Gasdermin D Is a Novel Prognostic Biomarker and Relates to TMZ Response in Glioblastoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Glioma Tissues
2.2. GlioVis Analysis
2.3. Immunohistochemical (IHC) Staining and Evaluation
2.4. Immunofluorescence Staining
2.5. Cells and Cell Culture
2.6. Western Blot
2.7. Statistical Analysis
3. Results
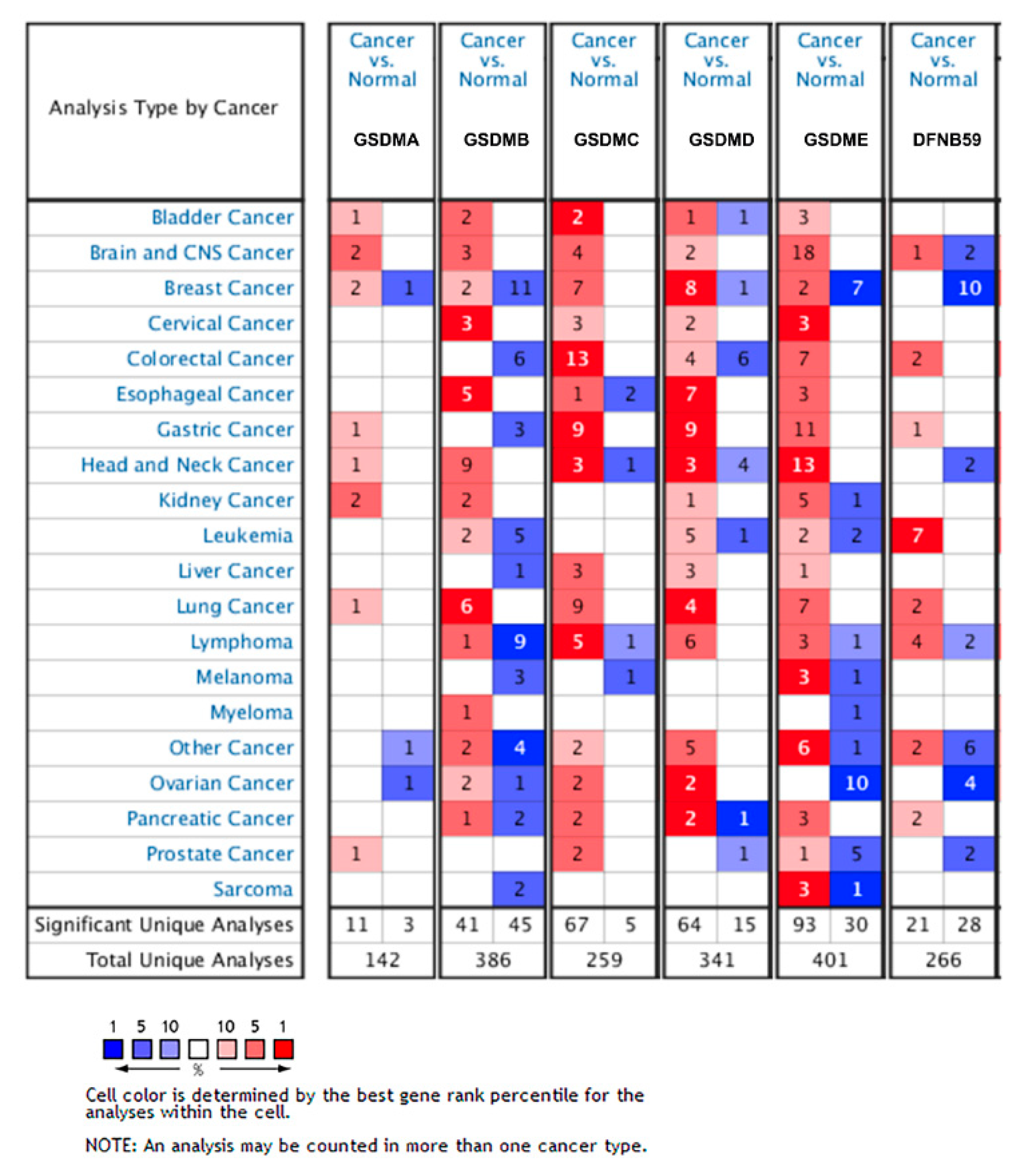
3.1. Transcriptional Level of GSDMs in Glioma
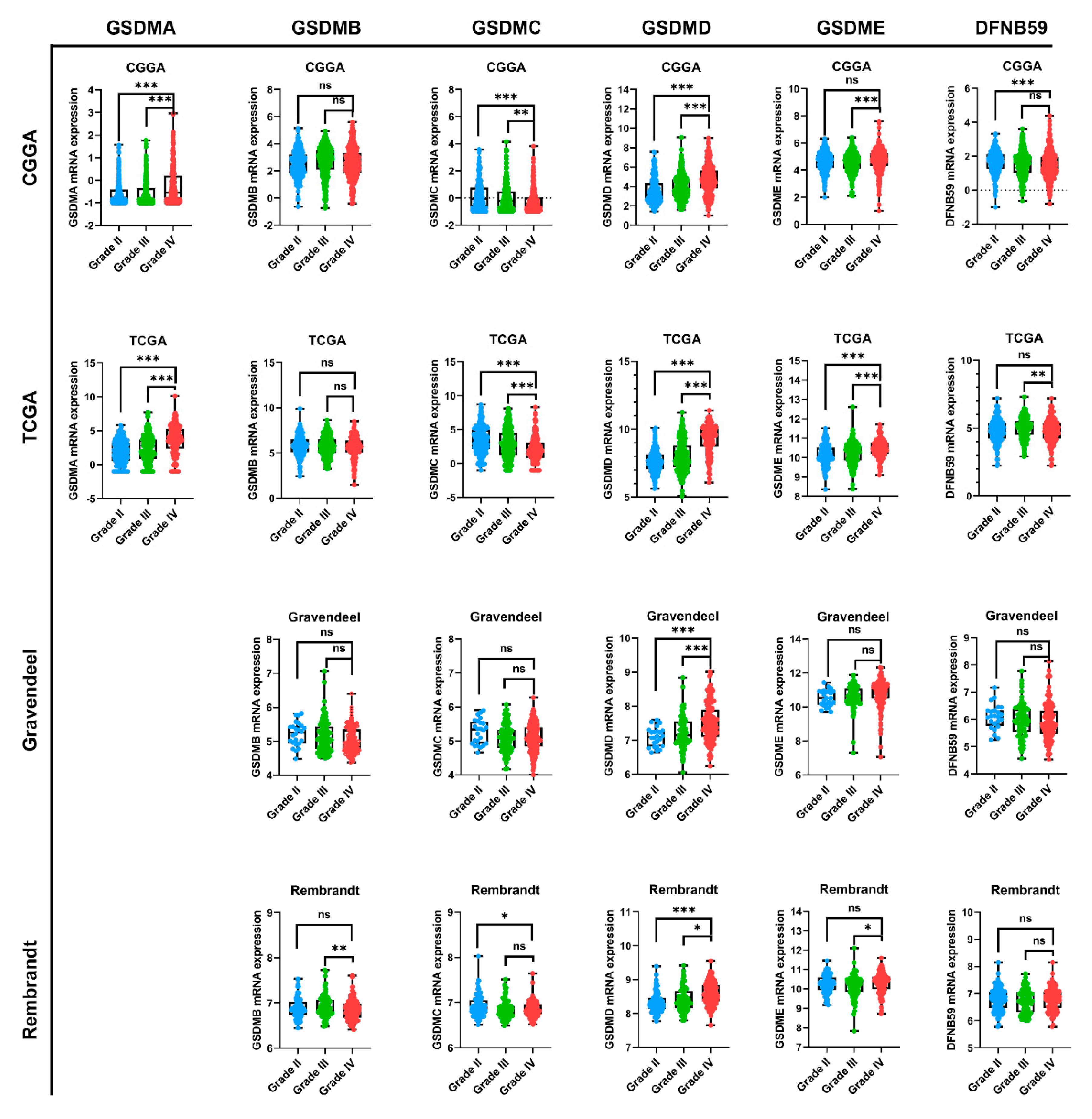
3.2. GSMDs mRNA Expression Associated with Glioma WHO Grade
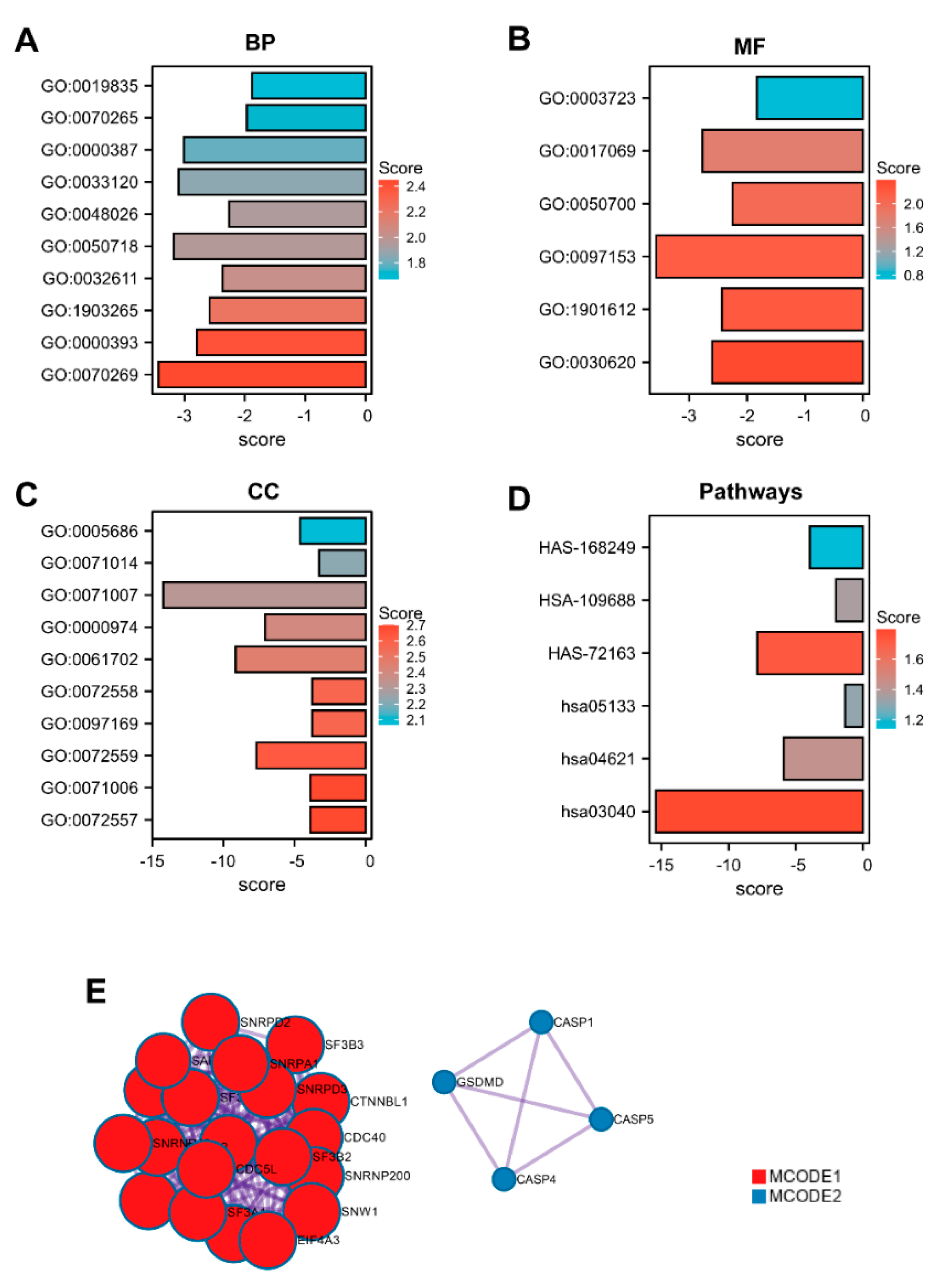
3.3. Gene Ontology Enrichment Analysis
3.4. Prognostic Role of GSMDs in Glioma
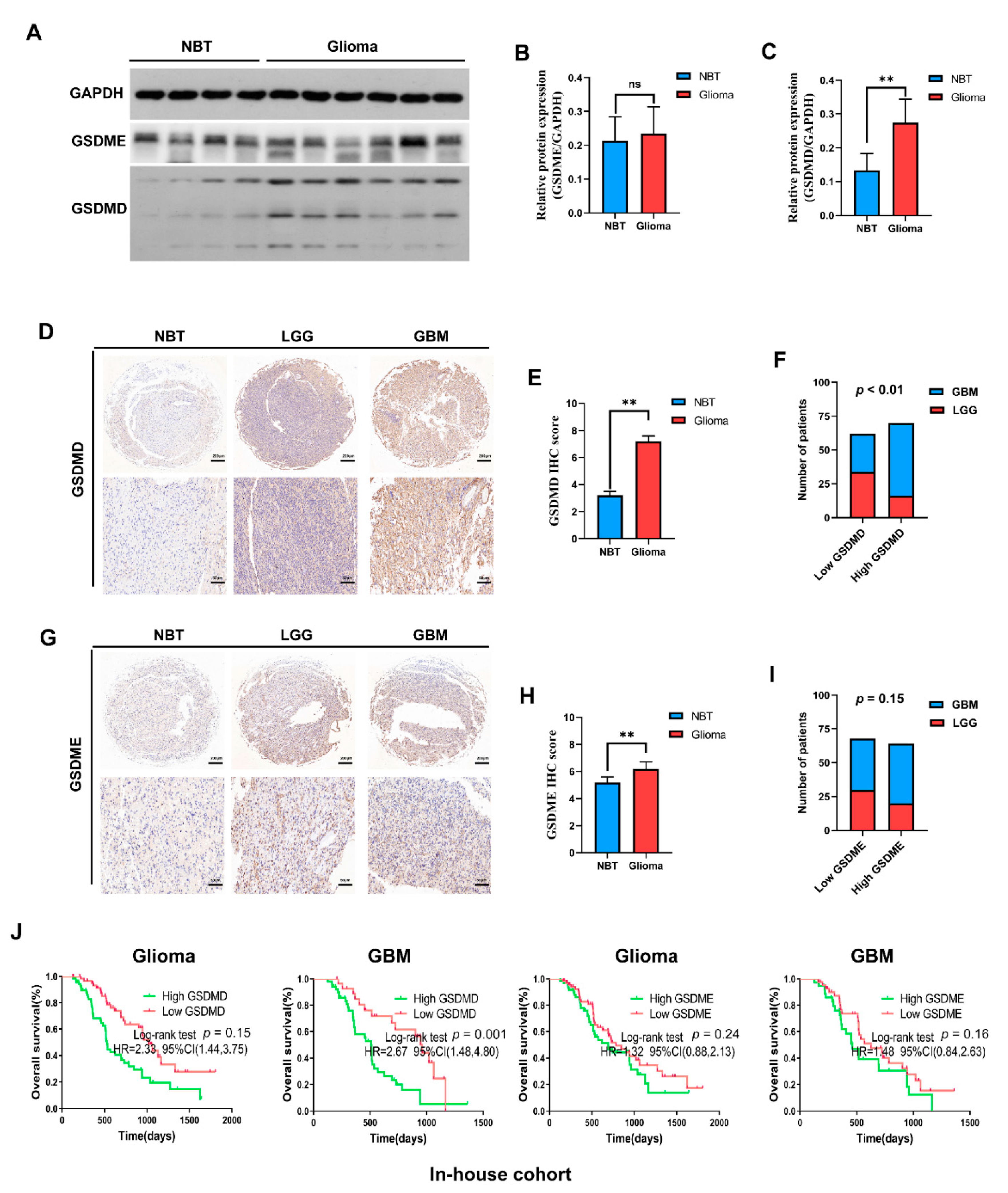
3.5. In-House Cohort Validation
3.6. High GSDMD Expression Associated with IDH Wildtype, 1p19q Codeletion and Subtypes
3.7. GSDMD Associated with Immune Cell Infiltration in Glioma
3.8. GSDMD Mediated TMZ-Induced Pyroptosis in Glioma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CL | classical |
| DMEM | Dulbecco’s modified Eagle’s medium |
| EMT | mesenchymal transition |
| ELISA | enzyme linked immunosorbent assay |
| GBM | glioblastoma multiforme |
| GSDMs | gasdermins |
| HER-2 | human epidermal growth factor receptor 2 |
| IHC | immunohistochemistry |
| IF | immunofluorescence |
| IL-1β | interleukin-1 beta |
| IDH | isocitrate dehydrogenase |
| LUAD | lung adenocarcinoma |
| LGG | lower grade glioma |
| ME | mesenchymal |
| PVDF | poly-vinylidene fluoride |
| PN | proneural |
| STRING | Search Tool for the Retrieval of Interacting Genes |
| TMZ | temozolomide |
| WB | Western blot |
References
- Vargo, M.M. Brain Tumors and Metastases. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 115–141. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro. Oncol. 2020, 22 (Suppl. 2), iv1–iv96. [Google Scholar] [CrossRef]
- Tan, A.C.; Ashley, D.M.; Lopez, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Jovcevska, I.; Kocevar, N.; Komel, R. Glioma and glioblastoma—How much do we (not) know? Mol. Clin. Oncol. 2013, 1, 935–941. [Google Scholar] [CrossRef] [Green Version]
- Saeki, N.; Kuwahara, Y.; Sasaki, H.; Satoh, H.; Shiroishi, T. Gasdermin (Gsdm) localizing to mouse Chromosome 11 is predominantly expressed in upper gastrointestinal tract but significantly suppressed in human gastric cancer cells. Mamm. Genome 2000, 11, 718–724. [Google Scholar] [CrossRef]
- Broz, P.; Pelegrin, P.; Shao, F. The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol. 2020, 20, 143–157. [Google Scholar] [CrossRef]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chen, Y.; Sun, Y.; Gao, Q.; Yu, B.; Jiang, X.; Guo, M. Gasdermin family: A promising therapeutic target for cancers and inflammation-driven diseases. J. Cell Commun. Signal. 2020, 14, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, W.; Shi, X.; Ding, J.; Liu, W.; He, H.; Wang, K.; Shao, F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 2017, 547, 99–103. [Google Scholar] [CrossRef]
- Zhou, C.B.; Fang, J.Y. The role of pyroptosis in gastrointestinal cancer and immune responses to intestinal microbial infection. Biochim. Biophys. Acta Rev. Cancer 2019, 1872, 1–10. [Google Scholar] [CrossRef]
- Jiang, M.; Qi, L.; Li, L.; Li, Y. The caspase-3/GSDME signal pathway as a switch between apoptosis and pyroptosis in cancer. Cell Death Discov. 2020, 6, 112. [Google Scholar] [CrossRef] [PubMed]
- Hergueta-Redondo, M.; Sarrio, D.; Molina-Crespo, A.; Megias, D.; Mota, A.; Rojo-Sebastian, A.; Garcia-Sanz, P.; Morales, S.; Abril, S.; Cano, A.; et al. Gasdermin-B promotes invasion and metastasis in breast cancer cells. PLoS ONE 2014, 9, e90099. [Google Scholar] [CrossRef] [Green Version]
- Eckel-Passow, J.E.; Lachance, D.H.; Molinaro, A.M.; Walsh, K.M.; Decker, P.A.; Sicotte, H.; Pekmezci, M.; Rice, T.; Kosel, M.L.; Smirnov, I.V.; et al. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. N. Engl. J. Med. 2015, 372, 2499–2508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molinaro, A.M.; Taylor, J.W.; Wiencke, J.K.; Wrensch, M.R. Genetic and molecular epidemiology of adult diffuse glioma. Nat. Rev. Neurol. 2019, 15, 405–417. [Google Scholar] [CrossRef]
- Reifenberger, G.; Wirsching, H.G.; Knobbe-Thomsen, C.B.; Weller, M. Advances in the molecular genetics of gliomas—Implications for classification and therapy. Nat. Rev. Clin. Oncol. 2017, 14, 434–452. [Google Scholar] [CrossRef] [PubMed]
- Behnan, J.; Finocchiaro, G.; Hanna, G. The landscape of the mesenchymal signature in brain tumours. Brain 2019, 142, 847–866. [Google Scholar] [CrossRef] [Green Version]
- Verhaak, R.G.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, K. Inflammasome-associated cell death: Pyroptosis, apoptosis, and physiological implications. Microbiol. Immunol. 2020, 64, 252–269. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Fox, D.; Man, S.M. Mechanisms of Gasdermin Family Members in Inflammasome Signaling and Cell Death. J. Mol. Biol. 2018, 430, 3068–3080. [Google Scholar] [CrossRef]
- Eder, K.; Kalman, B. The Dynamics of Interactions Among Immune and Glioblastoma Cells. Neuromol. Med. 2015, 17, 335–352. [Google Scholar] [CrossRef]
- Wei, J.; Chen, P.; Gupta, P.; Ott, M.; Zamler, D.; Kassab, C.; Bhat, K.P.; Curran, M.A.; de Groot, J.F.; Heimberger, A.B. Immune biology of glioma-associated macrophages and microglia: Functional and therapeutic implications. Neuro. Oncol. 2020, 22, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Chu, C.; Zhou, W.; Huang, Z.; Zhai, K.; Fang, X.; Huang, Q.; Zhang, A.; Wang, X.; Yu, X.; et al. Dual Role of WISP1 in maintaining glioma stem cells and tumor-supportive macrophages in glioblastoma. Nat. Commun. 2020, 11, 3015. [Google Scholar] [CrossRef]
- Hergueta-Redondo, M.; Sarrio, D.; Molina-Crespo, A.; Vicario, R.; Bernado-Morales, C.; Martinez, L.; Rojo-Sebastian, A.; Serra-Musach, J.; Mota, A.; Martinez-Ramirez, A.; et al. Gasdermin B expression predicts poor clinical outcome in HER2-positive breast cancer. Oncotarget 2016, 7, 56295–56308. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Xu, Z.; Chen, X.; Wang, X.; Zeng, S.; Qian, L.; Yang, X.; Ou, C.; Lin, W.; Gong, Z.; et al. Overexpression of GSDMC is a prognostic factor for predicting a poor outcome in lung adenocarcinoma. Mol. Med. Rep. 2020, 21, 360–370. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Qiu, X.; Xi, G.; Liu, H.; Zhang, F.; Lv, T.; Song, Y. Downregulation of GSDMD attenuates tumor proliferation via the intrinsic mitochondrial apoptotic pathway and inhibition of EGFR/Akt signaling and predicts a good prognosis in nonsmall cell lung cancer. Oncol. Rep. 2018, 40, 1971–1984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, R.; Wei, H.; Wang, S.; Huang, Z.; Chen, H.; Zhang, S.; Lin, J.; Zhong, G. Gasdermin D expression and clinicopathologic outcome in primary osteosarcoma patients. Int. J. Clin. Exp. Pathol. 2020, 13, 3149–3157. [Google Scholar]
- Wesseling, P.; Capper, D. WHO 2016 Classification of gliomas. Neuropathol. Appl. Neurobiol. 2018, 44, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Shirahata, M.; Ono, T.; Stichel, D.; Schrimpf, D.; Reuss, D.E.; Sahm, F.; Koelsche, C.; Wefers, A.; Reinhardt, A.; Huang, K.; et al. Novel, improved grading system(s) for IDH-mutant astrocytic gliomas. Acta Neuropathol. 2018, 136, 153–166. [Google Scholar] [CrossRef] [Green Version]
- Barritault, M.; Meyronet, D.; Ducray, F. Molecular classification of adult gliomas: Recent advances and future perspectives. Curr. Opin. Oncol. 2018, 30, 375–382. [Google Scholar] [CrossRef]
- Reinhardt, A.; Stichel, D.; Schrimpf, D.; Koelsche, C.; Wefers, A.K.; Ebrahimi, A.; Sievers, P.; Huang, K.; Casalini, M.B.; Fernandez-Klett, F.; et al. Tumors diagnosed as cerebellar glioblastoma comprise distinct molecular entities. Acta Neuropathol. Commun. 2019, 7, 163. [Google Scholar] [CrossRef]
- Lu, J.; Li, D.; Zeng, Y.; Wang, H.; Feng, W.; Qi, S.; Yu, L. IDH1 mutation promotes proliferation and migration of glioma cells via EMT induction. J. BUON 2019, 24, 2458–2464. [Google Scholar] [PubMed]
- Qian, Z.; Li, Y.; Fan, X.; Zhang, C.; Wang, Y.; Jiang, T.; Liu, X. Molecular and clinical characterization of IDH associated immune signature in lower-grade gliomas. Oncoimmunology 2018, 7, e1434466. [Google Scholar] [CrossRef] [Green Version]
- Bhat, K.; Balasubramaniyan, V.; Vaillant, B.; Ezhilarasan, R.; Hummelink, K.; Hollingsworth, F.; Wani, K.; Heathcock, L.; James, J.D.; Goodman, L.D.; et al. Mesenchymal differentiation mediated by NF-kappaB promotes radiation resistance in glioblastoma. Cancer Cell 2013, 24, 331–346. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Hu, B.; Hu, X.; Kim, H.; Squatrito, M.; Scarpace, L.; Decarvalho, A.C.; Lyu, S.; Li, P.; Li, Y.; et al. Tumor Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment. Cancer Cell 2017, 32, 42–56.e6. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Sborgi, L.; Ruhl, S.; Mulvihill, E.; Pipercevic, J.; Heilig, R.; Stahlberg, H.; Farady, C.J.; Muller, D.J.; Broz, P.; Hiller, S. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016, 35, 1766–1778. [Google Scholar] [CrossRef]
- Schneider, K.S.; Gross, C.J.; Dreier, R.F.; Saller, B.S.; Mishra, R.; Gorka, O.; Heilig, R.; Meunier, E.; Dick, M.S.; Cikovic, T.; et al. The Inflammasome Drives GSDMD-Independent Secondary Pyroptosis and IL-1 Release in the Absence of Caspase-1 Protease Activity. Cell Rep. 2017, 21, 3846–3859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evavold, C.L.; Ruan, J.; Tan, Y.; Xia, S.; Wu, H.; Kagan, J.C. The Pore-Forming Protein Gasdermin D Regulates Interleukin-1 Secretion from Living Macrophages. Immunity 2018, 48, 35–44.e6. [Google Scholar] [CrossRef] [Green Version]
- Neyns, B.; Tosoni, A.; Hwu, W.J.; Reardon, D.A. Dose-dense temozolomide regimens: Antitumor activity, toxicity, and immunomodulatory effects. Cancer 2010, 116, 2868–2877. [Google Scholar] [CrossRef]
- Hegi, M.E.; Liu, L.; Herman, J.G.; Stupp, R.; Wick, W.; Weller, M.; Mehta, M.P.; Gilbert, M.R. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J. Clin. Oncol. 2008, 26, 4189–4199. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, K.; Lin, X.; Yao, Z.; Wang, S.; Xiong, X.; Ning, Z.; Wang, J.; Xu, X.; Jiang, Y.; et al. Metformin induces human esophageal carcinoma cell pyroptosis by targeting the miR-497/PELP1 axis. Cancer Lett. 2019, 450, 22–31. [Google Scholar] [CrossRef] [PubMed]

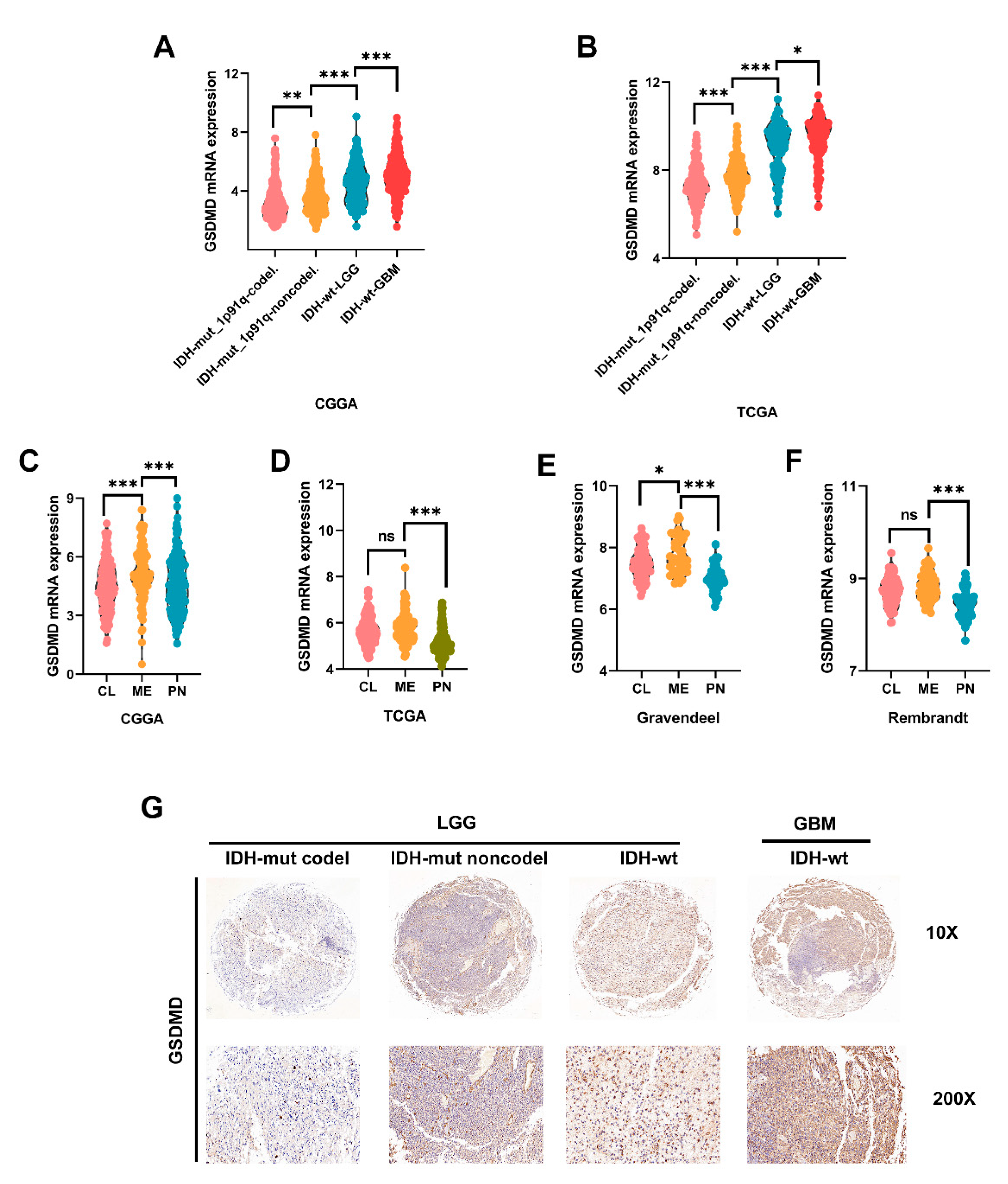
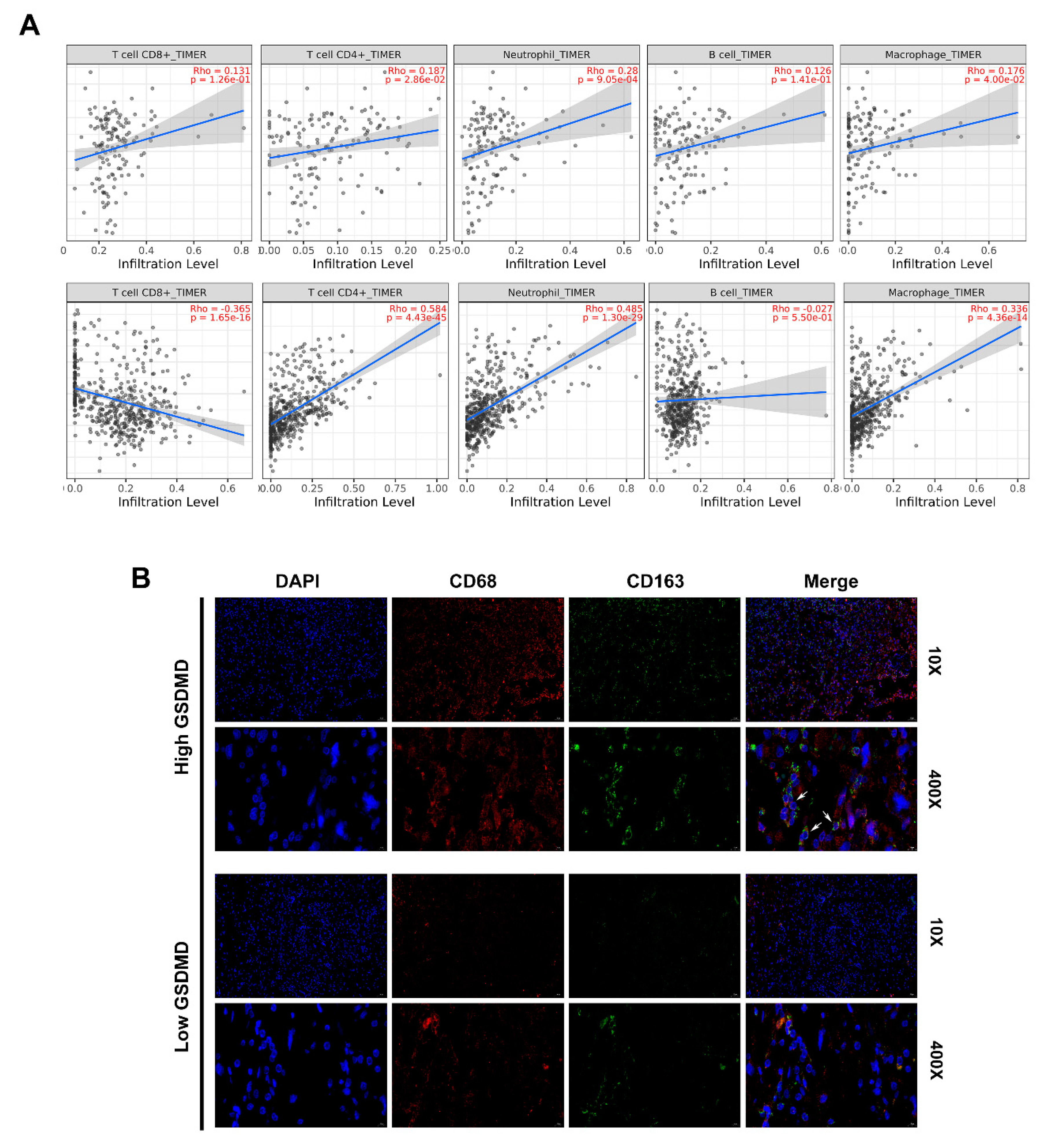
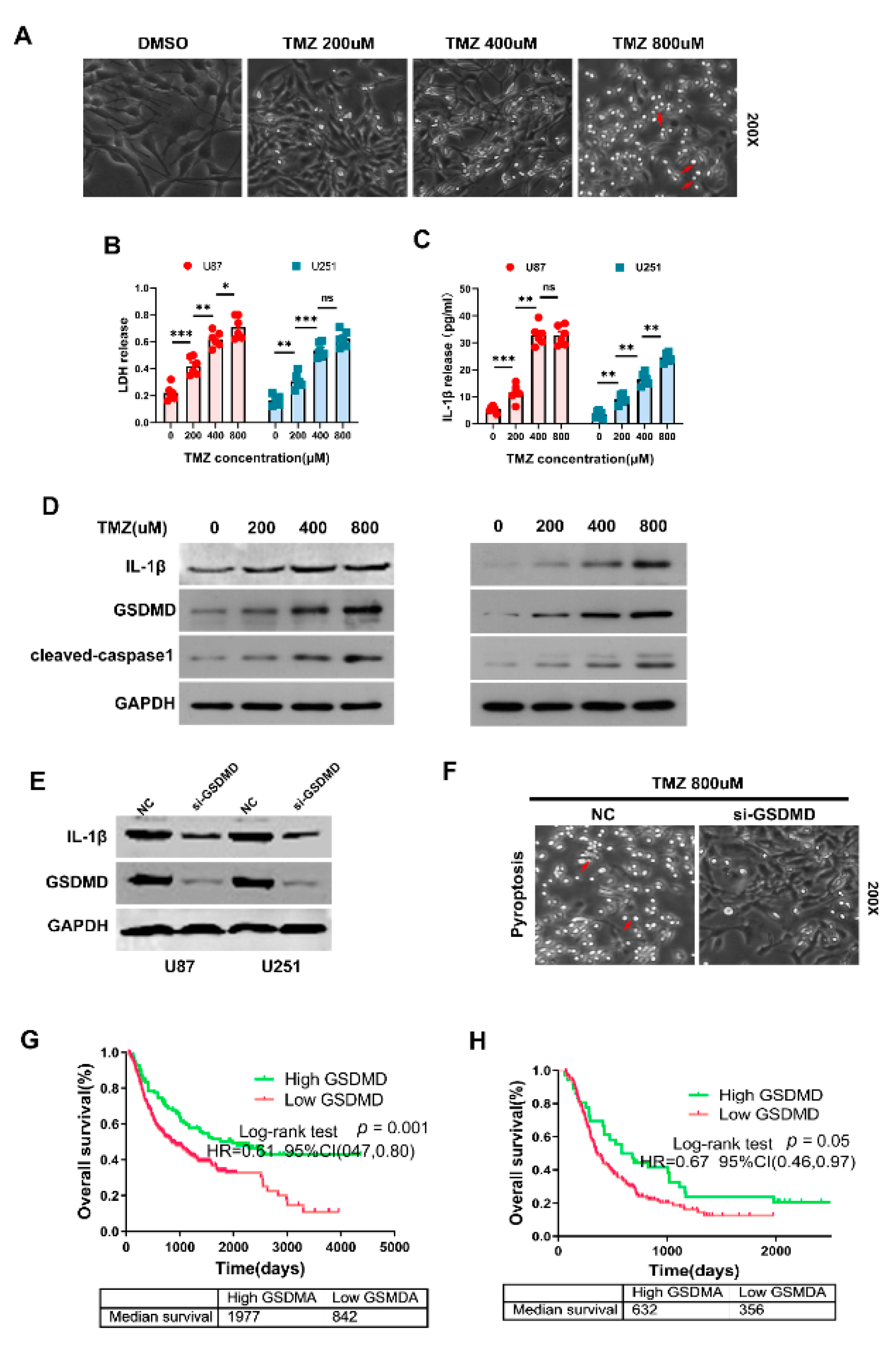
| Variables | Univariate Cox Regression | Multivariate Cox Regression | ||
|---|---|---|---|---|
| HR (95%CI) | p Value | HR (95%CI) | p Value | |
| Age (>60 years vs. ≤60 years) | 2.54 (1.77–3.66) | <0.001 | 2.31 (1.62–3.30) | <0.001 |
| Gender (Female vs. male) | 0.94 (0.69–1.30) | 0.72 | - | - |
| WHO Grade (IV vs. I-III) | 0.41 (0.27–0.64) | <0.001 | 0.37 (0.25–0.56) | <0.001 |
| IDH status (wild-type vs. mutant) | 3.99 (2.29–6.95) | <0.001 | 0.43 (0.23–0.82) | 0.01 |
| MGMT promoter (methy vs. unmethy) | 1.31 (0.92–1.88) | 0.13 | - | - |
| Subtypes (ME vs. others) | 0.46 (0.23–0.92) | 0.03 | - | - |
| GSDMA expression | 0.98 (0.92–1.06) | 0.72 | - | - |
| GSDMB expression | 1.12 (0.96–1.31) | 0.17 | - | - |
| GSDMC expression | 0.96 (0.88–1.05) | 0.40 | - | - |
| GSDMD expression | 1.10 (0.92–1.30) | 0.34 | - | - |
| GSDME expression | 1.45 (1.08–1.93) | 0.01 | 1.32 (1.03–1.70) | 0.03 |
| DFNB59 expression | 0.96 (0.88–1.05) | 0.06 | - | - |
| Variables | Univariate Cox Regression | Multivariate Cox Regression | ||
|---|---|---|---|---|
| HR (95%CI) | p Value | HR (95%CI) | p Value | |
| Age (>60 years vs. ≤60 years) | 0.96 (0.69–1.33) | 0.81 | - | - |
| Gender (Female vs. male) | 0.98 (0.78–1.23) | 0.78 | - | - |
| WHO Grade (IV vs. I–III) | 3.20 (2.40–4.24) | <0.001 | 3.25 (2.37–4.10) | <0.001 |
| IDH status (wildtype vs. mutant) | 2.47 (1.85–3.30) | <0.001 | 2.32 (1.75–3.08) | <0.001 |
| GSDMA expression | 0.92 (0.74–1.15) | 0.45 | - | - |
| GSDMB expression | 1.01 (0.99–1.03) | 0.56 | - | - |
| GSDMC expression | 0.96 (0.99–1.14) | 0.11 | - | - |
| GSDMD expression | 0.99 (0.99–1.00) | 0.02 | 0.99 (0.99–1.00) | 0.03 |
| GSDME expression | 1.01 (1.00–1.02) | 0.003 | 1.01 (1.00–1.02) | 0.002 |
| DFNB59 expression | 0.99 (0.92–1.06) | 0.76 | - | - |
| Factors | GSDMD Expression | ||
|---|---|---|---|
| Low | High | p Value | |
| Age (mean ± SD) | 55.82 ± 10.90 | 53.57 ± 13.16 | |
| Gender | 0.46 | ||
| Female | 27 | 35 | |
| Male | 35 | 35 | |
| Karnofsky score | 0.15 | ||
| >80 | 43 | 40 | |
| ≤70 | 19 | 30 | |
| Grade | 0.01 | ||
| I | 3 | 1 | |
| II | 11 | 3 | |
| III | 20 | 6 | |
| IV | 28 | 52 | |
| IDH1/2 status | <0.01 | ||
| Mutant | 22 | 6 | |
| Wildtype | 7 | 15 | |
| Chemotherapy | 35 | 46 | 0.28 |
| Radiotherapy | 42 | 51 | 0.52 |
| Immune Cells | TCGA | CGGA | |||||
|---|---|---|---|---|---|---|---|
| Markers | Cor | 95%CI | p Value | Cor | 95%CI | p Value | |
| CD8+ T cell | CD8A | 0.42 | 0.35–0.48 | <0.0001 | 0.25 | 0.17–0.32 | <0.0001 |
| CD8B | 0.41 | 0.35–0.48 | <0.0001 | 0.47 | 0.41–0.53 | <0.0001 | |
| T cell | CD3D | 0.62 | 0.57–0.67 | <0.0001 | 0.63 | 0.58–0.68 | <0.0001 |
| CD3E | 0.65 | 0.60–0.70 | <0.0001 | 0.44 | 0.37–0.50 | <0.0001 | |
| B cell | CD86 | 0.60 | 0.55–0.65 | <0.0001 | 0.46 | 0.40–0.52 | <0.0001 |
| CD79A | 0.30 | 0.22–0.36 | <0.0001 | 0.44 | 0.37–0.50 | <0.0001 | |
| CSF1R | 0.38 | 0.31–0.45 | <0.0001 | 0.26 | 0.19–0.33 | <0.0001 | |
| Monocyte | CCL2 | 0.56 | 0.50–0.61 | <0.0001 | 0.31 | 0.24–0.38 | <0.0001 |
| CD68 | 0.64 | 0.59–0.68 | <0.0001 | 0.45 | 0.39–0.51 | <0.0001 | |
| NOS2 | 0.15 | 0.07–0.23 | <0.0001 | 0.05 | 0.00–0.13 | 0.1561 | |
| macrophage | CD163 | 0.52 | 0.46–0.58 | <0.0001 | 0.42 | 0.35–0.48 | <0.0001 |
| IRF5 | 0.63 | 0.58–0.67 | <0.0001 | 0.44 | 0.37–0.50 | <0.0001 | |
| PTGS2 | 0.26 | 0.19–0.33 | <0.0001 | −0.07 | (−0.15)–0.01 | 0.0685 | |
| CEACAM8 | 0.08 | 0.00–0.15 | 0.0509 | - | - | - | |
| MS4A4A | 0.55 | 0.49–0.60 | <0.0001 | 0.38 | 0.32–0.45 | <0.0001 | |
| Neutrophil | ITGAM | 0.53 | 0.47–0.58 | <0.0001 | 0.43 | 0.36–0.49 | <0.0001 |
| CCR7 | 0.44 | 0.38–0.50 | <0.0001 | 0.21 | 0.13–0.28 | <0.0001 | |
| KIR2DL1 | 0.04 | 0.00–0.12 | 0.2825 | - | - | - | |
| KIR2DL3 | 0.13 | 0.05–0.20 | 0.0006 | - | - | - | |
| KIR2DL4 | 0.35 | 0.28–0.41 | <0.0001 | - | - | - | |
| T cell exhaustion | PDCD1 | 0.57 | 0.50–0.62 | <0.0001 | 0.46 | 0.40–0.52 | <0.0001 |
| CTLA4 | 0.50 | 0.44–0.56 | <0.0001 | 0.44 | 0.37–0.50 | <0.0001 | |
| LAG3 | 0.32 | 0.24–0.38 | <0.0001 | 0.60 | 0.54–0.64 | <0.0001 | |
| HAVCR2 | 0.64 | 0.59–0.68 | <0.0001 | 0.34 | 0.27–0.41 | <0.0001 | |
| BTLA | 0.35 | 0.28–0.42 | <0.0001 | 0.25 | 0.18–0.32 | <0.0001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Gao, L.; Zhu, X.; Geng, R.; Tao, X.; Xu, H.; Chen, Z. Gasdermin D Is a Novel Prognostic Biomarker and Relates to TMZ Response in Glioblastoma. Cancers 2021, 13, 5620. https://doi.org/10.3390/cancers13225620
Liu J, Gao L, Zhu X, Geng R, Tao X, Xu H, Chen Z. Gasdermin D Is a Novel Prognostic Biomarker and Relates to TMZ Response in Glioblastoma. Cancers. 2021; 13(22):5620. https://doi.org/10.3390/cancers13225620
Chicago/Turabian StyleLiu, Junhui, Lun Gao, Xiaonan Zhu, Rongxin Geng, Xiang Tao, Haitao Xu, and Zhibiao Chen. 2021. "Gasdermin D Is a Novel Prognostic Biomarker and Relates to TMZ Response in Glioblastoma" Cancers 13, no. 22: 5620. https://doi.org/10.3390/cancers13225620
APA StyleLiu, J., Gao, L., Zhu, X., Geng, R., Tao, X., Xu, H., & Chen, Z. (2021). Gasdermin D Is a Novel Prognostic Biomarker and Relates to TMZ Response in Glioblastoma. Cancers, 13(22), 5620. https://doi.org/10.3390/cancers13225620






