Endocrine Treatment for Breast Cancer Patients Revisited—History, Standard of Care, and Possibilities of Improvement
Simple Summary
Abstract
1. Introduction
2. History and the State of the Art of Endocrine Treatment
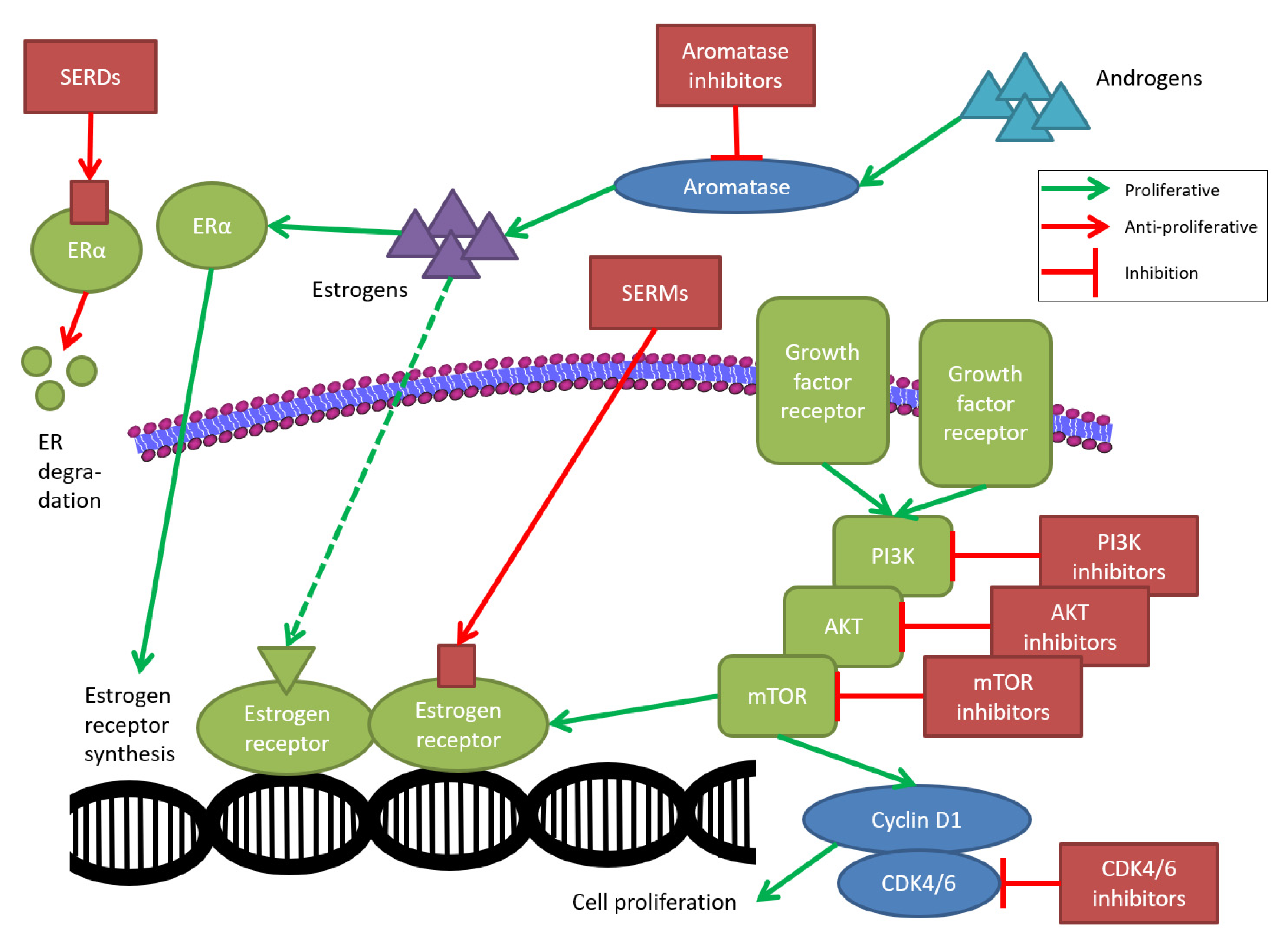
2.1. Tamoxifen
2.2. Aromatase Inhibitors
2.3. Fulvestrant
3. Modern Approaches in Advanced Breast Cancer
3.1. PI3K/AKT/mTOR Signaling Pathway
3.2. CDK4/6 Signaling Pathway
3.3. Selective Estrogen Receptor Downregulation
3.4. Germline BRCA1/2 Mutation
4. Adverse Events of and Adherence to Endocrine Treatment
4.1. Endocrine Treatment Adherence in Early Breast Cancer Patients
4.2. Endocrine Treatment Adherence in Advanced Breast Cancer Patients
5. Upcoming Improvements in the Treatment of Early Breast Cancer
5.1. CDK4/6 Inhibitors as a Possible New Cornerstone
5.2. CDK4/6 Inhibitors Instead of Neo-/Adjuvant Chemotherapy
5.3. Further Approaches
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Beatson, G. On the Treatment of Inoperable Cases of Carcinoma of the Mamma: Suggestions for a New Method of Treatment, with Illustrative Cases. Lancet 1896, 148, 162–165. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group; Davies, C.; Godwin, J.; Gray, R.; Clarke, M.; Cutter, D.; Darby, S.; McGale, P.; Pan, H.C.; Taylor, C.; et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet 2011, 378, 771–784. [Google Scholar] [PubMed]
- Hammond, M.E.H.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer. J. Clin. Oncol. 2010, 28, 2784–2795. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of Chemotherapy plus a Monoclonal Antibody against HER2 for Metastatic Breast Cancer That Overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Mouridsen, H.; Gershanovich, M.; Sun, Y.; Pérez-Carrión, R.; Boni, C.; Monnier, A.; Apffelstaedt, J.; Smith, R.; Sleeboom, H.P.; Jänicke, F.; et al. Superior Efficacy of Letrozole Versus Tamoxifen as First-Line Therapy for Postmenopausal Women with Advanced Breast Cancer: Results of a Phase III Study of the International Letrozole Breast Cancer Group. J. Clin. Oncol. 2001, 19, 2596–2606. [Google Scholar] [CrossRef]
- Nabholtz, J.; Buzdar, A.; Pollak, M.; Harwin, W.; Burton, G.; Mangalik, A.; Steinberg, M.; Webster, A.; Von Euler, M. Anastrozole Is Superior to Tamoxifen as First-Line Therapy for Advanced Breast Cancer in Postmenopausal Women: Results of a North American Multicenter Randomized Trial. J. Clin. Oncol. 2000, 18, 3758–3767. [Google Scholar] [CrossRef]
- Rugo, H.S.; Rumble, R.B.; Macrae, E.; Barton, D.L.; Connolly, H.K.; Dickler, M.N.; Fallowfield, L.; Fowble, B.; Ingle, J.N.; Jahanzeb, M.; et al. Endocrine Therapy for Hormone Receptor–Positive Metastatic Breast Cancer: American Society of Clinical Oncology Guideline. J. Clin. Oncol. 2016, 34, 3069–3103. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group; Dowsett, M.; Forbes, J.F.; Bradley, R.; Ingle, J.; Aihara, T.; Bliss, J.; Boccardo, F.; Coates, A.; Coombes, R.C.; et al. Aromatase inhibitors versus tamoxifen in early breast cancer: Patient-level meta-analysis of the randomised trials. Lancet 2015, 386, 1341–1352. [Google Scholar]
- Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer. Cochrane Database Syst. Rev. 2001, CD000486. [Google Scholar]
- Ditsch, N.; Kolberg-Liedtke, C.; Friedrich, M.; Jackisch, C.; Albert, U.-S.; Banys-Paluchowski, M.; Bauerfeind, I.; Blohmer, J.-U.; Budach, W.; Dall, P.; et al. AGO Recommendations for the Diagnosis and Treatment of Patients with Early Breast Cancer: Update 2021. Breast Care 2021, 16, 214–227. [Google Scholar] [CrossRef]
- Baum, M.; Budzar, A.U.; Cuzick, J.; Forbes, J.; Houghton, J.H.; Klijn, J.G.M.; Sahmoud, T. ATAC Trialists’ Group Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: First results of the ATAC randomised trial. Lancet 2002, 359, 2131–2139. [Google Scholar] [CrossRef] [PubMed]
- Thürlimann, B.; Keshaviah, A.; Coates, A.S.; Mouridsen, H.; Mauriac, L.; Forbes, J.F.; Paridaens, R.; Castiglione-Gertsch, M.; Gelber, R.D.; Rabaglio, M.; et al. A Comparison of Letrozole and Tamoxifen in Postmenopausal Women with Early Breast Cancer. N. Engl. J. Med. 2005, 353, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Colleoni, M.; Giobbie-Hurder, A.; Regan, M.M.; Thürlimann, B.; Mouridsen, H.; Mauriac, L.; Forbes, J.F.; Paridaens, R.; Láng, I.; Smith, I.; et al. Analyses Adjusting for Selective Crossover Show Improved Overall Survival with Adjuvant Letrozole Compared with Tamoxifen in the BIG 1-98 Study. J. Clin. Oncol. 2011, 29, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Coombes, R.C.; Hall, E.; Gibson, L.J.; Paridaens, R.; Jassem, J.; DeLozier, T.; Jones, S.E.; Alvarez, I.; Bertelli, G.; Ortmann, O.; et al. A Randomized Trial of Exemestane after Two to Three Years of Tamoxifen Therapy in Postmenopausal Women with Primary Breast Cancer. N. Engl. J. Med. 2004, 350, 1081–1092. [Google Scholar] [CrossRef]
- Jonat, W.; Gnant, M.; Boccardo, F.; Kaufmann, M.; Rubagotti, A.; Zuna, I.; Greenwood, M.; Jakesz, R. Effectiveness of switching from adjuvant tamoxifen to anastrozole in postmenopausal women with hormone-sensitive early-stage breast cancer: A meta-analysis. Lancet Oncol. 2006, 7, 991–996. [Google Scholar] [CrossRef]
- Brufsky, A.M.; Dickler, M.N. Estrogen Receptor-Positive Breast Cancer: Exploiting Signaling Pathways Implicated in Endocrine Resistance. Oncologist 2018, 23, 528–539. [Google Scholar] [CrossRef]
- Mills, J.N.; Rutkovsky, A.C.; Giordano, A. Mechanisms of resistance in estrogen receptor positive breast cancer: Overcoming resistance to tamoxifen/aromatase inhibitors. Curr. Opin. Pharmacol. 2018, 41, 59–65. [Google Scholar] [CrossRef]
- Cole, M.P.; Jones, C.; Todd, I.D.H. A New Anti-oestrogenic Agent in Late Breast Cancer: An Early Clinical Appraisal of ICI46474. Br. J. Cancer 1971, 25, 270–275. [Google Scholar] [CrossRef]
- Jordan, V.C. Tamoxifen: A most unlikely pioneering medicine. Nat. Rev. Drug Discov. 2003, 2, 205–213. [Google Scholar] [CrossRef]
- Davies, C.; Pan, H.; Godwin, J.; Gray, R.; Arriagada, R.; Raina, V.; Abraham, M.; Alencar, V.H.M.; Badran, A.; Bonfill, X.; et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013, 381, 805–816. [Google Scholar] [CrossRef]
- Gray, R.G.; Rea, D.; Handley, K.; Bowden, S.J.; Perry, P.; Earl, H.M.; Poole, C.J.; Bates, T.; Chetiyawardana, S.; Dewar, J.A.; et al. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6953 women with early breast cancer. J. Clin. Oncol. 2013, 31, 5. [Google Scholar] [CrossRef]
- Thomssen, C.; Balic, M.; Harbeck, N.; Gnant, M.S. Gallen/Vienna 2021: A Brief Summary of the Consensus Discussion on Customizing Therapies for Women with Early Breast Cancer. Breast Care 2021, 16, 135–143. [Google Scholar] [CrossRef]
- Pagani, O.; Francis, P.; Fleming, G.F.; Walley, B.A.; Viale, G.; Colleoni, M.; Láng, I.; Gómez, H.L.; Tondini, C.; Pinotti, G.; et al. Absolute Improvements in Freedom from Distant Recurrence to Tailor Adjuvant Endocrine Therapies for Premenopausal Women: Results from TEXT and SOFT. J. Clin. Oncol. 2020, 38, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Francis, P.A.; Pagani, O.; Fleming, G.F.; Walley, B.A.; Colleoni, M.; Láng, I.; Gómez, H.L.; Tondini, C.A.; Ciruelos, E.; Burstein, H.J.; et al. Tailoring Adjuvant Endocrine Therapy for Premenopausal Breast Cancer. N. Engl. J. Med. 2018, 379, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Regan, M.M.; Pagani, O.; Francis, P.; Walley, B.A.; Ribi, K.; Bernhard, J.; Luo, W.; Gómez, H.L.; Burstein, H.J.; et al. Treatment Efficacy, Adherence, and Quality of Life among Women Younger Than 35 Years in the International Breast Cancer Study Group TEXT and SOFT Adjuvant Endocrine Therapy Trials. J. Clin. Oncol. 2017, 35, 3113–3122. [Google Scholar] [CrossRef] [PubMed]
- Santen, R.J.; Brodie, H.; Simpson, E.R.; Siiteri, P.K.; Brodie, A. History of Aromatase: Saga of an Important Biological Mediator and Therapeutic Target. Endocr. Rev. 2009, 30, 343–375. [Google Scholar] [CrossRef]
- De Placido, S.; Gallo, C.; De Laurentiis, M.; Bisagni, G.; Arpino, G.; Sarobba, M.G.; Riccardi, F.; Russo, A.; Del Mastro, L.; Cogoni, A.A.; et al. Adjuvant anastrozole versus exemestane versus letrozole, upfront or after 2 years of tamoxifen, in endocrine-sensitive breast cancer (FATA-GIM3): A randomised, phase 3 trial. Lancet Oncol. 2018, 19, 474–485. [Google Scholar] [CrossRef]
- Smith, I.; Yardley, D.; Burris, H.A.; De Boer, R.; Amadori, D.; McIntyre, K.; Ejlertsen, B.; Gnant, M.; Jonat, W.; Pritchard, K.I.; et al. Comparative Efficacy and Safety of Adjuvant Letrozole Versus Anastrozole in Postmenopausal Patients with Hormone Receptor–Positive, Node-Positive Early Breast Cancer: Final Results of the Randomized Phase III Femara Versus Anastrozole Clinical Evaluation (FACE) Trial. J. Clin. Oncol. 2017, 35, 1041–1048. [Google Scholar] [CrossRef]
- Goss, P.E.; Ingle, J.N.; Pritchard, K.I.; Robert, N.J.; Muss, H.; Gralow, J.; Gelmon, K.; Whelan, T.; Strasser-Weippl, K.; Rubin, S.; et al. Extending Aromatase-Inhibitor Adjuvant Therapy to 10 Years. N. Engl. J. Med. 2016, 375, 209–219. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Z.; Xiang, Q.; Liu, Q.; Duan, X.; Liu, Y.; Cui, Y. Extended Adjuvant Therapy with Aromatase Inhibitors for Early Breast Cancer: A Meta-analysis of Randomized Controlled Trials. Clin. Breast Cancer 2019, 19, e578–e588. [Google Scholar] [CrossRef]
- Blok, E.J.; Kroep, J.R.; Meershoek-Klein Kranenbarg, E.; Duijm-de Carpentier, M.; Putter, H.; van den Bosch, J.; Maartense, E.; van Leeuwen-Stok, A.E.; Liefers, G.J.; Nortier, J.W.R.; et al. Optimal Duration of Extended Adjuvant Endocrine Therapy for Early Breast Cancer; Results of the IDEAL Trial (BOOG 2006-05). J. Natl. Cancer Inst. 2018, 110, 40–48. [Google Scholar] [CrossRef]
- Mamounas, E.P.; Bandos, H.; Lembersky, B.C.; Jeong, J.-H.; Geyer, C.E.; Rastogi, P.; Fehrenbacher, L.; Graham, M.L.; Chia, S.K.; Brufsky, A.M.; et al. Use of letrozole after aromatase inhibitor-based therapy in postmenopausal breast cancer (NRG Oncology/NSABP B-42): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 88–99. [Google Scholar] [CrossRef]
- Jerusalem, G.; Farah, S.; Courtois, A.; Chirgwin, J.; Aebi, S.; Karlsson, P.; Neven, P.; Hitre, E.; Graas, M.P.; Simoncini, E.; et al. Continuous versus intermittent extended adjuvant letrozole for breast cancer: Final results of randomized phase 3 SOLE (Study of Letrozole Extension) and SOLE Estrogen Substudy. Ann. Oncol. 2021, 32, 1256–1266. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.I.; Goodwin, A.; Wilcken, N. Fulvestrant for hormone-sensitive metastatic breast cancer. Cochrane Database Syst. Rev. 2017, 1, CD011093. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, Y.; Zhang, Y.; Zhang, S.; Li, W.; Guan, X.; Zhao, Y.; Gong, C.; Hu, X.; Zhang, J.; et al. Fulvestrant 500 mg Versus Exemestane in Postmenopausal Women with Metastatic Breast Cancer Resistant to Adjuvant Nonsteroidal Aromatase Inhibitors in Clinical Practice: A Multicenter Retrospective Study. Clin. Breast Cancer 2019, 19, e452–e458. [Google Scholar] [CrossRef]
- Robertson, J.F.R.; Jiang, Z.; Di Leo, A.; Ohno, S.; Pritchard, K.I.; Ellis, M.; Bradbury, I.; Campbell, C. A meta-analysis of clinical benefit rates for fulvestrant 500 mg vs. alternative endocrine therapies for hormone receptor-positive advanced breast cancer. Breast Cancer 2019, 26, 703–711. [Google Scholar] [CrossRef]
- Cardoso, F.; Costa, A.; Norton, L.; Senkus, E.; Aapro, M.; André, F.; Barrios, C.; Bergh, J.; Biganzoli, L.; Blackwell, K.; et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Breast 2014, 23, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Maass, N.; Ostermann, H.; Possinger, K.; Klein, P.; Tesch, H.; Mühlenhoff, L.; Bauerschlag, D. ACT-FASTER, a Prospective Cohort Study Exploring Treatment Patterns with Fulvestrant and Exemestane in Postmenopausal Patients with Advanced Hormone Receptor-Positive Breast Cancer under Real-Life Conditions in Germany. Breast Care 2019, 14, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xiong, Y.; Liao, C.; He, Y.; Duan, S.; Yi, F.; Wei, Y.; Zhang, W. Anastrozole plus fulvestrant vs. anastrozole alone for hormone receptor-positive advanced breast cancer: A meta-analysis of randomized controlled trials. Breast Cancer Res. Treat. 2020, 180, 269–278. [Google Scholar] [CrossRef]
- Beck, J.T.; Hortobagyi, G.N.; Campone, M.; Lebrun, F.; Deleu, I.; Rugo, H.S.; Pistilli, B.; Masuda, N.; Hart, L.; Melichar, B.; et al. Everolimus plus exemestane as first-line therapy in HR(+), HER2(−) advanced breast cancer in BOLERO-2. Breast Cancer Res. Treat. 2014, 143, 459–467. [Google Scholar] [CrossRef]
- Tesch, H.; Stoetzer, O.; Decker, T.; Kurbacher, C.M.; Marmé, F.; Schneeweiss, A.; Mundhenke, C.; Distelrath, A.; Fasching, P.A.; Lux, M.P.; et al. Efficacy and safety of everolimus plus exemestane in postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative locally advanced or metastatic breast cancer: Results of the single-arm, phase IIIB 4EVER trial. Int. J. Cancer 2019, 144, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Piccart, M.; Hortobagyi, G.N.; Campone, M.; Pritchard, K.I.; Lebrun, F.; Ito, Y.; Noguchi, S.; Perez, A.; Rugo, H.S.; Deleu, I.; et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: Overall survival results from BOLERO-2dagger. Ann. Oncol. 2014, 25, 2357–2362. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Zaiss, M.; Harper-Wynne, C.; Ferreira, M.; Dubey, S.; Chan, S.; Makris, A.; Nemsadze, G.; Brunt, A.M.; Kuemmel, S.; et al. Fulvestrant Plus Vistusertib vs Fulvestrant Plus Everolimus vs Fulvestrant Alone for Women with Hormone Receptor-Positive Metastatic Breast Cancer: The MANTA Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 1556–1564. [Google Scholar] [CrossRef]
- Yi, Z.; Liu, B.; Sun, X.; Rong, G.; Wang, W.; Li, H.; Guan, X.; Li, L.; Zhai, J.; Li, C.; et al. Safety and efficacy of sirolimus combined with endocrine therapy in patients with advanced hormone receptor-positive breast cancer and the exploration of biomarkers. Breast 2020, 52, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Im, S.-A.; Iwata, H.; Cortes, J.; DE Laurentiis, M.; Jiang, Z.; Arteaga, C.L.; Jonat, W.; Clemons, M.; Ito, Y.; et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 904–916. [Google Scholar] [CrossRef]
- Di Leo, A.; Johnston, S.; Lee, K.S.; Ciruelos, E.; Lønning, P.E.; Janni, W.; O’Regan, R.; Mouret-Reynier, M.A.; Kalev, D.; Egle, D.; et al. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018, 19, 87–100. [Google Scholar] [CrossRef]
- Campone, M.; Im, S.-A.; Iwata, H.; Clemons, M.; Ito, Y.; Awada, A.; Chia, S.; Jagiello-Gruszfeld, A.; Pistilli, B.; Tseng, L.-M.; et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant for postmenopausal, hormone receptor-positive, human epidermal growth factor receptor 2-negative, advanced breast cancer: Overall survival results from BELLE-2. Eur. J. Cancer 2018, 103, 147–154. [Google Scholar] [CrossRef]
- Dent, S.; Cortés, J.; Im, Y.-H.; Diéras, V.; Harbeck, N.; Krop, I.; Wilson, T.; Cui, N.; Schimmoller, F.; Hsu, J.; et al. Phase III randomized study of taselisib or placebo with fulvestrant in estrogen receptor-positive, PIK3CA-mutant, HER2-negative, advanced breast cancer: The SANDPIPER trial. Ann. Oncol. 2021, 32, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Andre, F.; Ciruelos, E.M.; Juric, D.; Loibl, S.; Campone, M.; Mayer, I.A.; Rubovszky, G.; Yamashita, T.; Kaufman, B.; Lu, Y.S.; et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: Final overall survival results from SOLAR-1. Ann. Oncol. 2021, 32, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Ciruelos, E.M.; Rugo, H.S.; Mayer, I.A.; Levy, C.; Forget, F.; Delgado Mingorance, J.I.; Safra, T.; Masuda, N.; Park, Y.H.; Juric, D.; et al. Patient-Reported Outcomes in Patients with PIK3CA-Mutated Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer from SOLAR-1. J. Clin. Oncol. 2021, 39, 2005–2015. [Google Scholar] [CrossRef]
- Andre, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef]
- Rugo, H.S.; Lerebours, F.; Ciruelos, E.; Drullinsky, P.; Ruiz-Borrego, M.; Neven, P.; Park, Y.H.; Prat, A.; Bachelot, T.; Juric, D.; et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): One cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 2021, 22, 489–498. [Google Scholar] [CrossRef]
- Burstein, H.J.; Somerfield, M.R.; Barton, D.L.; Dorris, A.; Fallowfield, L.J.; Jain, D.; Johnston, S.R.D.; Korde, L.A.; Litton, J.K.; Macrae, E.R.; et al. Endocrine Treatment and Targeted Therapy for Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer: ASCO Guideline Update. J. Clin. Oncol. 2021, JCO2101392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jang, H.; Nussinov, R. PI3K inhibitors: Review and new strategies. Chem. Sci. 2020, 11, 5855–5865. [Google Scholar] [CrossRef] [PubMed]
- Shor, R.E.; Dai, J.; Lee, S.; Pisarsky, L.; Matei, I.; Lucotti, S.; Lyden, D.; Bissell, M.J.; Ghajar, C.M. The PI3K/mTOR inhibitor Gedatolisib eliminates dormant breast cancer cells in organotypic culture, but fails to prevent metastasis in preclinical settings. Mol. Oncol. 2021. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 22 September 2021).
- Smyth, L.M.; Tamura, K.; Oliveira, M.; Ciruelos, E.M.; Mayer, I.A.; Sablin, M.P.; Biganzoli, L.; Ambrose, H.J.; Ashton, J.; Barnicle, A.; et al. Capivasertib, an AKT Kinase Inhibitor, as Monotherapy or in Combination with Fulvestrant in Patients with AKT1 (E17K)-Mutant, ER-Positive Metastatic Breast Cancer. Clin. Cancer Res. 2020, 26, 3947–3957. [Google Scholar] [CrossRef]
- Jones, R.H.; Casbard, A.; Carucci, M.; Cox, C.; Butler, R.; Alchami, F.; Madden, T.-A.; Bale, C.; Bezecny, P.; Joffe, J.; et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive breast cancer (FAKTION): A multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2020, 21, 345–357. [Google Scholar] [CrossRef]
- Turner, N.; Howell, S.; Jhaveri, K.; Gomez, H.; Toi, M.; Hu, X.; Loibl, S.; Rugo, H.S.; Ni, P.; De Bruin, E.; et al. 350TiP A phase III trial of capivasertib and fulvestrant versus placebo and fulvestrant in patients with HR+/HER2− breast cancer (CAPItello-291). Ann. Oncol. 2020, 31, S388–S389. [Google Scholar] [CrossRef]
- Dent, R.; Kim, S.-B.; Oliveira, M.; Barrios, C.; O’Shaughnessy, J.; Isakoff, S.J.; Saji, S.; Freitas-Junior, R.; Philco, M.; Bondarenko, I.; et al. Abstract GS3-04: Double-blind placebo (PBO)-controlled randomized phase III trial evaluating first-line ipatasertib (IPAT) combined with paclitaxel (PAC) for PIK3CA/AKT1/PTEN-altered locally advanced unresectable or metastatic triple-negative breast cancer (aTNBC): Primary results from IPATunity130 Cohort A. Cancer Res. 2020, 81, GS3-04. [Google Scholar] [CrossRef]
- Turner, N.; Dent, R.; O’Shaughnessy, J.; Kim, S.-B.; Isakoff, S.; Barrios, C.; Saji, S.; Bondarenko, I.; Nowecki, Z.; Lian, Q.; et al. 283MO Ipatasertib (IPAT) + paclitaxel (PAC) for PIK3CA/AKT1/PTEN-altered hormone receptor-positive (HR+) HER2-negative advanced breast cancer (aBC): Primary results from Cohort B of the IPATunity130 randomised phase III trial. Ann. Oncol. 2020, 31, S354–S355. [Google Scholar] [CrossRef]
- Finn, R.S.; Dering, J.; Conklin, D.; Kalous, O.; Cohen, D.J.; Desai, A.J.; Ginther, C.; Atefi, M.; Chen, I.; Fowst, C.; et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009, 11, R77. [Google Scholar] [CrossRef]
- Miller, T.W.; Balko, J.M.; Fox, E.M.; Ghazoui, Z.; Dunbier, A.; Anderson, H.; Dowsett, M.; Jiang, A.; Smith, R.A.; Maira, S.M.; et al. ERalpha-dependent E2F transcription can mediate resistance to estrogen deprivation in human breast cancer. Cancer Discov. 2011, 1, 338–351. [Google Scholar] [CrossRef]
- Turner, N.C.; Neven, P.; Loibl, S.; Andre, F. Advances in the treatment of advanced oestrogen-receptor-positive breast cancer. Lancet 2016, 389, 2403–2414. [Google Scholar] [CrossRef]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martin, M.; et al. Phase III Randomized Study of Ribociclib and Fulvestrant in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: MONALEESA-3. J. Clin. Oncol. 2018, 36, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.-A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; Turner, N.C.; Bondarenko, I.; Ro, J.; Im, S.-A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016, 17, 425–439. [Google Scholar] [CrossRef]
- Sledge, J.G.W.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. MONARCH 2: Abemaciclib in Combination with Fulvestrant in Women with HR+/HER2− Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J. Clin. Oncol. 2017, 35, 2875–2884. [Google Scholar] [CrossRef]
- Johnston, S.; Martin, M.; Di Leo, A.; Im, S.-A.; Awada, A.; Forrester, T.; Frenzel, M.; Hardebeck, M.C.; Cox, J.; Barriga, S.; et al. MONARCH 3 final PFS: A randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 2019, 5, 1–8. [Google Scholar] [CrossRef]
- Tripathy, D.; Im, S.-A.; Colleoni, M.; Franke, F.; Bardia, A.; Harbeck, N.; Hurvitz, S.A.; Chow, L.; Sohn, J.; Lee, K.S.; et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A randomised phase 3 trial. Lancet Oncol. 2018, 19, 904–915. [Google Scholar] [CrossRef]
- Hortobagyi, G.; Stemmer, S.; Burris, H.; Yap, Y.-S.; Sonke, G.; Paluch-Shimon, S.; Campone, M.; Petrakova, K.; Blackwell, K.; Winer, E.; et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 2018, 29, 1541–1547. [Google Scholar] [CrossRef]
- Giuliano, M.; Schettini, F.; Rognoni, C.; Milani, M.; Jerusalem, G.; Bachelot, T.; De Laurentiis, M.; Thomas, G.; De Placido, P.; Arpino, G.; et al. Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: A systematic review and network meta-analysis. Lancet Oncol. 2019, 20, 1360–1369. [Google Scholar] [CrossRef]
- Schneeweiss, A.; Ettl, J.; Luftner, D.; Beckmann, M.W.; Belleville, E.; Fasching, P.A.; Fehm, T.N.; Geberth, M.; Haberle, L.; Hadji, P.; et al. Initial experience with CDK4/6 inhibitor-based therapies compared to antihormone monotherapies in routine clinical use in patients with hormone receptor positive, HER2 negative breast cancer—Data from the PRAEGNANT research network for the first 2 years of drug availability in Germany. Breast 2020, 54, 88–95. [Google Scholar]
- Hartkopf, A.D.; Huober, J.; Volz, B.; Nabieva, N.; Taran, F.-A.; Schwitulla, J.; Overkamp, F.; Kolberg, H.-C.; Hadji, P.; Tesch, H.; et al. Treatment landscape of advanced breast cancer patients with hormone receptor positive HER2 negative tumors—Data from the German PRAEGNANT breast cancer registry. Breast 2018, 37, 42–51. [Google Scholar] [CrossRef]
- Sledge, G.W., Jr.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. The Effect of Abemaciclib Plus Fulvestrant on Overall Survival in Hormone Receptor-Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy-MONARCH 2: A Randomized Clinical Trial. JAMA Oncol. 2019, 6, 116–124. [Google Scholar] [CrossRef]
- Turner, N.C.; Slamon, D.J.; Ro, J.; Bondarenko, I.; Im, S.-A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2018, 379, 1926–1936. [Google Scholar] [CrossRef]
- Jimenez, M.M.; Zielinski, C.; Ruiz-Borrego, M.; Carrasco, E.; Ciruelos, E.; Muñoz, M.; Bermejo, B.; Margeli, M.; Csoszi, T.; Anton, A.; et al. 229MO Overall survival (OS) of palbociclib (P) plus endocrine therapy (ET) versus capecitabine (CAP) in hormone-receptor+/HER2− metastatic breast cancer (MBC) that progressed on aromatase inhibitors (AIs): Final results of the PEARL study. Ann. Oncol. 2021, 32, S457–S458. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.; Hart, L.; Campone, M.; Petrakova, K.; Winer, E.P.; Janni, W.; et al. Overall Survival Results from the Phase III MONALEESA-2 Trial of Postmenopausal Patients with HR+/HER2- Advanced Breast Cancer Treated with Endocrine Therapy +/− Ribociclib. Presented at 2021 European Society for Medical Oncology, Paris, France, 16–21 September 2021. [Google Scholar]
- Slamon, D.J.; Neven, P.; Chia, S.; Jerusalem, G.; De Laurentiis, M.; Im, S.; Petrakova, K.; Bianchi, G.V.; Martin, M.; Nusch, A.; et al. Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase 3 randomized MONALEESA-3 trial: Updated overall survival. Ann. Oncol. 2021, 32, 1015–1024. [Google Scholar] [CrossRef]
- Tripathy, D.; Im, S.-A.; Colleoni, M.; Franke, F.; Bardia, A.; Harbeck, N.; Hurvitz, S.; Chow, L.; Sohn, J.; Lee, K.S.; et al. Abstract PD2-04: Updated overall survival (OS) results from the phase III MONALEESA-7 trial of pre- or perimenopausal patients with hormone receptor positive/human epidermal growth factor receptor 2 negative (HR+/HER2−) advanced breast cancer (ABC) treated with endocrine therapy (ET) ± ribociclib. Cancer Res. 2021, 81, PD2-04. [Google Scholar]
- Sharifi, M.N.; Anandan, A.; Grogan, P.; O’Regan, R.M. Therapy after cyclin-dependent kinase inhibition in metastatic hormone receptor-positive breast cancer: Resistance mechanisms and novel treatment strategies. Cancer 2020, 126, 3400–3416. [Google Scholar] [CrossRef]
- Xu, B. Dalpiciclib Extends Progression-Free Survival in HR+/HER2- Advanced Breast Cancer. Oncologist 2021, 26 (Suppl. S3), S9–S10. [Google Scholar]
- van Kruchten, M.; de Vries, E.G.; Glaudemans, A.W.; van Lanschot, M.C.; van Faassen, M.; Kema, I.P.; Brown, M.; Schroder, C.P.; de Vries, E.F.; Hospers, G.A. Measuring residual estrogen receptor availability during fulvestrant therapy in patients with metastatic breast cancer. Cancer Discov. 2015, 5, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.C.; Crews, C.M. Induced protein degradation: An emerging drug discovery paradigm. Nat. Rev. Drug Discov. 2017, 16, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Shomali, M.; Cheng, J.; Sun, F.; Koundinya, M.; Guo, Z.; Hebert, A.T.; McManus, J.; Levit, M.N.; Hoffmann, D.; Courjaud, A.; et al. SAR439859, a Novel Selective Estrogen Receptor Degrader (SERD), Demonstrates Effective and Broad Antitumor Activity in Wild-Type and Mutant ER-Positive Breast Cancer Models. Mol. Cancer Ther. 2021, 20, 250–262. [Google Scholar] [CrossRef]
- Liang, J.; Zbieg, J.R.; Blake, R.A.; Chang, J.H.; Daly, S.; DiPasquale, A.G.; Friedman, L.S.; Gelzleichter, T.; Gill, M.; Giltnane, J.M.; et al. GDC-9545 (Giredestrant): A Potent and Orally Bioavailable Selective Estrogen Receptor Antagonist and Degrader with an Exceptional Preclinical Profile for ER+ Breast Cancer. J. Med. Chem. 2021, 64, 11841–11856. [Google Scholar] [CrossRef]
- Bardia, A.; Kaklamani, V.; Wilks, S.; Weise, A.; Richards, D.; Harb, W.; Osborne, C.; Wesolowski, R.; Karuturi, M.; Conkling, P.; et al. Phase I Study of Elacestrant (RAD1901), a Novel Selective Estrogen Receptor Degrader, in ER-Positive, HER2-Negative Advanced Breast Cancer. J. Clin. Oncol. 2021, 39, 1360–1370. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.S.; Moss, T.A.; Balazs, A.; Barlaam, B.; Breed, J.; Carbajo, R.J.; Chiarparin, E.; Davey, P.R.J.; Delpuech, O.; Fawell, S.; et al. Discovery of AZD9833, a Potent and Orally Bioavailable Selective Estrogen Receptor Degrader and Antagonist. J. Med. Chem. 2020, 63, 14530–14559. [Google Scholar] [CrossRef]
- Jhaveri, K.L.; Lim, E.; Hamilton, E.P.; Saura, C.; Meniawy, T.; Jeselsohn, R.; Beck, J.T.; Kaufman, P.A.; Sammons, S.; Banda, K.; et al. A first-in-human phase 1a/b trial of LY3484356, an oral selective estrogen receptor (ER) degrader (SERD) in ER+ advanced breast cancer (aBC) and endometrial endometrioid cancer (EEC): Results from the EMBER study. J. Clin. Oncol. 2021, 39, 1050. [Google Scholar] [CrossRef]
- Hamilton, E.P.; Patel, M.R.; Armstrong, A.C.; Baird, R.D.; Jhaveri, K.; Hoch, M.; Klinowska, T.; Lindemann, J.; Morgan, S.R.; Schiavon, G.; et al. A First-in-Human Study of the New Oral Selective Estrogen Receptor Degrader AZD9496 for ER+/HER2− Advanced Breast Cancer. Clin. Cancer Res. 2018, 24, 3510–3518. [Google Scholar] [CrossRef]
- Robertson, J.F.; Evans, A.; Henschen, S.; Kirwan, C.C.; Jahan, A.; Kenny, L.M.; Dixon, J.M.; Schmid, P.; Kothari, A.; Mohamed, O.; et al. A randomized, window of opportunity study comparing the effects of the novel oral SERD AZD9496 with fulvestrant in patients with ER+ HER2− primary breast cancer. Clin. Cancer Res. 2020, 26, 4242–4249. [Google Scholar] [CrossRef]
- Hurvitz, S.; Park, Y.; Bardia, A.; Quiroga, V.; López-Valverde, V.; Steinseifer, J.; Moore, H.; Spera, G.; Xue, C.; Fasching, P. LBA14 Neoadjuvant giredestrant (GDC-9545) + palbociclib (palbo) vs anastrozole (A) + palbo in post-menopausal women with oestrogen receptor-positive, HER2-negative, untreated early breast cancer (ER+/HER2– eBC): Interim analysis of the randomised, open-label, phase II coopERA BC study. Ann. Oncol. 2021, 32, S1285–S1286. [Google Scholar] [CrossRef]
- Chandarlapaty, S.; Linden, H.M.; Neven, P.; Petrakova, K.; Bardiz, A.; Kabos, P.; Braga, S.; Boni, V.; Pelekanou, V.; Ternès, N.; et al. AMEERA-1: Subgroup analyses of phase I/II study of amcenestrant (SAR439859), an oral selective estrogen receptor (ER) degrader (SERD), with palbociclib in postmenopausal women with ER+/ human epidermal growth factor receptor 2-negative (HER2–) advanced breast cancer (aBC). Presented at the 2021 European Society for Medical Oncology, Paris, France, 16–21 September 2021. [Google Scholar]
- Radius. Menarini Group and Radius Health Announce Positive Phase 3 Topline Results from the EMERALD Trial Evaluating Elacestrant in Breast Cancer. Press Release 20 October 2021. Available online: https://ir.radiuspharm.com/news-releases/news-release-details/menarini-group-and-radius-health-announce-positive-phase-3 (accessed on 20 October 2021).
- Fasching, P.A.; Yadav, S.; Hu, C.; Wunderle, M.; Haberle, L.; Hart, S.N.; Rubner, M.; Polley, E.C.; Lee, K.Y.; Gnanaolivu, R.D.; et al. Mutations in BRCA1/2 and Other Panel Genes in Patients with Metastatic Breast Cancer—Association with Patient and Disease Characteristics and Effect on Prognosis. J. Clin. Oncol. 2021, 39, 1619–1630. [Google Scholar] [CrossRef]
- Robson, M.; Im, S.-A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Gonçalves, A.; Lee, K.-H.; Fehrenbacher, L.; Yerushalmi, R.; Mina, L.A.; Martin, M.; et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N. Engl. J. Med. 2018, 379, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.; Tung, N.; Conte, P.; Im, S.-A.; Senkus, E.; Xu, B.; Masuda, N.; Delaloge, S.; Li, W.; Armstrong, A.; et al. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann. Oncol. 2019, 30, 558–566. [Google Scholar] [CrossRef]
- Litton, J.K.; Hurvitz, S.A.; Mina, L.A.; Rugo, H.S.; Lee, K.H.; Goncalves, A.; Diab, S.; Woodward, N.; Goodwin, A.; Yerushalmi, R.; et al. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: Final overall survival results from the EMBRACA trial. Ann. Oncol. 2020, 31, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Thill, M.; Friedrich, M.; Kolberg-Liedtke, C.; Albert, U.-S.; Banys-Paluchowski, M.; Bauerfeind, I.; Blohmer, J.-U.; Budach, W.; Dall, P.; Fallenberg, E.M.; et al. AGO Recommendations for the Diagnosis and Treatment of Patients with Locally Advanced and Metastatic Breast Cancer: Update 2021. Breast Care 2021, 16, 228–235. [Google Scholar] [CrossRef]
- Tesch, H.; Müller, V.; Wöckel, A.; Ettl, J.; Belleville, E.; Schütz, F.; Hartkopf, A.; Thill, M.; Huober, J.; Fasching, P.A.; et al. Update Breast Cancer 2020 Part 4—Advanced Breast Cancer. Geburtshilfe Frauenheilkd. 2020, 80, 1115–1122. [Google Scholar] [CrossRef]
- Stickeler, E.; Aktas, B.; Behrens, A.; Belleville, E.; Ditsch, N.; Fasching, P.A.; Fehm, T.N.; Hartkopf, A.D.; Jackisch, C.; Janni, W.; et al. Update Breast Cancer 2021 Part 1—Prevention and Early Stages. Geburtshilfe Frauenheilkd. 2021, 81, 526–538. [Google Scholar] [CrossRef] [PubMed]
- Huober, J.; Schneeweiss, A.; Hartkopf, A.D.; Müller, V.; Lux, M.P.; Janni, W.; Ettl, J.; Belleville, E.; Thill, M.; Fasching, P.A.; et al. Update Breast Cancer 2020 Part 3—Early Breast Cancer. Geburtshilfe Frauenheilkd. 2020, 80, 1105–1114. [Google Scholar] [CrossRef]
- Ditsch, N.; Stickeler, E.; Behrens, A.; Belleville, E.; Fasching, P.A.; Fehm, T.N.; Hartkopf, A.D.; Jackisch, C.; Janni, W.; Kolberg-Liedtke, C.; et al. Update Breast Cancer 2021 Part 2—Advanced Stages, Long-Term Consequences and Biomarkers. Geburtshilfe Frauenheilkd. 2021, 81, 539–548. [Google Scholar] [CrossRef]
- Fehm, T.N.; Stickeler, E.; Fasching, P.A.; Janni, W.; Kolberg-Liedtke, C.; Kolberg, H.-C.; Lüftner, D.; Müller, V.; Schütz, F.; Thomssen, C.; et al. Update Breast Cancer 2021 Part 3—Current Developments in the Treatment of Early Breast Cancer: Review and Assessment of Specialised Treatment Scenarios by an International Expert Panel. Geburtshilfe und Frauenheilkunde 2021, 81, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Nabieva, N.; Fehm, T.; Haberle, L.; de Waal, J.; Rezai, M.; Baier, B.; Baake, G.; Kolberg, H.C.; Guggenberger, M.; Warm, M.; et al. Influence of side-effects on early therapy persistence with letrozole in post-menopausal patients with early breast cancer: Results of the prospective EvAluate-TM study. Eur. J. Cancer 2018, 96, 82–90. [Google Scholar] [CrossRef]
- Stearns, V.; Chapman, J.-A.W.; Manuela, R.; Ellis, M.J.; Ingle, J.N.; Pritchard, K.I.; Budd, G.T.; Rabaglio, M.; Sledge, G.W.; Le Maitre, A.; et al. Treatment-Associated Musculoskeletal and Vasomotor Symptoms and Relapse-Free Survival in the NCIC CTG MA.27 Adjuvant Breast Cancer Aromatase Inhibitor Trial. J. Clin. Oncol. 2015, 33, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chlebowski, R.T.; Shi, J.; Barac, A.; Haque, R. Aromatase inhibitor and tamoxifen use and the risk of venous thromboembolism in breast cancer survivors. Breast Cancer Res. Treat. 2019, 174, 785–794. [Google Scholar] [CrossRef]
- van Hellemond, I.E.G.; Smorenburg, C.H.; Peer, P.G.M.; Swinkels, A.C.P.; Seynaeve, C.M.; van der Sangen, M.J.C.; Kroep, J.R.; de Graaf, H.; Honkoop, A.H.; Erdkamp, F.L.G.; et al. Assessment and management of bone health in women with early breast cancer receiving endocrine treatment in the DATA study. Int. J. Cancer. 2019, 145, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Hadji, P.; Jackisch, C.; Bolten, W.; Blettner, M.; Hindenburg, H.J.; Klein, P.; Konig, K.; Kreienberg, R.; Rief, W.; Wallwiener, D.; et al. COMPliance and Arthralgia in Clinical Therapy: The COMPACT trial, assessing the incidence of arthralgia, and compliance within the first year of adjuvant anastrozole therapy. Ann. Oncol. 2014, 25, 372–377. [Google Scholar] [CrossRef]
- Chirgwin, J.H.; Giobbie-Hurder, A.; Coates, A.S.; Price, K.N.; Ejlertsen, B.; Debled, M.; Gelber, R.D.; Goldhirsch, A.; Smith, I.; Rabaglio, M.; et al. Treatment Adherence and Its Impact on Disease-Free Survival in the Breast International Group 1-98 Trial of Tamoxifen and Letrozole, Alone and in Sequence. J. Clin. Oncol. 2016, 34, 2452–2459. [Google Scholar] [CrossRef]
- Henry, N.L.; Azzouz, F.; Desta, Z.; Li, L.; Nguyen, A.T.; Lemler, S.; Hayden, J.; Tarpinian, K.; Yakim, E.; Flockhart, D.A.; et al. Predictors of Aromatase Inhibitor Discontinuation as a Result of Treatment-Emergent Symptoms in Early-Stage Breast Cancer. J. Clin. Oncol. 2012, 30, 936–942. [Google Scholar] [CrossRef]
- Lombard, J.M.; Zdenkowski, N.; Wells, K.; Beckmore, C.; Reaby, L.; Forbes, J.F.; Chirgwin, J. Aromatase inhibitor induced musculoskeletal syndrome: A significant problem with limited treatment options. Support. Care Cancer 2016, 24, 2139–2146. [Google Scholar] [CrossRef]
- Onesti, C.E.; Jerusalem, G. CDK4/6 inhibitors in breast cancer: Differences in toxicity profiles and impact on agent choice. A systematic review and meta-analysis. Expert Rev. Anticancer Ther. 2021, 21, 283–298. [Google Scholar] [CrossRef]
- Goetz, M.P.; Martin, M.; Tokunaga, E.; Park, I.H.; Huober, J.; Toi, M.; Stoffregen, C.; Shekarriz, S.; Andre, V.; Gainford, M.C.; et al. Health-Related Quality of Life in MONARCH 3: Abemaciclib plus an Aromatase Inhibitor as Initial Therapy in HR+, HER2− Advanced Breast Cancer. Oncologist 2020, 25, e1346–e1354. [Google Scholar] [CrossRef]
- Verma, S.; O’Shaughnessy, J.; Burris, H.A.; Campone, M.; Alba, E.; Chandiwana, D.; Dalal, A.A.; Sutradhar, S.; Monaco, M.; Janni, W. Health-related quality of life of postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer treated with ribociclib + letrozole: Results from MONALEESA-2. Breast Cancer Res. Treat. 2018, 170, 535–545. [Google Scholar] [CrossRef]
- Rugo, H.; Diéras, V.; Gelmon, K.; Finn, R.; Slamon, D.; Martin, M.; Neven, P.; Shparyk, Y.; Mori, A.; Lu, D.; et al. Impact of palbociclib plus letrozole on patient-reported health-related quality of life: Results from the PALOMA-2 trial. Ann. Oncol. 2018, 29, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Nabieva, N.; Kellner, S.; Fehm, T.; Häberle, L.; de Waal, J.; Rezai, M.; Baier, B.; Baake, G.; Kolberg, H.-C.; Guggenberger, M.; et al. Influence of patient and tumor characteristics on early therapy persistence with letrozole in postmenopausal women with early breast cancer: Results of the prospective Evaluate-TM study with 3941 patients. Ann. Oncol. 2018, 29, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Hadji, P.; Ziller, V.; Kyvernitakis, I.; Bauer, M.; Haas, G.; Schmidt, N.; Kostev, K. Persistence in patients with breast cancer treated with tamoxifen or aromatase inhibitors: A retrospective database analysis. Breast Cancer Res. Treat. 2013, 138, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Hershman, D.L.; Shao, T.; Kushi, L.; Buono, D.; Tsai, W.Y.; Fehrenbacher, L.; Kwan, M.; Gomez, S.L.; Neugut, A.I. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res. Treat. 2011, 126, 529–537. [Google Scholar] [CrossRef]
- Partridge, A.H.; LaFountain, A.; Mayer, E.; Taylor, B.S.; Winer, E.; Asnis-Alibozek, A. Adherence to Initial Adjuvant Anastrozole Therapy among Women with Early-Stage Breast Cancer. J. Clin. Oncol. 2008, 26, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.A.; Roy, A.; Burrell, A.; Fairchild, C.J.; Fuldeore, M.J.; Ollendorf, D.A.; Wong, P.K. Medication Compliance and Persistence: Terminology and Definitions. Value Health 2008, 11, 44–47. [Google Scholar] [CrossRef]
- Hershman, D.L.; Kushi, L.; Shao, T.; Buono, D.; Kershenbaum, A.; Tsai, W.-Y.; Fehrenbacher, L.; Gomez, S.L.; Miles, S.; Neugut, A.I. Early Discontinuation and Nonadherence to Adjuvant Hormonal Therapy in a Cohort of 8769 Early-Stage Breast Cancer Patients. J. Clin. Oncol. 2010, 28, 4120–4128. [Google Scholar] [CrossRef]
- Brito, C.; Portela, M.C.; De Vasconcellos, M.T.L. Adherence to hormone therapy among women with breast cancer. BMC Cancer 2014, 14, 397. [Google Scholar] [CrossRef]
- Kemp, A.; Preen, D.B.; Saunders, C.; Boyle, F.; Bulsara, M.; Malacova, E.; Roughead, E.E. Early discontinuation of endocrine therapy for breast cancer: Who is at risk in clinical practice? Springerplus 2014, 3, 282. [Google Scholar] [CrossRef] [PubMed]
- Wigertz, A.; Ahlgren, J.; Holmqvist, M.; Fornander, T.; Adolfsson, J.; Lindman, H.; Bergkvist, L.; Lambe, M. Adherence and discontinuation of adjuvant hormonal therapy in breast cancer patients: A population-based study. Breast Cancer Res. Treat. 2012, 133, 367–373. [Google Scholar] [CrossRef]
- Tinari, N.; Fanizza, C.; Romero, M.; Gambale, E.; Moscetti, L.; Vaccaro, A.; Seminara, P.; Longo, F.; Gori, S.; Vici, P.; et al. Identification of Subgroups of Early Breast Cancer Patients at High Risk of Nonadherence to Adjuvant Hormone Therapy: Results of an Italian Survey. Clin. Breast Cancer 2015, 15, e131–e137. [Google Scholar] [CrossRef]
- Addeo, R.; Iodice, P.; Maiorino, L.; Febbraro, A.; Incoronato, P.; Pisano, A.; Bianco, M.; Mabilia, R.; Riccardi, F.; Del Prete, S. Acceptance and Adherence of Oral Endocrine Therapy in Women with Metastatic Breast Cancer: Exacampania Group Study. Breast J. 2015, 21, 326–328. [Google Scholar] [CrossRef]
- Guth, U.; Huang, D.J.; Schotzau, A.; Schmid, S.M. Use of Palliative Endocrine Therapy in Patients with Hormone Receptor-Positive Distant Metastatic Breast Cancer: How Often, How Effective, How Long? Oncology 2016, 90, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wallwiener, M.; Nabieva, N.; Feisst, M.; Fehm, T.; De Waal, J.; Rezai, M.; Baier, B.; Baake, G.; Kolberg, H.C.; Guggenberger, M.; et al. Patient and tumor characteristics and their influence on therapy persistence with letrozole in patients with advanced breast cancer. BMC Cancer 2018, submitted. [Google Scholar]
- Neugut, A.I.; Zhong, X.; Wright, J.D.; Accordino, M.; Yang, J.; Hershman, D.L. Nonadherence to Medications for Chronic Conditions and Nonadherence to Adjuvant Hormonal Therapy in Women with Breast Cancer. JAMA Oncol. 2016, 2, 1326–1332. [Google Scholar] [CrossRef] [PubMed]
- Nabieva, N.; Häberle, L.; Brucker, S.Y.; Janni, W.; Volz, B.; Loehberg, C.R.; Hartkopf, A.; Walter, C.; Baake, G.; Fridman, A.; et al. The influence of preexisting joint and muscle/limb pain on the development of musculoskeletal adverse events during letrozole treatment in early breast cancer patients—Final results from the PreFace study. Int. J. Cancer 2018, submitted. [Google Scholar]
- Robertson, J.F.R.; Bondarenko, I.; Trishkina, E.; Dvorkin, M.; Panasci, L.; Manikhas, A.; Shparyk, Y.; Cardona-Huerta, S.; Cheung, K.-L.; Philco-Salas, M.J.; et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): An international, randomised, double-blind, phase 3 trial. Lancet 2016, 388, 2997–3005. [Google Scholar] [CrossRef]
- Wallwiener, M.; Nabieva, N.; Feisst, M.; Fehm, T.; de Waal, J.; Rezai, M.; Baier, B.; Baake, G.; Kolberg, H.C.; Guggenberger, M.; et al. Influence of patient and tumor characteristics on therapy persistence with letrozole in postmenopausal women with advanced breast cancer: Results of the prospective observational EvAluate-TM study. BMC Cancer 2019, 19, 611. [Google Scholar] [CrossRef]
- Loibl, S.; Marmé, F.; Martin, M.; Untch, M.; Bonnefoi, H.; Kim, S.-B.; Bear, H.; McCarthy, N.; Olivé, M.M.; Gelmon, K.; et al. Palbociclib for Residual High-Risk Invasive HR-Positive and HER2-Negative Early Breast Cancer—The Penelope-B Trial. J. Clin. Oncol. 2021, 39, 1518–1530. [Google Scholar] [CrossRef]
- Mayer, E.L.; Dueck, A.C.; Martin, M.; Rubovszky, G.; Burstein, H.J.; Bellet-Ezquerra, M.; Miller, K.D.; Zdenkowski, N.; Winer, E.P.; Pfeiler, G.; et al. Palbociclib with adjuvant endocrine therapy in early breast cancer (PALLAS): Interim analysis of a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2021, 22, 212–222. [Google Scholar] [CrossRef]
- O’Shaughnessy, J.A.; Johnston, S.; Harbeck, N.; Toi, M.; Im, Y.-H.; Reinisch, M.; Shao, Z.; Lehtinen, P.L.K.; Huang, C.-S.; Tryakin, A.; et al. Abstract GS1-01: Primary outcome analysis of invasive disease-free survival for monarchE: Abemaciclib combined with adjuvant endocrine therapy for high risk early breast cancer. Cancer Res. 2021, 81, GS1-01. [Google Scholar] [CrossRef]
- Harbeck, N.; Rastogi, P.; Martin, M.; Tolaney, S.; Shao, Z.; Fasching, P.; Huang, C.; Jaliffe, G.; Tryakin, A.; Goetz, M.; et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: Updated efficacy and Ki-67 analysis from the monarchE study. Ann. Oncol. 2021. [Google Scholar] [CrossRef]
- Slamon, D.J.; Fasching, P.A.; Patel, R.; Verma, S.; Hurvitz, S.A.; Chia, S.K.L.; Crown, J.; Martin, M.; Barrios, C.H.; Spera, G.; et al. NATALEE: Phase III study of ribociclib (RIBO) + endocrine therapy (ET) as adjuvant treatment in hormone receptor–positive (HR+), human epidermal growth factor receptor 2–negative (HER2–) early breast cancer (EBC). J. Clin. Oncol. 2019, 37, TPS597. [Google Scholar] [CrossRef]
- Prat, A.; Saura, C.; Pascual, T.; Hernando, C.; Munoz, M.; Pare, L.; Farré, B.G.; Fernández, P.L.; Galván, P.; Chic, N.; et al. Ribociclib plus letrozole versus chemotherapy for postmenopausal women with hormone receptor-positive, HER2-negative, luminal B breast cancer (CORALLEEN): An open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2020, 21, 33–43. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Martin, M.; Press, M.F.; Chan, D.; Fernandez-Abad, M.; Petru, E.; Rostorfer, R.; Guarneri, V.; Huang, C.-S.; Barriga, S.; et al. Potent Cell-Cycle Inhibition and Upregulation of Immune Response with Abemaciclib and Anastrozole in neoMONARCH, Phase II Neoadjuvant Study in HR+/HER2− Breast Cancer. Clin. Cancer Res. 2020, 26, 566–580. [Google Scholar] [CrossRef] [PubMed]
- Cottu, P.; D’Hondt, V.; Dureau, S.; Lerebours, F.; Desmoulins, I.; Heudel, P.; Duhoux, F.; Levy, C.; Mouret-Reynier, M.-A.; Dalenc, F.; et al. Letrozole and palbociclib versus chemotherapy as neoadjuvant therapy of high-risk luminal breast cancer. Ann. Oncol. 2018, 29, 2334–2340. [Google Scholar] [CrossRef]
- Ma, C.X.; Gao, F.; Luo, J.; Northfelt, D.W.; Goetz, M.; Forero, A.; Hoog, J.; Naughton, M.; Ademuyiwa, F.; Suresh, R.; et al. NeoPalAna: Neoadjuvant Palbociclib, a Cyclin-Dependent Kinase 4/6 Inhibitor, and Anastrozole for Clinical Stage 2 or 3 Estrogen Receptor-Positive Breast Cancer. Clin. Cancer Res. 2017, 23, 4055–4065. [Google Scholar] [CrossRef]
- Harbeck, N.; Gluz, O.; Christgen, M.; Graeser, M.; Hilpert, F.; Krauss, K.; Thill, M.; Warm, M.; Müller, V.; Braun, M.W.; et al. ADAPTcycle: Adjuvant dynamic marker-adjusted personalized therapy (ADAPT) comparing endocrine therapy plus ribociclib versus chemotherapy in intermediate-risk HR+/HER2− early breast cancer (EBC). J. Clin. Oncol. 2020, 38, TPS601. [Google Scholar] [CrossRef]
- Prat, A.; Chaudhury, A.; Solovieff, N.; Paré, L.; Martinez, D.; Chic, N.; Martínez-Sáez, O.; Brasó-Maristany, F.; Lteif, A.; Taran, T.; et al. Correlative Biomarker Analysis of Intrinsic Subtypes and Efficacy Across the MONALEESA Phase III Studies. J. Clin. Oncol. 2021, 39, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- Saura, C.; Hlauschek, D.; Oliveira, M.; Zardavas, D.; Jallitsch-Halper, A.; de la Peña, L.; Nuciforo, P.; Ballestrero, A.; Dubsky, P.; Lombard, J.M.; et al. Neoadjuvant letrozole plus taselisib versus letrozole plus placebo in postmenopausal women with oestrogen receptor-positive, HER2-negative, early-stage breast cancer (LORELEI): A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2019, 20, 1226–1238. [Google Scholar] [CrossRef]
- Mayer, I.A.; Prat, A.; Egle, D.; Blau, S.; Fidalgo, J.A.P.; Gnant, M.; Fasching, P.A.; Colleoni, M.; Wolff, A.; Winer, E.P.; et al. A Phase II Randomized Study of Neoadjuvant Letrozole Plus Alpelisib for Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer (NEO-ORB). Clin. Cancer Res. 2019, 25, 2975–2987. [Google Scholar] [CrossRef] [PubMed]
- Sanofi. Sanofi Partnering with Leading Academic Cooperative Groups to Study Amcenestrant in the Adjuvant Setting for Patients with Estrogen Receptor Positive Breast Cancer. Press Release 4 June 2021. Available online: https://www.sanofi.com/en/media-room/press-releases/2021/2021-06-04-07-00-00-2241809 (accessed on 13 October 2021).
- TRIO. TRIO Enrols First Patient in Global Phase 3 Giredestrant Early Breast Cancer TriaL. Press Release 31 August 2021. Available online: https://www.globenewswire.com/en/news-release/2021/08/31/2289261/0/en/TRIO-Enrols-First-Patient-in-Global-Phase-3-Giredestrant-Early-Breast-Cancer-Trial.html (accessed on 13 October 2021).
- Schiewer, M.J.; Goodwin, J.F.; Han, S.; Brenner, J.C.; Augello, M.A.; Dean, J.L.; Liu, F.; Planck, J.L.; Ravindranathan, P.; Chinnaiyan, A.M.; et al. Dual Roles of PARP-1 Promote Cancer Growth and Progression. Cancer Discov. 2012, 2, 1134–1149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, Y.; Wang, L.; Luo, X.; Huang, K.; Wang, C.; Du, M.; Liu, F.; Luo, T.; Huang, D.; et al. Poly(ADP-ribose) Polymerase 1 Is a Key Regulator of Estrogen Receptor α-dependent Gene Transcription. J. Biol. Chem. 2013, 288, 11348–11357. [Google Scholar] [CrossRef]
- Tutt, A.N.; Garber, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.D.; de Azambuja, E.; Fielding, A.; Balmaña, J.; et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N. Engl. J. Med. 2021, 384, 2394–2405. [Google Scholar] [CrossRef]
| Treatment Combination | Study Name | Sample Size | Randomization | Median PFS in Months | Median OS in Months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| With CDK4/6 Inhibitor | Without CDK4/6 Inhibitor | HR | 95% CI | Statistically Significant as per Protocol | With CDK4/6 Inhibitor | Without CDK4/6 Inhibitor | HR | 95% CI | Statistically Significant as per Protocol | ||||
| ET +/− abemaciclib | MONARCH-2 [68,75] | 669 | 2:1 | 16.4 | 9.3 | 0.55 | 0.45–0.68 | Yes | 46.7 | 37.3 | 0.76 | 0.61–0.95 | Yes |
| MONARCH-3 [69] | 493 | 2:1 | 28.2 | 14.8 | 0.54 | 0.42–0.70 | Yes | Not reported yet | |||||
| ET +/− dalpiciclib | DAWNA-1 [82] | 361 | 2:1 | 13.6 1 | 7.7 1 | 0.45 1 | 0.32–0.64 1 | Yes 1 | Not reported yet | ||||
| ET +/− palbociclib | PALOMA-2 [66] | 666 | 2:1 | 24.8 | 14.5 | 0.58 | 0.46–0.72 | Yes | Not reported yet | ||||
| PALOMA-3 [67,76] | 521 | 2:1 | 9.5 | 4.6 | 0.46 | 0.36–0.59 | Yes | 34.9 | 28.0 | 0.81 | 0.64–1.03 | No | |
| ET +/− ribociclib | MONALEESA-2 [71,78] | 668 | 1:1 | 25.3 | 16.0 | 0.57 | 0.46–0.70 | Yes | 63.9 | 51.4 | 0.76 | 0.63–0.93 | Yes |
| MONALEESA-3 [65,79] | 726 | 2:1 | 20.5 | 12.8 | 0.59 | 0.48–0.73 | Yes | 53.7 | 41.5 | 0.73 | 0.59–0.90 | Yes | |
| MONALEESA-7 [70,80] | 672 | 1:1 | 23.8 | 13.0 | 0.55 | 0.44–0.69 | Yes | 58.7 | 48.0 | 0.76 | 0.61–0.96 | Yes | |
| Substance Name | Original Substance Code | Trial Program |
|---|---|---|
| Amcenestrant [85] | SAR439859 | AMEERA |
| Camizestrant [88] | AZD9833 | SERENA |
| Elacestrant [87] | RAD1901 | EMERALD |
| Giredestrant [86] | GDC-9545 | persevERA (aBC) and lidERA (eBC) |
| - 1 | LY3484356 [89] | EMBER |
| CDK4/6 Inhibitor | Abemaciclib | Dalpiciclib | Palbociclib 1 | Ribociclib | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study name | ADAPTlate | CARABELA | monarchE | POETIC-A | SHR6390-III-303 | Appalaches | POLAR | TRAK-ER | ADAPTcycle | LEADER | NATALEE |
| Study code | NCT04565054 | NCT04293393 | NCT03155997 | NCT04584853 | NCT04842617 | NCT03609047 | NCT03820830 | NCT04985266 | NCT04055493 | NCT03285412 | NCT03701334 |
| Phase | III | II | III | III | III | II | III | II | III | II | III |
| Brief study summary | Adjuvant abemaciclib + SOC ET vs. SOC ET | Neoadjuvant abemaciclib + SOC ET vs. chemotherapy | Adjuvant abemaciclib + SOC ET vs. SOC ET | Adjuvant abemaciclib + SOC ET vs. SOC ET | Adjuvant dalpiciclib + SOC ET vs. SOC ET | Adjuvant palbociclib + SOC ET vs. chemotherapy followed by SOC ET | Adjuvant palbociclib + SOC ET vs. SOC ET | Adjuvant palbociclib + fulvestrant vs. SOC ET | Adjuvant ribociclib + SOC ET vs. chemotherapy followed by SOC ET | Adjuvant intermittent ribociclib + SOC ET vs. continuous ribociclib + SOC ET | Adjuvant ribociclib + SOC ET vs. SOC ET |
| Main patient population criteria | High risk | High and intermediate risk | High risk | High risk | High risk | High risk | Local/regional recurrence | High risk | Intermediate risk | High and intermediate risk | High and intermediate risk |
| Pre- and postmenopausal | Pre- and postmenopausal | Pre- and postmenopausal | Postmenopausal | Node-positive | ≥70 years old | Pre- and postmenopausal | Pre- and postmenopausal | Pre- and postmenopausal | MRD based on ctDNA | Pre- and postmenopausal | |
| Female only | Female only | Male patients allowed | Female only | Pre- and postmenopausal | Male patients allowed | Male patients allowed | Male patients allowed | Female only | Pre- and postmenopausal | Male patients allowed | |
| Female only | |||||||||||
| Randomization | 1:1 | 1:1 | 1:1 | 1:1 | 1:1 | 2:1 | 1:1 | 1:1 | 3:2 | 1:1 | 1:1 |
| Duration of CDK4/6i intake | 2 years | 1 year | 2 years | 2 years | - 2 | 2 years | 3 years | 2 years | 2 years | 1 year | 3 years |
| Number of patients | 1250 | 200 | 5637 | 2500 | 4350 | 366 | 400 | 1100 | 1670 | 231 | 5101 |
| Primary endpoint | iDFS | RCB | iDFS | iDFS | iDFS | D-RFI | iDFS | ctDNA, iDFS | iDFS | Safety | iDFS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nabieva, N.; Fasching, P.A. Endocrine Treatment for Breast Cancer Patients Revisited—History, Standard of Care, and Possibilities of Improvement. Cancers 2021, 13, 5643. https://doi.org/10.3390/cancers13225643
Nabieva N, Fasching PA. Endocrine Treatment for Breast Cancer Patients Revisited—History, Standard of Care, and Possibilities of Improvement. Cancers. 2021; 13(22):5643. https://doi.org/10.3390/cancers13225643
Chicago/Turabian StyleNabieva, Naiba, and Peter A. Fasching. 2021. "Endocrine Treatment for Breast Cancer Patients Revisited—History, Standard of Care, and Possibilities of Improvement" Cancers 13, no. 22: 5643. https://doi.org/10.3390/cancers13225643
APA StyleNabieva, N., & Fasching, P. A. (2021). Endocrine Treatment for Breast Cancer Patients Revisited—History, Standard of Care, and Possibilities of Improvement. Cancers, 13(22), 5643. https://doi.org/10.3390/cancers13225643









