Cannabidiol Suppresses Angiogenesis and Stemness of Breast Cancer Cells by Downregulation of Hypoxia-Inducible Factors-1α
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Cell Culture
2.2. Generation of Sphere-Forming Cells
2.3. Reagents and Antibodies
2.4. Cell Proliferation Assay
2.5. Immunoblotting
2.6. Invasion Assay
2.7. Migration Assay (Wound Healing Assay)
2.8. Transient Transfection
2.9. Immunofluorescence (IF) Assay
2.10. Real-Time Polymerase Chain Reaction (RT-PCR)
2.11. Co-Immunoprecipitation (Co-IP)
2.12. Enzyme-Linked Immunosorbent Assay (ELISA) for VEGF
2.13. HUVEC Tube-Formation Assay
2.14. Clonogenic Assay
2.15. Detection of CD44 and CD24 by Flow Cytometry (FACs) Analysis
2.16. Statistical Analysis
3. Results
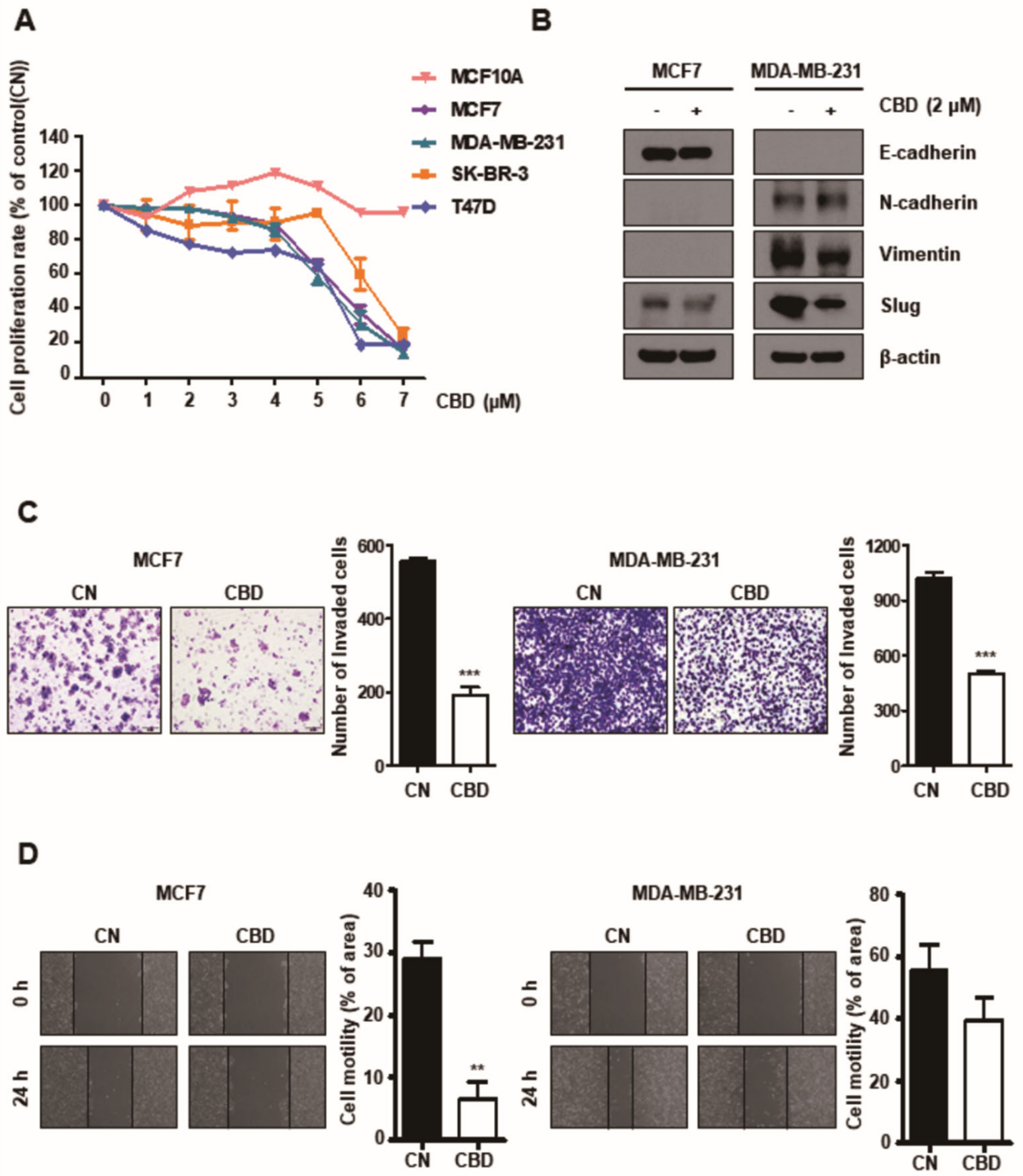
3.1. CBD Reduces Proliferation, Invasion, and Migration of Breast Cancer Cells
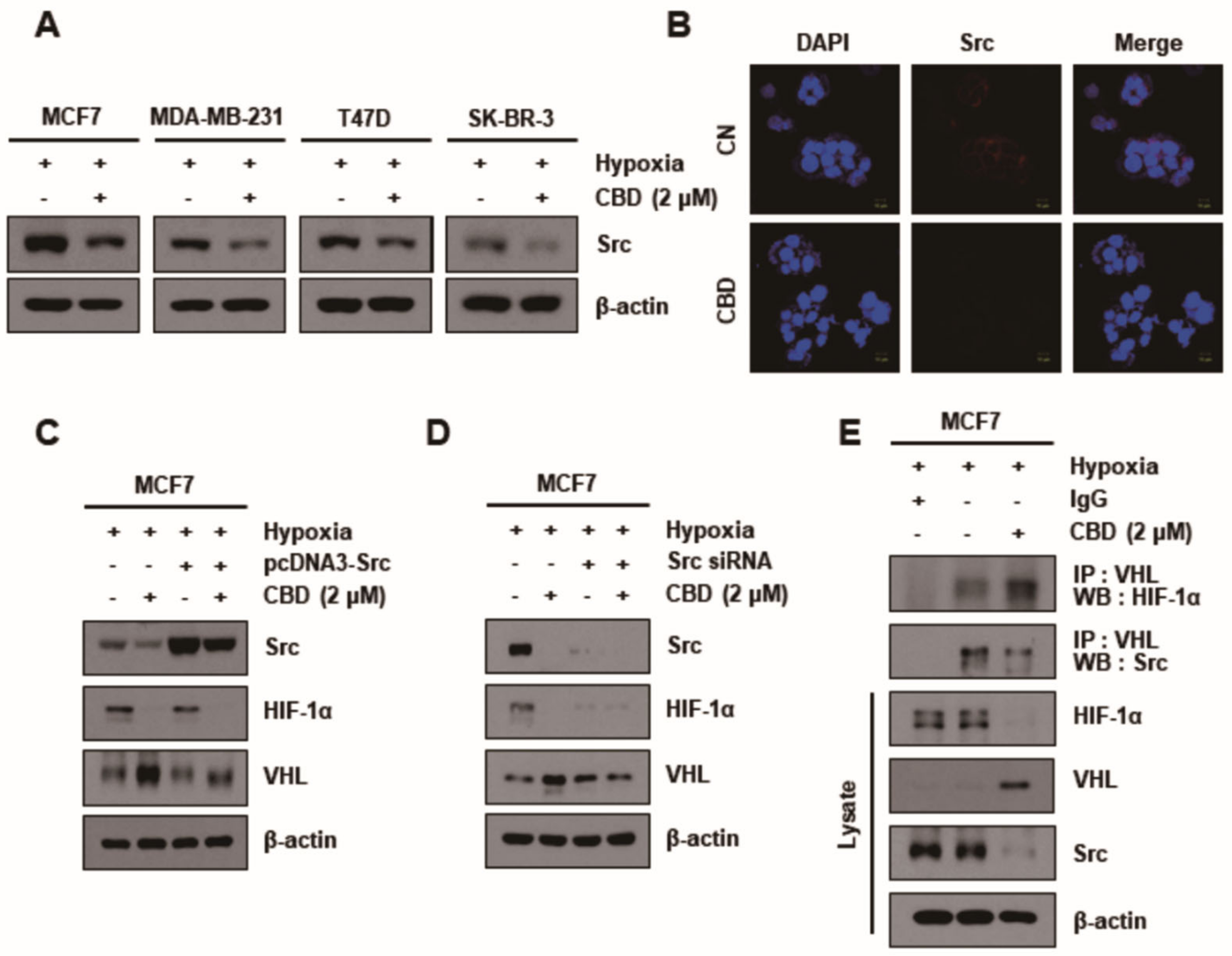
3.2. CBD Decreases HIF-1α by Upregulating the Ubiquitination of HIF-1α
3.3. CBD Modulates the Expression Pattern of Kinases in MCF7 Cells
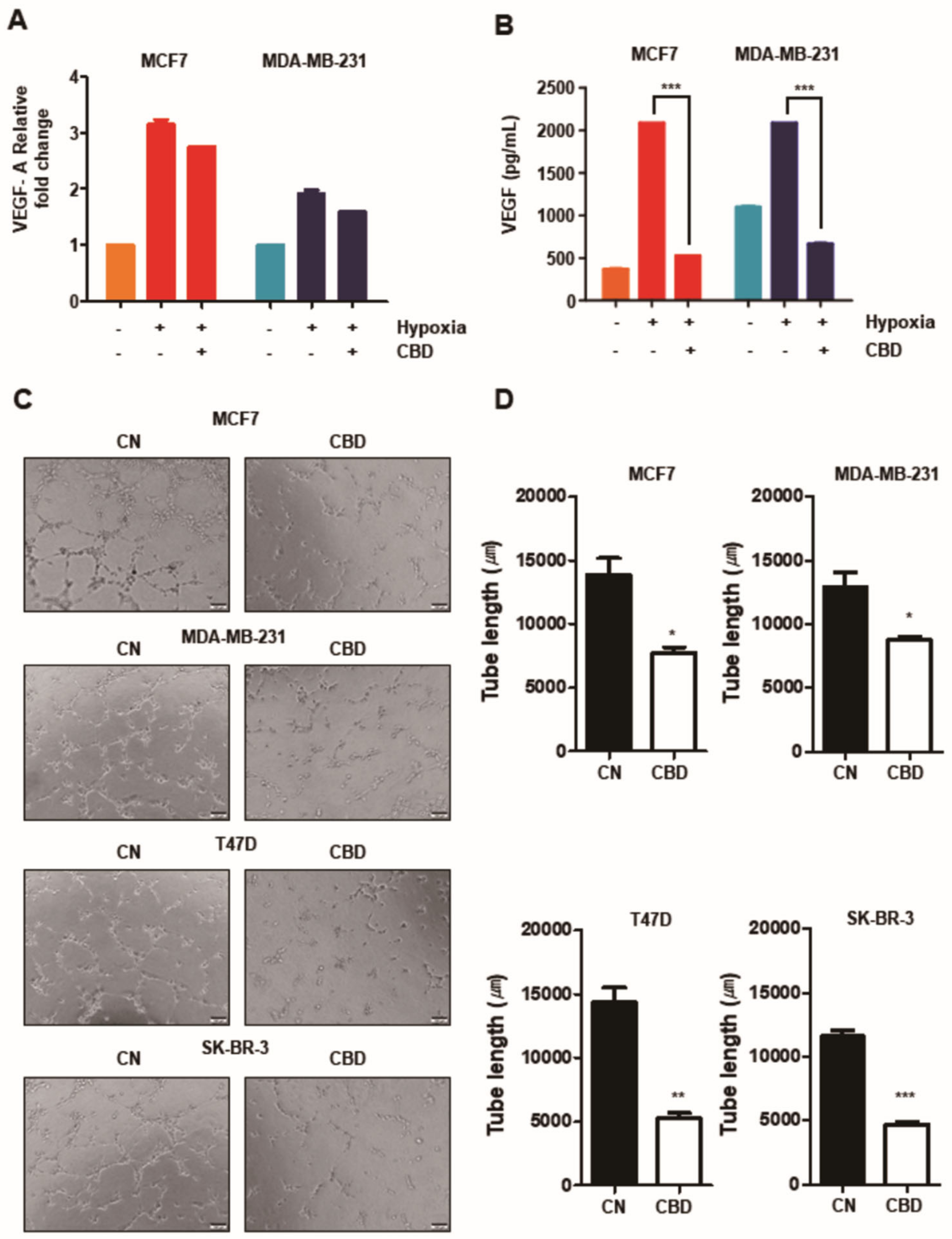
3.4. CBD Inhibits Angiogenesis in Breast Cancer
3.5. CBD Reduces Stemness of Breast Cancer
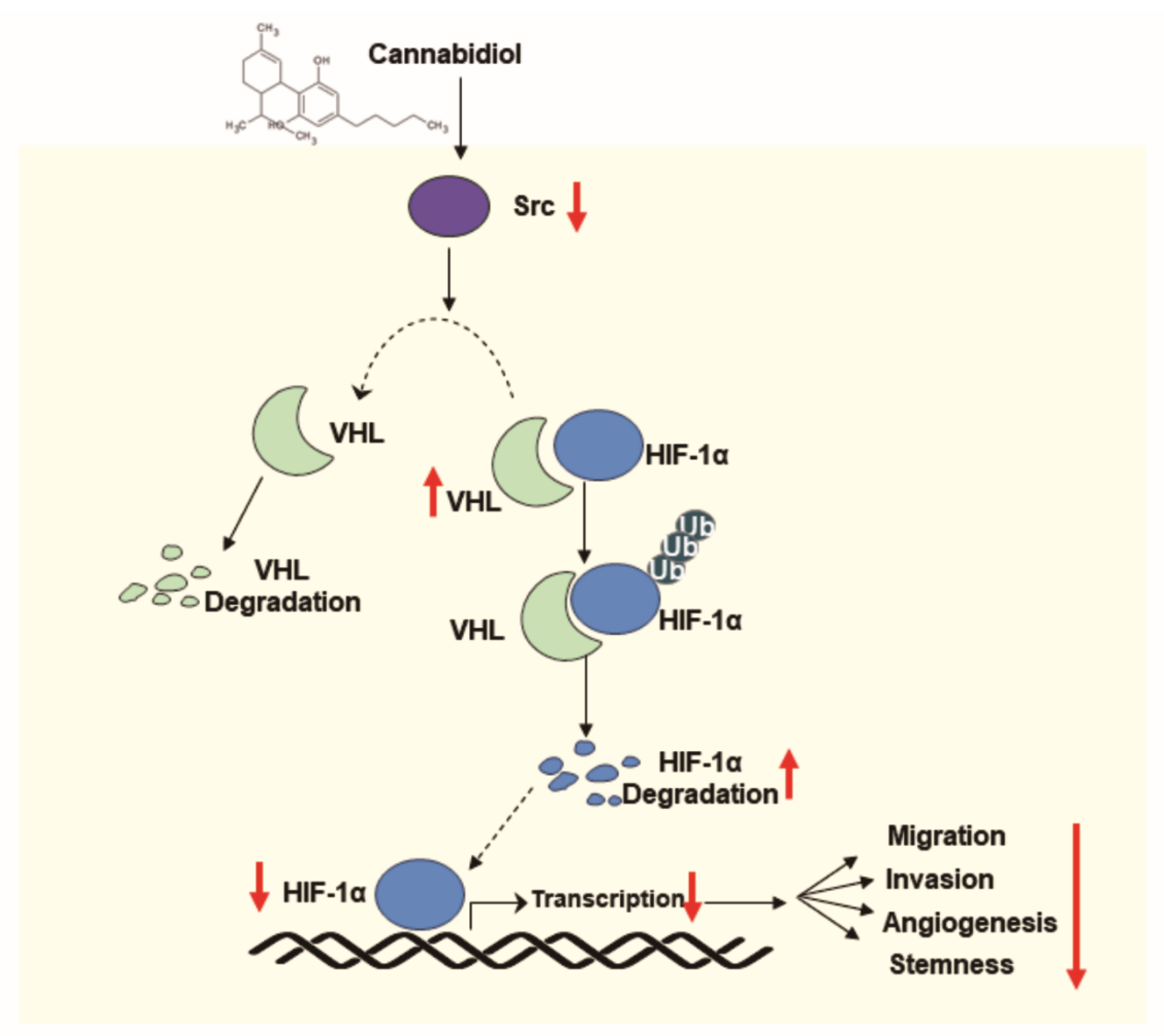
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Izzo, A.A.; Borrelli, F.; Capasso, R.; Di Marzo, V.; Mechoulam, R. Non-psychotropic plant cannabinoids: New therapeutic opportunities from an ancient herb. Trends. Pharmacol. Sci. 2009, 30, 515–527. [Google Scholar] [CrossRef]
- Pisanti, S.; Malfitano, A.M.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.C.; Fiore, D.; et al. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol. Ther. 2017, 175, 133–150; [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Kuzontkoski, P.M.; Groopman, J.E.; Prasad, A. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol. Cancer Ther. 2011, 10, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Melck, D.; Rueda, D.; Galve-Roperh, I.; De Petrocellis, L.; Guzmán, M.; Di Marzo, V. Involvement of the cAMP/protein kinase A pathway and of mitogen-activated protein kinase in the anti-proliferative effects of anandamide in human breast cancer cells. FEBS. Lett. 1999, 463, 235–240. [Google Scholar] [CrossRef]
- Javid, F.A.; Phillips, R.M.; Afshinjavid, S.; Verde, R.; Ligresti, A. Cannabinoid pharmacology in cancer research: A new hope for cancer patients? Eur. J. Pharmacol. 2016, 775, 1–14. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Melck, D.; Palmisano, A.; Bisogno, T.; Laezza, C.; Bifulco, M.; Di Marzo, V. The endogenous cannabinoid anandamide inhibits human breast cancer cell proliferation. Proc. Natl. Acad. Sci. USA 1998, 95, 8375–8380. [Google Scholar] [CrossRef]
- Hsu, L.H.; Chu, N.M.; Lin, Y.F.; Kao, S.H. G-Protein Coupled Estrogen Receptor in Breast Cancer. Int. J. Mol. Sci. 2019, 20, 306. [Google Scholar] [CrossRef] [PubMed]
- Pisanti, S.; Picardi, P.; Prota, L.; Proto, M.C.; Laezza, C.; McGuire, P.G.; Morbidelli, L.; Gazzerro, P.; Ziche, M.; Das, A.; et al. Genetic and pharmacologic inactivation of cannabinoid CB1 receptor inhibits angiogenesis. Blood 2011, 117, 5541–5550. [Google Scholar] [CrossRef] [PubMed]
- Hegde, V.L.; Singh, U.P.; Nagarkatti, P.S.; Nagarkatti, M. Critical Role of Mast Cells and Peroxisome Proliferator-Activated Receptor γ in the Induction of Myeloid-Derived Suppressor Cells by Marijuana Cannabidiol In Vivo. J. Immunol. 2015, 194, 5211–5222. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.W.; Tong, G.H.; Liu, Y. Cancer stem cells and hypoxia-inducible factors (Review). Int. J. Oncol. 2018, 53, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Schito, L.; Semenza, G.L. Hypoxia-Inducible Factors: Master Regulators of Cancer Progression. Trends. Cancer 2016, 2, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Dynamic regulation of stem cell specification and maintenance by hypoxia-inducible factors. Mol. Aspects. Med. 2016, 47–48, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Mei, W.; Lin, X.; Kapoor, A.; Gu, Y.; Zhao, K.; Tang, D. The Contributions of Prostate Cancer Stem Cells in Prostate Cancer Initiation and Metastasis. Cancers 2019, 11, 434. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.R.; Semenza, G.L. Oxygen Sensing and Homeostasis. Physiology 2015, 30, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Liu, B.; Ding, M.; Zhou, J.; Yang, C.; Chen, Y. Hypoxia and cancer related pathology. Cancer. Lett. 2020, 486, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Solinas, M.; Massi, P.; Cinquina, V.; Valenti, M.; Bolognini, D.; Gariboldi, M.; Monti, E.; Rubino, T.; Parolaro, D. Cannabidiol, a Non-Psychoactive Cannabinoid Compound, Inhibits Proliferation and Invasion in U87-MG and T98G Glioma Cells through a Multitarget Effect. PLoS ONE 2013, 8, e76918. [Google Scholar] [CrossRef]
- Xiang, L.; Semenza, G.L. Hypoxia-inducible factors promote breast cancer stem cell specification and maintenance in response to hypoxia or cytotoxic chemotherapy. Adv. Cancer Res. 2019, 141, 175–212. [Google Scholar] [CrossRef]
- Alexander, A.; Smith, P.F.; Rosengren, R.J. Cannabinoids in the treatment of cancer. Cancer. Lett. 2009, 285, 6–12. [Google Scholar] [CrossRef]
- Garcia-Morales, L.; Castillo, A.M.; Tapia Ramirez, J.; Zamudio-Meza, H.; Dominguez-Robles, M.D.C.; Meza, I. CBD Reverts the Mesenchymal Invasive Phenotype of Breast Cancer Cells Induced by the Inflammatory Cytokine IL-1beta. Int. J. Mol. Sci. 2020, 21, 2429. [Google Scholar] [CrossRef]
- Corrado, C.; Fontana, S. Hypoxia and HIF Signaling: One Axis with Divergent Effects. Int. J. Mol. Sci. 2020, 21, 5611. [Google Scholar] [CrossRef] [PubMed]
- Slemc, L.; Kunej, T. Transcription factor HIF1A: Downstream targets, associated pathways, polymorphic hypoxia response element (HRE) sites, and initiative for standardization of reporting in scientific literature. Tumour. Biol. 2016, 37, 14851–14861. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, J.A.; Jiang, B.H.; Iyer, N.V.; Agani, F.; Leung, S.W.; Koos, R.D.; Semenza, G.L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 1996, 16, 4604–4613. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, P.H.; Wiesener, M.S.; Chang, G.W.; Clifford, S.C.; Vaux, E.C.; Cockman, M.E.; Wykoff, C.C.; Pugh, C.W.; Maher, E.R.; Ratcliffe, P.J. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999, 399, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Kaelin, W.G. Proline hydroxylation and gene expression. Annu. Rev. Biochem. 2005, 74, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Mahon, P.C.; Hirota, K.; Semenza, G.L. FIH-1: A novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001, 15, 2675–2686. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.R.; Semenza, G.L. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol. Rev. 2012, 92, 967–1003. [Google Scholar] [CrossRef] [PubMed]
- Snell, C.E.; Turley, H.; McIntyre, A.; Li, D.; Masiero, M.; Schofield, C.J.; Gatter, K.C.; Harris, A.L.; Pezzella, F. Proline-hydroxylated hypoxia-inducible factor 1α (HIF-1α) upregulation in human tumours. PLoS ONE 2014, 9, e88955. [Google Scholar] [CrossRef]
- Chou, M.T.; Anthony, J.; Bjorge, J.D.; Fujita, D.J. The von Hippel-Lindau Tumor Suppressor Protein Is Destabilized by Src: Implications for Tumor Angiogenesis and Progression. Genes Cancer 2010, 1, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, D.; Tsiokas, L.; Zhou, X.M.; Foster, D.; Brugge, J.S.; Sukhatme, V.P. Hypoxic induction of human vascular endothelial growth factor expression through c-Src activation. Nature 1995, 375, 577–581. [Google Scholar] [CrossRef]
- Ellis, L.M.; Staley, C.A.; Liu, W.; Fleming, R.Y.; Parikh, N.U.; Bucana, C.D.; Gallick, G.E. Down-regulation of vascular endothelial growth factor in a human colon carcinoma cell line transfected with an antisense expression vector specific for c-src. J. Biol. Chem. 1998, 273, 1052–1057. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Lee, T.; Lee, N.; Yang, E.G.; Lee, C.; Lee, J.; Moon, E.-Y.; Ha, J.; Park, H. Src activates HIF-1α not through direct phosphorylation of HIF-1α specific prolyl-4 hydroxylase 2 but through activation of the NADPH oxidase/Rac pathway. Carcinogenesis 2011, 32, 703–712. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Semenza, G.L. Regulation of the breast cancer stem cell phenotype by hypoxia-inducible factors. Clin. Sci. 2015, 129, 1037–1045. [Google Scholar] [CrossRef]
- Mathieu, J.; Zhang, Z.; Zhou, W.; Wang, A.J.; Heddleston, J.M.; Pinna, C.M.; Hubaud, A.; Stadler, B.; Choi, M.; Bar, M.; et al. HIF induces human embryonic stem cell markers in cancer cells. Cancer. Res. 2011, 71, 4640–4652. [Google Scholar] [CrossRef]
- Zhao, Z.; Song, Z.; Liao, Z.; Liu, Z.; Sun, H.; Lei, B.; Chen, W.; Dang, C. PKM2 promotes stemness of breast cancer cell by through Wnt/β-catenin pathway. Tumour. Biol. 2016, 37, 4223–4234. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Chen, I.; Shimoda, L.A.; Park, Y.; Zhang, C.; Tran, L.; Zhang, H.; Semenza, G.L. Chemotherapy-Induced Ca(2+) Release Stimulates Breast Cancer Stem Cell Enrichment. Cell. Rep. 2017, 18, 1946–1957. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Tran, L.; Park, Y.; Chen, I.; Lan, J.; Xie, Y.; Semenza, G.L. Reciprocal regulation of DUSP9 and DUSP16 expression by HIF1 controls ERK and p38 MAP kinase activity and mediates chemotherapy-induced breast cancer stem cell enrichment. Cancer Res. 2018, 78, 4191–4202. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef] [PubMed]
- Abraham, B.K.; Fritz, P.; McClellan, M.; Hauptvogel, P.; Athelogou, M.; Brauch, H. Prevalence of CD44+/CD24−/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clin. Cancer Res. 2005, 11, 1154–1159. [Google Scholar] [PubMed]
- Lü, X.; Xu, K.; Lü, H.; Yin, Y.; Ma, C.; Liu, Y.; Li, H.; Suo, Z. CD44(+)/CD24(−) cells are transit progenitors and do not determine the molecular subtypes and clinical parameters in breast carcinomas. Ultrastruct. Pathol. 2011, 35, 72–78. [Google Scholar] [CrossRef]
- Nogi, H.; Suzuki, M.; Kamio, M.; Kato, K.; Kawase, K.; Toriumi, Y.; Takeyama, H.; Fukushima, H.; Morikawa, T.; Uchida, K. Impact of CD44+CD24- cells on non-sentinel axillary lymph node metastases in sentinel node-positive breast cancer. Oncol. Rep. 2011, 25, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, S.; Vieira, A.F.; Gerhard, R.; Leitão, D.; Pinto, R.; Cameselle-Teijeiro, J.F.; Milanezi, F.; Schmitt, F.; Paredes, J. Breast cancer stem cell markers CD44, CD24 and ALDH1: Expression distribution within intrinsic molecular subtype. J. Clin. Pathol. 2011, 64, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Song, J.; Jiang, Y.; Yu, C.; Ma, Z. Predictive value of CD44 and CD24 for prognosis and chemotherapy response in invasive breast ductal carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 11287–11295. [Google Scholar] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, M.J.; Kim, B.G.; Kim, W.Y.; Lee, D.-H.; Yun, H.K.; Jeong, S.; Park, S.H.; Kim, B.R.; Kim, J.L.; Kim, D.Y.; et al. Cannabidiol Suppresses Angiogenesis and Stemness of Breast Cancer Cells by Downregulation of Hypoxia-Inducible Factors-1α. Cancers 2021, 13, 5667. https://doi.org/10.3390/cancers13225667
Jo MJ, Kim BG, Kim WY, Lee D-H, Yun HK, Jeong S, Park SH, Kim BR, Kim JL, Kim DY, et al. Cannabidiol Suppresses Angiogenesis and Stemness of Breast Cancer Cells by Downregulation of Hypoxia-Inducible Factors-1α. Cancers. 2021; 13(22):5667. https://doi.org/10.3390/cancers13225667
Chicago/Turabian StyleJo, Min Jee, Bu Gyeom Kim, Woo Young Kim, Dae-Hee Lee, Hye Kyeong Yun, Soyeon Jeong, Seong Hye Park, Bo Ram Kim, Jung Lim Kim, Dae Yeong Kim, and et al. 2021. "Cannabidiol Suppresses Angiogenesis and Stemness of Breast Cancer Cells by Downregulation of Hypoxia-Inducible Factors-1α" Cancers 13, no. 22: 5667. https://doi.org/10.3390/cancers13225667
APA StyleJo, M. J., Kim, B. G., Kim, W. Y., Lee, D.-H., Yun, H. K., Jeong, S., Park, S. H., Kim, B. R., Kim, J. L., Kim, D. Y., Lee, S. I., & Oh, S. C. (2021). Cannabidiol Suppresses Angiogenesis and Stemness of Breast Cancer Cells by Downregulation of Hypoxia-Inducible Factors-1α. Cancers, 13(22), 5667. https://doi.org/10.3390/cancers13225667





