Renal Safety Profile of EGFR Targeted Therapies: A Study from VigiBase® the WHO Global Database of Individual Case Safety Reports
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Design
2.3. Statistical Analysis
3. Results
3.1. Description of the Studied Cases
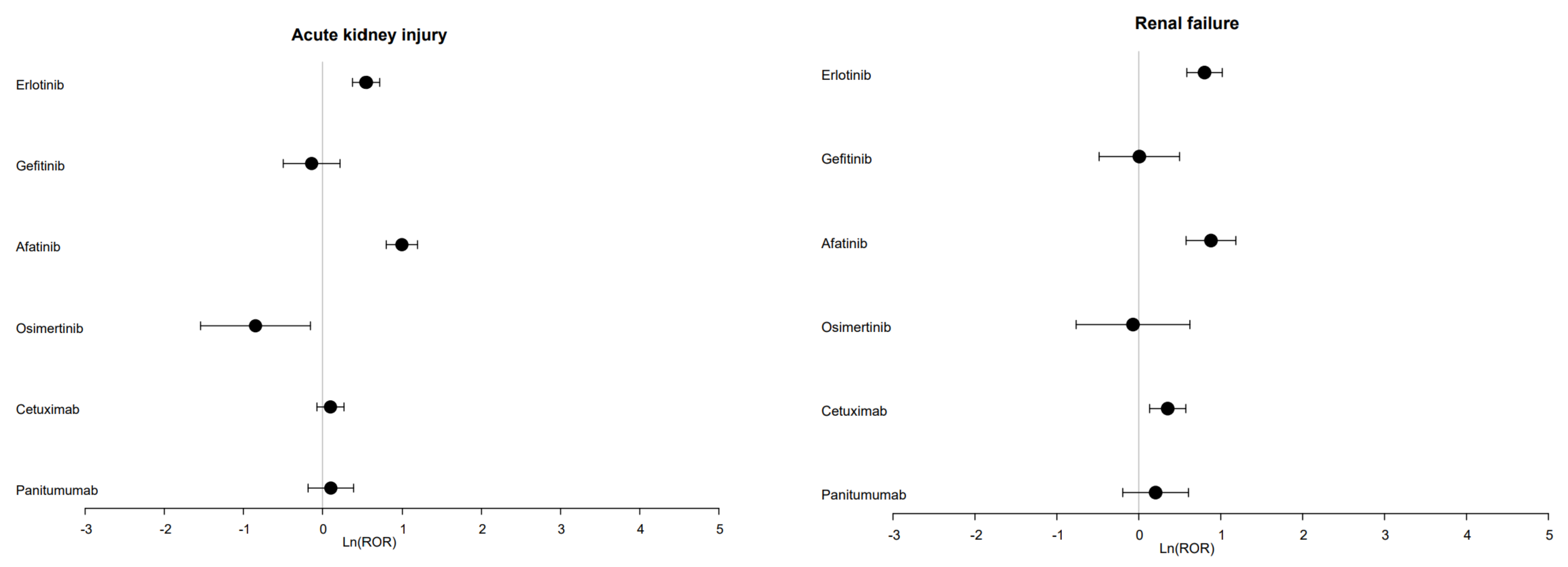
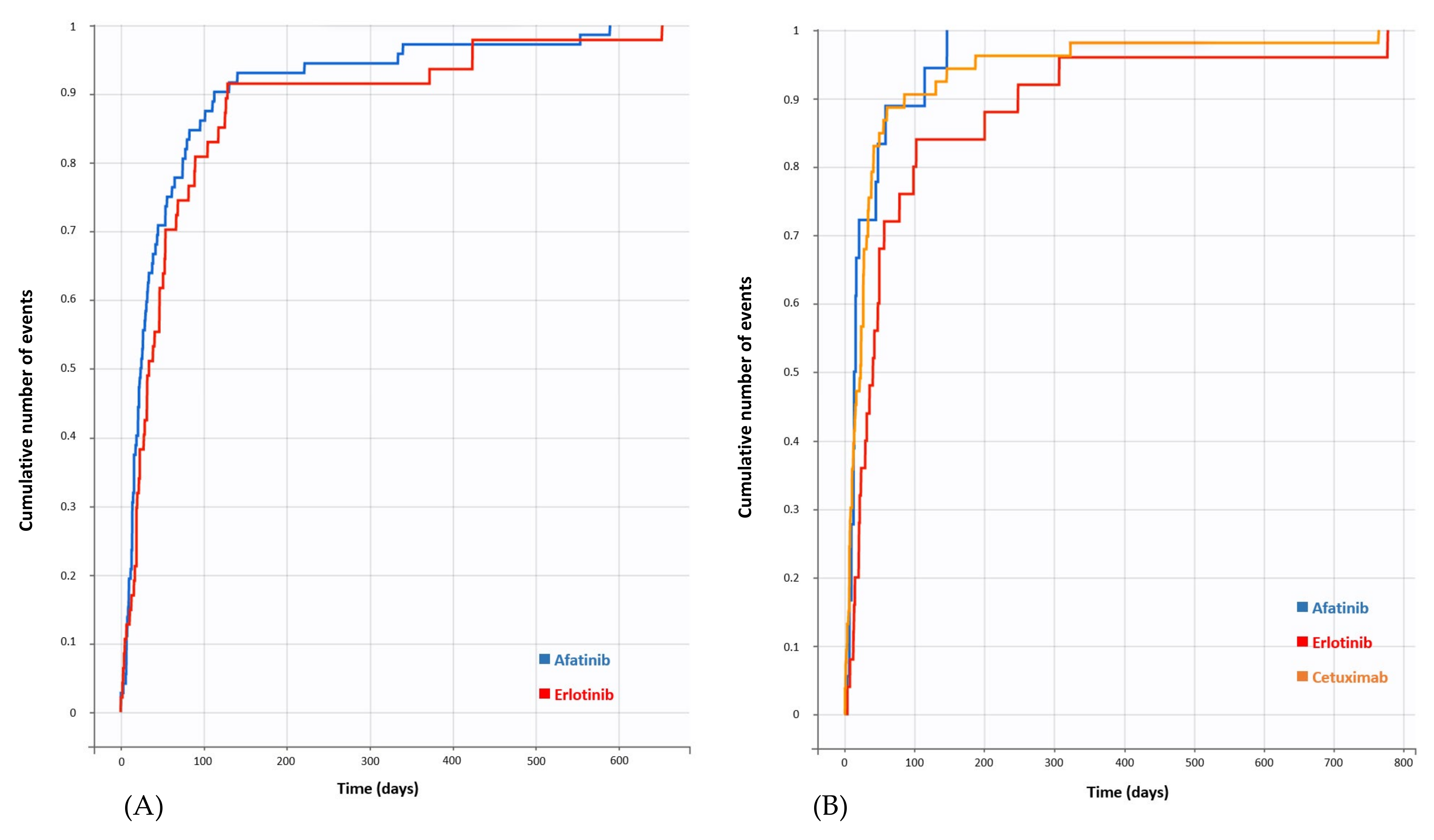
3.2. Disproportionality Analysis and Time to Onset (TTO)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scaltriti, M.; Baselga, J. The Epidermal Growth Factor Receptor Pathway: A Model for Targeted Therapy. Clin. Cancer Res. 2006, 12, 5268–5272. [Google Scholar] [CrossRef]
- Food and Drug Administration FDA-Approved Drugs. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm (accessed on 24 May 2021).
- European Medicines Agency Medicines. Available online: https://www.ema.europa.eu/en/medicines (accessed on 24 May 2021).
- Guardiola, S.; Varese, M.; Sánchez-Navarro, M.; Giralt, E. A Third Shot at EGFR: New Opportunities in Cancer Therapy. Trends Pharmacol. Sci. 2019, 40, 941–955. [Google Scholar] [CrossRef]
- Karachaliou, N.; Fernandez-Bruno, M.; Bracht, J.W.P.; Rosell, R. EGFR first- and second-generation TKIs—There is still place for them in EGFR-mutant NSCLC patients. Transl. Cancer Res. 2018, 8, S23–S47. [Google Scholar] [CrossRef]
- Tan, C.-S.; Kumarakulasinghe, N.B.; Huang, Y.-Q.; Ang, Y.L.E.; Choo, J.R.-E.; Goh, B.-C.; Soo, R.A. Third generation EGFR TKIs: Current data and future directions. Mol. Cancer 2018, 17, 29. [Google Scholar] [CrossRef] [PubMed]
- Wieduwilt, M.J.; Moasser, M.M. The epidermal growth factor receptor family: Biology driving targeted therapeutics. Cell. Mol. Life Sci. 2008, 65, 1566–1584. [Google Scholar] [CrossRef] [PubMed]
- Bonni, A.; Brunet, A.; West, A.E.; Datta, S.R.; Takasu, M.A.; Greenberg, M.E. Cell Survival Promoted by the Ras-MAPK Signaling Pathway by Transcription-Dependent and -Independent Mechanisms. Science 1999, 286, 1358–1362. [Google Scholar] [CrossRef] [PubMed]
- LoPiccolo, J.; Blumenthal, G.M.; Bernstein, W.B.; Dennis, P.A. Targeting the PI3K/Akt/mTOR pathway: Effective combinations and clinical considerations. Drug Resist. Updat. 2008, 11, 32–50. [Google Scholar] [CrossRef]
- Hennessy, B.T.; Smith, D.L.; Ram, P.; Lu, Y.; Mills, G.B. Exploiting the PI3K/AKT Pathway for Cancer Drug Discovery. Nat. Rev. Drug Discov. 2005, 4, 988–1004. [Google Scholar] [CrossRef]
- Spano, J.-P.; Milano, G.; Rixe, C.; Fagard, R. JAK/STAT signalling pathway in colorectal cancer: A new biological target with therapeutic implications. Eur. J. Cancer 2006, 42, 2668–2670. [Google Scholar] [CrossRef]
- Harandi, A.; Zaidi, A.S.; Stocker, A.M.; Laber, D. Clinical Efficacy and Toxicity of Anti-EGFR Therapy in Common Cancers. J. Oncol. 2009, 2009, 567486. [Google Scholar] [CrossRef]
- Ni Ding, P.; Lord, S.J.; Gebski, V.; Links, M.; Bray, V.; Gralla, R.J.; Yang, J.C.-H.; Lee, C.K. Risk of Treatment-Related Toxicities from EGFR Tyrosine Kinase Inhibitors: A Meta-analysis of Clinical Trials of Gefitinib, Erlotinib, and Afatinib in Advanced EGFR -Mutated Non–Small Cell Lung Cancer. J. Thorac. Oncol. 2017, 12, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, F.A.; Pereira, J.R.; Ciuleanu, T.E.; Tan, E.H.; Hirsh, V.; Thongprasert, S.; Campos, D.; Maoleekoonpiroj, S.; Smylie, M.; Martins, R.; et al. Erlotinib in Previously Treated Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2005, 353, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Jonker, D.J.; O’Callaghan, C.J.; Karapetis, C.; Zalcberg, J.R.; Tu, D.; Au, H.-J.; Berry, S.R.; Krahn, M.; Price, T.; Simes, R.J.; et al. Cetuximab for the Treatment of Colorectal Cancer. N. Engl. J. Med. 2007, 357, 2040–2048. [Google Scholar] [CrossRef] [PubMed]
- Thatcher, N.; Chang, A.; Parikh, P.; Pereira, J.R.; Ciuleanu, T.E.; von Pawel, J.; Thongprasert, S.; Tan, E.H.; Pemberton, K.; Archer, V.; et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: Results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 2005, 366, 1527–1537. [Google Scholar] [CrossRef]
- Yang, J.C.-H.; Schuler, M.H.; Yamamoto, N.; O’Byrne, K.J.; Hirsh, V.; Mok, T.; Geater, S.L.; Orlov, S.V.; Tsai, C.-M.; Boyer, M.J.; et al. LUX-Lung 3: A randomized, open-label, phase III study of afatinib versus pemetrexed and cisplatin as first-line treatment for patients with advanced adenocarcinoma of the lung harboring EGFR-activating mutations. J. Clin. Oncol. 2012, 30, LBA7500. [Google Scholar] [CrossRef]
- Soria, J.-C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef]
- Giusti, R.M.; Cohen, M.H.; Keegan, P.; Pazdur, R. FDA Review of a Panitumumab (Vectibix™) Clinical Trial for First-Line Treatment of Metastatic Colorectal Cancer. Oncologist 2009, 14, 284–290. [Google Scholar] [CrossRef]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the Human Tissue-specific Expression by Genome-wide Integration of Transcriptomics and Antibody-based Proteomics. Mol. Cell. Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Gesualdo, L.; Di Paolo, S.; Calabró, A.; Milani, S.; Maiorano, E.; Ranieri, E.; Pannarale, G.; Schena, F.P. Expression of epidermal growth factor and its receptor in normal and diseased human kidney: An immunohistochemical and in situ hybridization study. Kidney Int. 1996, 49, 656–665. [Google Scholar] [CrossRef]
- Sis, B.; Sarioglu, S.; Çelik, A.; Zeybel, M.; Soylu, A.; Bora, S. Epidermal growth factor receptor expression in human renal allograft biopsies: An immunohistochemical study. Transpl. Immunol. 2004, 13, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Izzedine, H. Toxicités rénales des thérapies ciblées en oncologie. Néphrologie Thérapeutique 2020, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Izzedine, H.; Perazella, M.A. Adverse kidney effects of epidermal growth factor receptor inhibitors. Nephrol. Dial. Transplant. 2017, 32, 1089–1097. [Google Scholar] [CrossRef]
- Cosmai, L.; Gallieni, M.; Liguigli, W.; Porta, C. Renal toxicity of anticancer agents targeting vascular endothelial growth factor (VEGF) and its receptors (VEGFRs). J. Nephrol. 2016, 30, 171–180. [Google Scholar] [CrossRef]
- Jhaveri, K.D.; Wanchoo, R.; Sakhiya, V.; Ross, D.W.; Fishbane, S. Adverse Renal Effects of Novel Molecular Oncologic Targeted Therapies: A Narrative Review. Kidney Int. Rep. 2016, 2, 108–123. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. What Is VigiBase? Available online: https://www.who-umc.org/vigibase/vigibase/ (accessed on 28 April 2021).
- World Health Organization. Open Access to the WHO Global Pharmacovigilance Data Base. Available online: https://www.who.int/medicines/news/glob_pharmvig_database_qa/en/ (accessed on 28 April 2021).
- Lindquist, M. VigiBase, the WHO Global ICSR Database System: Basic Facts. Ther. Innov. Regul. Sci. 2008, 42, 409–419. [Google Scholar] [CrossRef]
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Welcome to the ICH MedDRA Website. Available online: https://www.meddra.org/how-to-use/support-documentation/english/welcome (accessed on 28 April 2021).
- World Health Organization. VigiMethods. Available online: https://www.who-umc.org/vigibase/vigilyze/vigimethods/ (accessed on 5 May 2021).
- European Medicines Agency. Screening for Adverse Reactions in EudraVigilance; European Medicines Agency: London, UK, 2016. [Google Scholar]
- Faillie, J.-L. Les études cas–non cas: Principe, méthodes, biais et interprétations. Therapies 2018, 73, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Playford, R.J.; Hanby, A.M.; Gschmeissner, S.; Peiffer, L.P.; Wright, N.A.; McGarrity, T. The epidermal growth factor receptor (EGF-R) is present on the basolateral, but not the apical, surface of enterocytes in the human gastrointestinal tract. Gut 1996, 39, 262–266. [Google Scholar] [CrossRef]
- Van Sebille, Y.Z.; Gibson, R.; Wardill, H.; Bowen, J. ErbB small molecule tyrosine kinase inhibitor (TKI) induced diarrhoea: Chloride secretion as a mechanistic hypothesis. Cancer Treat. Rev. 2015, 41, 646–652. [Google Scholar] [CrossRef]
- Rugo, H.S.; Di Palma, J.A.; Tripathy, D.; Bryce, R.; Moran, S.; Olek, E.; Bosserman, L. The characterization, management, and future considerations for ErbB-family TKI-associated diarrhea. Breast Cancer Res. Treat. 2019, 175, 5–15. [Google Scholar] [CrossRef]
- Kim, Y.; Quach, A.; Das, S.; Barrett, K.E. Potentiation of calcium-activated chloride secretion and barrier dysfunction may underlie EGF receptor tyrosine kinase inhibitor-induced diarrhea. Physiol. Rep. 2020, 8, e14490. [Google Scholar] [CrossRef]
- Davis, M.I.; Hunt, J.P.; Herrgard, S.; Ciceri, P.; Wodicka, L.M.; Pallares, G.; Hocker, M.; Treiber, D.K.; Zarrinkar, P.P. Comprehensive analysis of kinase inhibitor selectivity. Nat. Biotechnol. 2011, 29, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Brotelle, T.; Bay, J.-O. La voie de signalisation PI3K-AKT-mTOR: Description, développement thérapeutique, résistances, marqueurs prédictifs/pronostiques et applications thérapeutiques en cancérologie. Bull Cancer 2016, 103, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Wright, K.L.; Huang, M.; Song, L.; Haura, E.; Turkson, J.; Zhang, S.; Wang, T.; Sinibaldi, D.; Coppola, D.; et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 2002, 21, 2000–2008. [Google Scholar] [CrossRef] [PubMed]
- Hayman, S.R.; Leung, N.; Grande, J.P.; Garovic, V.D. VEGF Inhibition, Hypertension, and Renal Toxicity. Curr. Oncol. Rep. 2012, 14, 285–294. [Google Scholar] [CrossRef]
- Meiracker, A.H.V.D.; Danser, A.J. Mechanisms of Hypertension and Renal Injury During Vascular Endothelial Growth Factor Signaling Inhibition. Hypertension 2016, 68, 17–23. [Google Scholar] [CrossRef][Green Version]
- Flamant, M.; Bollée, G.; Hénique, C.; Tharaux, P.-L. Epidermal growth factor: A new therapeutic target in glomerular disease. Nephrol. Dial. Transplant. 2012, 27, 1297–1304. [Google Scholar] [CrossRef]
- Rayego-Mateos, S.; Rodrigues-Diez, R.; Morgado-Pascual, J.L.; Valentijn, F.; Valdivielso, J.M.; Goldschmeding, R.; Ruiz-Ortega, M. Role of Epidermal Growth Factor Receptor (EGFR) and Its Ligands in Kidney Inflammation and Damage. Mediat. Inflamm. 2018, 2018, 8739473. [Google Scholar] [CrossRef]
- Alatawi, Y.; Hansen, R.A. Empirical estimation of under-reporting in the U.S. Food and Drug Administration Adverse Event Reporting System (FAERS). Expert Opin. Drug Saf. 2017, 16, 761–767. [Google Scholar] [CrossRef]
| Parameters | n | % |
|---|---|---|
| Patient’s | 989 | |
| Age, years, median (Q1–Q3) | 68 (60–74) | |
| Sex, Female/Male | 402/587 | 40.6/59.4 |
| Seriousness | ||
| Serious | 950 | 96.1 |
| Not serious | 31 | 3.1 |
| Unknown | 8 | 0.8 |
| Outcome | ||
| Death | 82 | 8.3 |
| Not recovered/not resolved | 116 | 11.7 |
| Recovered/resolved with sequelae | 15 | 1.5 |
| Recovered/resolved | 419 | 42.4 |
| Unknown | 357 | 36.1 |
| Top 5 reporting countries | ||
| United States of America | 326 | 33.0 |
| Japan | 181 | 18.3 |
| Germany | 139 | 14.1 |
| France | 81 | 8.2 |
| Belgium | 35 | 3.5 |
| Tyrosine kinase inhibitors and monoclonal antibodies of interest | 999 | |
| Erlotinib | 303 | 30.3 |
| Gefitinib | 75 | 7.5 |
| Afatinib | 199 | 19.9 |
| Osimertinib | 31 | 3.1 |
| Cetuximab | 290 | 29.0 |
| Panitumumab | 101 | 10.1 |
| Top 10 reporting MedDRA PT events of interest | 1079 | |
| Acute kidney injury | 458 | 42.4 |
| Renal failure | 252 | 23.4 |
| Renal impairment | 117 | 10.8 |
| Renal disorder | 41 | 3.8 |
| Chronic kidney disease | 20 | 1.9 |
| Prerenal failure | 15 | 1.4 |
| Fluid retention | 15 | 1.4 |
| Thrombotic microangiopathy | 15 | 1.4 |
| Nephrotic syndrome | 14 | 1.3 |
| Renal tubular necrosis | 12 | 1.1 |
| Characteristics of the Drugs | Erlotinib n = 303 | Gefitinib n = 75 | Afatinib n = 199 | Osimertinib n = 31 | Cetuximab n = 290 | Panitumumab n = 101 |
|---|---|---|---|---|---|---|
| Reporting year, n (%) | ||||||
| Before 2010 | 61 (20.1) | 24 (32.0) | 0 (0.0) | 0 (0.0) | 86 (29.7) | 4 (4.0) |
| 2010 | 32 (10.6) | 2 (2.7) | 3 (1.5) | 0 (0.0) | 20 (6.9) | 8 (7.9) |
| 2011 | 35 (11.6) | 7 (9.3) | 3 (1.5) | 0 (0.0) | 22 (7.6) | 9 (8.9) |
| 2012 | 23 (7.6) | 2 (2.7) | 8 (4.0) | 0 (0.0) | 7 (2.4) | 1 (1.0) |
| 2013 | 27 (8.9) | 5 (6.7) | 18 (9.0) | 0 (0.0) | 12 (4.1) | 8 (7.9) |
| 2014 | 41 (13.5) | 10 (13.3) | 11 (5.5) | 0 (0.0) | 41 (14.1) | 8 (7.9) |
| 2015 | 17 (5.6) | 4 (5.3) | 46 (23.1) | 0 (0.0) | 25 (8.6) | 9 (8.9) |
| 2016 | 20 (6.6) | 6 (8.0) | 37 (18.6) | 1 (3.2) | 13 (4.5) | 16 (15.8) |
| 2017 | 23 (7.6) | 5 (6.7) | 18 (9.0) | 6 (19.4) | 23 (7.9) | 13 (12.9) |
| 2018 | 12 (4.0) | 3 (4.0) | 31 (15.6) | 15 (48.4) | 15 (5.2) | 14 (13.9) |
| 2019 | 6 (2.0) | 5 (6.7) | 13 (6.5) | 6 (19.4) | 20 (6.9) | 5 (5.0) |
| 2020 | 6 (2.0) | 2 (2.7) | 11 (5.5) | 3 (9.7) | 6 (2.1) | 6 (5.9) |
| Top 3 reported indications (MedDRA PT), n (%) | Non-small cell lung cancer 78 (25.7) Lung neoplasm malignant 20 (6.6) Lung adenocarcinoma 17 (5.6) | Lung adenocarcinoma 12 (16.0) Non-small cell lung cancer 11 (14.7) Lung neoplasm malignant 4 (5.3) | Non-small cell lung cancer 55 (27.6) Lung adenocarcinoma 35 (17.6) Malignant neoplasm of bronchus and lung 18 (9.0) | Non-small cell lung cancer 18 (58.1) Lung cancer 3 (9.7) Non-small cell lung cancer metastatic 2 (6.5) | Non-small cell lung cancer 23 (7.9) Metastatic colorectal cancer 22 (7.6) Colorectal cancer 17 (5.9) | Colon cancer 17 (16.8) Metastatic colorectal cancer 15 (14.9) Colorectal cancer 10 (9.9) |
| Unknown indication, n (%) | 32 (10.6) | 14 (18.7) | 20 (10.1) | 3 (9.7) | 27 (9.3) | 5 (5.0) |
| Top 5 reported adverse drug reactions (MedDRA PT), n (%) | Acute kidney injury 139 (45.9) Renal failure 83 (27.4) Renal impairment 26 (8.6) Renal disorder 15 (5.0) Thrombotic microangiopathy 12 (4.0) | Acute kidney injury 30 (40.0) Renal failure 16 (21.3) Renal impairment 9 (12.0) Renal disorder 3 (4.0) Nephrotic syndrome 3 (4.0) | Acute kidney injury 101 (50.8) Renal failure 42 (21.1) Renal impairment 26 (13.1) Renal disorder 11 (5.5) Prerenal failure 10 (5.0) | Renal impairment 13 (41.9) Renal failure 8 (25.8) Acute kidney injury 8 (25.8) Renal disorder 2 (6.5) Nephrotic syndrome 1 (3.2) | Acute kidney injury 133 (45.9) Renal failure 79 (27.2) Renal impairment 32 (11.0) Renal disorder 8 (2.8) Renal tubular disorder 4 (1.4) | Acute kidney injury 47 (46.5) Renal failure 24 (23.8) Renal impairment 11 (10.9) Nephrotic syndrome 4 (4.0) Nephropathy 3 (3.0) |
| Variation | Acute Kidney Injury | Renal Failure | ||
|---|---|---|---|---|
| Number of AKI Cases | Top 5 Associated ADRs, n (% of AKI Cases) | Number of RF Cases | Top 5 Associated ADRs, n (% of RF Cases) | |
| Erlotinib | 139 | Diarrhoea, 50 (36.0) Dehydration, 33 (23.7) Vomiting, 23 (16.5) Nausea, 19 (13.7) Anaemia, 16 (11.5) | 83 | Diarrhoea, 25 (30.1) Dehydration, 13 (15.7) Rash, 9 (10.8) Vomiting, 9 (10.8) Dyspnoea, 9 (10.8) |
| Afatinib | 101 | Diarrhoea, 56 (55.4) Dehydration, 20 (19.8) Vomiting, 19 (18.8) Decreased appetite, 13 (12.9) Nausea, 10 (9.9) | 42 | Diarrhoea, 35 (83.3) Dehydration, 19 (45.2) Vomiting, 8 (19.0) Rash, 7 (16.7) Nausea, 5 (11.9) |
| Cetuximab | - | 79 | Diarrhoea, 20 (25.3) Dehydration, 13 (16.5) Sepsis, 9 (11.4) Fatigue, 8 (10.1) Blood creatinine increased, 7 (8.9) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crosnier, A.; Abbara, C.; Cellier, M.; Lagarce, L.; Babin, M.; Bourneau-Martin, D.; Briet, M. Renal Safety Profile of EGFR Targeted Therapies: A Study from VigiBase® the WHO Global Database of Individual Case Safety Reports. Cancers 2021, 13, 5907. https://doi.org/10.3390/cancers13235907
Crosnier A, Abbara C, Cellier M, Lagarce L, Babin M, Bourneau-Martin D, Briet M. Renal Safety Profile of EGFR Targeted Therapies: A Study from VigiBase® the WHO Global Database of Individual Case Safety Reports. Cancers. 2021; 13(23):5907. https://doi.org/10.3390/cancers13235907
Chicago/Turabian StyleCrosnier, Alexandre, Chadi Abbara, Morgane Cellier, Laurence Lagarce, Marina Babin, Delphine Bourneau-Martin, and Marie Briet. 2021. "Renal Safety Profile of EGFR Targeted Therapies: A Study from VigiBase® the WHO Global Database of Individual Case Safety Reports" Cancers 13, no. 23: 5907. https://doi.org/10.3390/cancers13235907
APA StyleCrosnier, A., Abbara, C., Cellier, M., Lagarce, L., Babin, M., Bourneau-Martin, D., & Briet, M. (2021). Renal Safety Profile of EGFR Targeted Therapies: A Study from VigiBase® the WHO Global Database of Individual Case Safety Reports. Cancers, 13(23), 5907. https://doi.org/10.3390/cancers13235907






