On Efficacy of Microwave Ablation in the Thermal Treatment of an Early-Stage Hepatocellular Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Numerical Method and Simulation Conditions
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ananthakrishnan, A.; Gogineni, V.; Kia Saeian, K. Epidemiology of Primary and Secondary Liver Cancers. Semin. Intervent. Radiol. 2006, 23, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Vonlaufen, A.; Phillips, P.A.; Xu, Z.; Goldstein, D.; Pirola, R.C.; Wilson, J.S.; Apte, M.V. Pancreatic stellate cells and pancreatic cancer cells: An unholy alliance. Cancer Res. 2008, 68, 7707–7710. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Wang, X.W. Cancer stem cells in the development of liver cancer. J. Clin. Investig. 2013, 123, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, W.; Jia, Y.; Yu, Q.; Grau, G.E.; Peng, L.; Ran, Y.; Yang, Z.; Deng, H.; Lou, J. Single-cell clones of liver cancer stem cells have the potential of differentiating into different types of tumor cells. Cell Death Dis. 2013, 4, e857. [Google Scholar] [CrossRef] [PubMed]
- Willacy, H. Primary Liver Cancer. 2018. Available online: https://patient.info/cancer/primary-liver-cancer-leaflet (accessed on 8 August 2021).
- Pichardo, G. Understanding Liver Cancer–The Basics. 2019. Available online: https://www.webmd.com/cancer/understanding-liver-cancer-basic-information (accessed on 10 August 2021).
- Petrick, J.L.; McGlynn, K.A. The changing epidemiology of primary liver cancer. Curr. Epidemiol. Rep. 2019, 6, 104–111. [Google Scholar] [CrossRef]
- Markman, M. Liver Cancer. 2021. Available online: https://www.cancercenter.com/cancer-types/liver-cancer (accessed on 10 August 2021).
- Balogh, J.; Victor, D.; Asham, E.H.; Burroughs, S.G.; Boktour, M.; Saharia, A.; Li, X.; Ghobrial, R.M.; Monsour, H.P., Jr. Hepatocellular carcinoma: A review. J. Hepatocell. Carcinoma 2016, 3, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Kumar, R.; Pandey, A.K. Hepatocellular Carcinoma: Causes, Mechanism of Progression and Biomarkers. Curr. Chem. Genom. Transl. Med. 2018, 12, 9–26. [Google Scholar] [CrossRef]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Crespo, M.; Leiva, M.; Sabio, G. Circadian Clock and Liver Cancer. Cancers 2021, 13, 3631. [Google Scholar] [CrossRef]
- Fabrizi, F.; Cerutti, R.; Alfieri, C.M.; Ridruejo, E. An Update on Hepatocellular Carcinoma in Chronic Kidney Disease. Cancers 2021, 13, 3617. [Google Scholar] [CrossRef] [PubMed]
- Shira, K.; Ebata, T.; Oda, K.; Nishio, X.; Nagasaka, T.; Nimura, Y.; Nagino, M. Perineural Invasion Is a Prognostic Factor in Intrahepatic Cholangiocarcinoma. World J. Surg. 2008, 32, 2395–2402. [Google Scholar] [CrossRef] [PubMed]
- Schnater, J.M.; Kohler, S.E.; Lamers, W.H.; von Schweinitz, D.; Aronson, D.C. Where Do We Stand with Hepatoblastoma? A Review. Cancer 2003, 98, 668–678. [Google Scholar] [CrossRef]
- Van den Brand, M.; Flucke, U.E.; Bult, P.; Weemaes, C.M.R.; van Deuren, M. Angiosarcoma in a patient with immunodeficiency, centromeric region instability, facial anomalies (ICF) syndrome. Am. J. Med Genet. Part A 2011, 155, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Rademaker, J.; Widjaja, A.; Galansk, M. Hepatic hemangiosarcoma: Imaging findings and differential diagnosis. Eur. Radiol. 2000, 10, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.; Jiang, J.Y.; Goggins, W.B.; Liang, M.; Fang, Y.; Fung, F.D.; Leung, C.; Wang, H.H.; Wong, G.L.; Wong, V.W.; et al. International incidence and mortality trends of liver cancer: A global profile. Sci. Rep. 2017, 7, 45846. [Google Scholar] [CrossRef] [PubMed]
- Sarveazad, A.; Agah, S.; Babahajian, A.; Amini, N.; Bahardoust, M. Predictors of 5 year survival rate in hepatocellular carcinoma patients. J. Res. Med. Sci. 2019, 24, 86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Zhang, L.; Yao, L.; Xue, X.; Zhang, S.; Li, X.; Chen, Y.; Pang, P.; Sun, D.; et al. Radiomics predict postoperative survival of patients with primary liver cancer with different pathological types. Ann. Transl. Med. 2020, 8, 820. [Google Scholar] [CrossRef]
- Kim, Y.A.; Kang, D.; Moon, H.; Sinn, D.; Kang, M.; Woo, S.M.; Chang, Y.J.; Park, B.; Kong, S.Y.; Guallar, E.; et al. Survival in untreated hepatocellular carcinoma: A national cohort study. PLoS ONE 2021, 16, e0246143. [Google Scholar] [CrossRef]
- Chong, C.C.N.; Lee, K.F.; Chu, C.M.; Chan, A.W.H.; Wong, J.; Chan, S.L.; Lok, H.T.; Fung, A.K.Y.; Fong, A.K.Y.; Cheung, Y.S.; et al. Microwave ablation provides better survival than liver resection for hepatocellular carcinoma in patients with borderline liver function: Application of ALBI score to patient selection. HPB 2018, 20, 546–554. [Google Scholar] [CrossRef]
- Glassberg, M.B.; Ghosh, S.; Clymer, J.W.; Wright, G.W.J.; Ferko, N.; Amaral, J.F. Microwave ablation compared with hepatic resection for the treatment of hepatocellular carcinoma and liver metastases: A systematic review and meta-analysis. World J. Surg. Oncol. 2019, 17, 98. [Google Scholar] [CrossRef]
- Hui, T.C.; Kwan, J.; Pua, U. Advanced Techniques in the Percutaneous Ablation of Liver Tumours. Diagnostics 2021, 11, 585. [Google Scholar] [CrossRef]
- Afaghi, P.; Lapolla, M.A.; Ghandi, K. Percutaneous microwave ablation applications for liver tumors: Recommendations for COVID-19 patients. Heliyon 2021, 7, e06454. [Google Scholar] [CrossRef]
- Suresh, D.; Srinivas, A.N.; Kumar, D.P. Etiology of Hepatocellular Carcinoma: Special Focus on Fatty Liver Disease. Front. Oncol. 2020, 10, 601710. [Google Scholar] [CrossRef] [PubMed]
- Pons, F.; Varela, M.; Llovet, J.M. Staging systems in hepatocellular carcinoma. HPB 2005, 7, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Koulouris, A.; Tsagkaris, C.; Spyrou, V.; Pappa, E.; Troullinou, A.; Nikolaou, M. Hepatocellular Carcinoma: An Overview of the Changing Landscape of Treatment Options. J. Hepatocell. Carcinoma 2021, 8, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Di Sandro, S.; Benuzzi, L.; Lauterio, A.; Botta, F.; De Carlis, R.; Najjar, M.; Centonze, L.; Danieli, M.; Pezzoli, I.; Rampoldi, A.; et al. Single Hepatocellular Carcinoma approached by curative-intent treatment: A propensity score analysis comparing radiofrequency ablation and liver resection. Eur. J. Surg. Oncol. 2019, 45, 1691–1699. [Google Scholar] [CrossRef]
- Imajo, K.; Ogawa, Y.; Yoneda, M.; Saito, S.; Nakajima, A. A review of conventional and newer generation microwave ablation systems for hepatocellular carcinoma. J. Med. Ultrason. 2020, 47, 265–277. [Google Scholar] [CrossRef]
- Violi, N.V.; Duran, R.; Guiu, B.; Cercueil, J.P.; Aubé, C.; Digklia, A.; Pache, I.; Deltenre, P.; Knebel, J.F.; Denys, A. Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: A randomised controlled phase 2 trial. Lancet Gastroenterol. Hepatol. 2018, 3, 317–325. [Google Scholar] [CrossRef]
- Crocetti, L.; Scalise, P.; Bozzi, E.; Campani, D.; Rossi, P.; Cervelli, R.; Bargellini, I.; Ghinolfi, D.; De Simone, P.; Cioni, R. Microwave Ablation of Very-Early- and Early-Stage HCC: Efficacy Evaluation by Correlation with Histology after Liver Transplantation. Cancers 2021, 13, 3420. [Google Scholar] [CrossRef]
- Kalra, N.; Gupta, P.; Chawla, Y.; Khandelwal, N. Locoregional treatment for hepatocellular carcinoma: The best is yet to come. World J. Radiol. 2015, 7, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, R.; Posa, A.; Mariappan, M.; Spiliopoulos, S. Locoregional treatments for hepatocellular carcinoma: Current evidence and future directions. World J. Gastroenterol. 2019, 25, 4614–4628. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Kang, Y.K.; Kim, T.Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.M.; Matilla, A.; et al. Nivolumab (NIVO) + ipilimumab (IPI) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): Results from CheckMate 040. J. Clin. Oncol. 2019, 37, 4012. [Google Scholar] [CrossRef]
- Abdelaziz, A.O.; Nabeel, M.M.; Elbaz, T.M.; Shousha, H.I.; Hassan, E.M.; Mahmoud, S.H.; Rashed, N.A.; Ibrahim, M.M.; Abdelmaksoud, A.H. Microwave ablation versus transarterial chemoembolization in large hepatocellular carcinoma: Prospective analysis. Scand. J. Gastroenterol. 2015, 50, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, N.; Okoh, A.; Yigitbas, H.; Yazici, P.; Ali, N.; Berber, E. Laparoscopic microwave thermosphere ablation of malignant liver tumors: An analysis of 53 cases. J. Surg. Oncol. 2016, 113, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.; Ikeda, Y.; Kawanaka, H.; Okuyama, T.; Kawasaki, K.; Eguchi, D.; Korenaga, D.; Takenaka, K. Efficacy of surgical microwave therapy in patients with unresectable hepatocellular carcinoma. Ann. Surg. Oncol. 2011, 18, 3650–3656. [Google Scholar] [CrossRef]
- Berber, E. Laparoscopic microwave thermosphere ablation of malignant liver tumors: An initial clinical evaluation. Surg. Endosc. 2016, 30, 692–698. [Google Scholar] [CrossRef]
- Facciorusso, A.; Abd El Aziz, M.A.; Tartaglia, N.; Ramai, D.; Mohan, B.P.; Cotsoglou, C.; Pusceddu, S.; Giacomelli, L.; Ambrosi, A.; Sacco, R. Microwave Ablation Versus Radiofrequency Ablation for Treatment of Hepatocellular Carcinoma: A Meta-Analysis of Randomized Controlled Trials. Cancers 2020, 12, 3796. [Google Scholar] [CrossRef]
- Poggi, G.; Tosoratti, N.; Montagna, B.; Picchi, C. Microwave ablation of hepatocellular carcinoma. World J. Hepatol. 2015, 7, 2578–2589. [Google Scholar] [CrossRef]
- Simon, C.J.; Dupuy, D.E.; Mayo-Smith, W.W. Microwave ablation: Principles and applications. Radiographics 2005, 25, S69–S83. [Google Scholar] [CrossRef]
- Kuroda, H.; Nagasawa, T.; Fujiwara, Y.; Sato, H.; Abe, T.; Kooka, Y.; Endo, K.; Oikawa, T.; Sawara, K.; Takikawa, Y. Comparing the Safety and Efficacy of Microwave Ablation Using Thermosphere™ Technology versus Radiofrequency Ablation for Hepatocellular Carcinoma: A Propensity Score-Matched Analysis. Cancers 2021, 13, 1295. [Google Scholar] [CrossRef]
- Lerardi, A.M.; Mangano, A.; Floridi, C.; Dionigi, G.; Biondi, A.; Duka, E.; Lucchina, N.; Lianos, G.D.; Carrafiello, G. A newsystem of microwave ablation at 2450 MHz: Preliminary experience. Updates Surg. 2015, 67, 39–45. [Google Scholar] [CrossRef]
- Kapoor, H.; Nisiewicz, M.J.; Jayavarapu, R.; Gedaly, R.; Raissi, D. Early outcomes with single-antenna high-powered percutaneous microwave ablation for primary and secondary hepatic malignancies: Safety, effectiveness, and predictors of ablative failure. J. Clin. Imaging Sci. 2020, 10, 10. [Google Scholar] [CrossRef]
- Prakash, P.; Converse, M.; Webster, J.G.M.; Mahvi, D.M. An Optimal Sliding Choke Antenna for Hepatic Microwave Ablation. IEEE Trans. Bio-Med. Eng. 2009, 56, 2470–2476. [Google Scholar] [CrossRef]
- Chetboun, M.; Kianmanesh, R.; Sommacale, D.; Diebold, M.D.; Piardi, T. Complete necrosis after microwave thermosphere ablation of liver metastases from colorectal cancer, histological proof of efficacy. J. Surg. Oncol. 2016, 113, 843–844. [Google Scholar] [CrossRef]
- Hojjatollah, F.; Punit, P. Antenna Designs for Microwave Tissue Ablation. Crit. Rev. Biomed. Eng. 2018, 46, 495–521. [Google Scholar] [CrossRef]
- Suwa, K.; Seki, T.; Aoi, K.; Yamashina, M.; Murata, M.; Yamashiki, N.; Nishio, A.; Shimatani, M.; Naganuma, M. Efficacy of microwave ablation versus radiofrequency ablation for hepatocellular carcinoma: A propensity score analysis. Abdom. Radiol. 2021, 46, 3790–3797. [Google Scholar] [CrossRef] [PubMed]
- Alonzo, M.; Bos, A.; Bennett, S.; Ferral, H. The Emprint™ Ablation System with Thermosphere™ Technology: One of the Newer Next-GenerationMicrowave AblationTechnologies. Semin. Interv. Radiol. 2015, 32, 335–338. [Google Scholar] [CrossRef]
- Hendriks, P.; Berkhout, W.E.M.; Kaanen, C.I.; Sluijter, J.H.; Visser, I.J.; van den Dobbelsteen, J.J.; de Geus-Oei, L.F.; Webb, A.G.; Burgmans, M.C. Performance of the Emprint and Amica Microwave Ablation Systems in ex vivo Porcine Livers: Sphericity and Reproducibility Versus Size. Cardio Vasc. Interv. Radiol. 2021, 44, 952–958. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, G.; Qiu, B. Theoretical evaluation of the treatment effectiveness of a novel coaxial multi-slot antenna for conformal microwave ablation of tumors. Int. J. Heat Mass Transf. 2015, 90, 81–91. [Google Scholar] [CrossRef]
- Ge, M.; Jiang, H.; Huang, X.; Zhou, Y.; Zhi, D.; Zhao, G.; Chen, Y.; Wang, L.; Qiu, B. A multi-slot coaxial microwave antenna for liver tumor ablation. Phys. Med. Biol. 2018, 6, 175011. [Google Scholar] [CrossRef]
- Radmilović-Radjenović, M.; Radjenović, D.; Radjenović, B. Finite element analysis of the effect of microwave ablation on the liver, lung, kidney, and bone malignant tissues. Europhys. Lett. 2021, 135, 3500. [Google Scholar] [CrossRef]
- Chiang, J.; Wang, P.; Brace, C.L. Computational modelling of microwave tumour ablations. Int. J. Hyperth. 2013, 29, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Rattanadecho, P.; Keangin, P. Numerical study of heat transfer and blood flow in two-layered porous liver tissue during microwave ablation process using single and double slot antenna. Int. J. Heat Mass Tranf. 2013, 58, 457–470. [Google Scholar] [CrossRef]
- Rubio, M.F.; López, G.D.; Perezgasga, F.V.; García, F.F.; Hernández, A.V.; Salas, L.L. Computer Modeling for Microwave Ablation in Breast Cancer Using a Coaxial Slot Antenna. Int. J. Thermophys. 2015, 36, 2687–2704. [Google Scholar] [CrossRef]
- Reinhardt, M.; Brandmaier, P.; Seider, D.; Kolesnik, M.; Jenniskens, S.; Sequeiros, R.B.; Eibisberger, M.; Voglreiterg, P.; Ronan Flanagan, R.; Mariappan, P.; et al. A prospective development study of software-guided radio-frequency ablation of primary and secondary liver tumors: Clinical intervention modelling, planning and proof for ablation cancer treatment (ClinicIMPPACT). Contemp. Clin. Trials Commun. 2017, 8, 25–32. [Google Scholar] [CrossRef]
- Wongkedsada, T.; Phasukkit, P. Microwave Ablation Multi-Antennas Operation studying for Hepatic Cancer Microwave Ablation Treatment system using 3D-Finite Element Analysis. In Proceedings of the 12th Biomedical Engineering International Conference (BMEiCON2019), Ubon Ratchathani, Thailand, 19–22 November 2019. [Google Scholar]
- Lin, J.C.; Hirai, S.; Chiang, C.L.; Hsu, W.L.; Su, J.L.; Wang, Y.J. Computer simulation and experimental studies of SAR distributions of interstitial arrays of sleeved-slot microwave antennas for hyperthermia treatment of brain tumors. IEEE Trans. Microw. Theory Tech. 2000, 48, 2191–2198. [Google Scholar]
- Maini, S.; Marwaha, A. Modeling and simulation of novel antenna for the treatment of hepatocellular carcinoma using finite element method. Electromagn. Biol. Med. 2013, 32, 373–381. [Google Scholar] [CrossRef]
- Neagu, V. A study of microwave ablation antenna optimization. In Proceedings of the 2017 E-Health and Bioengineering Conference (EHB), Sinaia, Romania, 22–24 June 2017. [Google Scholar]
- Towoju, O.; Ishola, F.; Sanni, T.; Olatunji, O. Investigation of Influence of Coaxial Antenna Slot Positioning on Thermal Efficiency in Microwave Ablation using COMSOL. J. Phys. Conf. Ser. 2019, 1378, 032066. [Google Scholar] [CrossRef]
- Selmi, M.; Bin Dukhyil, A.A.; Belmabrouk, H. Numerical Analysis of Human Cancer Therapy Using Microwave Ablation. Appl. Sci. 2020, 10, 211. [Google Scholar] [CrossRef]
- Radmilović-Radjenović, M.; Sabo, M.; Prnova, M.; Šoltes, L.; Radjenović, B. Finite Element Analysis of the Microwave Ablation Method for Enhanced Lung Cancer Treatment. Cancers 2021, 13, 3500. [Google Scholar] [CrossRef]
- Paruch, M. Mathematical Modeling of Breast Tumor Destruction Using Fast Heating during Radiofrequency Ablation. Materials 2020, 13, 136. [Google Scholar] [CrossRef]
- Tehrani, M.H.H.; Soltani, M.; Kashkooli, F.M.; Raahemifar, K. Use of microwave ablation for thermal treatment of solid tumors with different shapes and sizes—A computational approach. PLoS ONE 2020, 15, e0233219. [Google Scholar] [CrossRef] [PubMed]
- Cazacu, D.I. Modeling and Simulation of Microwave Ablation of Liver Tumors. Ph.D. Thesis, Jacobs University, Bremen, Germany, 2019. Available online: https://opus.jacobs-university.de/frontdoor/index/index/docId/887 (accessed on 12 July 2021).
- 3D-IRCADb. Available online: https://www.ircad.fr/research/3dircadb/ (accessed on 17 August 2021).
- Comsol Multiphysics. 1986–2020. Burlington (MA): COMSOL, Inc. Available online: www.comsol.com (accessed on 17 July 2021).
- Heat Transfer Modeling Software for Analyzing Thermal Effects. Available online: www.comsol.com (accessed on 17 July 2021).
- Sun, Y.Y.; Cheng, Z.G.; Dong, L.; Zhang, G.M.; Wang, Y.; Liang, P. Comparison of temperature curve and ablation zone between 915-and 2450-MHz cooled-shaft microwave antenna: Results in ex vivo porcine livers. Eur. J. Radiol. 2012, 81, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Ibitoye, A.Z.; Nwoye, E.O.; Aweda, A.M.; Oremosu, A.A.; Anunobi, C.C.; Akanmu, N.O. Microwave ablation of ex vivo bovine tissues using a dual slot antenna with a floating metallic sleeve. Int. J. Hyperther. 2016, 32, 923–930. [Google Scholar] [CrossRef][Green Version]
- Yang, D.S.; Bertram, J.M.; Converse, M.C.; O’Rourke, A.P.; Webster, J.G.; Hagness, S.C.; Will, J.A.; Mahvi, D.M. A floating sleeve antenna yields localized hepatic microwave ablation. IEEE Trans. Bio-Med. Eng. 2006, 53, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Luyen, H.; Hagness, S.C.; Behdad, N. Reduced-Diameter Designs of Coax-Fed Microwave Ablation Antennas Equipped with Baluns. IEEE Antennas Wirel. Propag. Lett. 2017, 16, 1385–1388. [Google Scholar] [CrossRef]
- Gas, P. Optimization of multi-slot coaxial antennas for microwave thermotherapy based on the S11-parameter analysis. Biocybern. Biomed. Eng. 2017, 37, 78–93. [Google Scholar] [CrossRef]
- Simanovskii, D.M.; Mackanos, M.A.; Irani, A.R.; O’Connell-Rodwell, C.E.; Contag, C.H.; Schwettman, H.A.; Palanker, D.V. Cellular tolerance to pulsed hyperthermia. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2006, 74, 11915. [Google Scholar] [CrossRef]
- Hossan, M.R.; Dutta, P. Effects of temperature dependent properties in electromagnetic heating. Int. J. Heat Mass Transf. 2012, 55, 3412–3422. [Google Scholar] [CrossRef]
- Ji, Z.; Brace, C.L. Expanded modeling of temperature-dependent dielectric properties for microwave thermal ablation. Phys. Med. Biol. 2011, 56, 5249–5264. [Google Scholar] [CrossRef]
- O’Rourke, A.P.; Lazebnik, M.; Bertram, J.M.; Converse, M.C.; Hagness, S.C.; Webster, J.G.; Mahvi, D.M. Dielectric properties of human normal, malignant and cirrhotic liver tissue: In vivo and ex vivo measurements from 0.5 to 20 GHz using a precision open-ended coaxial probe. Phys. Med. Biol. 2007, 52, 4707–4719. [Google Scholar] [CrossRef] [PubMed]
- Prakash, P. Theoretical Modeling for Hepatic Microwave Ablation. Open Biomed. Eng. J. 2010, 4, 27–38. [Google Scholar] [CrossRef]
- Pennes, H.H. Analysis of tissue and arterial blood temperatures in the resting human forearm. J. Appl. Physiol. 1948, 1, 93–122. [Google Scholar] [CrossRef] [PubMed]
- Keangin, P.; Rattanadecho, P.; Wessapan, T. An analysis of heat transfer in liver tissue during microwave ablation using single and double slot antenna. Int. Commun. Heat Mass Transf. 2011, 38, 757–766. [Google Scholar] [CrossRef]
- Cavagnaro, M.; Pinto, R.; Lopresto, V. Numerical models to evaluate the temperature increase induced by ex vivo microwave thermal ablation. Phys. Med. Biol. 2015, 60, 3287–3311. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, P.R.; Rossetto, F.; Prakash, M.; Neuman, D.G.; Lee, T. Phantom and animal tissues for modelling the electrical properties of human liver. Int. J. Hyperth. 2003, 19, 89–101. [Google Scholar] [CrossRef]
- Yang, D.; Converse, M.C.; Mahvi, D.M.; Webster, J.G. Expanding the Bioheat Equation to Include Tissue Internal Water Evaporation during Heating. IEEE Trans. Biomed. Eng. 2007, 54, 1382–1388. [Google Scholar] [CrossRef] [PubMed]
- Diller, K.R.; Pearce, J.A. Issues in modeling thermal alterations in tissues. Ann. N. Y. Acad. Sci. 1999, 888, 153–164. [Google Scholar] [CrossRef]
- Berjano, E.J. Theoretical modeling for radiofrequency ablation: State-of-the-art and challenges for the future. BioMed. Eng. OnLine 2006, 5, 24. [Google Scholar] [CrossRef]
- Izzo, F.; Granata, V.; Grassi, R.; Fusco, R.; Palaia, R.; Delrio, P.; Carrafiello, G.; Azoulay, D.; Petrillo, A.; Curley, S.A. Radiofrequency Ablation and Microwave Ablation in Liver Tumors: An Update. Oncologist 2019, 24, e990–e1005. [Google Scholar] [CrossRef] [PubMed]
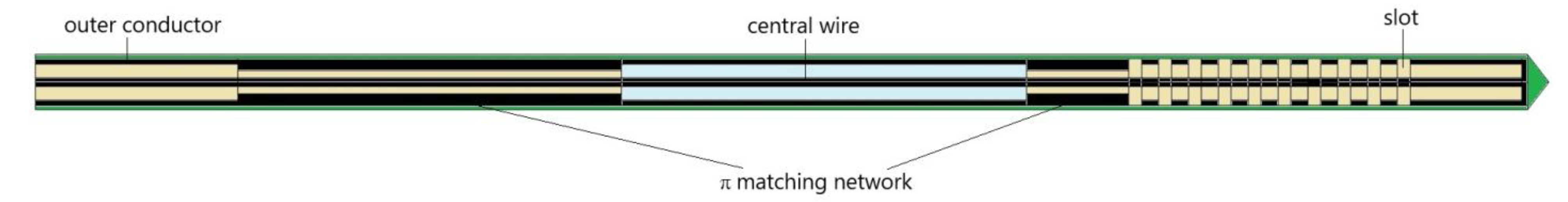
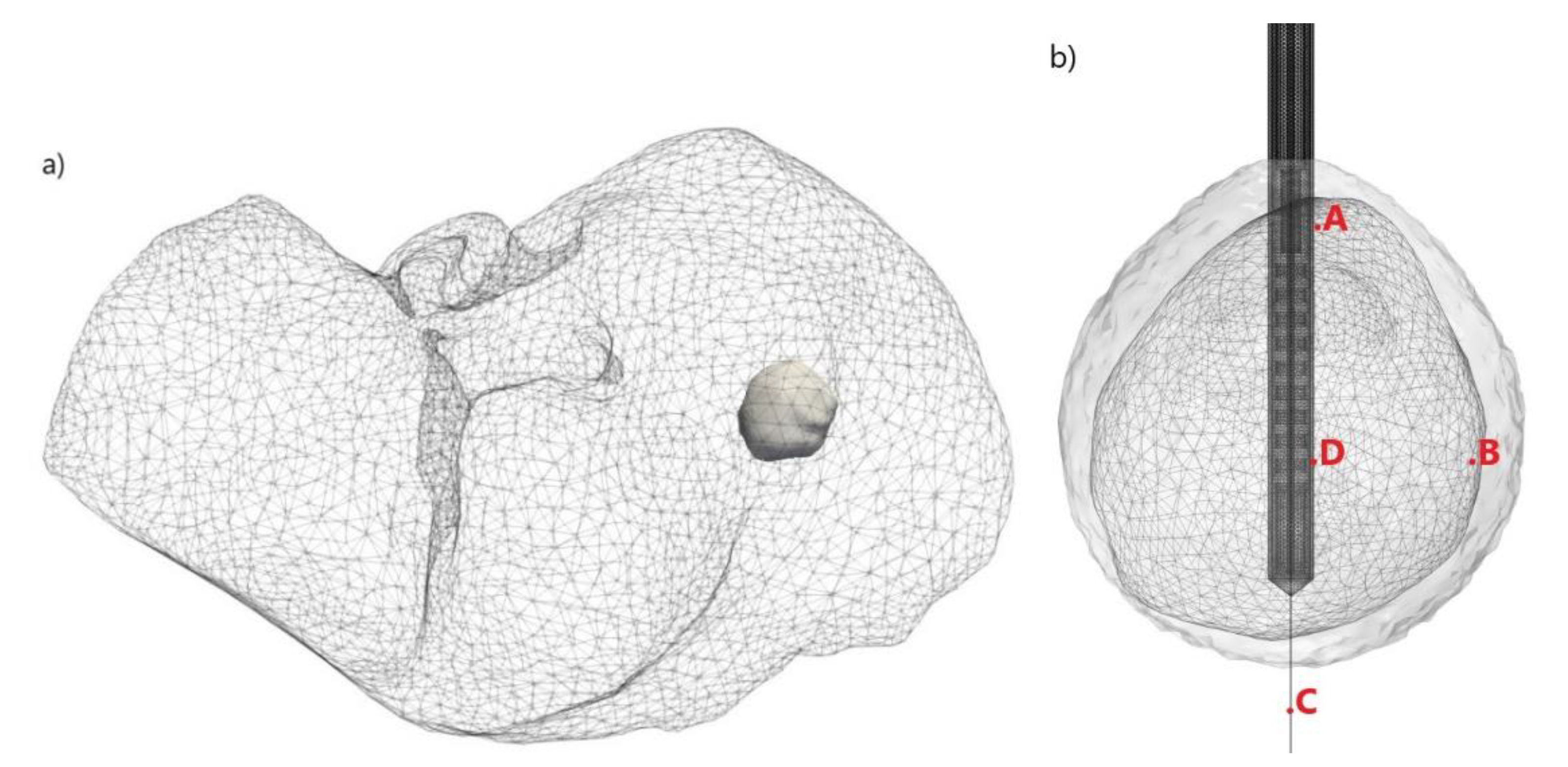
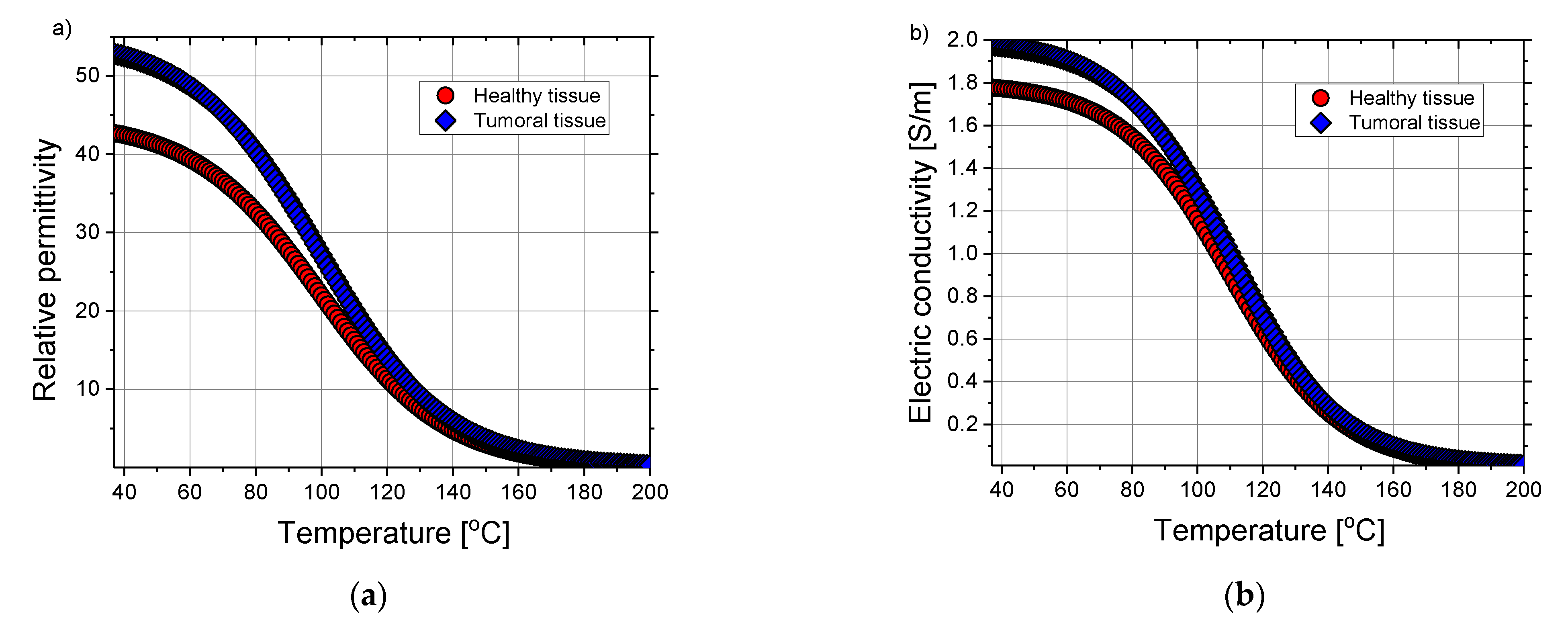
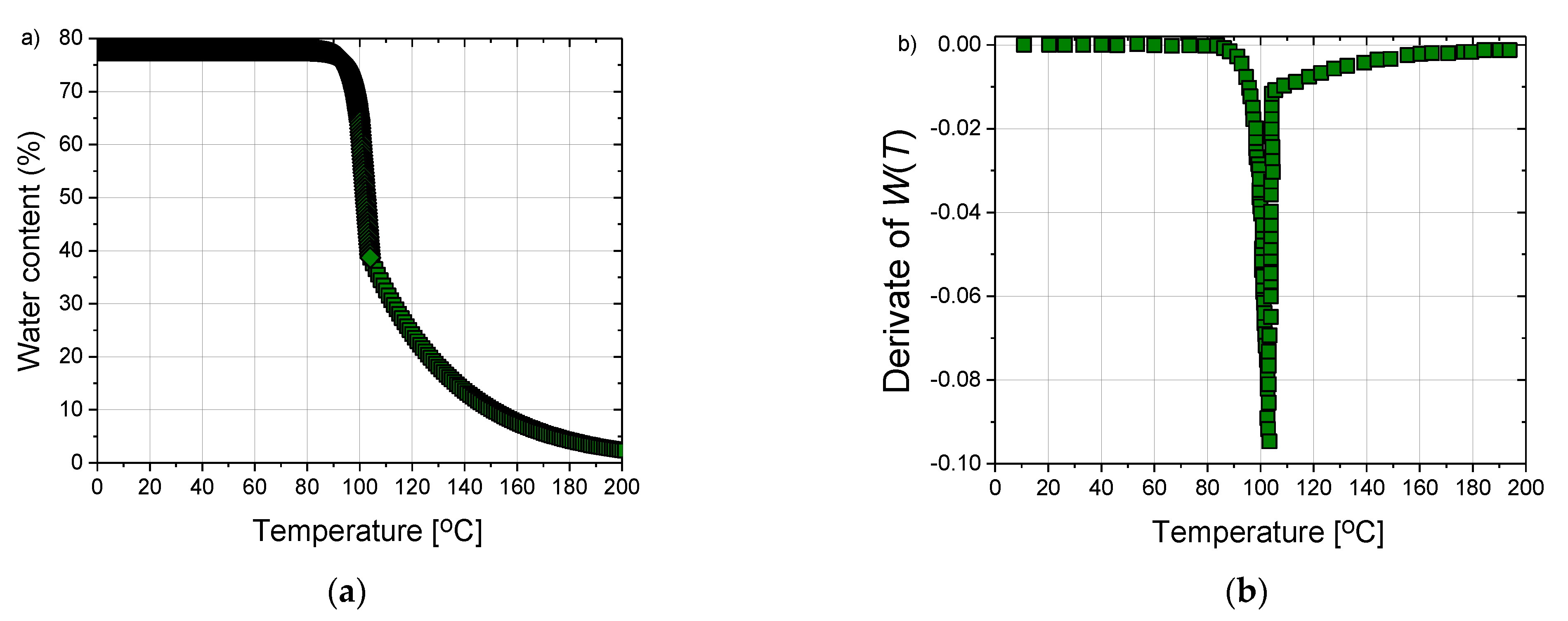

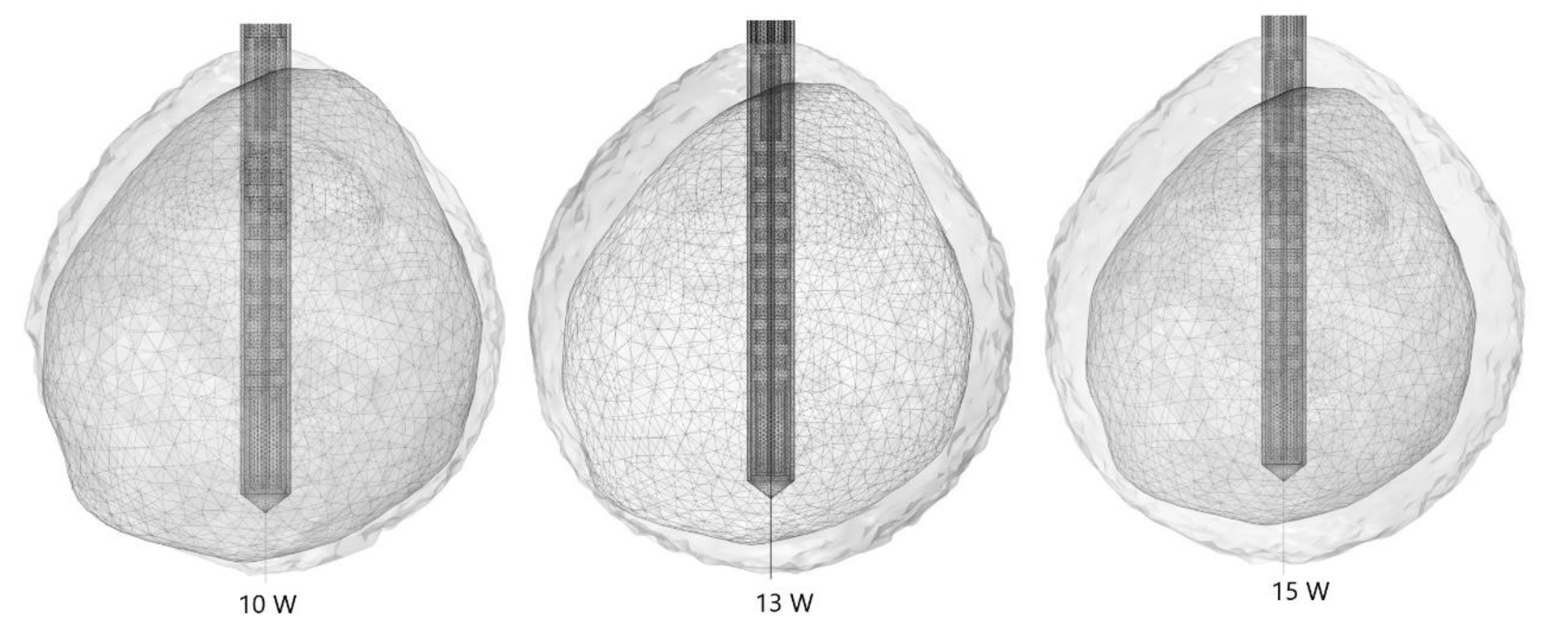
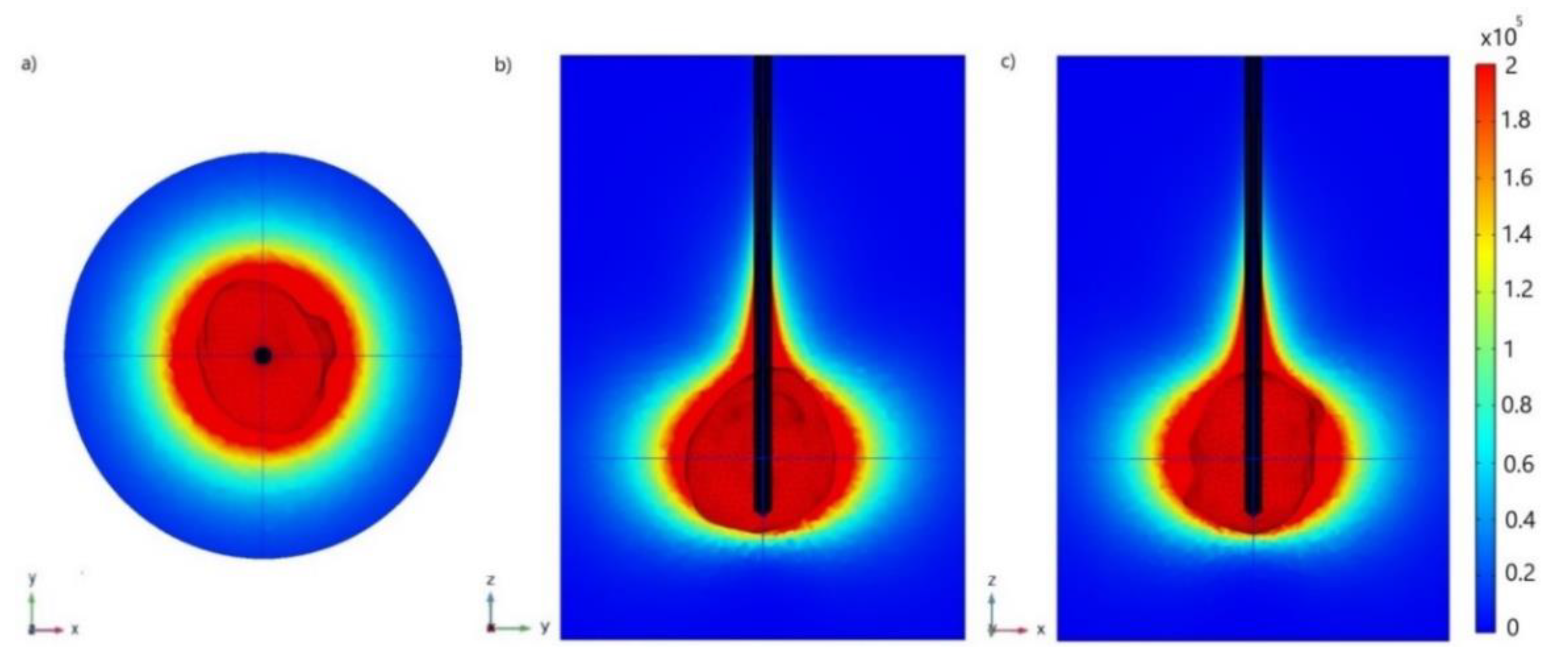


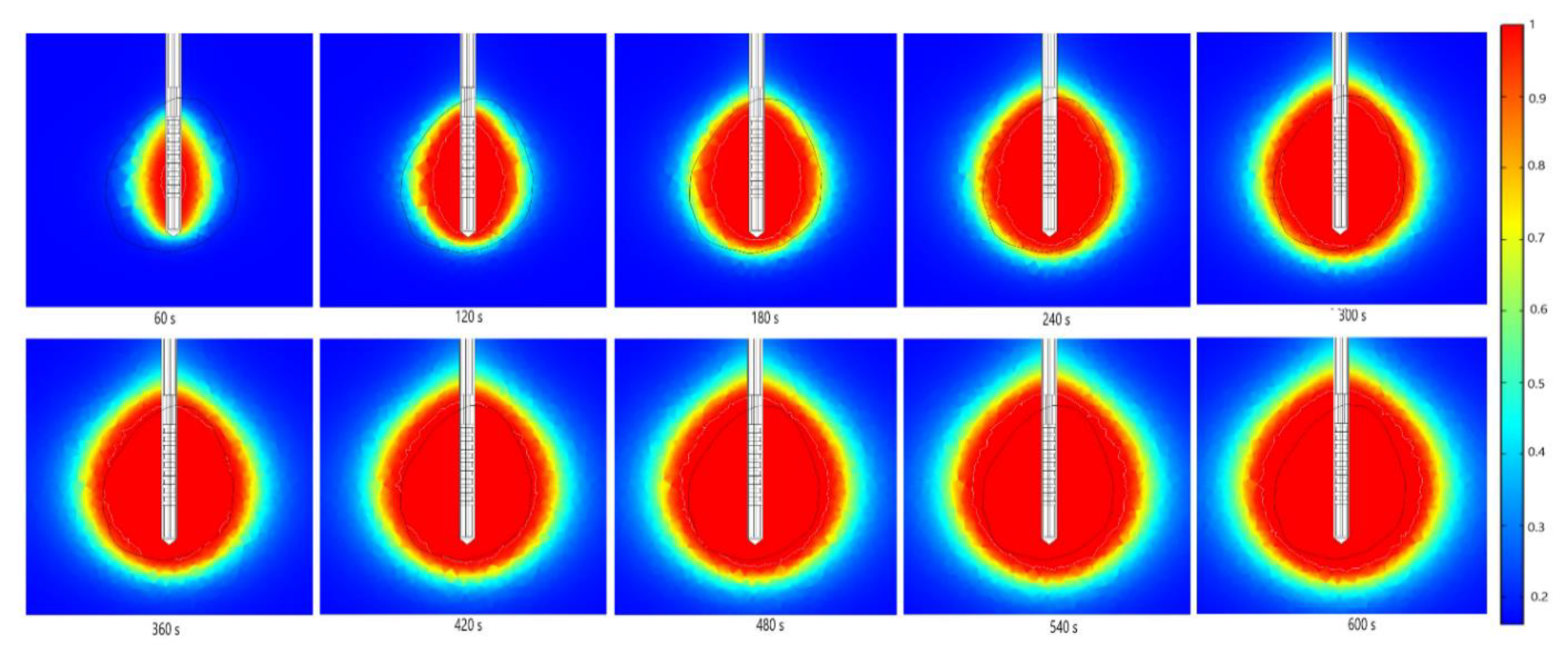

| Parameter | Value |
|---|---|
| Tissue properties | |
| Density | 1079 kg/m3 |
| Thermal conductivity | 0.52 W/m °C |
| Specific heat | 3540 J/kg °C |
| Tumor properties | |
| Density | 1040 kg/m3 |
| Thermal conductivity | 0.57 W/m °C |
| Specific heat | 3960 J/kg °C |
| Blood properties | |
| Density | 1060 kg/m3 |
| Thermal conductivity | 0.5 W/m °C |
| Specific heat | 3600 J/kg °C |
| Temperature | 37 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radjenović, B.; Sabo, M.; Šoltes, L.; Prnova, M.; Čičak, P.; Radmilović-Radjenović, M. On Efficacy of Microwave Ablation in the Thermal Treatment of an Early-Stage Hepatocellular Carcinoma. Cancers 2021, 13, 5784. https://doi.org/10.3390/cancers13225784
Radjenović B, Sabo M, Šoltes L, Prnova M, Čičak P, Radmilović-Radjenović M. On Efficacy of Microwave Ablation in the Thermal Treatment of an Early-Stage Hepatocellular Carcinoma. Cancers. 2021; 13(22):5784. https://doi.org/10.3390/cancers13225784
Chicago/Turabian StyleRadjenović, Branislav, Martin Sabo, Lukaš Šoltes, Marta Prnova, Pavel Čičak, and Marija Radmilović-Radjenović. 2021. "On Efficacy of Microwave Ablation in the Thermal Treatment of an Early-Stage Hepatocellular Carcinoma" Cancers 13, no. 22: 5784. https://doi.org/10.3390/cancers13225784
APA StyleRadjenović, B., Sabo, M., Šoltes, L., Prnova, M., Čičak, P., & Radmilović-Radjenović, M. (2021). On Efficacy of Microwave Ablation in the Thermal Treatment of an Early-Stage Hepatocellular Carcinoma. Cancers, 13(22), 5784. https://doi.org/10.3390/cancers13225784






