Simple Summary
Anti-EGFR-related skin toxicity has been described as a predictive biomarker of response in patients with RAS wild-type (WT) metastatic colorectal cancer (mCRC). With the CAVE mCRC trial we previously provided the first evidence of the activity of cetuximab plus avelumab as rechallenge treatment in pretreated chemo-refractory RAS WT mCRC. Nowadays, skin toxicity remains the only confirmed clinical biomarker of response to anti-EGFR treatment in mCRC. The role of skin toxicity has not yet been explored in a rechallenge setting. In this paper we provide a post-hoc analysis of the CAVE mCRC trial that investigated the role of skin toxicity as a predictive biomarker of activity of cetuximab plus avelumab treatment and its correlation with different clinico-molecular variables on survival at the univariate and multivariate levels. High-grade skin toxicity, together to the circulating tumor DNA RAS/BRAF/EGFR wild-type status were the only variables with an impact on PFS and OS.
Abstract
The single-arm phase II CAVE mCRC trial evaluated the combination of cetuximab plus avelumab as rechallenge strategy in RAS wild-type (WT) metastatic colorectal cancer (mCRC) patients, with clinical response to first-line anti-EGFR-based chemotherapy, who progressed and received a subsequent line of therapy. The correlation of skin toxicity (ST) and different clinico-molecular variables with overall survival (OS), progression-free survival (PFS) and response rate (RR) was assessed at univariate and multivariate analysis. A total of 33/77 (42.9%) patients experienced grade 2–3 ST and displayed median OS (mOS) of 17.8 months (CI 95%, 14.9–20.6); whereas 44/77 (57.1%) patients with grade 0–1 ST exhibited mOS of 8.2 months (CI 95%, 5.5–10.9), (hazard ratio (HR), 0.51; CI 95%, 0.29–0.89; p = 0.019). Median PFS (mPFS) was 4.6 months (CI 95%, 3.4–5.7) in patients with grade 2–3 ST, compared to patients with grade 0–1 ST with mPFS of 3.4 months (CI 95%, 2.7–4.1; HR, 0.49; CI 95%, 0.3–0.8; p = 0.004). Grade 2–3 ST (HR, 0.51; CI 95%, 0.29–0.89; p = 0.019) and RAS/BRAF/EGFR WT circulating tumor DNA (ctDNA) (HR, 0.50; CI 95%, 0.27–0.9; p = 0.019) had a statistically significant effect on OS at univariate analysis. At the multivariate analysis, RAS/BRAF/EGFR WT ctDNA status maintained statistical significance (HR, 0.49; CI 95%, 0.27–0.9; p = 0.023), whereas there was a trend towards ST grade 2–3 (HR, 0.54; CI 95%, 0.29–1.01; p = 0.054). Skin toxicity is a promising biomarker to identify patients with mCRC that could benefit of anti-EGFR rechallenge.
1. Introduction
Different studies have shown promising activity of reintroduction of anti-epidermal growth factor receptor (EGFR) drugs in patients with RAS wild-type (RAS WT) metastatic colorectal cancer (mCRC), that obtained clinical benefit by first-line therapy with anti-EGFR drugs, then became resistant and progressed to second-line treatment [1,2,3,4,5,6]. This treatment strategy is called rechallenge. The biological rational relies on the arising of RAS mutant (RAS MT) cells during treatment with EGFR inhibitors (EGFRi), that lead to progression of disease (PD). During a subsequent EGFR-free therapeutic window, the acquired selected RAS MT clones progressively decay, while sensitive cells proliferate restoring vulnerability to EGFRi [7,8,9].
CAVE mCRC is a single-arm phase II study, in which 77 patients with refractory RAS WT mCRC received cetuximab plus the immune checkpoint inhibitor (ICI) avelumab, as rechallenge strategy [5]. In the intention-to-treat population (ITT population) the primary endpoint was met, with an improvement in median overall survival (mOS) [5]. The highest benefit was observed in patients with RAS, BRAF, and EGFR wild-type tumor assessed by basal plasma circulating tumor DNA (ctDNA) analysis [5].
So far, beside WT ctDNA at liquid biopsy analysis, several potential biomarkers have been investigated to predict the response to EGFRi rechallenge with discordant results [4,6,10].
The insurgence of skin toxicity (ST) is the most frequent adverse drug-related reaction (ADR) associated with EGFRi [11,12,13,14]. The physio-pathological mechanism of ST is due to the role of the EGFR pathway in the homeostasis of healthy tissues including skin and adnexal [11,12]. Therefore, EGFRi dysregulates keratinocyte proliferation, differentiation, migration, production of pro-inflammatory cytokines, and immune infiltration. Interestingly, strong evidence suggests a correlation between the appearance and intensity of ST and clinical outcome [15,16,17,18,19,20]. In mCRC robust data coming from large phase II/III clinical trials showed that patients treated with the monoclonal antibodies (mAbs) cetuximab and panitumumab had significant improvement in response rate (RR) and overall survival (OS) in case of insurgence of ST of grade 2 or higher [17,18,19,20]. In this scenario, in the absence of predictive biomarkers of response to rechallenge with EGFRi, we sought to address the impact of ST in terms of OS, PFS, and RR in the CAVE mCRC study.
2. Materials and Methods
2.1. Study Design and Patient Population
CAVE mCRC is a non-profit, single-arm, academic, open-label, phase II trial [5]. Patients enrolled in the study had: histologically confirmed mCRC with RAS (NRAS and KRAS, exon 2, 3 and 4) WT tumors, obtained a complete (CR) or partial response (PR) during a first-line treatment with an anti-EGFR-based regimen and should have progressed, and received at least one subsequent line of therapy with an interval of more than 4 months from last dose of the anti-EGFR drug. Additional inclusion and exclusion criteria are described in the full protocol available online [5].
This trial is registered with Eudract.ema.europa.eu, EudraCT number: 2017-004392-32 and ClinicalTrial.gov identifier: NCT04561336.
2.2. Patient Monitoring and Response Assessment
Clinical monitoring of patient safety was constantly assessed, and toxicity was graded using the National Cancer Institute-Common Toxicity Criteria (NCI-CTC) for adverse events, version 4.03. With the term skin toxicity, we included acneiform, erythematosus, maculo-papular rash, and cutaneous xerosis.
Tumor evaluation was assessed according to RECIST criteria, version 1.1, by using spiral or conventional CT scan, radiography, or MRI, if required. Tumor measurements were performed at baseline, and every 8 weeks for 40 weeks and every 12 weeks thereafter. Patients were followed until progression, regardless of whether study treatment was discontinued or delayed. Radiological results were evaluated by local investigators and confirmed by coordinating center investigators. All data, toxicities, and serious adverse events were collected in the electronic case report form. All patients provided written informed consent before entering the trial. The study was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization and Good Clinical Practice guidelines.
2.3. qPCR Analysis of Plasma Samples
Plasma specimens of 67 out of 77 patients were collected at baseline and were suitable for ctDNA evaluation of KRAS, NRAS, BRAF, and EGFR extracellular domain S492R mutations by using the automated Idylla TM qPCR-based platform.
The results of the analyses were visualized using the on-line tool Idylla TM Explore (idyllaexplore.biocartis.com, last access 30 May 2020). The protocol has been previously validated and is fully described elsewhere [21].
2.4. Statistical Analysis
Several clinical factors were evaluated for their correlation with median mPFS, mOS, and overall response rate (ORR). PFS and OS were calculated using the Kaplan–Meier method. The chi-square and Fisher tests were used to assess the correlation between clinical variables and PFS, OS, and ORR at univariate and multivariate analysis. Statistical analyses were performed using the SPSS package (v.23).
3. Results
Between August 2018 and February 2020, 77 patients were enrolled in CAVE mCRC trial and received the combination of avelumab plus cetuximab as rechallenge therapy. The main patient characteristics are listed in Table S1. ITT population survival outcomes have been previously described [5].
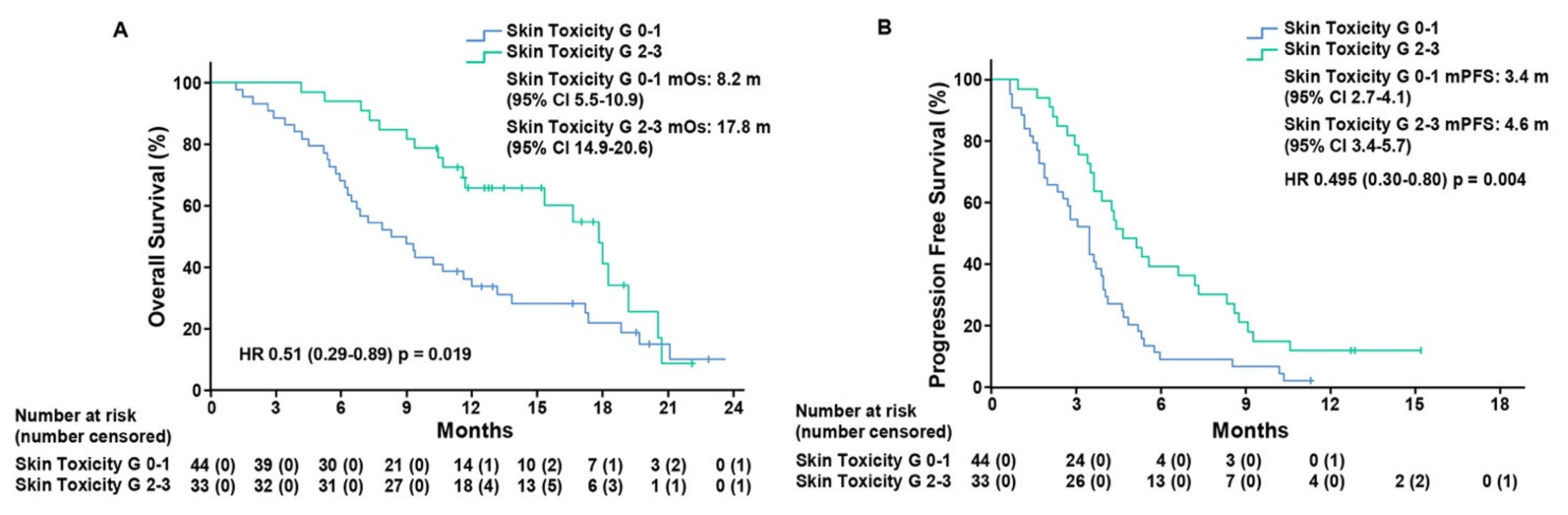
To assess the predictive value of ST as a biomarker of response to treatment, we performed a post-hoc analysis of patients that was based on the grading of skin toxicity. Thirty-three (42.9%) patients, that experienced grade 2–3 ST, presented mOS of 17.8 months (CI 95%, 14.9–20.6) as compared to 44 (57.1%) patients with grade 0–1 ST, who displayed mOS of 8.2 months (CI 95%, 5.5–10.9), (hazard ratio (HR), 0.51; CI 95%, 0.29–0.89; p = 0.019)). mPFS was 4.6 months (CI 95%, 3.4–5.7) in patients with grade 2–3 ST as compared to patients with grade 0–1 ST, who exhibited mPFS of 3.4 months (CI 95%, 2.7–4.1), (HR, 0.49; CI 95%, 0.3–0.8; p = 0.004) (Figure 1).
Figure 1.
Kaplan–Meier estimates of overall survival (A) and progression-free survival (B) in the intention-to-treat population according to skin toxicity grade 0–1 and grade 2–3. Grade, G; median overall survival, mOS; median progression-free survival, mPFS; months, m.
To further investigate the impact of skin toxicity on patient outcome, we did univariate and multivariate analyses with different clinical variables. At univariate analysis for PFS, besides skin toxicity, another three variables were associated with an improvement in PFS: number of metastatic sites ≤2 (HR, 0.54; CI 95%, 0.33–0.87; p = 0.013); surgery of primary tumor (HR, 0.58; CI 95%, 0.36–0.94; p = 0.028); and RAS/BRAF/EGFR WT ctDNA status at baseline liquid biopsy analysis (HR, 0,41; CI 95%, 0.23–0.75; p = 0.004). However, at multivariate analysis for PFS none of the analyzed parameters retained statistical significance (Table 1). Interestingly, grade 2–3 ST (HR, 0.51; CI 95%, 0.29–0.89; p = 0.019) and RAS/BRAF/EGFR WT ctDNA (HR, 0.50; CI 95%, 0.27–0.9; p = 0.019) were the only variables that had a statistically significant effect on OS at univariate analysis (Table 2). Of note, at the multivariate analysis RAS/BRAF/EGFR WT ctDNA status maintained statistical significance (HR, 0.49; CI 95%, 0.27–0.9; p = 0.023), whereas there was a trend towards ST grade 2–3 (HR, 0.54; CI 95%, 0.29–1.01; p = 0.054).

Table 1.
Association of clinical variables with median progression-free survival by univariate and multivariate analysis.

Table 2.
Association of clinical variables with median overall survival by univariate and multivariate analysis.
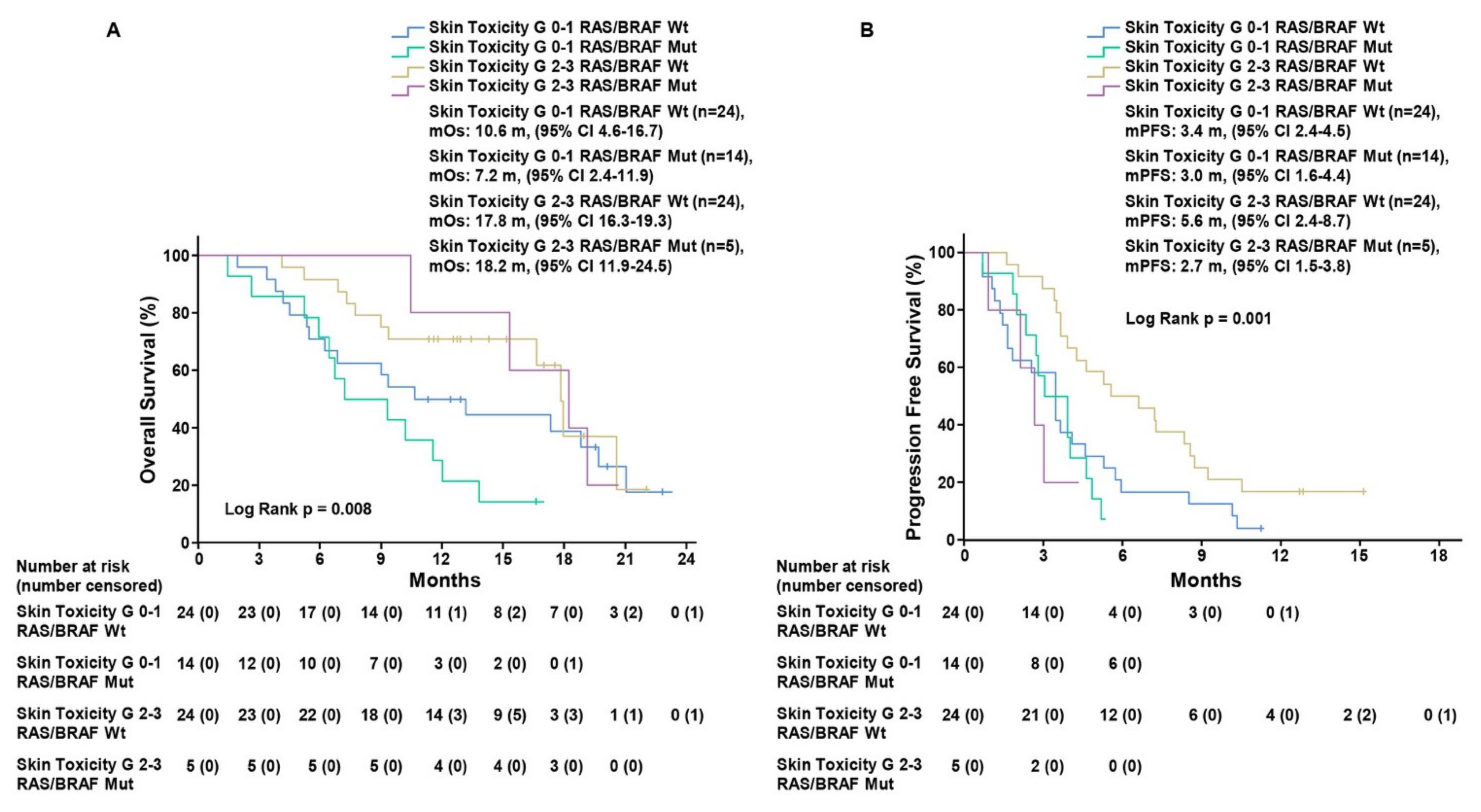
We also evaluated RR and disease-control rate (DCR) in each defined subgroup (Table 3). In the 33 patients with grade 2–3 ST, 1 (3%) CR, 2 (6.1%) PR, and 24 (72.7%) SD were observed, with DCR of 81.8%. On the other hand, in the 44 patients with grade 0–1 ST, we found 0 CR, 3 (6.8%) PR, 20 (45.5%) SD, with 52.3% DCR. To date, the only recognized clinical biomarker of response to rechallenge strategy with anti-EGFR drugs is represented by the absence of mutations in RAS/BRAF/EGFR genes [2,3,5,6]. Therefore, we assessed the impact of ST in the 67 patients with basal RAS/BRAF/EGFR ctDNA analysis, divided into four subgroups: RAS/BRAF/EGFR WT ST 2–3, RAS/BRAF/EGFR WT ST 0–1, RAS/BRAF/EGFR mutant ST 2–3, and RAS/BRAF/EGFR mutant ST 0–1. Kaplan–Meier analysis showed that mOS was 17.8 months (CI 95%, 16.3–19.3) in the 24 patients with RAS/BRAF/EGFR WT ctDNA at baseline with grade 2–3 ST as compared to mOS of 10.6 months (CI 95%, 4.6–16.6), (HR, 0.64; CI 95%, 0.30–1.39; p = 0.26) for those 24 patients with grade 0–1 ST. Regarding the subgroup of 19 patients with RAS/BRAF/EGFR mutant ctDNA at baseline, mOS was 18.2 months (CI 95%, 11.9–24.5) for 5 patients that experienced grade 2–3 ST as compared with 7.2 months (CI 95% 2.4–11.9), (HR, 0.17; CI 95%, 0.038–0.81; p = 0.026) for 14 patients with grade 0–1 ST (log-rank p = 0.001) (Figure 2, Figure S1 and Figure S2). mPFS was 5.6 months (CI 95%, 2.4–8.7) in RAS/BRAF/EGFR WT patients with grade 2–3 ST as compared to patients with grade 0–1 ST, who exhibited mPFS of 3.4 months (CI 95%, 2.4–4.5), (HR, 0.49; CI 95%, 0.26–0.9; p = 0.021). For the 19 patients with, RAS/BRAF/EGFR mutant tumor, mPFS was 2.7 months (CI 95% 1.5–3.8) for 5 patients with grade 2–3 ST and 3.0 months (CI 95% 1.6–4.4) for 14 patients with grade 0–1 ST (HR, 1.93; CI 95%, 0.65–5.72; p = 0.23) (log-rank p = 0.008) (Figure 2, Figure S1 and Figure S2).

Table 3.
Association of clinical variables with overall response rate.
Figure 2.
Kaplan–Meier estimates of overall survival (A) and progression-free survival (B) in patients with RAS/BRAF/EGFR wild-type and mutant circulating DNA according to skin toxicity grade 0–1 and grade 2–3. Grade, G; median overall survival, mOS; median progression-free survival, mPFS; months, m.
4. Discussion
Dermatologic toxicities such as acneiform rash, dry skin, pruritus, erythema, and paronychia have been reported in more than 90% patients receiving anti-EGFR monoclonal antibodies for the treatment of mCRC, although the majority of these toxicities are of mild grade [22]. Acneiform rash is the most frequent dermatologic ADR that could negatively impact treatment outcome, not only due to the risk of more intense adverse events such as infections and pruritus, which could lead to dose modifications and interruptions, but also because of the psychological impact on patient everyday life [11]. The correlation between EGFR blockade-related ST and response to treatment has been largely investigated in phase II and phase III clinical trials and, to date, ST represents the only clinical marker of response to cetuximab or panitumumab treatment [17]. Conversely, the EVEREST trial, which aimed to consider dose escalation of cetuximab in patients experiencing no skin toxicity after 21 days of treatment with the scope of achieving better outcomes, showed that the increase in grade 2 ST after cetuximab dose intensification did not correlate with improved OS. Interestingly, RR was higher only in patients with KRAS WT tumors [23]. Recently, a post-hoc analysis of the FIRE3 trial showed that the occurrence of grade 2 ST and of early tumor shrinkage were the only factors associated with improved survival in mCRC RAS WT patients treated with chemotherapy plus cetuximab compared with bevacizumab [20].
Except for an analysis conducted by Santini and colleagues, which reported a significant correlation between ST experienced during first-line cetuximab treatment and irinotecan plus cetuximab rechallenge in KRAS WT patients, the potential predictive role of ST that occurs during rechallenge treatment has not been investigated [1]. In the CAVE mCRC trial ST was a common adverse event that was related to cetuximab treatment with 33/77 patients having grade 2–3 ST and 44/77 presenting grade 0–1 ST. The occurrence of grade 2–3 ST is a strong predictor of patient outcome. In fact, in the ITT population, grade 2–3 versus grade 0–1 ST was correlated with an improvement in OS (HR, 0.51; CI 95%, 0.29–0.89; p = 0.019) and in PFS (HR, 0.49; CI 95%, 0.3–0.8; p = 0.004).
Different clinical factors have been investigated to identify the population that could really benefit from anti-EGFR rechallenge. So far, the absence of resistance mutations at liquid biopsy analysis is the only available biomarker to predict response to EGFRi rechallenge. Interestingly, in the CAVE mCRC trial, ST and RAS/BRAF/EGFR WT ctDNA were the only two parameters associated with a statistically significant increase in overall survival at univariate analysis. Multivariate analysis confirmed the value of RAS/BRAF/EGFR WT ctDNA status, with a trend towards statistical significance for skin toxicity. Moreover, patients with grade 2–3 ST had DCR of 81.8% as compared to 52.3% in the subgroup with grade 0–1 ST, with only six patients experiencing PD at first evaluation. These are very promising data that have to be put in the context of a chemorefractory mCRC disease setting, in which responses to regorafenib and trifluoridin/tipiracil are relatively rare and DCR is approximately 40% [24,25].
Of note, when comparing survival outcomes of the four defined subgroups: RAS/BRAF/EGFR WT ST 2-3, RAS/BRAF/EGFR WT ST 0–1, RAS/BRAF/EGFR mutant ST 2-3, and RAS/BRAF/EGFR mutant ST 0–1, even for patients with RAS/BRAF/EGFR WT tumors at basal plasma ctDNA analysis the highest benefit was observed in those that experienced grade 2–3 ST, with improvement in mPFS of approximately 2 months (5.6 months) compared with the other three subgroups. On the other hand, regarding OS, when experiencing a high-grade ST, patients with RAS/BRAF/EGFR mutant tumors present similar outcomes (18.2 months) with RAS/BRAF/EGFR WT (17.8 months), vs. 10.6 months and 7.2 months in patients experiencing grade 0–1 ST with RAS/BRAF/EGFR WT and mutant tumors, respectively. Considering the efficacy of treatment in RAS/BRAF/EGFR-mutated patients we could speculate on the contribution of avelumab to an anti-EGFR treatment as rechallenge strategy. In fact, we know that the combination of an anti-EGFR and an anti-PDL1 antibody could enhance the ADCC and therefore providing better efficacy that a single agent anti-EGFR drug. However, the data show a discrepancy between OS and PFS results, with a clear advantage in OS for patients with RAS/BRAF/EGFR mutant experiencing a high-grade ST that is not reflected in PFS, which remains poor and comparable to the subgroups of patients with G0–1 skin toxicity, independently from the RAS/BRAF/EGFR WT or mutant status. Subsequent lines of treatment and the small sample of RAS/BRAF/EGFR mutant patients (5 patients with G2-3 ST, 14 patients with G0–1 ST) could have affected the PFS results. Thus, the nature of our study (single-arm, small sample of patients) limits us in solving the doubt about OS-PFS discrepancy.
The CAVE mCRC represents, so far, the first evidence of the activity of an anti-EGFR monoclonal antibody in combination with an immune checkpoint inhibitor as rechallenge treatment in pretreated chemo-refractory RAS WT mCRC. The rational of the potential effectiveness of this novel combination derives from the induction of antibody-dependent cell-mediated cytotoxicity (ADCC) in enhancing antitumor activity from the IgG1 iso-type monoclonal antibodies avelumab and cetuximab [26,27,28]. In this respect, preclinical and clinical data from our group demonstrated how this combination increased ADCC in human non-small-cell lung cancer (NCSLC) cell lines and determined NK-cell-driven ADCC in chemo-refractory NSCLC patients in a proof-of-concept study [29].
Acneiform rash is a result of EGFRi at skin level, which could be related to the recruitment of immune cells and the consequent inflammatory response [30,31]. Specifically, blocking EGFR with mAbs leads to the release of type I interferon (IFN) in keratinocytes, which causes improved antigen presentation in the tumor microenvironment with T cell recruitment. On the other hand, IFN modulates immune response by activating cytokines and chemokines (TNF alpha, IL-1 Beta, IL-6, and IL-10) [32]. All these mechanisms could be the effectors of ST, with loss of antimicrobial response and damage to the epithelial barrier. Our study has different limitations. Due to the single-arm design it is very difficult to discern the impact of avelumab in the development of skin toxicity. Although cetuximab skin toxicity, with acneiform rash being the principal clinical manifestation, has been extensively described in mCRC, there is poor evidence regarding anti PD-L1 immune-related skin toxicity in the same setting, considering that, unfortunately, only a few patients with an MSI tumor (almost 5%) could really benefit from CPI treatment [17,18,19,20,21,26]. However, immune-related skin toxicity is well described in other solid tumors and has been reported with a wide range of clinical manifestations. In the CAVE mCRC trial, the principal clinical manifestation of skin toxicity that has been reported is an acneiform rash, that we have correlated with anti-EGFR activity. Moreover, the onset of cutaneous toxicity has been between cycle 2 and cycle 3 for most of our patients (75 out of 77), while CPI-related dermatologic adverse events seem to have a delayed onset. Future randomized analyses with a cetuximab single-agent control arm will clarify how avelumab contributes to ST.
5. Conclusions
With the limitations of the single-arm non-randomized design of the trial, the CAVE mCRC study demonstrates a potential role of ST as predictive biomarker of response to anti-EGFR retreatment. In the future, the implementation of translational analyses to be integrated with plasma ctDNA analysis in the context of a randomized and larger trial is necessary to confirm the potential role of ST as a surrogate of response.
Supplementary Materials
The followings are available online at https://www.mdpi.com/article/10.3390/cancers13225715/s1, Table S1: Baseline characteristics, Figure S1: Kaplan–Meier estimates of overall survival (A) and progression-free survival (B) in patients with RAS/BRAF/EGFR wild-type circulating DNA according to skin toxicity grade 0–1 and grade 2–3, Figure S2: Kaplan–Meier estimates of overall survival (A) and progression-free survival (B) in patients with RAS/BRAF/EGFR mutant circulating DNA according to skin toxicity grade 0–1 and grade 2–3.
Author Contributions
G.M. and D.C. wrote the original draft. G.M., D.C., F.C., and E.M. (Erika Martinelli) performed the conceptualization and methodology of the study; D.C., S.N., T.T., F.P., A.A., E.M. (Evaristo Maiello), T.L., C.C., G.S., C.P., E.M. (Erika Martinelli), F.C., and G.M. enrolled and treated patients and collected the clinical data; D.C., G.M., L.E., N.N., E.M. (Erika Martinelli), F.C., and V.F. analyzed and interpreted the data. All the authors revised and approved the final version. All authors have read and agreed to the published version of the manuscript.
Funding
Two research grants, that partially covered the costs of the study, were provided by Merck and by Regione Campania (I-Cure Research Project, Grant number: Cup 21C17000030007).
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board/Ethics Committee) of Università Vanvitelli di Napoli, AOU Vanvitelli-AORN Ospedali dei Colli (Protocol Code: 427; date of approval: 05/06/2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: ErM has served as an advisor and speaker for AstraZeneca, Amgen, Bayer, Merck-Serono, Roche, Sanofi, Servier, and Pierre Fabre. TT has served as an advisor and speaker for Roche, Merck-Serono, Sanofi, Servier, Novartis, and Bayer. FP has served as advisor/speaker for Amgen, Roche, Lilly, Sanofi, Merck-Serono, Bayer, and Servier and received a research grants from BMS. AA has served as an advisor and speaker for Amgen and Servier. NN has served as an advisor and speaker for MSD, Qiagen, Biocartis, Incyte, Roche, BMS, MERCK, Thermo Fisher, Boehringer Ingelheim, AstraZeneca, Sanofi, Eli Lilly, and Bayer. EvM has served as an advisor and speaker for AstraZeneca, Eli Lilly, Servier, Sanofi Genzyme, Roche, Merck, Eisai, and Pfizer. TL has served as speaker for Servier. CC has served as an advisor for Roche, Bayer, and Agmen; as a speaker for Roche, Bayer, Agmen, and Servier; and has received research funding form Merck-Serono. GS has served as an advisor for Amgen and Servier. CP has served as an advisor and speaker for MSD, Bayer, AstraZeneca, Roche, Merck-Serono, Lilly, Servier, Sanofi, and Astellas. FC has served as an advisor and speaker for Roche, Amgen, Merck-Serono, Pfizer, Sanofi, Bayer, Servier, BMS, Cellgene, and Lilly and has received institutional research grants form Bayer, Roche, Merck-Serono, Amgen, AstraZeneca, and Takeda. GM, VF, SN, LE, and DC declare no conflict of interest.
References
- Santini, D.; Vincenzi, B.; Addeo, R.; Garufi, C.; Masi, G.; Scartozzi, M.; Mancuso, A.; Frezza, A.M.; Venditti, O.; Imperatori, M.; et al. Cetuximab rechallenge in metastatic colorectal cancer patients: How to come away from acquired resistance? Ann. Oncol. 2017, 23, 2313–2318, Erratum in 2017, 28, 2906. [Google Scholar] [CrossRef]
- Cremolini, C.; Rossini, D.; Dell’Aquila, E.; Lonardi, S.; Conca, E.; Del Re, M.; Busico, A.; Pietrantonio, F.; Danesi, R.; Aprile, G.; et al. Rechallenge for Patients With RAS and BRAF Wild-Type Metastatic Colorectal Cancer With Acquired Resistance to First-line Cetuximab and Irinotecan: A Phase 2 Single-Arm Clinical Trial. JAMA Oncol. 2019, 5, 343–350. [Google Scholar] [CrossRef]
- Sunakawa, Y.; Nakamura, M.; Ishizaki, M.; Kataoka, M.; Satake, H.; Kitazono, M.; Yanagisawa, H.; Kawamoto, Y.; Kuramochi, H.; Ohori, H.; et al. RAS Mutations in Circulating Tumor DNA and Clinical Outcomes of Rechallenge Treatment With Anti-EGFR Antibodies in Patients With Metastatic Colorectal Cancer. JCO Precis. Oncol. 2020, 4, 898–911. [Google Scholar] [CrossRef]
- Masuishi, T.; Tsuji, A.; Kotaka, M.; Nakamura, M.; Kochi, M.; Takagane, A.; Shimada, K.; Denda, T.; Segawa, Y.; Tanioka, H.; et al. Phase 2 study of irinotecan plus cetuximab rechallenge as third-line treatment in KRAS wild-type metastatic colorectal cancer: JACCRO CC-08. Br. J. Cancer 2020, 123, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, E.; Martini, G.; Famiglietti, V.; Troiani, T.; Napolitano, S.; Pietrantonio, F.; Ciardiello, D.; Terminiello, M.; Borrelli, C.; Vitiello, P.P.; et al. Cetuximab Rechallenge Plus Avelumab in Pretreated Patients With RAS Wild-type Metastatic Colorectal Cancer: The Phase 2 Single-Arm Clinical CAVE Trial. JAMA Oncol. 2021, 7, 1529–1535. [Google Scholar] [CrossRef]
- Ciardiello, D.; Martini, G.; Famiglietti, V.; Napolitano, S.; De Falco, V.; Troiani, T.; Latiano, T.P.; Ros, J.; Elez Fernandez, E.; Vitiello, P.P.; et al. Biomarker-Guided Anti-Egfr Rechallenge Therapy in Metastatic Colorectal Cancer. Cancers 2021, 13, 1941. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, E.; Ciardiello, D.; Martini, G.; Troiani, T.; Cardone, C.; Vitiello, P.P.; Normanno, N.; Rachiglio, A.M.; Maiello, E.; Latiano, T.; et al. Implementing anti-epidermal growth factor receptor (EGFR) therapy in metastatic colorectal cancer: Challenges and future perspectives. Ann. Oncol. 2020, 31, 30–40. [Google Scholar] [CrossRef]
- Goldberg, R.M.; Montagut, C.; Wainberg, Z.A.; Ronga, P.; Audhuy, F.; Taieb, J.; Stintzing, S.; Siena, S.; Santini, D. Optimising the use of cetuximab in the continuum of care for patients with metastatic colorectal cancer. ESMO Open 2018, 3, e000353. [Google Scholar] [CrossRef]
- Parseghian, C.M.; Loree, J.M.; Morris, V.K.; Liu, X.; Clifton, K.K.; Napolitano, S.; Henry, J.T.; Pereira, A.A.; Vilar, E.; Johnson, B. Anti-EGFR-resistant clones decay exponentially after progression: Implications for anti-EGFR re-challenge. Ann. Oncol. 2019, 30, 243–249. [Google Scholar] [CrossRef]
- Rossini, D.; Germani, M.M.; Pagani, F.; Pellino, A.; Dell’Aquila, E.; Bensi, M.; Liscia, N.; Moretto, R.; Boccaccino, A.; Prisciandaro, M.; et al. Retreatment With Anti-EGFR Antibodies in Metastatic Colorectal Cancer Patients: A Multi-institutional Analysis. Clin. Colorectal. Cancer 2020, 19, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Lacouture, M.E. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat. Rev. Cancer 2006, 6, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Parmar, S.; Schumann, C.; Rüdiger, S.; Boeck, S.; Heinemann, V.; Kächele, V.; Seeringer, A.; Paul, T.; Seufferlein, T.; Stingl, J.C. Pharmacogenetic predictors for EGFR-inhibitor-associated skin toxicity. Pharm. J. 2013, 13, 181–188. [Google Scholar] [CrossRef][Green Version]
- Lacouture, M.E.; Sibaud, V.; Gerber, P.A.; Van den Hurk, C.; Fernández-Peñas, P.; Santini, D.; Jahn, F.; Jordan, K. Prevention and Management of Dermatological Toxicities Related to Anticancer Agents: ESMO Clinical Practice Guidelines†. Ann. Oncol. 2021, 32, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Lupu, I.; Voiculescu, V.M.; Bacalbasa, N.; Prie, B.E.; Cojocaru, I.; Giurcaneanu, C. Cutaneous adverse reactions specific to epidermal growth factor receptor inhibitors. J. Med. Life 2015, 8, 57–61. [Google Scholar] [PubMed]
- Wacker, B.; Nagrani, T.; Weinberg, J.; Witt, K.; Clark, G.; Cagnoni, P.J. Correlation between Development of Rash and Efficacy in Patients Treated with the Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Erlotinib in Two Large Phase III Studies. Clin. Cancer Res. 2007, 13, 3913. [Google Scholar] [CrossRef]
- Herbst, R.S.; Arquette, M.; Shin, D.M.; Dicke, K.; Vokes, E.E.; Azarnia, N.; Hong, W.K.; Kies, M.S. Phase II multicenter study of the epidermal growth factor receptor antibody cetuximab and cisplatin for recurrent and refractory squamous cell carcinoma of the head and neck. J. Clin. Oncol. 2005, 23, 5578–5587. [Google Scholar] [CrossRef]
- Orditura, M.; De Vita, F.; Galizia, G.; Lieto, E.; Vecchione, L.; Vitiello, F.; Martinelli, E.; Ciardiello, F. Correlation between efficacy and skin rash occurrence following treatment with the epidermal growth factor receptor inhibitor cetuximab: A single institution retrospective analysis. Oncol. Rep. 2009, 21, 1023–1028. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cunningham, D.; Humblet, Y.; Siena, S.; Khayat, D.; Bleiberg, H.; Santoro, A.; Bets, D.; Mueser, M.; Harstrick, A.; Verslype, C.; et al. Cetuximab Monotherapy and Cetuximab plus Irinotecan in Irinotecan-Refractory Metastatic Colorectal Cancer. N. Engl. J. Med. 2004, 351, 337–345. [Google Scholar] [CrossRef]
- Lenz, H.J.; Van Cutsem, E.; Khambata-Ford, S.; Mayer, R.J.; Gold, P.; Stella, P.; Mirtsching, B.; Cohn, A.L.; Pippas, A.W.; Azarnia, N.; et al. Multicenter phase II and translational study of cetuximab in metastatic colorectal carcinoma refractory to irinotecan, oxaliplatin, and fluoropyrimidines. J. Clin. Oncol. 2006, 24, 4914–4921. [Google Scholar] [CrossRef] [PubMed]
- Holch, J.W.; Held, S.; Stintzing, S.; Fischer von Weikersthal, L.; Decker, T.; Kiani, A.; Kaiser, F.; Heintges, T.; Kahl, C.; Kullmann, F.; et al. Relation of cetuximab-induced skin toxicity and early tumor shrinkage in metastatic colorectal cancer patients: Results of the randomized phase 3 trial FIRE-3 (AIO KRK0306). Ann. Oncol. 2020, 31, 72–78. [Google Scholar] [CrossRef]
- Vitiello, P.P.; De Falco, V.; Giunta, E.F.; Ciardiello, D.; Cardone, C.; Vitale, P.; Zanaletti, N.; Borrelli, C.; Poliero, L.; Terminiello, M.; et al. Clinical Practice Use of Liquid Biopsy to Identify RAS/BRAF Mutations in Patients with Metastatic Colorectal Cancer (mCRC): A Single Institution Experience. Cancers 2019, 11, 1504. [Google Scholar] [CrossRef] [PubMed]
- Price, T.J.; Peeters, M.; Kim, T.W.; Li, J.; Cascinu, S.; Ruff, P.; Suresh, A.S.; Thomas, A.; Tjulandin, S.; Zhang, K.; et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): A randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol. 2014, 15, 569–579. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Tejpar, S.; Vanbeckevoort, D.; Peeters, M.; Humblet, Y.; Gelderblom, H.; Vermorken, J.B.; Viret, F.; Glimelius, B.; Gallerani, E.; et al. Intrapatient cetuximab dose escalation in metastatic colorectal cancer according to the grade of early skin reactions: The randomized EVEREST study. J. Clin. Oncol. 2012, 30, 2861–2868. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A.; Van Cutsem, E.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef]
- Mayer, R.J.; Van Cutsem, E.; Falcone, A.; Yoshino, T.; Garcia-Carbonero, R.; Mizunuma, N.; Yamazaki, K.; Shimada, Y.; Tabernero, J.; Komatsu, Y.; et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N. Engl. J. Med. 2015, 372, 1909–1919. [Google Scholar] [CrossRef]
- Ciardiello, D.; Vitiello, P.P.; Cardone, C.; Martini, G.; Troiani, T.; Martinelli, E.; Ciardiello, F. Immunotherapy of colorectal cancer: Challenges for therapeutic efficacy. Cancer Treat. Rev. 2019, 76, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Bourhis, J.; Stein, A.; Paul de Boer, J.; Van Den Eynde, M.; Gold, K.A.; Stintzing, S.; Becker, J.C.; Moran, M.; Schroeder, A.; Pennock; et al. Avelumab and cetuximab as a therapeutic combination: An overview of scientific rationale and current clinical trials in cancer. Cancer Treat. Rev. 2021, 97, 102172. [Google Scholar] [CrossRef]
- Ferris, R.L.; Lenz, H.J.; Trotta, A.M.; García-Foncillas, J.; Schulten, J.; Audhuy, F.; Merlano, M.; Milano, G. Rationale for combination of therapeutic antibodies targeting tumor cells and immune checkpoint receptors: Harnessing innate and adaptive immunity through IgG1 isotype immune effector stimulation. Cancer Treat. Rev. 2018, 63, 48–60. [Google Scholar] [CrossRef]
- Fasano, M.; Della Corte, C.M.; Di Liello, R.; Barra, G.; Sparano, F.; Viscardi, G.; Iacovino, M.L.; Paragliola, F.; Famiglietti, V.; Ciaramella, E.; et al. Induction of natural killer antibody-dependent cell cytotoxicity and of clinical activity of cetuximab plus avelumab in non-small cell lung cancer. ESMO Open 2020, 5, e000753. [Google Scholar] [CrossRef]
- Lulli, D.; Carbone, M.L.; Pastore, S. Epidermal growth factor receptor inhibitors trigger a type I interferon response in human skin. Oncotarget 2016, 7, 47777–47793. [Google Scholar] [CrossRef] [PubMed]
- Gurule, N.J.; Heasley, L.E. Linking tyrosine kinase inhibitor-mediated inflammation with normal epithelial cell homeostasis and tumor therapeutic responses. Cancer Drug Resist. 2018, 1, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Mascia, F.; Lam, G.; Keith, C.; Garber, C.; Steinberg, S.M.; Kohn, E.; Yuspa, S.H. Genetic ablation of epidermal EGFR reveals the dynamic origin of adverse effects of anti-EGFR therapy. Sci. Transl. Med. 2013, 5, 199ra110. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).