Changes in Blood Biomarkers of Angiogenesis and Immune Modulation after Radiation Therapy and Their Association with Outcomes in Thoracic Malignancies
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Treatment
2.3. Response Evaluation and Toxicity
2.4. Blood Biomarkers
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Longitudinal Assessment of Blood Biomarkers
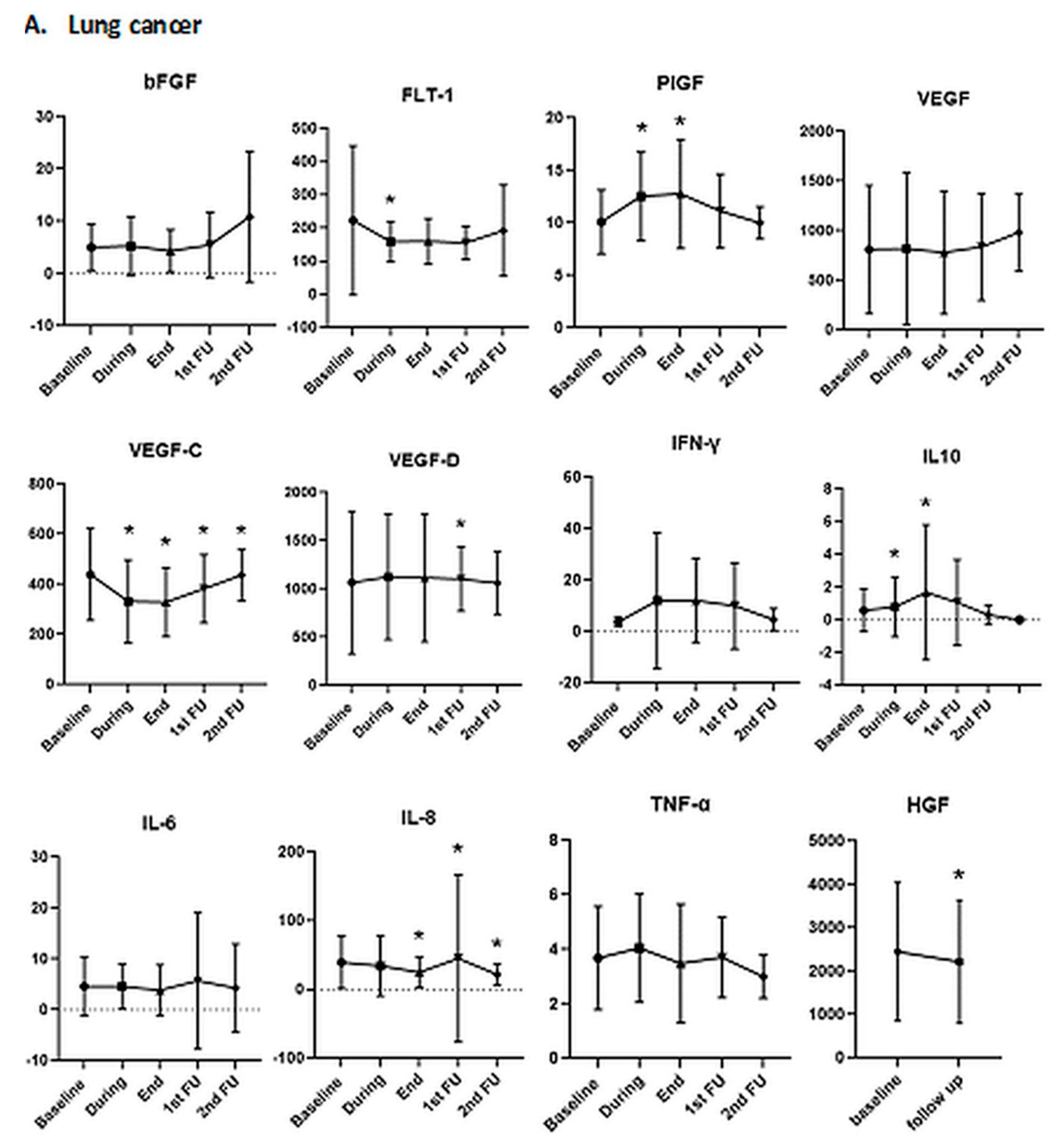
3.2.1. Association of Biomarkers with Tumor Histology
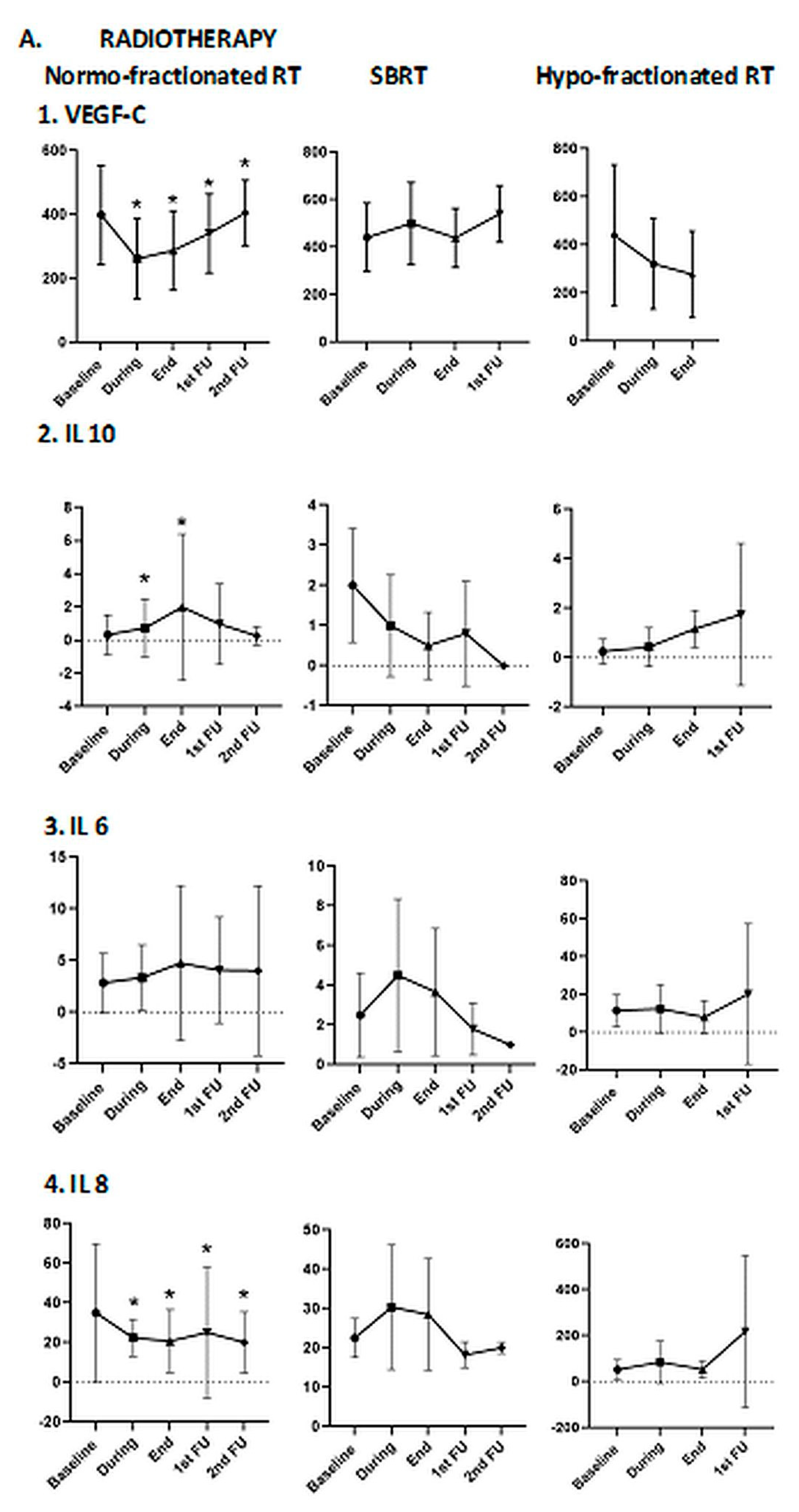
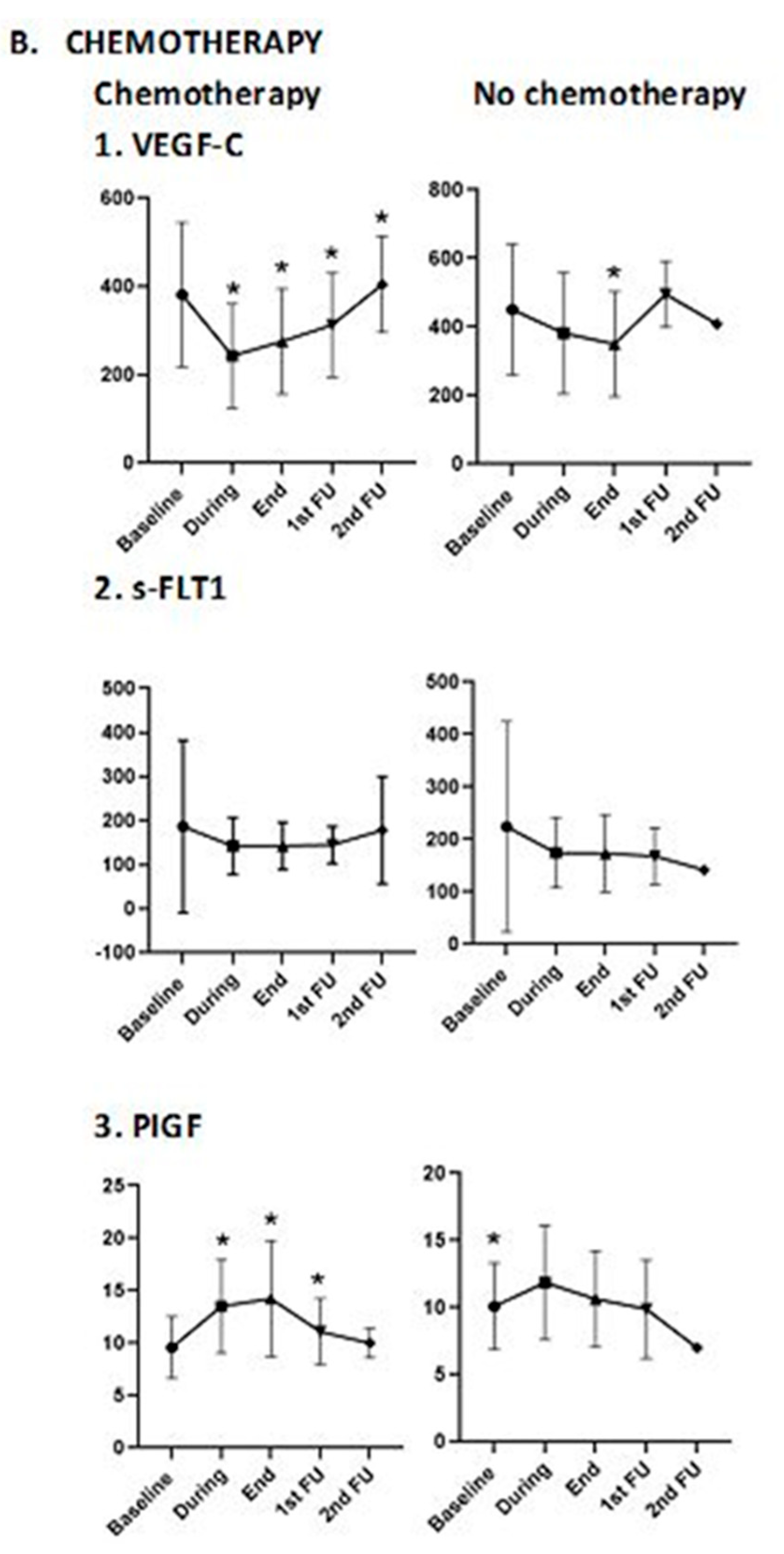
3.2.2. Association of Blood Biomarkers with Treatment
3.2.3. Correlation between Blood Biomarkers and Survival
3.2.4. Correlation between Blood Biomarkers and Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef]
- Formenti, S.C.; Demaria, S. Radiation therapy to convert the tumor into an in situ vaccine. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 879–880. [Google Scholar] [CrossRef]
- Vatner, R.E.; Cooper, B.T.; Vanpouille-Box, C.; Demaria, S.; Formenti, S.C. Combinations of immunotherapy and radiation in cancer therapy. Front. Oncol. 2014, 4, 325. [Google Scholar] [CrossRef]
- Eckert, F.; Gaipl, U.S.; Niedermann, G.; Hettich, M.; Schilbach, K.; Huber, S.M.; Zips, D. Beyond checkpoint inhibition—Immunotherapeutical strategies in combination with radiation. Clin. Transl. Radiat. Oncol. 2017, 2, 29–35. [Google Scholar] [CrossRef][Green Version]
- Ko, E.C.; Raben, D.; Formenti, S.C. The Integration of Radiotherapy with Immunotherapy for the Treatment of Non-Small Cell Lung Cancer. Clin. Cancer Res. 2018, 24, 5792–5806. [Google Scholar] [CrossRef] [PubMed]
- Rödel, F.; Frey, B.; Multhoff, G.; Gaipl, U. Contribution of the immune system to bystander and non-targeted effects of ionizing radiation. Cancer Lett. 2015, 356, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Golden, E.B.; Formenti, S.C. Is tumor (R)ejection by the immune system the “5th R” of radiobiology? Oncoimmunology 2014, 3, e28133. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Itasaka, S.; Harada, H.; Hiraoka, M. Microenvironment and radiation therapy. Biomed. Res. Int. 2013, 2013, 685308. [Google Scholar] [CrossRef] [PubMed]
- Golden, E.B.; Pellicciotta, I.; Demaria, S.; Barcellos-Hoff, M.H.; Formenti, S.C. The convergence of radiation and immunogenic cell death signaling pathways. Front. Oncol. 2012, 2, 88. [Google Scholar] [CrossRef]
- Allaoui, R.; Bergenfelz, C.; Mohlin, S.; Hagerling, C.; Salari, K.; Werb, Z.; Anderson, R.L.; Ethier, S.P.; Jirström, K.; Påhlman, S.; et al. Cancer-associated fibroblast-secreted CXCL16 attracts monocytes to promote stroma activation in triple-negative breast cancers. Nat. Commun. 2016, 7, 13050. [Google Scholar] [CrossRef]
- De Ruysscher, D.; Jin, J.; Lautenschlaeger, T.; She, J.X.; Liao, Z.; Kong, F.S. Blood-based biomarkers for precision medicine in lung cancer: Precision radiation therapy. Transl. Lung Cancer Res. 2017, 6, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Ozpiskin, O.M.; Zhang, L.; Li, J.J. Immune targets in the tumor microenvironment treated by radiotherapy. Theranostics 2019, 9, 1215–1231. [Google Scholar] [CrossRef]
- Dovedi, S.J.; Adlard, A.L.; Lipowska-Bhalla, G.; McKenna, C.; Jones, S.; Cheadle, E.J.; Stratford, I.J.; Poon, E.; Morrow, M.; Stewart, R.; et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014, 74, 5458–5468. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, E.; Lhuillier, C.; Vanpouille-Box, C.; Pilones, K.A.; García-Martínez, E.; Rudqvist, N.P.; Formenti, S.C.; Demaria, S. Barriers to Radiation-Induced in Situ Tumor Vaccination. Front. Immunol. 2017, 8, 229. [Google Scholar] [CrossRef]
- Theelen, W.; Peulen, H.; Lalezari, F.; Vries, J.D.; Langen, J.D.; Aerts, J.; Monkhorst, K.; Baas, P. Randomized phase II study of pembrolizumab after stereotactic body radiotherapy (SBRT) versus pembrolizumab alone in patients with advanced non-small cell lung cancer: The PEMBRO-RT study. J. Clin. Oncol. 2018, 36, 9023. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.J.; Ajani, J.A.; Kuzdzal, J.; Zander, T.; Van Cutsem, E.; Piessen, G.; Mendez, G.; Feliciano, J.; Motoyama, S.; Lièvre, A.; et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N. Engl. J. Med. 2021, 384, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Gkika, E.; Vach, W.; Adebahr, S.; Schimek-Jasch, T.; Brenner, A.; Brunner, T.B.; Kaier, K.; Prasse, A.; Muller-Quernheim, J.; Grosu, A.L.; et al. Is serum level of CC chemokine ligand 18 a biomarker for the prediction of radiation induced lung toxicity (RILT)? PLoS ONE 2017, 12, e0185350. [Google Scholar] [CrossRef]
- Nestle, U.; Adebahr, S.; Kaier, K.; Gkika, E.; Schimek-Jasch, T.; Hechtner, M.; Momm, F.; Gaertner, J.; Becker, G.; Grosu, A.L. Quality of life after pulmonary stereotactic fractionated radiotherapy (SBRT): Results of the phase II STRIPE trial. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2020, 148, 82–88. [Google Scholar] [CrossRef]
- Heist, R.S.; Duda, D.G.; Sahani, D.V.; Ancukiewicz, M.; Fidias, P.; Sequist, L.V.; Temel, J.S.; Shaw, A.T.; Pennell, N.A.; Neal, J.W.; et al. Improved tumor vascularization after anti-VEGF therapy with carboplatin and nab-paclitaxel associates with survival in lung cancer. Proc. Natl. Acad. Sci. USA 2015, 112, 1547–1552. [Google Scholar] [CrossRef]
- Hong, T.S.; Grassberger, C.; Yeap, B.Y.; Jiang, W.; Wo, J.Y.; Goyal, L.; Clark, J.W.; Crane, C.H.; Koay, E.J.; Dima, S.; et al. Pretreatment plasma HGF as potential biomarker for susceptibility to radiation-induced liver dysfunction after radiotherapy. NPJ Precis. Oncol. 2018, 2, 22. [Google Scholar] [CrossRef]
- Dranoff, G. Cytokines in cancer pathogenesis and cancer therapy. Nat. Rev. Cancer 2004, 4, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Tracey, K.J.; Cerami, A. Tumor necrosis factor: A pleiotropic cytokine and therapeutic target. Annu. Rev. Med. 1994, 45, 491–503. [Google Scholar] [CrossRef]
- Nam, J.-S.; Suchar, A.M.; Kang, M.-J.; Stuelten, C.H.; Tang, B.; Michalowska, A.M.; Fisher, L.W.; Fedarko, N.S.; Jain, A.; Pinkas, J.; et al. Bone sialoprotein mediates the tumor cell-targeted prometastatic activity of transforming growth factor beta in a mouse model of breast cancer. Cancer Res. 2006, 66, 6327–6335. [Google Scholar] [CrossRef]
- Salem, A.; Mistry, H.; Backen, A.; Hodgson, C.; Koh, P.; Dean, E.; Priest, L.; Haslett, K.; Trigonis, I.; Jackson, A.; et al. Cell Death, Inflammation, Tumor Burden, and Proliferation Blood Biomarkers Predict Lung Cancer Radiotherapy Response and Correlate with Tumor Volume and Proliferation Imaging. Clin. Lung Cancer 2018, 19, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Ao, X.; Zhao, L.; Davis, M.A.; Lubman, D.M.; Lawrence, T.S.; Kong, F.M. Radiation produces differential changes in cytokine profiles in radiation lung fibrosis sensitive and resistant mice. J. Hematol. Oncol. 2009, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Łukaszewicz-Zając, M.; Mroczko, B.; Kozłowski, M.; Nikliński, J.; Laudański, J.; Szmitkowski, M. Higher importance of interleukin 6 than classic tumor markers (carcinoembryonic antigen and squamous cell cancer antigen) in the diagnosis of esophageal cancer patients. Dis. Esophagus 2012, 25, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Yousif, N.G.; Al-Amran, F.G.; Hadi, N.; Lee, J.; Adrienne, J. Expression of IL-32 modulates NF-κB and p38 MAP kinase pathways in human esophageal cancer. Cytokine 2013, 61, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.A.; Nisar, S.; Maacha, S.; Carneiro-Lobo, T.C.; Akhtar, S.; Siveen, K.S.; Wani, N.A.; Rizwan, A.; Bagga, P.; Singh, M.; et al. Cytokine-chemokine network driven metastasis in esophageal cancer; promising avenue for targeted therapy. Mol. Cancer 2021, 20, 2. [Google Scholar] [CrossRef] [PubMed]
- Ryan, B.M.; Pine, S.R.; Chaturvedi, A.K.; Caporaso, N.; Harris, C.C. A combined prognostic serum interleukin-8 and interleukin-6 classifier for stage 1 lung cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. J. Thorac. Oncol. 2014, 9, 1494–1503. [Google Scholar] [CrossRef]
- Nishimoto, N.; Sasai, M.; Shima, Y.; Nakagawa, M.; Matsumoto, T.; Shirai, T.; Kishimoto, T.; Yoshizaki, K. Improvement in Castleman’s disease by humanized anti-interleukin-6 receptor antibody therapy. Blood 2000, 95, 56–61. [Google Scholar] [CrossRef]
- Liao, C.; Yu, Z.; Guo, W.; Liu, Q.; Wu, Y.; Li, Y.; Bai, L. Prognostic value of circulating inflammatory factors in non-small cell lung cancer: A systematic review and meta-analysis. Cancer Biomark. Sect. A Dis. Markers 2014, 14, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.A.; Jenkins, B.J. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat. Rev. Immunol. 2018, 18, 773–789. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.; Putoczki, T.L. Targeting IL-11 signaling in colon cancer. Oncotarget 2013, 4, 1860–1861. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin. Immunol. 2014, 26, 54–74. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lee, H.; Herrmann, A.; Buettner, R.; Jove, R. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat. Rev. Cancer 2014, 14, 736–746. [Google Scholar] [CrossRef]
- Li, N.; Grivennikov, S.I.; Karin, M. The unholy trinity: Inflammation, cytokines, and STAT3 shape the cancer microenvironment. Cancer Cell 2011, 19, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Tye, H.; Kennedy, C.L.; Najdovska, M.; McLeod, L.; McCormack, W.; Hughes, N.; Dev, A.; Sievert, W.; Ooi, C.H.; Ishikawa, T.-O.; et al. STAT3-Driven Upregulation of TLR2 Promotes Gastric Tumorigenesis Independent of Tumor Inflammation. Cancer Cell 2012, 22, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, X. Association between serum cytokines and progression of breast cancer in Chinese population. Medicine 2017, 96, e8840. [Google Scholar] [CrossRef]
- Laino, A.S.; Woods, D.; Vassallo, M.; Qian, X.; Tang, H.; Wind-Rotolo, M.; Weber, J. Serum interleukin-6 and C-reactive protein are associated with survival in melanoma patients receiving immune checkpoint inhibition. J. Immunother. Cancer 2020, 8, e000842. [Google Scholar] [CrossRef]
- Keegan, A.; Ricciuti, B.; Garden, P.; Cohen, L.; Nishihara, R.; Adeni, A.; Paweletz, C.; Supplee, J.; Jänne, P.A.; Severgnini, M.; et al. Plasma IL-6 changes correlate to PD-1 inhibitor responses in NSCLC. J. Immunother. Cancer 2020, 8, e000678. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, V.; Agolli, L.; Visco, V.; Monaco, F.; Muni, R.; Spagnoli, A.; Campanella, B.; Valeriani, M.; Minniti, G.; Osti, M.F.; et al. Cytokines, fatigue, and cutaneous erythema in early stage breast cancer patients receiving adjuvant radiation therapy. BioMed Res. Int. 2014, 2014, 523568. [Google Scholar] [CrossRef]
- Muraro, E.; Furlan, C.; Avanzo, M.; Martorelli, D.; Comaro, E.; Rizzo, A.; Fae, D.A.; Berretta, M.; Militello, L.; Del Conte, A.; et al. Local High-Dose Radiotherapy Induces Systemic Immunomodulating Effects of Potential Therapeutic Relevance in Oligometastatic Breast Cancer. Front. Immunol. 2017, 8, 1476. [Google Scholar] [CrossRef]
- Alfaro, C.; Sanmamed, M.F.; Rodríguez-Ruiz, M.E.; Teijeira, Á.; Oñate, C.; González, Á.; Ponz, M.; Schalper, K.A.; Pérez-Gracia, J.L.; Melero, I. Interleukin-8 in cancer pathogenesis, treatment and follow-up. Cancer Treat. Rev. 2017, 60, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.; Debnath, B.; Neamati, N. Role of the CXCL8-CXCR1/2 Axis in Cancer and Inflammatory Diseases. Theranostics 2017, 7, 1543–1588. [Google Scholar] [CrossRef] [PubMed]
- Bakouny, Z.; Choueiri, T.K. IL-8 and cancer prognosis on immunotherapy. Nat. Med. 2020, 26, 650–651. [Google Scholar] [CrossRef]
- Schalper, K.A.; Carleton, M.; Zhou, M.; Chen, T.; Feng, Y.; Huang, S.-P.; Walsh, A.M.; Baxi, V.; Pandya, D.; Baradet, T.; et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat. Med. 2020, 26, 688–692. [Google Scholar] [CrossRef]
- Yuen, K.C.; Liu, L.-F.; Gupta, V.; Madireddi, S.; Keerthivasan, S.; Li, C.; Rishipathak, D.; Williams, P.; Kadel, E.E.; Koeppen, H.; et al. High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nat. Med. 2020, 26, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; Demaria, S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef]
- Rube, C.E.; Palm, J.; Erren, M.; Fleckenstein, J.; Konig, J.; Remberger, K.; Rube, C. Cytokine plasma levels: Reliable predictors for radiation pneumonitis? PLoS ONE 2008, 3, e2898. [Google Scholar] [CrossRef]
- Rube, C.E.; Wilfert, F.; Palm, J.; Konig, J.; Burdak-Rothkamm, S.; Liu, L.; Schuck, A.; Willich, N.; Rube, C. Irradiation induces a biphasic expression of pro-inflammatory cytokines in the lung. Strahlentherapie und Onkologie Organ der Deutschen Rontgengesellschaft 2004, 180, 442–448. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, L.; Ji, W.; Wang, X.; Zhu, X.; Hayman, J.A.; Kalemkerian, G.P.; Yang, W.; Brenner, D.; Lawrence, T.S.; et al. Elevation of plasma TGF-beta1 during radiation therapy predicts radiation-induced lung toxicity in patients with non-small-cell lung cancer: A combined analysis from Beijing and Michigan. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Stenmark, M.H.; Cai, X.W.; Shedden, K.; Hayman, J.A.; Yuan, S.; Ritter, T.; Ten Haken, R.K.; Lawrence, T.S.; Kong, F.M. Combining physical and biologic parameters to predict radiation-induced lung toxicity in patients with non-small-cell lung cancer treated with definitive radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, e217–e222. [Google Scholar] [CrossRef] [PubMed]
- Sprung, C.N.; Forrester, H.B.; Siva, S.; Martin, O.A. Immunological markers that predict radiation toxicity. Cancer Lett. 2015, 368, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Mazeron, R.; Etienne-Mastroianni, B.; Perol, D.; Arpin, D.; Vincent, M.; Falchero, L.; Martel-Lafay, I.; Carrie, C.; Claude, L. Predictive factors of late radiation fibrosis: A prospective study in non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 38–43. [Google Scholar] [CrossRef]
- Fu, X.L.; Huang, H.; Bentel, G.; Clough, R.; Jirtle, R.L.; Kong, F.M.; Marks, L.B.; Anscher, M.S. Predicting the risk of symptomatic radiation-induced lung injury using both the physical and biologic parameters V(30) and transforming growth factor beta. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 899–908. [Google Scholar] [CrossRef]
- Moriconi, F.; Christiansen, H.; Raddatz, D.; Dudas, J.; Hermann, R.M.; Rave-Fränk, M.; Sheikh, N.; Saile, B.; Hess, C.F.; Ramadori, G. Effect of radiation on gene expression of rat liver chemokines: In vivo and in vitro studies. Radiat. Res. 2008, 169, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, H.; Saile, B.; Neubauer-Saile, K.; Tippelt, S.; Rave-Fränk, M.; Hermann, R.M.; Dudas, J.; Hess, C.F.; Schmidberger, H.; Ramadori, G. Irradiation leads to susceptibility of hepatocytes to TNF-alpha mediated apoptosis. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2004, 72, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Rubin, P.; Williams, J.; Hernady, E.; Smudzin, T.; Okunieff, P. Circulating IL-6 as a predictor of radiation pneumonitis. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 641–648. [Google Scholar] [CrossRef]
- Hart, J.P.; Broadwater, G.; Rabbani, Z.; Moeller, B.J.; Clough, R.; Huang, D.; Sempowski, G.A.; Dewhirst, M.; Pizzo, S.V.; Vujaskovic, Z.; et al. Cytokine profiling for prediction of symptomatic radiation-induced lung injury. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 1448–1454. [Google Scholar] [CrossRef]
- Bodelon, C.; Polley, M.Y.; Kemp, T.J.; Pesatori, A.C.; McShane, L.M.; Caporaso, N.E.; Hildesheim, A.; Pinto, L.A.; Landi, M.T. Circulating levels of immune and inflammatory markers and long versus short survival in early-stage lung cancer. Ann. Oncol. 2013, 24, 2073–2079. [Google Scholar] [CrossRef] [PubMed]
- Enewold, L.; Mechanic, L.E.; Bowman, E.D.; Zheng, Y.L.; Yu, Z.; Trivers, G.; Alberg, A.J.; Harris, C.C. Serum concentrations of cytokines and lung cancer survival in African Americans and Caucasians. Cancer Epidemiol. Biomark. Prev. 2009, 18, 215–222. [Google Scholar] [CrossRef] [PubMed]


| Variable | Number of Patients (%) |
|---|---|
| Gender | |
| Male | 38 (68%) |
| Female | 18 (32%) |
| COPD | |
| COPD Gold 3–4 * | 35 (62.5%) |
| COPD Gold 0–2 | 8 (14.3%) |
| Tumor type | |
| Lung cancer | 41 (73.3%) |
| Esophageal cancer | 13 (23.1%) |
| other | 2 (3.6%) |
| Histology | |
| Squamous cell carcinoma | 24 (42.9%) |
| Adenocarcinoma | 20 (37.5%) |
| Small cell lung cancer | 3 (5.4%) |
| Large cell carcinoma | 2 (3.6%) |
| other | 7 (12.5%) |
| Tumor stage | |
| T1 | 8 (14.3%) |
| T2 | 16 (28.6%) |
| T3 | 22 (39.3%) |
| T4 | 10 (17.9%) |
| N0 | 17 (30.4%) |
| N1 | 7 (12.5%) |
| N2 | 23 (41.1%) |
| N3 | 8 (14.3%) |
| M0 | 49 (87.5%) |
| M1 | 7 (12.5%) |
| Type of treatment | |
| Adjuvant | 8 (14.3%) |
| Concurrent chemoradiation | 35 (62.5%) |
| Stereotactic body radiotherapy | 6 (10.7%) |
| Palliative radiotherapy | 7 (12.5%) |
| Chemotherapy | |
| Yes | 35 (62.5%) |
| No | 21 (37.5%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkika, E.; Adebahr, S.; Brenner, A.; Schimek-Jasch, T.; Radicioni, G.; Exner, J.-P.; Rühle, A.; Spohn, S.K.B.; Popp, I.; Zamboglou, C.; et al. Changes in Blood Biomarkers of Angiogenesis and Immune Modulation after Radiation Therapy and Their Association with Outcomes in Thoracic Malignancies. Cancers 2021, 13, 5725. https://doi.org/10.3390/cancers13225725
Gkika E, Adebahr S, Brenner A, Schimek-Jasch T, Radicioni G, Exner J-P, Rühle A, Spohn SKB, Popp I, Zamboglou C, et al. Changes in Blood Biomarkers of Angiogenesis and Immune Modulation after Radiation Therapy and Their Association with Outcomes in Thoracic Malignancies. Cancers. 2021; 13(22):5725. https://doi.org/10.3390/cancers13225725
Chicago/Turabian StyleGkika, Eleni, Sonja Adebahr, Anton Brenner, Tanja Schimek-Jasch, Gianluca Radicioni, Jan-Philipp Exner, Alexander Rühle, Simon K. B. Spohn, Ilinca Popp, Constantinos Zamboglou, and et al. 2021. "Changes in Blood Biomarkers of Angiogenesis and Immune Modulation after Radiation Therapy and Their Association with Outcomes in Thoracic Malignancies" Cancers 13, no. 22: 5725. https://doi.org/10.3390/cancers13225725
APA StyleGkika, E., Adebahr, S., Brenner, A., Schimek-Jasch, T., Radicioni, G., Exner, J.-P., Rühle, A., Spohn, S. K. B., Popp, I., Zamboglou, C., Sprave, T., Firat, E., Niedermann, G., Nicolay, N. H., Nestle, U., Grosu, A.-L., & Duda, D. G. (2021). Changes in Blood Biomarkers of Angiogenesis and Immune Modulation after Radiation Therapy and Their Association with Outcomes in Thoracic Malignancies. Cancers, 13(22), 5725. https://doi.org/10.3390/cancers13225725









