Can Naloxegol Therapy Improve Quality of Life in Patients with Advanced Cancer?
Abstract
:Simple Summary
Abstract
1. Introduction
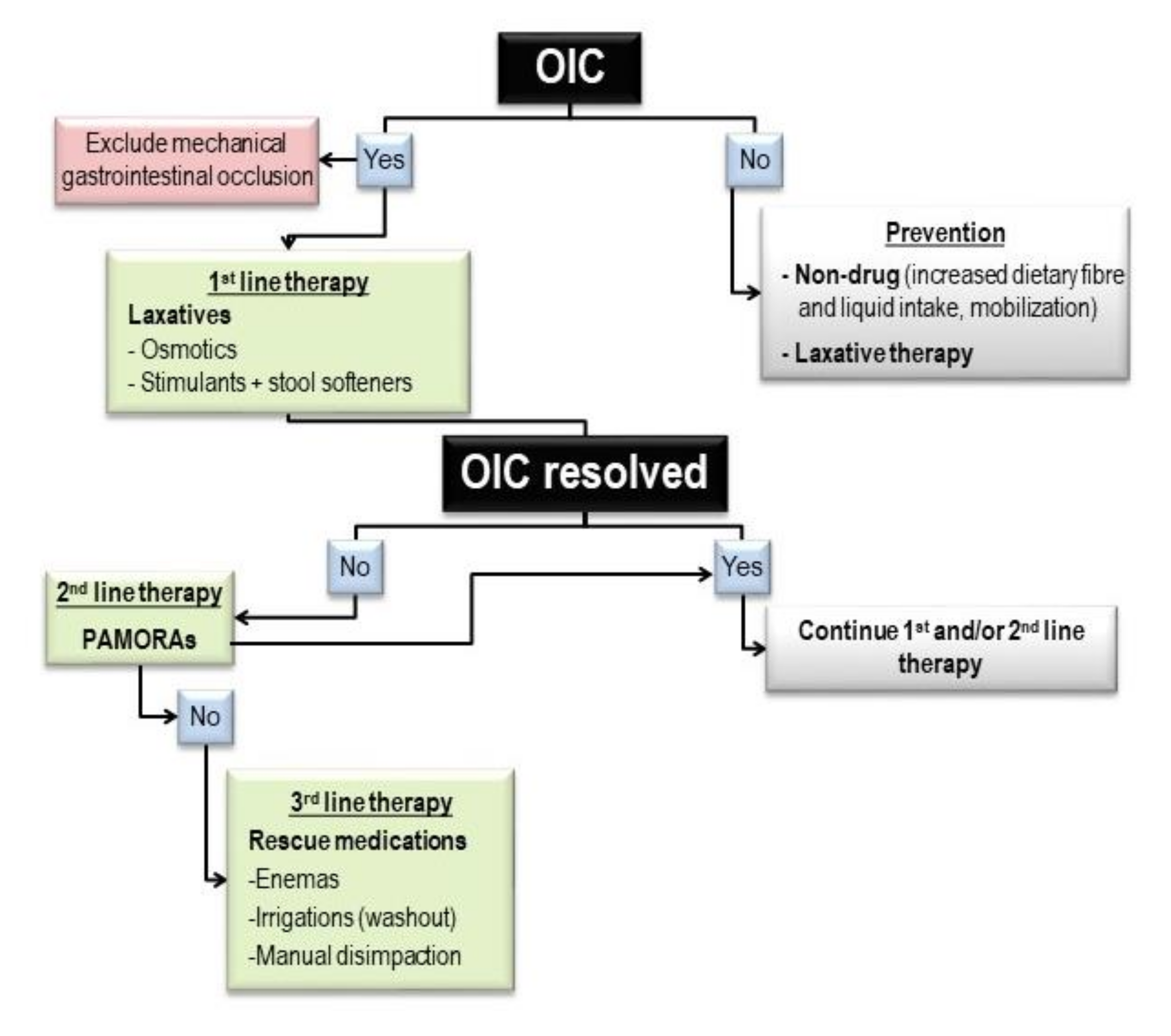
2. Materials and Methods
2.1. Setting
2.2. Study Design and Patients
2.3. Endpoints
- The objective constipation, assessed by the number of weekly spontaneous bowel movements (defined as a stool not induced by rescue medications) [29];
- The subjective perception of bowel function, assessed by the Bowel Function Index (BFI) [30], consisting of 3 numerical scales (from 0 to 100) to easily estimate OIC from the patient’s perspective: the difficulty of evacuation, the feeling of incomplete bowel evacuation and a personal judgment on constipation. The final score was calculated as the average of the scores obtained for each of the 3 scales. A BFI score greater than 30 indicated the presence of OIC [31]. The BFI was administered to patients every week;
- The intensity of the background pain, assessed by the Numerical Rating Scale (NRS) [32] every week.
2.4. Statistical Analysis
2.5. Power Analysis
3. Results
3.1. Patients and Clinical Features
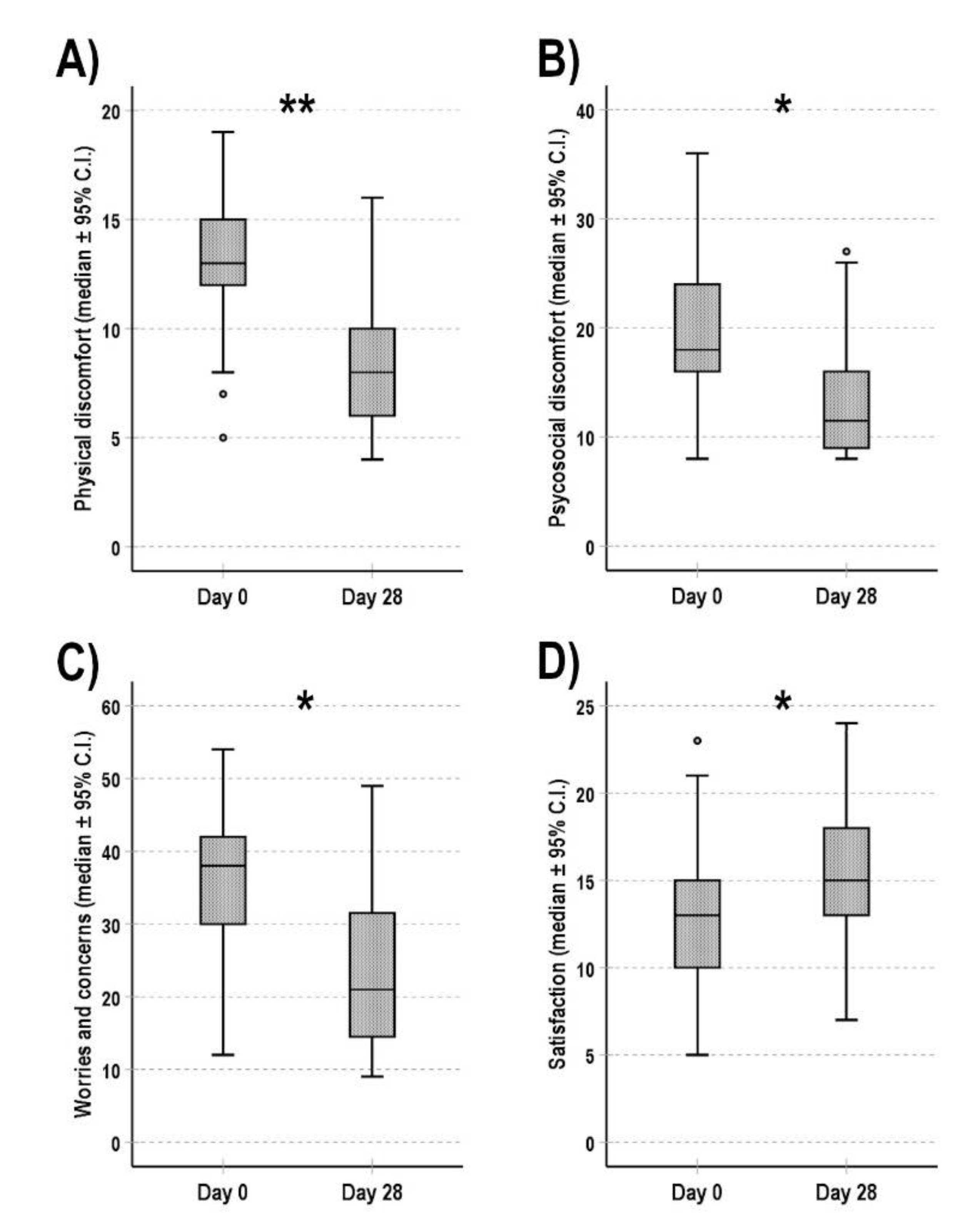
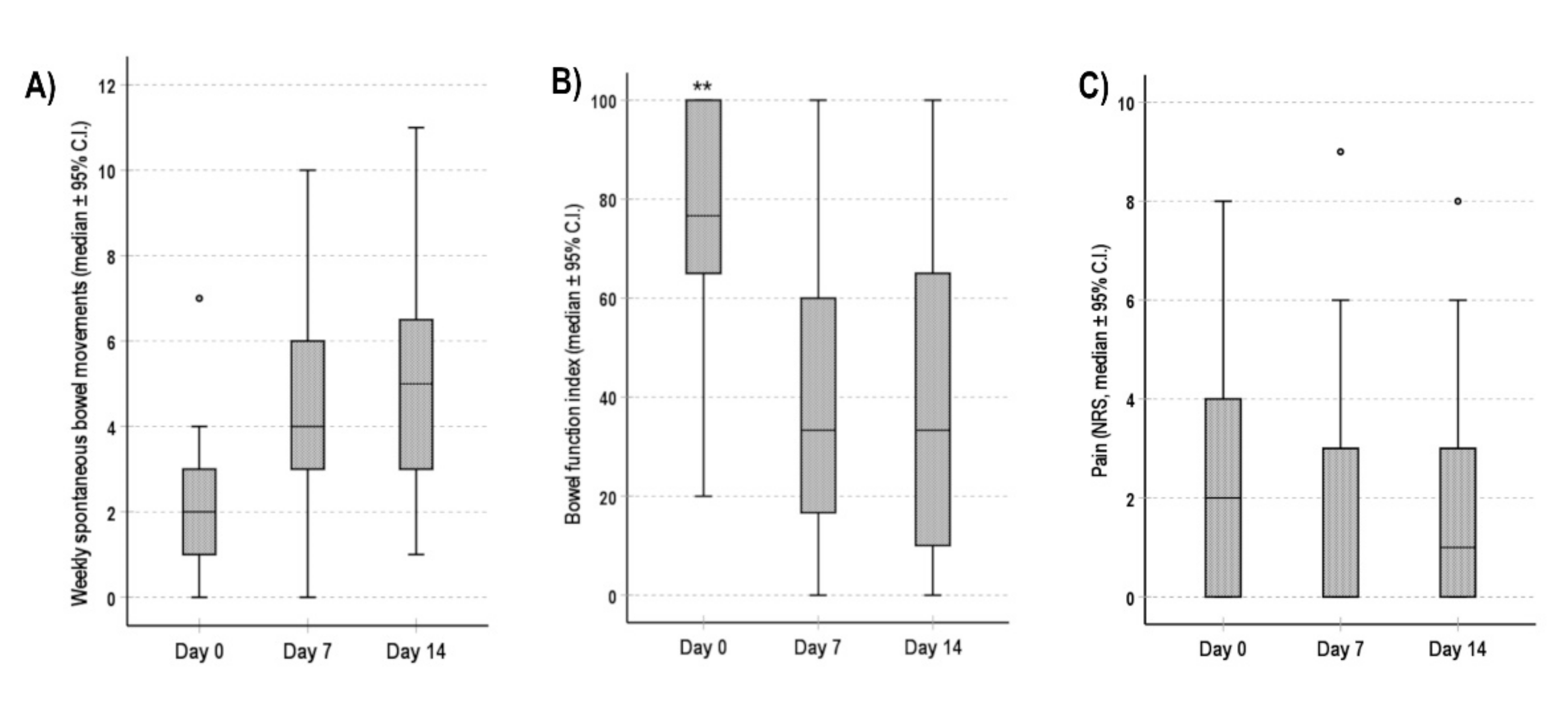
3.2. Constipation-Related Quality of Life
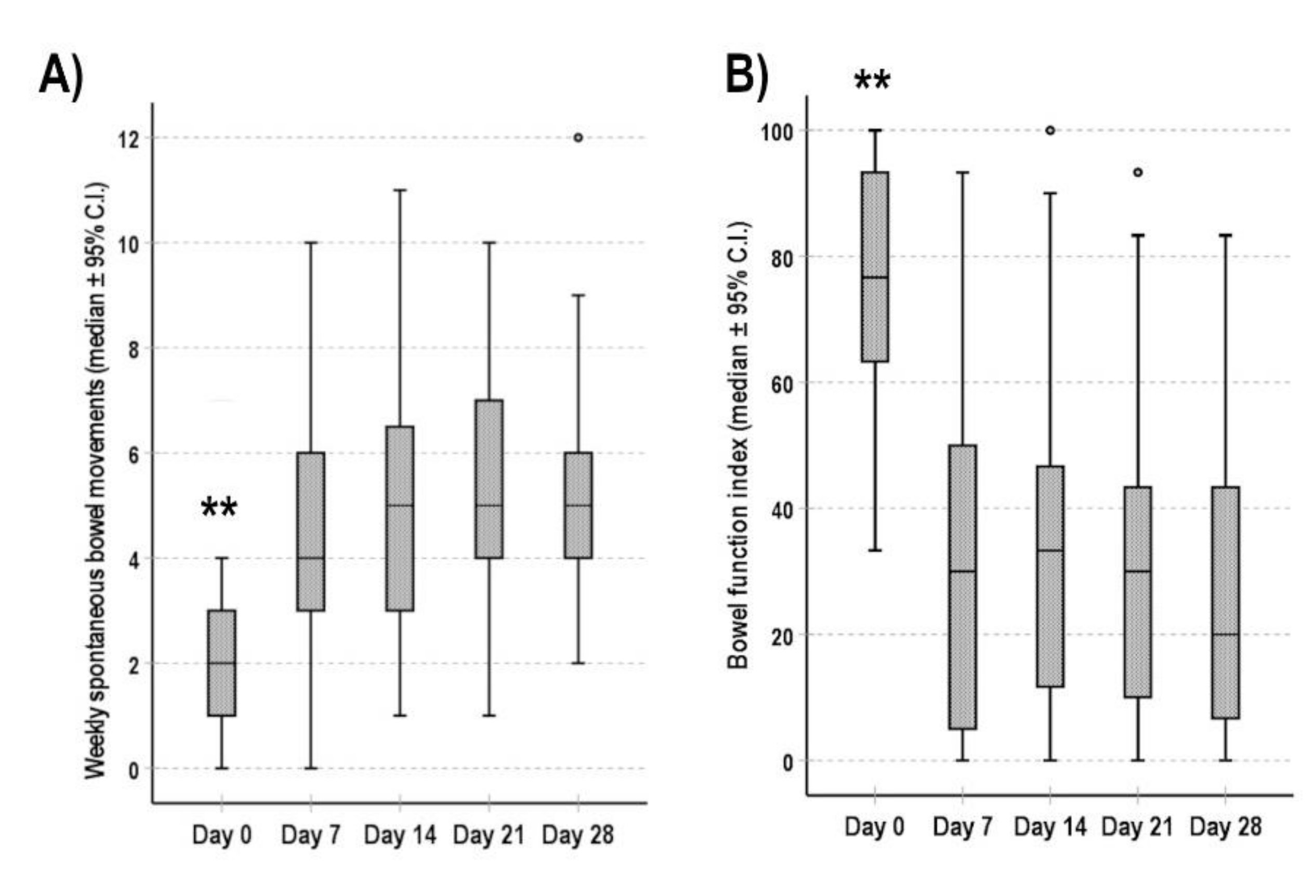
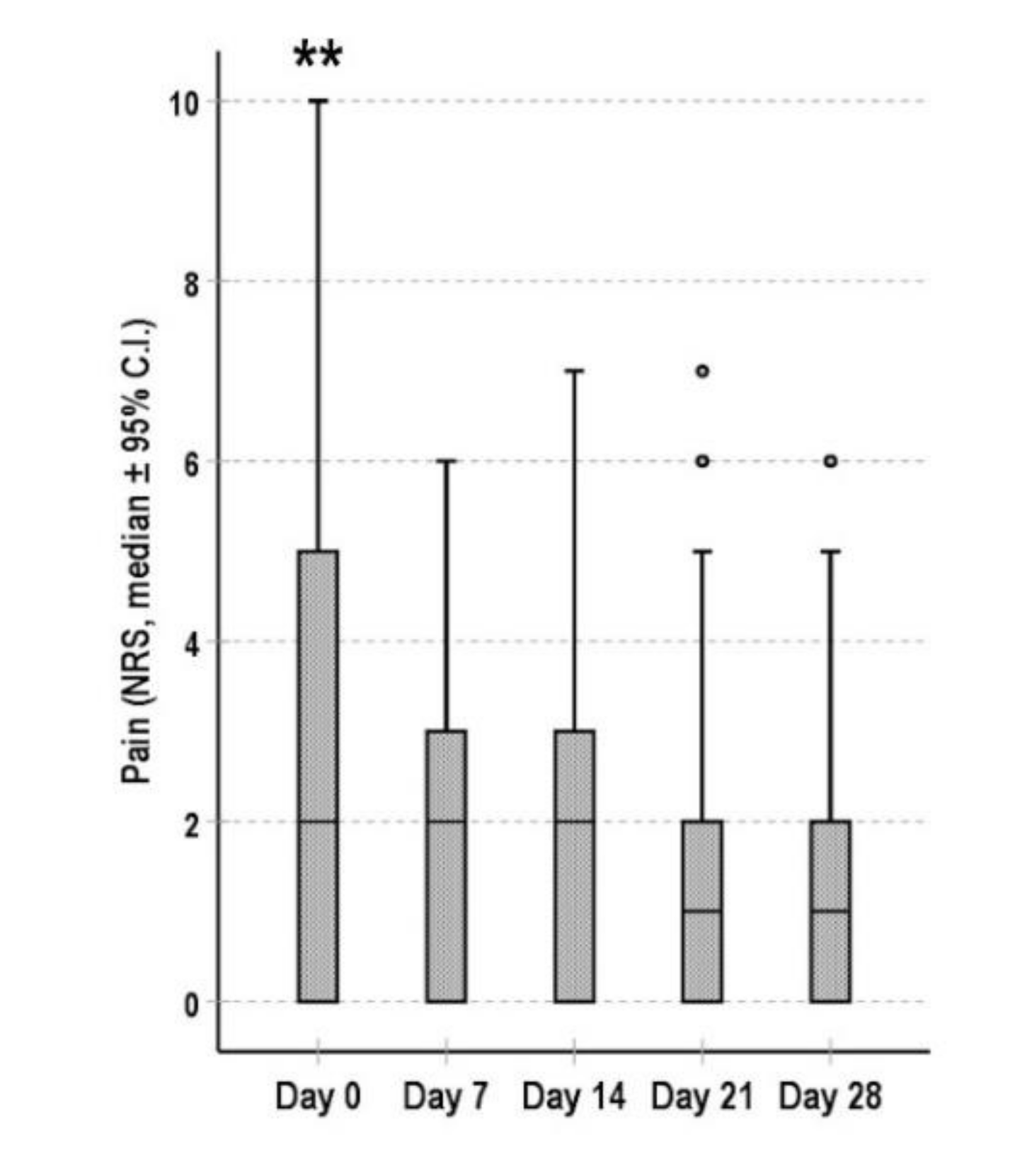
3.3. Bowel Function and Pain
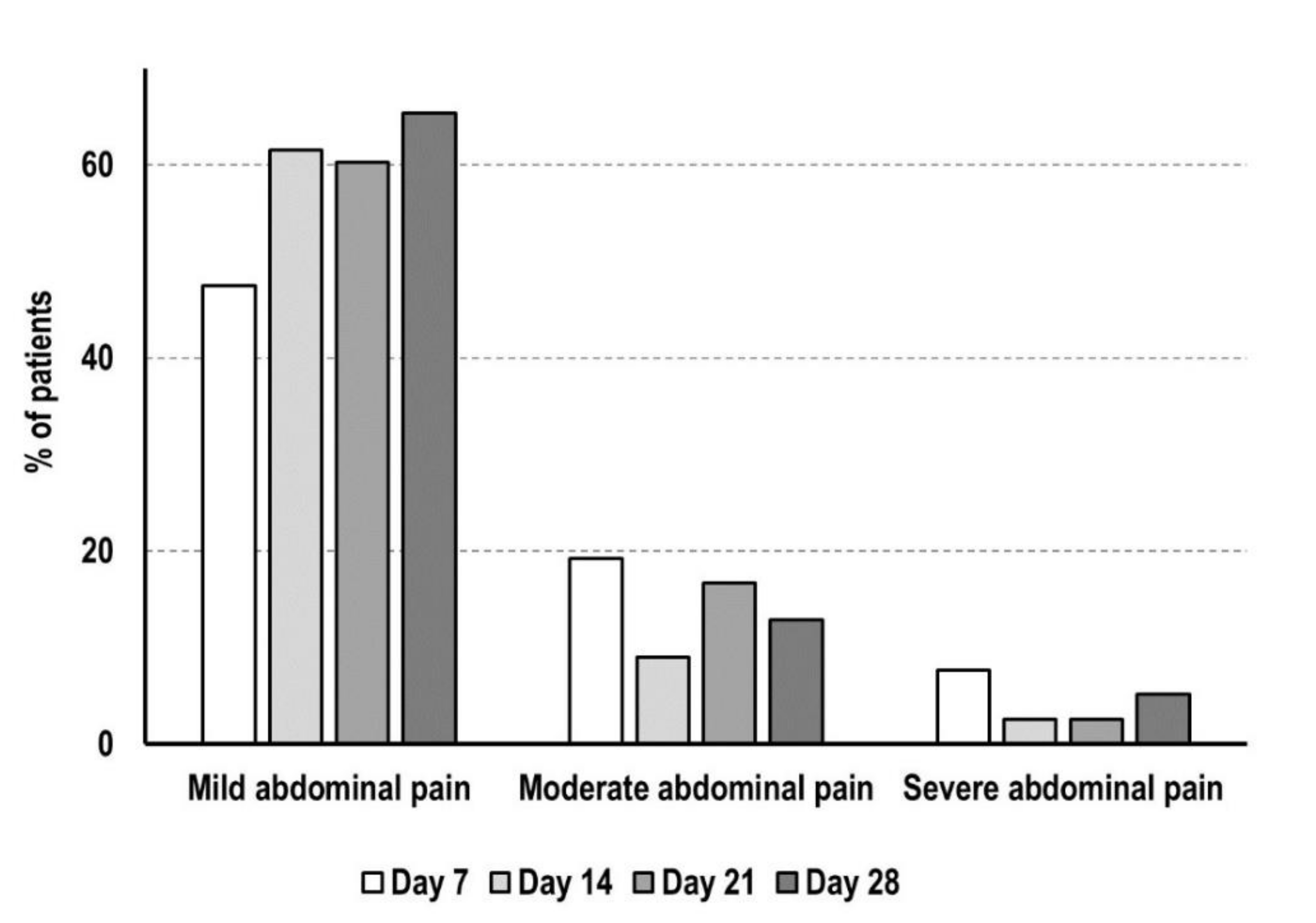
3.4. Adverse Reactions
3.5. Dropout Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vadivelu, N.; Kai, A.M.; Kodumudi, V.; Sramcik, J.; Kaye, A.D. The Opioid Crisis: A Comprehensive Overview. Curr. Pain Headache Rep. 2018, 22, 16. [Google Scholar] [CrossRef]
- Brock, C.; Olesen, S.S.; Olesen, A.E.; Frøkjaer, J.B.; Andresen, T.; Drewes, A.M. Opioid-Induced Bowel Dysfunction. Drugs 2012, 72, 1847–1865. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.D.; Galligan, J.J. Function of opioids in the enteric nervous system. Neurogastroenterol. Motil. 2004, 16, 17–28. [Google Scholar] [CrossRef]
- Kaufman, P.N.; Krevsky, B.; Malmud, L.S.; Maurer, A.H.; Somers, M.B.; Siegel, J.A.; Fisher, R.S. Role of opiate receptors in the regulation of colonic transit. Gastroenterology 1988, 94, 1351–1356. [Google Scholar] [CrossRef]
- Azizi, Z.; Anbardan, S.J.; Daryani, N.E. A review of the clinical manifestations, pathophysiology and management of opioid bowel dysfunction and narcotic bowel syndrome. Middle East J. Dig. Dis. 2014, 6, 5. [Google Scholar] [CrossRef]
- Glare, P.; Walsh, D.; Sheehan, D. The Adverse Effects of Morphine: A Prospective Survey of Common Symptoms During Repeated Dosing for Chronic Cancer Pain. Am. J. Hosp. Palliat. Med. 2006, 23, 229–235. [Google Scholar] [CrossRef]
- Larkin, P.J.; Cherny, N.I.; La Carpia, D.; Guglielmo, M.; Ostgathe, C.; Scotté, F.; Ripamonti, C.I. Diagnosis, assessment and management of constipation in advanced cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29, iv111–iv125. [Google Scholar] [CrossRef] [PubMed]
- Bell, T.J.; Panchal, S.J.; Miaskowski, C.; Bolge, S.C.; Milanova, T.; Williamson, R. The Prevalence, Severity, and Impact of Opioid-Induced Bowel Dysfunction: Results of a US and European Patient Survey (PROBE 1). Pain Med. 2009, 10, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Sykes, N.P. The relationship between opioid use and laxative use in terminally ill cancer patients. Palliat. Med. 1998, 12, 375–382. [Google Scholar] [CrossRef]
- Emmanuel, A.; Johnson, M.; McSkimming, P.; Dickerson, S. Laxatives Do Not Improve Symptoms of Opioid-Induced Constipation: Results of a Patient Survey. Pain Med. 2017, 18, 1932–1940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenner, D.M.; Stern, E.; Cash, B.D. Opioid-Related Constipation in Patients with Non-cancer Pain Syndromes: A Review of Evidence-Based Therapies and Justification for a Change in Nomenclature. Curr. Gastroenterol. Rep. 2017, 19, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Coyne, K.S.; Margolis, M.K.; Yeomans, K.; King, F.R.; Chavoshi, S.; Payne, K.A.; LoCasale, R.J. Opioid-Induced Constipation Among Patients with Chronic Noncancer Pain in the United States, Canada, Germany, and the United Kingdom: Laxative Use, Response, and Symptom Burden Over Time. Pain Med. 2015, 16, 1551–1565. [Google Scholar] [CrossRef] [Green Version]
- Green, A.F. Comparative effects of analgesics on pain threshold, respiratory frequency and gastrointestinal propulsion. Br. J. Pharmacol. Chemother. 1959, 14, 26–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drossman, D.A.; Hasler, W.L. Rome IV—Functional GI disorders: Disorders of gut-brain interaction. Gastroenterology 2016, 150, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- Lacy, B.E.; Mearin, F.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407.e5. [Google Scholar] [CrossRef] [Green Version]
- Laugsand, E.A.; Jakobsen, G.; Kaasa, S.; Klepstad, P. Inadequate symptom control in advanced cancer patients across Europe. Support. Care Cancer 2011, 19, 2005–2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larkin, P.J.; Sykes, N.P.; Centeno, C.; Ellershaw, J.E.; Elsner, F.; Eugene, B.; Gootjes, J.R.G.; Nabal, M.; Noguera, A.; Ripamonti, C.; et al. The management of constipation in palliative care: Clinical practice recommendations. Palliat. Med. 2008, 22, 796–807. [Google Scholar] [CrossRef]
- Mesía, R.; Virizuela Echaburu, J.A.; Gómez, J.; Sauri, T.; Serrano, G.; Pujol, E. Opioid-Induced Constipation in Oncological Patients: New Strategies of Management. Curr. Treat. Options Oncol. 2019, 20, 91. [Google Scholar] [CrossRef] [Green Version]
- Fallon, M.; Giusti, R.; Aielli, F.; Hoskin, P.; Rolke, R.; Sharma, M.; Ripamonti, C.I. Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29, iv166–iv191. [Google Scholar] [CrossRef]
- Farmer, A.D.; Drewes, A.M.; Chiarioni, G.; De Giorgio, R.; O’Brien, T.; Morlion, B.; Tack, J. Pathophysiology and management of opioid-induced constipation: European expert consensus statement. United Eur. Gastroenterol. J. 2019, 7, 7–20. [Google Scholar] [CrossRef] [Green Version]
- Rossi, M.; Casale, G.; Badiali, D.; Aielli, F.; Aloe Spiriti, M.A.; Arcioni, R.; Bordin, F.; Ferrara, M.; Morelli Sbarra, G.; Corcione, A.; et al. Opioid-induced bowel dysfunction: Suggestions from a multidisciplinary expert Board. Support. Care Cancer 2019, 27, 4083–4090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijayvargiya, P.P.; Camilleri, M.; Vijayvargiya, P.P.; Erwin, P.; Murad, M.H. Systematic review with meta-analysis: Efficacy and safety of treatments for opioid-induced constipation. Aliment. Pharmacol. Ther. 2020, 52, 37–53. [Google Scholar] [CrossRef]
- Cobo Dols, M.; Beato Zambrano, C.; Cabezón Gutiérrez, L.; Chicas Sett, R.; Blancas López-Barajas, M.I.; García Navalón, F.; Fírvida Pérez, J.L.; Serrano Bermúdez, G.; Togores Torres, P.; Delgado Mingorance, I.; et al. Efficacy of naloxegol on symptoms and quality of life related to opioid-induced constipation in patients with cancer: A 3-month follow-up analysis. BMJ Support. Palliat. Care 2021, 11, 25–31. [Google Scholar] [CrossRef]
- Dols, M.C.; Zambrano, C.B.; Cabezón-Gutiérrez, L.; Chicas-Sett, R.; López-Barajas, M.I.B.; Navalón, F.J.G.; Pérez, J.L.F.; Serrano Bermúdez, G.; Torres, P.T.; Mingorance, I.D.; et al. One-year efficacy and safety of naloxegol on symptoms and quality of life related to opioid-induced constipation in patients with cancer: KYONAL study. BMJ Support. Palliat. Care 2021, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Farmer, A.D.; Holt, C.B.; Downes, T.J.; Ruggeri, E.; Del Vecchio, S.; De Giorgio, R. Pathophysi0ology, diagnosis, and management of opioid-induced constipation. Lancet Gastroenterol. Hepatol. 2018, 3, 203–212. [Google Scholar] [CrossRef]
- Agenzia Italiana del Farmaco (AIFA). Nota 90. Gazz. Uff. Repubb. Ital. 2018. Serie generale n. 28. [Google Scholar]
- Nethical Vitaever—Innovation for Home Care. Available online: https://www.vitaever.com/ (accessed on 1 September 2021).
- Marquis, P.; De La Loge, C.; Dubois, D.; McDermott, A.; Chassany, O. Development and validation of the Patient Assessment of Constipation Quality of Life questionnaire. Scand. J. Gastroenterol. 2005, 40, 540–551. [Google Scholar] [CrossRef]
- Mueller-Lissner, S.; Kamm, M.A.; Wald, A.; Hinkel, U.; Koehler, U.; Richter, E.; Bubeck, J. Multicenter, 4-Week, Double-Blind, Randomized, Placebo-Controlled Trial of Sodium Picosulfate in Patients With Chronic Constipation. Am. J. Gastroenterol. 2010, 105, 897–903. [Google Scholar] [CrossRef]
- Ducrotté, P.; Caussé, C. The bowel function index: A new validated scale for assessing opioid-induced constipation. Curr. Med. Res. Opin. 2012, 28, 457–466. [Google Scholar] [CrossRef]
- Ueberall, M.; Müller-Lissner, S.; Buschmann-Kramm, C.; Bosse, B. The Bowel Function Index for Evaluating Constipation in Pain Patients: Definition of a Reference Range for a Non-Constipated Population of Pain Patients. J. Int. Med. Res. 2011, 39, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Paice, J.A.; Cohen, F.L. Validity of a verbally administered numeric rating scale to measure cancer pain intensity. Cancer Nurs. 1997, 20, 88–93. [Google Scholar] [CrossRef]
- Casadio, M.; Biasco, G.; Abernethy, A.; Bonazzi, V.; Pannuti, R.; Pannuti, F. The National Tumor Association Foundation (ANT): A 30 year old model of home palliative care. BMC Palliat. Care 2010, 9, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zavagli, V.; Raccichini, M.; Ostan, R.; Franchini, L.; Bonazzi, A.; Varani, S.; Pannuti, R. The ANT Home Care Model in Palliative and End-of-Life Care. An Investigation on Family Caregivers’ Satisfaction with the Services Provided. Transl. Med. UniSa 2021, 23, 22. [Google Scholar] [CrossRef]
- Chey, W.D.; Webster, L.; Sostek, M.; Lappalainen, J.; Barker, P.N.; Tack, J. Naloxegol for opioid-induced constipation in patients with noncancer pain. N. Engl. J. Med. 2014, 370, 2387–2396. [Google Scholar] [CrossRef] [PubMed]
- Webster, L.; Chey, W.D.; Tack, J.; Lappalainen, J.; Diva, U.; Sostek, M. Randomised clinical trial: The long-term safety and tolerability of naloxegol in patients with pain and opioid-induced constipation. Aliment. Pharmacol. Ther. 2014, 40, 771–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webster, L.; Dhar, S.; Eldon, M.; Masuoka, L.; Lappalainen, J.; Sostek, M. A phase 2, double-blind, randomized, placebo-controlled, dose-escalation study to evaluate the efficacy, safety, and tolerability of naloxegol in patients with opioid-induced constipation. Pain 2013, 154, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Tack, J.; Lappalainen, J.; Diva, U.; Tummala, R.; Sostek, M. Efficacy and safety of naloxegol in patients with opioid-induced constipation and laxative-inadequate response. United Eur. Gastroenterol. J. 2015, 3, 471–480. [Google Scholar] [CrossRef]
- Garnock-Jones, K.P. Naloxegol: A review of its use in patients with opioid-induced constipation. Drugs 2015, 75, 419–425. [Google Scholar] [CrossRef]
- Hale, M.; Wild, J.; Reddy, J.; Yamada, T.; Arjona Ferreira, J.C. Naldemedine versus placebo for opioid-induced constipation (COMPOSE-1 and COMPOSE-2): Two multicentre, phase 3, double-blind, randomised, parallel-group trials. Lancet Gastroenterol. Hepatol. 2017, 2, 555–564. [Google Scholar] [CrossRef]
- Weber, H.C. Opioid-induced constipation in chronic noncancer pain. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 11–17. [Google Scholar] [CrossRef]
- Bull, J.; Bonsignore, L.; Massie, L.; Riggs, A.; Knotkova, H.; Wellman, C.; Portenoy, R. Challenges in Recruiting Patients to a Controlled Feasibility Study of a Drug for Opioid-Induced Constipation: Lessons from the Population with Advanced Cancer. J. Pain Symptom Manag. 2019, 57, e5–e8. [Google Scholar] [CrossRef] [PubMed]
| Patients, n (%) | 150 |
|---|---|
| Men | 77 (51.3%) |
| Women | 73 (48.7%) |
| Age (mean ± St. Dev.) | 72.7 ± 11.1 |
| Geographical area, n (%) | |
| Northern Italy | 74 (49.3%) |
| Central Italy | 10 (6.7%) |
| Southern Italy | 66 (44.0%) |
| KPS (mean ± St. Dev) | 48.9 ± 13.1 |
| Survival (days), (median; 95% C.I.) | 83 (65–101) |
| Tumor primary site, n (%) | |
| Gastrointestinal | 41 (27.3%) |
| Lung | 33 (22.0%) |
| Genitourinary | 31 (20.7%) |
| Breast | 15 (10.0%) |
| Head/neck | 9 (6.0%) |
| Other a | 21 (14.0%) |
| Metastasis, n (%) | 121 (80.7%) |
| Opioid therapy, n (%) | |
| Fentanyl | 79 (52.6%) |
| Oxycodone | 37 (24.7%) |
| Buprenorphine | 10 (6.7%) |
| Morphine | 12 (8.0%) |
| Other b | 12 (8.0%) |
| Morphine equivalent dose, mean ± St. Dev. (mg/die) | 42.1 ± 36.8 |
| Duration of opioid therapy, mean ± St. Dev. (days) | 82 ± 154 |
| Laxative therapy, n (%) | |
| Osmotic | 66 (44.0%) |
| Combined | 54 (36.0%) |
| Emollient | 17 (11.3%) |
| Stimulant | 13 (8.7%) |
| Duration of laxative therapy, mean ± St. Dev. (days) | 56 ± 115 |
| Motivation for Dropping Out | Total, n (%) | Within Day 7 | Within Day 14 | Within Day 21 | Within Day 28 |
|---|---|---|---|---|---|
| Death | 21 (29.2%) | 9 | 6 | 4 | 2 |
| Severe clinical worsening | 13 (18.0%) | 3 | 3 | 7 | / |
| Serious adverse reaction | 14 (19.4%) | 13 | / | / | 1 |
| Refusal for other reasons | 10 (13.9%) | 7 | 2 | 1 | / |
| Non-adherence to therapy | 9 (12.5%) | 4 | 3 | / | 2 |
| Worsening of constipation | 5 (7.0%) | / | 2 | 2 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostan, R.; Gambino, G.; Malavasi, I.; Ronga, G.; Solipaca, M.; Spunghi, M.; Varani, S.; Pannuti, R.; Ruggeri, E. Can Naloxegol Therapy Improve Quality of Life in Patients with Advanced Cancer? Cancers 2021, 13, 5736. https://doi.org/10.3390/cancers13225736
Ostan R, Gambino G, Malavasi I, Ronga G, Solipaca M, Spunghi M, Varani S, Pannuti R, Ruggeri E. Can Naloxegol Therapy Improve Quality of Life in Patients with Advanced Cancer? Cancers. 2021; 13(22):5736. https://doi.org/10.3390/cancers13225736
Chicago/Turabian StyleOstan, Rita, Giuseppe Gambino, Italo Malavasi, Gianluca Ronga, Maria Solipaca, Michela Spunghi, Silvia Varani, Raffaella Pannuti, and Enrico Ruggeri. 2021. "Can Naloxegol Therapy Improve Quality of Life in Patients with Advanced Cancer?" Cancers 13, no. 22: 5736. https://doi.org/10.3390/cancers13225736
APA StyleOstan, R., Gambino, G., Malavasi, I., Ronga, G., Solipaca, M., Spunghi, M., Varani, S., Pannuti, R., & Ruggeri, E. (2021). Can Naloxegol Therapy Improve Quality of Life in Patients with Advanced Cancer? Cancers, 13(22), 5736. https://doi.org/10.3390/cancers13225736






