic Review" Cancers 13, no. 23: 5960. https://doi.org/10.3390/cancers13235960
APA StylePalmisciano, P., Haider, A. S., Sabahi, M., Nwagwu, C. D., Bin Alamer, O., Scalia, G., Umana, G. E., Cohen-Gadol, A. A., El Ahmadieh, T. Y., Yu, K., & Pathmanaban, O. N. (2021). Primary Skull Base Chondrosarcomas: A Systematic Review. Cancers, 13(23), 5960. https://doi.org/10.3390/cancers13235960








