Locoregional Therapies for Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Hepatocellular Carcinoma
1.2. Portal Vein Thrombosis
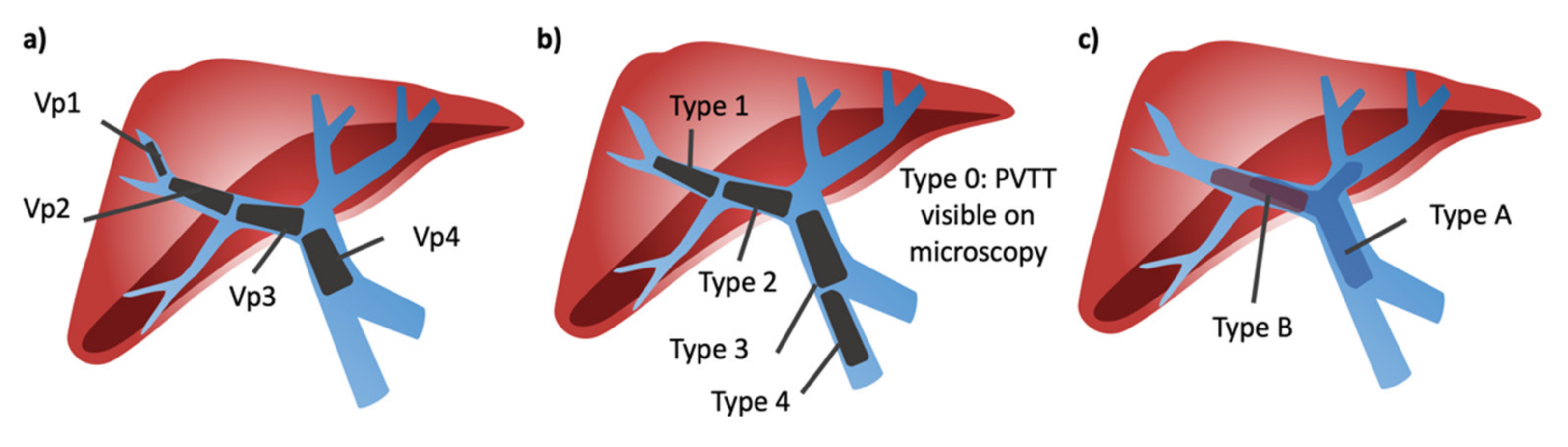
1.3. Portal Vein Tumor Thrombus Classification
2. Locoregional Therapies
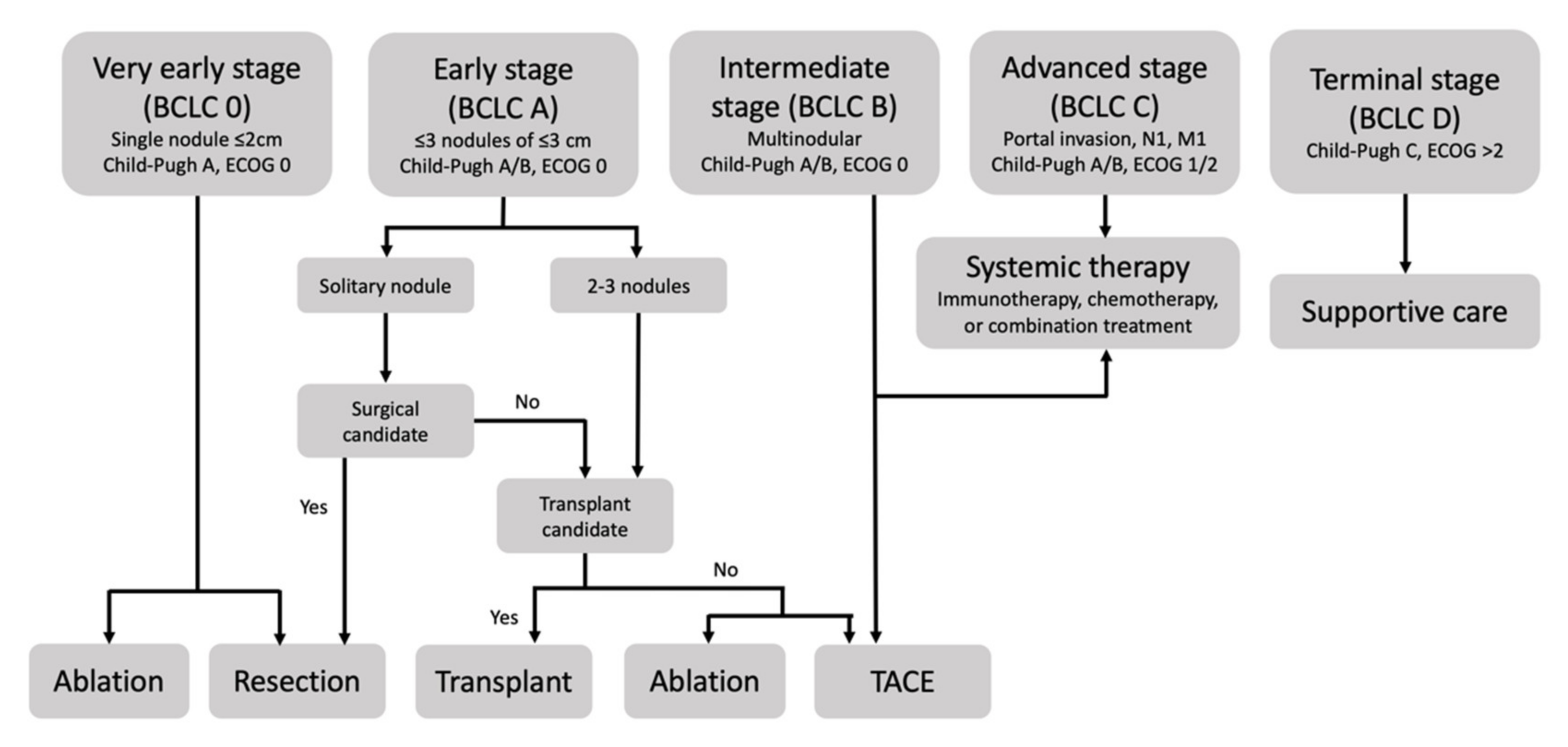
2.1. Patient Selection
2.2. Transarterial Chemoembolization
2.3. Transarterial Radioembolization
2.4. Ablation
3. Other Approaches to HCC with PVTT
3.1. Radiotherapy
3.2. Hepatic Intra-Arterial Infusion
3.3. Resection
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2021, 7, 1–28. [Google Scholar]
- Zhang, B.H.; Yang, B.H.; Tang, Z.Y. Randomized controlled trial of screening for hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2004, 130, 417–422. [Google Scholar] [CrossRef]
- Llovet, J.M.; Fuster, J.; Bruix, J. The Barcelona approach: Diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transplant. 2004, 10, S115–S120. [Google Scholar] [CrossRef]
- Valla, D.C.; Condat, B. Portal vein thrombosis in adults: Pathophysiology, pathogenesis and management. J. Hepatol. 2000, 32, 865–871. [Google Scholar] [CrossRef]
- Mantaka, A.; Augoustaki, A.; Kouroumalis, E.A.; Samonakis, D.N. Portal vein thrombosis in cirrhosis: Diagnosis, natural history, and therapeutic challenges. Ann. Gastroenterol. 2018, 31, 315. [Google Scholar] [CrossRef] [PubMed]
- Cerrito, L.; Annicchiarico, B.E.; Iezzi, R.; Gasbarrini, A.; Pompili, M.; Ponziani, F.R. Treatment of hepatocellular carcinoma in patients with portal vein tumor thrombosis: Beyond the known frontiers. World J. Gastroenterol. 2019, 25, 4360. [Google Scholar] [CrossRef]
- Lim, J.; Kim, H.I.; Kim, E.; Kim, J.; An, J.; Chang, S.; Kim, S.O.; Lee, H.C.; Lee, Y.S.; Shim, J.H. Variceal bleeding is aggravated by portal venous invasion of hepatocellular carcinoma: A matched nested case-control study. BMC Cancer 2021, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Hepatobiliary Cancers (Version 5.2021). Available online: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf (accessed on 28 October 2021).
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Ikai, I.; Kudo, M.; Arii, S.; Omata, M.; Kojiro, M.; Sakamoto, M.; Takayasu, K.; Hayashi, N.; Makuuchi, M.; Matsuyama, Y.; et al. Report of the 18th follow-up survey of primary liver cancer in Japan. Hepatol. Res. 2010, 40, 1043–1059. [Google Scholar] [CrossRef]
- Shi, J.; Lai, E.C.H.; Li, N.; Guo, W.X.; Xue, J.; Lau, W.Y.; Wu, M.C.; Cheng, S.Q. A new classification for hepatocellular carcinoma with portal vein tumor thrombus. J. Hepato-Biliary-Pancreat. Sci. 2011, 18, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, X.; Wang, S.; Wen, H. Surgical treatment for hepatocellular carcinoma with portal vein tumor thrombus: A novel classification. World J. Surg. Oncol. 2015, 13, 86. [Google Scholar] [CrossRef] [Green Version]
- Venerito, M.; Pech, M.; Canbay, A.; Donghia, R.; Guerra, V.; Chatellier, G.; Pereira, H.; Gandhi, M.; Malfertheiner, P.; Chow, P.K.H.; et al. NEMESIS: Noninferiority, individual-patient metaanalysis of selective internal radiation therapy with 90Y resin microspheres versus sorafenib in advanced hepatocellular Carcinoma. J. Nucl. Med. 2020, 61, 1736–1742. [Google Scholar] [CrossRef]
- Helmberger, T.; Golfieri, R.; Pech, M.; Pfammatter, T.; Arnold, D.; Cianni, R.; Maleux, G.; Munneke, G.; Pellerin, O.; Peynircioglu, B.; et al. Clinical Application of Trans-Arterial Radioembolization in Hepatic Malignancies in Europe: First Results from the Prospective Multicentre Observational Study CIRSE Registry for SIR-Spheres Therapy (CIRT). Cardiovasc. Intervent. Radiol. 2021, 44, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Guo, R.P.; Lai, E.C.H.; Zhang, Y.J.; Lau, W.Y.; Chen, M.S.; Shi, M. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: A prospective comparative study. Ann. Surg. Oncol. 2011, 18, 413–420. [Google Scholar] [CrossRef]
- Zhu, K.; Chen, J.; Lai, L.; Meng, X.; Zhou, B.; Huang, W.; Cai, M.; Shan, H. Hepatocellular carcinoma with portal vein tumor thrombus: Treatment with transarterial chemoembolization combined with sorafenib—A retrospective controlled study. Radiology 2014, 272, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.M.; Ryoo, B.-Y.; Lee, S.J.; Kim, J.H.; Shin, J.H.; An, J.H.; Lee, H.C.; Lim, Y.-S. Efficacy and Safety of Transarterial Chemoembolization plus External Beam Radiotherapy vs. Sorafenib in Hepatocellular Carcinoma with Macroscopic Vascular Invasion: A Randomized Clinical Trial. JAMA Oncol. 2018, 4, 661. [Google Scholar] [CrossRef]
- Garin, E.; Rolland, Y.; Edeline, J.; Icard, N.; Lenoir, L.; Laffont, S.; Mesbah, H.; Breton, M.; Sulpice, L.; Boudjema, K.; et al. Personalized dosimetry with intensification using90Y-loaded glass microsphere radioembolization induces prolonged overall survival in hepatocellular carcinoma patients with portal vein thrombosis. J. Nucl. Med. 2015, 56, 339–346. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Lu, Y.; Wang, C.; Bai, W.; Qu, J.; Chen, Y.; Chang, X.; An, L.; Zhou, L.; Zeng, Z.; et al. Cryotherapy is Associated with Improved Clinical Outcomes of Sorafenib Therapy for Advanced Hepatocellular Carcinoma. Cell Biochem. Biophys. 2012, 63, 159–169. [Google Scholar] [CrossRef] [Green Version]
- Giorgio, A.; Merola, M.G.; Montesarchio, L.; Merola, F.; Santoro, B.; Coppola, C.; Gatti, P.; Amendola, F.; Di Sarno, A.; Calvanese, A.; et al. Sorafenib combined with radio-frequency ablation compared with sorafenib alone in treatment of hepatocellular carcinoma invading portal vein: A western randomized controlled trial. Anticancer Res. 2016, 36, 6179–6183. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Sun, W.; Chen, J.; Li, W.; Shen, Y.; Guo, X.; Teng, Y.; Liu, X.; Sun, S.; Wei, J.; et al. Percutaneous Radiofrequency Ablation Combined With Transarterial Chemoembolization Plus Sorafenib for Large Hepatocellular Carcinoma Invading the Portal Venous System: A Prospective Randomized Study. Front. Oncol. 2020, 10, 2278. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Zheng, J.; Sun, B.; Lu, N. Microwave ablation of hepatocellular carcinoma with portal vein tumor thrombosis after transarterial chemoembolization: A prospective study. Hepatol. Int. 2016, 10, 175–184. [Google Scholar] [CrossRef]
- Guan, Y.-S.; He, Q.; Wang, M.-Q. Transcatheter Arterial Chemoembolization: History for More than 30 Years. ISRN Gastroenterol. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouvry, C.; Palard, X.; Edeline, J.; Ardisson, V.; Loyer, P.; Garin, E.; Lepareur, N. Transarterial radioembolization (TARE) agents beyond 90 Y-microspheres. Biomed. Res. Int. 2018, 2018, 1435302. [Google Scholar] [CrossRef] [Green Version]
- Renzulli, M.; Peta, G.; Vasuri, F.; Marasco, G.; Caretti, D.; Bartalena, L.; Spinelli, D.; Giampalma, E.; D’Errico, A.; Golfieri, R. Standardization of conventional chemoembolization for hepatocellular carcinoma. Ann. Hepatol. 2021, 22, 100278. [Google Scholar] [CrossRef]
- Liu, Y.S.; Lin, C.Y.; Chuang, M.T.; Lin, C.Y.; Tsai, Y.S.; Wang, C.K.; Ou, M.C. Five-year outcome of conventional and drug-eluting transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. BMC Gastroenterol. 2018, 18, 1–9. [Google Scholar] [CrossRef]
- Llovet, J.M.; Real, M.I.; Montaña, X.; Planas, R.; Coll, S.; Aponte, J.; Ayuso, C.; Sala, M.; Muchart, J.; Solà, R.; et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: A randomised controlled trial. Lancet 2002, 359, 1734–1739. [Google Scholar] [CrossRef]
- Lo, C.M.; Ngan, H.; Tso, W.K.; Liu, C.L.; Lam, C.M.; Poon, R.T.P.; Fan, S.T.; Wong, J. Randomized controlled trial of transarterial Lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002, 35, 1164–1171. [Google Scholar] [CrossRef]
- Pinter, M.; Hucke, F.; Graziadei, I.; Vogel, W.; Maieron, A.; Königsberg, R.; Stauber, R.; Grünberger, B.; Müller, C.; Kölblinger, C.; et al. Advanced-stage hepatocellular carcinoma: Transarterial chemoembolization versus sorafenib. Radiology 2012, 263, 590–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorodetski, B.; Chapiro, J.; Schernthaner, R.; Duran, R.; Lin, M.; Lee, H.; Lenis, D.; Stuart, E.A.; Nonyane, B.A.S.; Pekurovsky, V.; et al. Advanced-stage hepatocellular carcinoma with portal vein thrombosis: Conventional versus drug-eluting beads transcatheter arterial chemoembolization. Eur. Radiol. 2016, 27, 526–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, G.E.; Lee, J.H.; Kim, H.Y.; Hwang, S.Y.; Kim, J.S.; Chung, J.W.; Yoon, J.H.; Lee, H.S.; Kim, Y.J. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology 2011, 258, 627–634. [Google Scholar] [CrossRef] [Green Version]
- Xue, T.-C.; Xie, X.-Y.; Zhang, L.; Yin, X.; Zhang, B.-H.; Ren, Z.-G. Transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus: A meta-analysis. BMC Gastroenterol. 2013, 13, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.P.; Wang, K.; Wang, M.; Yang, G.; Ye, X.F.; Wu, M.C.; Cheng, S.Q. Transarterial chemoembolization (TACE) combined with sorafenib versus TACE for hepatocellular carcinoma with portal vein tumor thrombus: A systematic review and meta-analysis. Oncotarget 2017, 8, 29416–29427. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Li, L.; Li, B.; Wang, W. Safety and efficacy of endovascular implantation of a portal vein stent combined with iodine-125 seed-strips followed by transcatheter arterial chemoembolization with sorafenib for the treatment of hepatocellular carcinoma with portal vein tumor thrombosis. Br. J. Radiol. 2020, 93, 20190279. [Google Scholar] [CrossRef] [PubMed]
- Mikell, J.K.; Dewaraja, Y.K.; Owen, D. Transarterial Radioembolization for Hepatocellular Carcinoma and Hepatic Metastases: Clinical Aspects and Dosimetry Models. Semin. Radiat. Oncol. 2020, 30, 68–76. [Google Scholar] [CrossRef]
- Riaz, A.; Awais, R.; Salem, R. Side Effects of Yttrium-90 Radioembolization. Front. Oncol. 2014, 4, 198. [Google Scholar] [CrossRef] [Green Version]
- Gil-Alzugaray, B.; Chopitea, A.; Iñarrairaegui, M.; Bilbao, J.I.; Rodriguez-Fraile, M.; Rodriguez, J.; Benito, A.; Dominguez, I.; D’Avola, D.; Herrero, J.I.; et al. Prognostic factors and prevention of radioembolization-induced liver disease. Hepatology 2013, 57, 1078–1087. [Google Scholar] [CrossRef]
- Salem, R.; Lewandowski, R.J.; Mulcahy, M.F.; Riaz, A.; Ryu, R.K.; Ibrahim, S.; Atassi, B.; Baker, T.; Gates, V.; Miller, F.H.; et al. Radioembolization for Hepatocellular Carcinoma Using Yttrium-90 Microspheres: A Comprehensive Report of Long-term Outcomes. Gastroenterology 2010, 138, 52–64. [Google Scholar] [CrossRef]
- Salem, R.; Gordon, A.C.; Mouli, S.; Hickey, R.; Kallini, J.; Gabr, A.; Mulcahy, M.F.; Baker, T.; Abecassis, M.; Miller, F.H.; et al. Y90 Radioembolization Significantly Prolongs Time to Progression Compared with Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology 2016, 151, 1155–1163.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilgard, P.; Hamami, M.; Fouly, A.E.; Scherag, A.; MüLler, S.; Ertle, J.; Heusner, T.; Cicinnati, V.R.; Paul, A.; Bockisch, A.; et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology 2010, 52, 1741–1749. [Google Scholar] [CrossRef]
- Sangro, B.; Carpanese, L.; Cianni, R.; Golfieri, R.; Gasparini, D.; Ezziddin, S.; Paprottka, P.M.; Fiore, F.; Van Buskirk, M.; Ignacio Bilbao, J.; et al. Survival after Yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: A European evaluation. Hepatology 2011, 54, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Vilgrain, V.; Pereira, H.; Assenat, E.; Guiu, B.; Ilonca, A.D.; Pageaux, G.P.; Sibert, A.; Bouattour, M.; Lebtahi, R.; Allaham, W.; et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): An open-label randomised controlled phase 3 trial. Lancet Oncol. 2017, 18, 1624–1636. [Google Scholar] [CrossRef]
- Chow, P.K.H.; Gandhi, M.; Tan, S.B.; Khin, M.W.; Khasbazar, A.; Ong, J.; Choo, S.P.; Cheow, P.C.; Chotipanich, C.; Lim, K.; et al. SIRveNIB: Selective internal radiation therapy versus sorafenib in Asia-Pacific patients with hepatocellular carcinoma. J. Clin. Oncol. 2018, 36, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Ricke, J.; Klümpen, H.J.; Amthauer, H.; Bargellini, I.; Bartenstein, P.; de Toni, E.N.; Gasbarrini, A.; Pech, M.; Peck-Radosavljevic, M.; Popovič, P.; et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J. Hepatol. 2019, 71, 1164–1174. [Google Scholar] [CrossRef] [PubMed]
- Kwee, S.A.; Wong, L.L.; Sato, M.M.; Acoba, J.D.; Rho, Y.S.; Srivastava, A.; Landsittel, D.P. Transarterial Radioembolization for Hepatocellular Carcinoma with Major Vascular Invasion: A Nationwide Propensity Score–Matched Analysis with Target Trial Emulation. J. Vasc. Interv. Radiol. 2021, 32, 1258–1266.e6. [Google Scholar] [CrossRef] [PubMed]
- Garin, E.; Tselikas, L.; Guiu, B.; Chalaye, J.; Edeline, J.; De Baere, T.; Assenat, E.; Tacher, V.; Robert, C.; Terroir-Cassou-Mounat, M.; et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): A randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 17–29. [Google Scholar] [CrossRef]
- Garin, E.; Lenoir, L.; Edeline, J.; Laffont, S.; Mesbah, H.; Porée, P.; Sulpice, L.; Boudjema, K.; Mesbah, M.; Guillygomarc’H, A.; et al. Boosted selective internal radiation therapy with 90Y-loaded glass microspheres (B-SIRT) for hepatocellular carcinoma patients: A new personalized promising concept. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1057–1068. [Google Scholar] [CrossRef] [Green Version]
- Salem, R.; Johnson, G.E.; Kim, E.; Riaz, A.; Bishay, V.; Boucher, E.; Fowers, K.; Lewandowski, R.; Padia, S.A. Yttrium-90 Radioembolization for the Treatment of Solitary, Unresectable HCC: The LEGACY Study. Hepatology 2021, 74, 2342–2352. [Google Scholar] [CrossRef] [PubMed]
- Dodd, G.D.; Napier, D.; Schoolfield, J.D.; Hubbard, L. Percutaneous radiofrequency ablation of hepatic tumors: Postablation syndrome. Am. J. Roentgenol. 2005, 185, 51–57. [Google Scholar] [CrossRef]
- Bertot, L.C.; Sato, M.; Tateishi, R.; Yoshida, H.; Koike, K. Mortality and complication rates of percutaneous ablative techniques for the treatment of liver tumors: A systematic review. Eur. Radiol. 2011, 21, 2584–2596. [Google Scholar] [CrossRef]
- Llovet, J.M.; De Baere, T.; Kulik, L.; Haber, P.K.; Greten, T.F.; Meyer, T.; Lencioni, R. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 293–313. [Google Scholar] [CrossRef]
- Giorgio, A.; Calisti, G.; Montesarchio, L.; Scognamiglio, U.; Matteucci, P.; Coppola, C.; Scarano, F.; Amendola, F.; Giorgio, V. Hepatocellular carcinoma invading portal venous system in cirrhosis: Long-term results of percutaneous radiofrequency ablation of both the nodule and portal vein tumor thrombus. A case control study-PubMed. Anticancer Res. 2014, 34, 6785–6790. [Google Scholar] [PubMed]
- Mitin, T.; Zietman, A.L. Promise and pitfalls of heavy-particles therapy. J. Clin. Oncol. 2014, 32, 2855–2863. [Google Scholar] [CrossRef] [Green Version]
- Bujold, A.; Massey, C.A.; Kim, J.J.; Brierley, J.; Cho, C.; Wong, R.K.S.; Dinniwell, R.E.; Kassam, Z.; Ringash, J.; Cummings, B.; et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J. Clin. Oncol. 2013, 31, 1631–1639. [Google Scholar] [CrossRef] [Green Version]
- Hong, T.S.; Wo, J.Y.; Yeap, B.Y.; Ben-Josef, E.; McDonnell, E.I.; Blaszkowsky, L.S.; Kwak, E.L.; Allen, J.N.; Clark, J.W.; Goyal, L.; et al. Multi-institutional phase II study of high-dose hypofractionated proton beam therapy in patients with localized, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J. Clin. Oncol. 2016, 34, 460–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.F.; Lo, C.H.; Lee, M.S.; Lin, C.S.; Dai, Y.H.; Shen, P.C.; Chao, H.L.; Huang, W.Y. Stereotactic ablative radiotherapy versus conventionally fractionated radiotherapy in the treatment of hepatocellular carcinoma with portal vein invasion: A retrospective analysis. Radiat. Oncol. 2019, 14, 180. [Google Scholar] [CrossRef]
- Kim, T.H.; Koh, Y.H.; Kim, B.H.; Kim, M.J.; Lee, J.H.; Park, B.; Park, J.W. Proton beam radiotherapy vs. radiofrequency ablation for recurrent hepatocellular carcinoma: A randomized phase III trial. J. Hepatol. 2021, 74, 603–612. [Google Scholar] [CrossRef]
- Kudo, M.; Kawamura, Y.; Hasegawa, K.; Tateishi, R.; Kariyama, K.; Shiina, S.; Toyoda, H.; Imai, Y.; Hiraoka, A.; Ikeda, M.; et al. Management of Hepatocellular Carcinoma in Japan: JSH Consensus Statements and Recommendations 2021 Update. Liver Cancer 2021, 10, 181–223. [Google Scholar] [CrossRef] [PubMed]
- Ueshima, K.; Ogasawara, S.; Ikeda, M.; Yasui, Y.; Terashima, T.; Yamashita, T.; Obi, S.; Sato, S.; Aikata, H.; Ohmura, T.; et al. Hepatic Arterial Infusion Chemotherapy versus Sorafenib in Patients with Advanced Hepatocellular Carcinoma. Liver Cancer 2020, 9, 583–595. [Google Scholar] [CrossRef]
- He, M.; Li, Q.; Zou, R.; Shen, J.; Fang, W.; Tan, G.; Zhou, Y.; Wu, X.; Xu, L.; Wei, W.; et al. Sorafenib Plus Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin vs. Sorafenib Alone for Hepatocellular Carcinoma with Portal Vein Invasion: A Randomized Clinical Trial. JAMA Oncol. 2019, 5, 953. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Chung, W.J.; Bae, S.H.; Song, D.S.; Song, M.J.; Kim, Y.S.; Yim, H.J.; Jung, Y.K.; Suh, S.J.; Park, J.Y.; et al. Randomized, prospective, comparative study on the effects and safety of sorafenib vs. hepatic arterial infusion chemotherapy in patients with advanced hepatocellular carcinoma with portal vein tumor thrombosis. Cancer Chemother. Pharmacol. 2018, 82, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Lee, H.W.; Park, J.Y.; Kim, S.U.; Kim, D.Y.; Ahn, S.H.; Han, K.-H.; Seong, J.; Won, J.Y.; Han, D.H.; et al. Appraisal of Long-Term Outcomes of Liver-Directed Concurrent Chemoradiotherapy for Hepatocellular Carcinoma with Major Portal Vein Invasion. J. Hepatocell. Carcinoma 2020, 7, 403. [Google Scholar] [CrossRef]
- Kim, B.K.; Kim, D.Y.; Byun, H.K.; Choi, H.J.; Beom, S.-H.; Lee, H.W.; Kim, S.U.; Park, J.Y.; Ahn, S.H.; Seong, J.; et al. Efficacy and Safety of Liver-Directed Concurrent Chemoradiotherapy and Sequential Sorafenib for Advanced Hepatocellular Carcinoma: A Prospective Phase 2 Trial. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 106–115. [Google Scholar] [CrossRef]
- Roayaie, S.; Jibara, G.; Taouli, B.; Schwartz, M. Resection of hepatocellular carcinoma with macroscopic vascular invasion. Ann. Surg. Oncol. 2013, 20, 3754–3760. [Google Scholar] [CrossRef]
- Kokudo, T.; Hasegawa, K.; Matsuyama, Y.; Takayama, T.; Izumi, N.; Kadoya, M.; Kudo, M.; Ku, Y.; Sakamoto, M.; Nakashima, O.; et al. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J. Hepatol. 2016, 65, 938–943. [Google Scholar] [CrossRef] [Green Version]
- Mähringer-Kunz, A.; Steinle, V.; Kloeckner, R.; Schotten, S.; Hahn, F.; Schmidtmann, I.; Hinrichs, J.B.; DüBer, C.; Galle, P.R.; Lang, H.; et al. The impact of portal vein tumor thrombosis on survival in patients with hepatocellular carcinoma treated with different therapies: A cohort study. PLoS ONE 2021, 16, e0249426. [Google Scholar] [CrossRef]
- Peng, B.G.; He, Q.; Li, J.P.; Zhou, F. Adjuvant transcatheter arterial chemoembolization improves efficacy of hepatectomy for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am. J. Surg. 2009, 198, 313–318. [Google Scholar] [CrossRef]
- Li, W.; Godzik, A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef] [Green Version]
- Liang, L.J.; Hu, W.J.; Yin, X.Y.; Zhou, Q.; Peng, B.G.; Li, D.M.; Lu, M.-D. Adjuvant intraportal venous chemotherapy for patients with hepatocellular carcinoma and portal vein tumor thrombi following hepatectomy plus portal thrombectomy. World J. Surg. 2008, 32, 627–631. [Google Scholar] [CrossRef] [PubMed]
| Study | Type | Size | PVTT | Treatment | Outcomes |
|---|---|---|---|---|---|
| Luo 2011 [17] | Prospective | 164 | Vp1–Vp4 | TACE | 12- and 24-mos. OS of 30.9% and 9.2%, downstaging in 10.7% |
| Zhu 2014 [18] | Retrospective | 91 | Vp2, Vp3 | TACE + sorafenib | OS 14 mos |
| Yoon 2018 [19] | RCT | 90 | Vp 2–Vp4 | TACE + ERBT | OS 13.8 mos, downstaging in 11.1% |
| Venerito 2020 [15] | Meta-analysis | 1243 | Vp 2–Vp4 | TARE | Non-inferiority of TACE to sorafenib |
| Garin 2015 [20] | RCT | 41 | Vp 2–Vp4 | Personalized Dosimetry TARE | OS 22.9 mos, downstaging in 12.2% |
| Yang 2012 [21] | RCT | 104 | Vp 1–Vp4 | Cryotherapy + sorafenib | OS 12.5 mos |
| Giorgio 2016 [22] | RCT | 99 | Vp4 | RFA + sorafenib | 1-, 3-, and 5-year OS: 63%, 30%, and 20% |
| Ding 2020 [23] | Prospective | 80 | Vp1–Vp3 | RFA + TACE + sorafenib | OS 15.3 mos |
| Long 2016 [24] | Prospective | 109 | Vp2–Vp4 | MWA after TACE | OS 13.5 mos |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zane, K.E.; Makary, M.S. Locoregional Therapies for Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis. Cancers 2021, 13, 5430. https://doi.org/10.3390/cancers13215430
Zane KE, Makary MS. Locoregional Therapies for Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis. Cancers. 2021; 13(21):5430. https://doi.org/10.3390/cancers13215430
Chicago/Turabian StyleZane, Kylie E., and Mina S. Makary. 2021. "Locoregional Therapies for Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis" Cancers 13, no. 21: 5430. https://doi.org/10.3390/cancers13215430
APA StyleZane, K. E., & Makary, M. S. (2021). Locoregional Therapies for Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis. Cancers, 13(21), 5430. https://doi.org/10.3390/cancers13215430






