Lack of Benefit of Extending Temozolomide Treatment in Patients with High Vascular Glioblastoma with Methylated MGMT
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Information
2.2. Magnetic Resonance Imaging
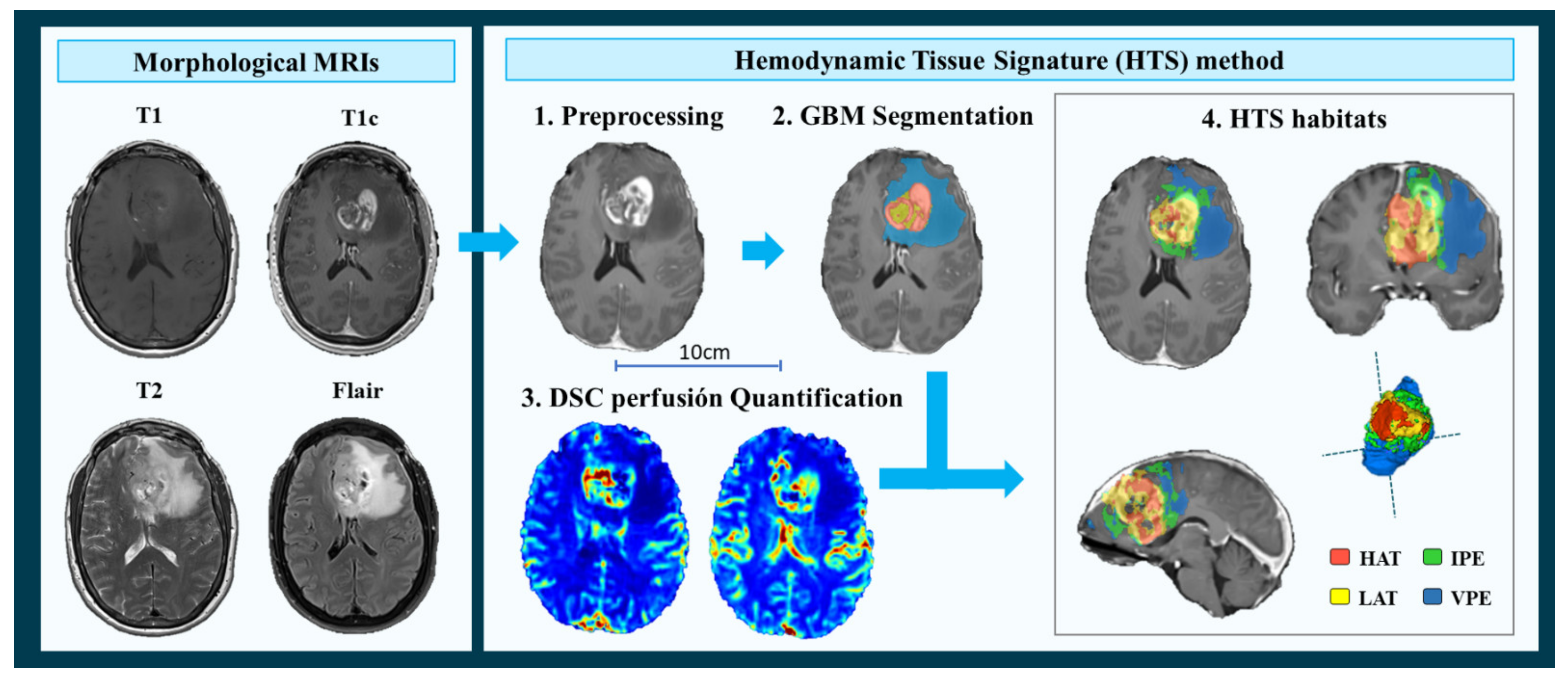
2.3. MRI Processing and Vascular Marker Calculation
- MRI Pre-processing. This phase includes voxel isotropic resampling of all MR images, correction of the magnetic field in homogeneities and noise, rigid intra-patient MRI registration, and skull-stripping.
- Glioblastoma tissue segmentation. It is performed using an unsupervised segmentation method, which implements a state-of-the-art deep-learning 3D convolutional neural network (CNN), which takes as input the T1c, T2, and Flair MRIs. This method is based on Directional Class Adaptive Spatially Varying Finite Mixture Model, or DCA-SVFMM, which consists of a clustering algorithm that combines Gaussian mixture modeling with continuous Markov Random Fields to take advantage of the self-similarity and local redundancy of the images.
- DSC perfusion quantification. In this phase, biomarkers such as the relative cerebral blood volume (rCBV) maps, as well as relative cerebral blood flow (rCBF) or Mean Transit Time (MTT), are calculated for each patient. T1-weighted leakage effects are automatically corrected using the Boxerman method [51], while gamma-variate curve fitting is employed to correct for T2 extravasation phase. rCBV maps are calculated by numerical integration of the area under the gamma-variate curve. The Arterial Input Function (AIF) is automatically quantified with a divide and conquer algorithm.
- Hemodynamic Tissue Signature and Vascular Habitats. The HTS provides an automated unsupervised method to describe the heterogeneity of the enhancing tumor and edema tissues, in terms of the angiogenic process located at these regions. We consider four sub-compartments for the glioblastoma, two within the active tumor: High Angiogenic Tumor habitat (HAT) and Low Angiogenic Tumor habitat (LAT); and two within the edema: Infiltrated Peripheral Edema habitat (IPE) and Vasogenic Peripheral Edema habitat (VPE). These four habitats are obtained by the unsupervised analysis of perfusion patterns, which is carried out through the Directional Class Adaptative Spatially Varying Finite Mixture Model (DCA-SVFMM) algorithm. Such algorithm is an extension of the classic FMM specially focused on image data, which incorporates a continuous Markov Random Field on the spatial coefficients of the model to capture the self-similarity and local redundancy of the images. The clustering consists of two stages: (a) a two-class clustering of the whole enhancing tumor and edema ROIs and (b) a two-class clustering performed using only the rCBV and rCBF data within the ROIs obtained in stage a to detect the different vascular behaviors expressed by the glioma.
2.4. Moderate- and High-Vascular Groups
- (I).
- The threshold proposed in the literature (49) (th = 10.7). It was calculated as the median rCBVmax of 96 patients included in an international multicenter study.
- (II).
- The median rCBVmax of the current study cohort (th = 9.1). Calculated from the 123 patients included in the present study.
- (III).
- The combined threshold of both cohorts (th = 9.8). It is calculated considering the 219 patients from two independent multicenter studies.
2.5. DNA Extraction and Assessment of MGMT Methylation
2.6. Statistical Analyses
2.6.1. Dataset Description: Differences between Methylated and Unmethylated MGMT Groups
2.6.2. Association between MGMT Methylation, Tumor Vascularity and Patient Survival
2.6.3. Survival Differences between Groups According to Tumor Vascularity and MGMT Methylation Status
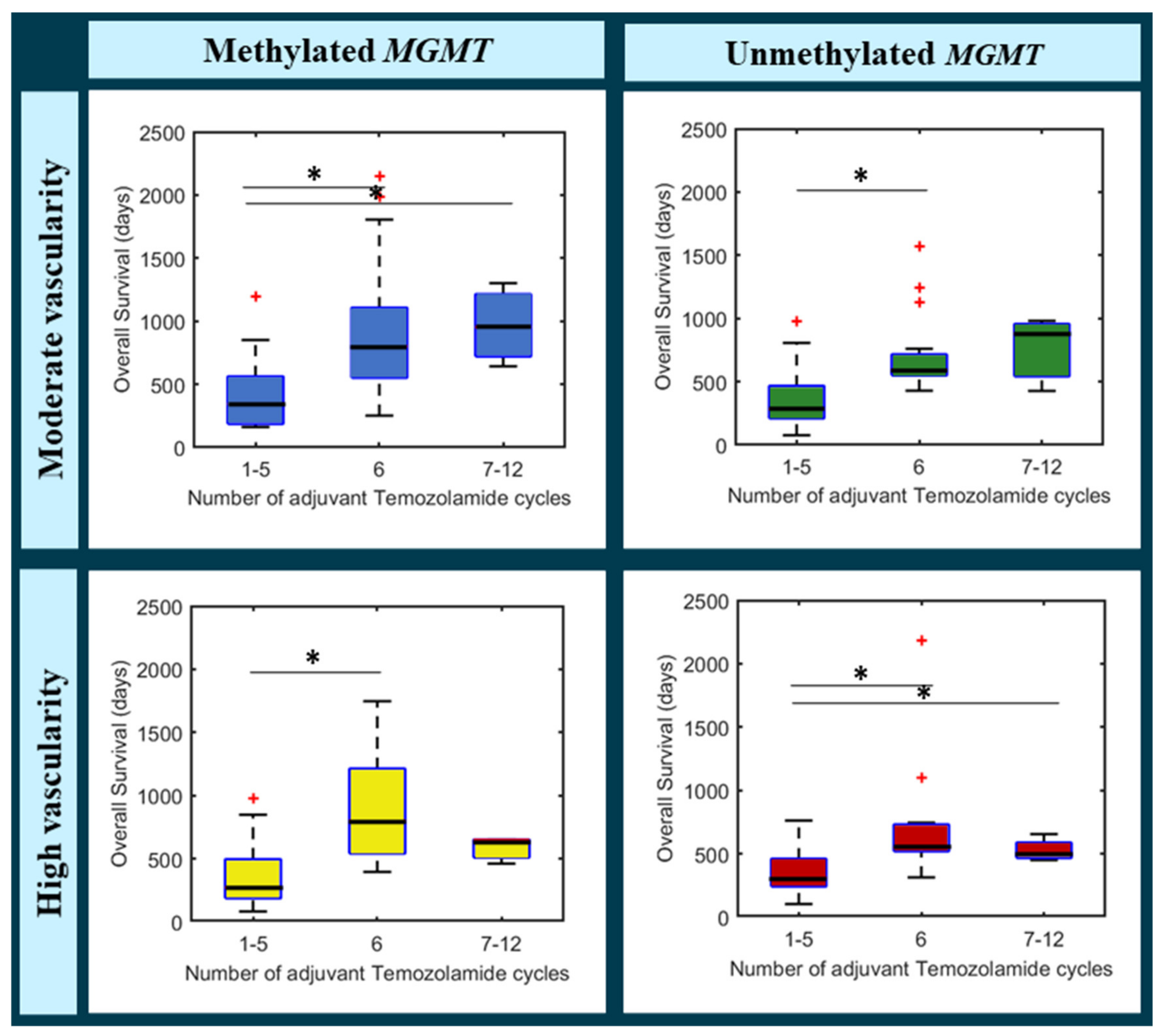
2.6.4. Benefit of Adjuvant Temozolomide Cycles in Different Groups of Glioblastoma Patients
3. Results
3.1. Study Cohort
3.2. Lack of Benefit of Temozolomide for MGMT Methylated Patients with High Vascular Tumors
3.2.1. Uniparametric Cox Regression Analysis
3.2.2. Kaplan Meier and Log Rank Test
3.3. Benefit of Adjuvant Temozolomide Cycles in Different Groups of Glioblastoma Patients
Multiparametric Cox Regression Analysis
- (a)
- Moderate vascularity + methylated MGMT
- (b)
- Moderate vascularity + unmethylated MGMT
- (c)
- High vascularity + methylated MGMT
- (d)
- High vascularity + unmethylated MGMT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gately, L.; McLachlan, S.; Dowling, A.; Philip, J. Life beyond a diagnosis of glioblastoma: A systematic review of the literature. J. Cancer Surviv. 2017, 11, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; Van Den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Soeda, A.; Hara, A.; Kunisada, T.; Yoshimura, S.I.; Iwama, T.; Park, D.M. The evidence of glioblastoma heterogeneity. Sci. Rep. 2015, 27, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Brown, T.J.; Brennan, M.C.; Li, M.; Church, E.W.; Brandmeir, N.J.; Rakszawski, K.L.; Patel, A.S.; Rizk, E.B.; Suki, D.; Sawaya, R.; et al. Association of the Extent of Resection With Survival in Glioblastoma: A Systematic Review and Meta-analysis. JAMA Oncol. 2016, 2, 1460–1469. [Google Scholar] [CrossRef] [Green Version]
- Lacroix, M.; Abi-Said, D.; Fourney, D.R.; Gokaslan, Z.L.; Shi, W.; DeMonte, F.; Lang, F.F.; McCutcheon, I.E.; Hassenbusch, S.J.; Holland, E.; et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prog-nosis, extent of resection, and survival. J. Neurosurg. 2001, 95, 190–198. [Google Scholar] [CrossRef] [Green Version]
- Molinaro, A.M.; Hervey-Jumper, S.; Morshed, R.A.; Young, J.; Han, S.J.; Chunduru, P.; Zhang, Y.; Phillips, J.J.; Shai, A.; Lafontaine, M.; et al. Association of Maximal Extent of Resection of Contrast-Enhanced and Non–Contrast-Enhanced Tumor with Survival Within Molecular Subgroups of Patients With Newly Diagnosed Glioblastoma. JAMA Oncol. 2020, 6, 495–503. [Google Scholar] [CrossRef]
- Shonka, N.A.; Aizenberg, M.R. Extent of Resection in Glioblastoma. J. Oncol. Pract. 2017, 13, 641–642. [Google Scholar] [CrossRef] [Green Version]
- Sanai, N.; Polley, M.Y.; McDermott, M.W.; Parsa, A.T.; Berger, M.S. An extent of resection threshold for newly diagnosed glioblastomas. J. Neurosurg. 2011, 115, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Stummer, W. Extent of resection and survival in glioblastoma multiforme: Identification of and adjustment for bias. Neurosurgery 2009, 64, E1206. [Google Scholar] [CrossRef]
- Vogelbaum, M.A. Does Extent of Resection of a Glioblastoma Matter? Neurosurgery 2012, 59, 79–81. [Google Scholar] [CrossRef] [Green Version]
- Perry, J.R.; Laperriere, N.; O’Callaghan, C.J.; Brandes, A.A.; Menten, J.; Phillips, C.; Fay, M.; Nishikawa, R.; Cairncross, J.G.; Roa, W.; et al. Short-Course Radiation plus Temozolomide in Elderly Patients with Gli-oblastoma. N. Engl. J. Med. 2017, 376, 1027–1037. [Google Scholar] [CrossRef] [Green Version]
- Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; De Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef] [Green Version]
- Balana, C.; Vaz, M.A.; Sepúlveda, J.M.; Mesia, C.; Del Barco, S.; Pineda, E.; Muñoz-Langa, J.; Estival, A.; Peñas, R.D.L.; Fuster, J.; et al. A phase II randomized, multicenter, open-label trial of continuing adjuvant temozolomide beyond 6 cycles in patients with glioblastoma (GEINO 14-01). Neuro-Oncology 2020, 22, 1851–1861. [Google Scholar] [CrossRef]
- Bhandari, M.; Gandhi, A.K.; Devnani, B.; Kumar, P.; Sharma, D.N.; Julka, P.K. Comparative Study of Adjuvant Temozolomide Six Cycles Versus Extended 12 Cycles in Newly Diagnosed Glioblastoma Multiforme. J. Clin. Diagn. Res. 2017, 11, XC04–XC08. [Google Scholar] [CrossRef]
- Chen, S.; Visintini, S. Extended Dosing (12 Cycles) of Adjuvant Temozolomide in Adults with Newly Diagnosed High Grade Gliomas: A Review of Clinical Effectiveness, Cost-Effectiveness, and Guidelines; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2018. [Google Scholar]
- Barnett, A.; Knusel, K.; Ali, A.; Bamashmos, A.S.; Sagar, S.; Ahluwalia, M.S. Efficacy of Extended Adjuvant Temozolomide Cycle Duration in Newly Diagnosed Glioblastoma: Four-year experience of a single major tertiary care institution (P2.6-035). Neurology 2019, 92 (Suppl. 15), P2.6-035. [Google Scholar]
- Urgoiti, G.B.R.; Singh, A.D.; Easaw, J.C. Extended adjuvant temozolomide for treatment of newlydiagnosed glio-blastoma multiforme. J. Neurooncol. 2012, 108, 173–177. [Google Scholar] [CrossRef]
- Hirono, S.; Hasegawa, Y.; Sakaida, T.; Uchino, Y.; Hatano, K.; Iuchi, T. Feasibility study of finalizing the extended adjuvant temozolomide based on methionine positron emission tomography (Met-PET) findings in patients with glioblastoma. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, M.R.; Wang, M.; Aldape, K.D.; Stupp, R.; Hegi, M.E.; Jaeckle, K.A.; Armstrong, T.S.; Wefel, J.S.; Won, M.; Blumenthal, D.T.; et al. Dose-dense temozolomide for newly diagnosed glioblastoma: A randomized phase III clinical trial. J. Clin. Oncol. 2013, 31, 4085. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A Randomized Trial of Bevacizumab for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef] [Green Version]
- Chinot, O.L.; Wick, W.; Mason, W.; Henriksson, R.; Saran, F.; Nishikawa, R.; Carpentier, A.F.; Hoang-Xuan, K.; Kavan, P.; Cernea, D.; et al. Bevacizumab plus Radiotherapy–Temozolomide for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014, 370, 709–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weller, M.; Butowski, N.; Tran, D.D.; Recht, L.D.; Lim, M.; Hirte, H.; Ashby, L.; Mechtler, L.; Goldlust, S.A.; Iwamoto, F.; et al. Rindopepimut with temozolomide for patients with newly diagnosed, EG-FRvIII-expressing glioblastoma (ACT IV): A randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017, 18, 1373–1385. [Google Scholar] [CrossRef] [Green Version]
- Stupp, R.; Taillibert, S.; Kanner, A.A.; Read, W.; Steinberg, D.M.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients with Glioblastoma. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lassman, A.B.; Joanta-Gomez, A.E.; Pan, P.C.; Wick, W. Current usage of tumor treating fields for glioblastoma. Neuro-Oncol. Adv. 2020, 2, vdaa069. [Google Scholar] [CrossRef]
- Fabian, D.; Guillermo Prieto Eibl, M.D.P.; Alnahhas, I.; Sebastian, N.; Giglio, P.; Puduvalli, V.; Gonzalez, J.; Palmer, J.D. Treatment of Glioblastoma (GBM) with the Addition of Tumor-Treating Fields (TTF): A Review. Cancers 2019, 11, 174. [Google Scholar] [CrossRef] [Green Version]
- Rominiyi, O.; Vanderlinden, A.; Clenton, S.J.; Bridgewater, C.; Al-Tamimi, Y.; Collis, S.J. Tumour treating fields therapy for glioblastoma: Current advances and future directions. Br. J. Cancer 2021, 124, 697–709. [Google Scholar] [CrossRef]
- Mehta, M.; Wen, P.; Nishikawa, R.; Reardon, D.; Peters, K. Critical review of the addition of tumor treating fields (TTFields) to the existing standard of care for newly diagnosed glioblastoma patients. Crit. Rev. Oncol. 2017, 111, 60–65. [Google Scholar] [CrossRef]
- Liu, Y.; Strawderman, M.S.; Warren, K.T.; Richardson, M.; Serventi, J.N.; Mohile, N.A.; Milano, M.T.; Walter, K.A. Clinical Efficacy of Tumor Treating Fields for Newly Diagnosed Glioblastoma. Anticancer Res. 2020, 40, 5801–5806. [Google Scholar] [CrossRef]
- Davies, A.M.; Weinberg, U.; Palti, Y. Tumor treating fields: A new frontier in cancer therapy. Ann. N. Y. Acad. Sci. 2013, 1291, 86–95. [Google Scholar] [CrossRef]
- Blumenthal, D.T.; Gorlia, T.; Gilbert, M.R.; Kim, M.M.; Nabors, L.B.; Mason, W.P.; Hegi, M.; Zhang, P.; Gkolfinopoulos, V.; Perry, J.R.; et al. Is more better? The impact of extended adjuvant temozolomide in newly diagnosed glioblastoma: A secondary analysis of EORTC and NRG Oncology/RTOG. Neuro-Oncology 2017, 19, 1119–1126. [Google Scholar] [CrossRef]
- Gramatzki, D.; Kickingereder, P.; Hentschel, B.; Felsberg, J.; Herrlinger, U.; Schackert, G.; Tonn, J.C.; Westphal, M.; Sabel, M.; Schlegel, U.; et al. Limited role for extended maintenance temozolomide for newly diagnosed glioblastoma. Neurology 2017, 88, 1422–1430. [Google Scholar] [CrossRef]
- Balaña, C.; Vaz, M.A.; López, D.; De La Peñas, R.; Garcia-Bueno, J.M.; Molina-Garrido, M.J.; Sepúlveda, J.M.; Cano, J.M.; Buges, C.; Sanz, S.M.; et al. Should we continue temozolomide beyond six cycles in the adjuvant treatment of glioblastoma without an evidence of clinical benefit? A cost analysis based on prescribing patterns in Spain. Clin. Transl. Oncol. 2013, 16, 273–279. [Google Scholar] [CrossRef]
- Weller, M.; Bent, M.V.D.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2020, 18, 170–186. [Google Scholar] [CrossRef]
- Sinigaglia, M.; Assi, T.; Besson, F.L.; Ammari, S.; Edjlali, M.; Feltus, W.; Rozenblum-Beddok, L.; Zhao, B.; Schwartz, L.H.; Mokrane, F.-Z.; et al. Imaging-guided precision medicine in glioblastoma patients treated with immune checkpoint modulators: Research trend and future directions in the field of imaging biomarkers and artificial intelligence. EJNMMI Res. 2019, 9, 1–20. [Google Scholar] [CrossRef]
- Colombo, M.C.; Giverso, C.; Faggiano, E.; Boffano, C.; Acerbi, F.; Ciarletta, P. Towards the Personalized Treatment of Glioblastoma: Integrating Pa-tient-Specific Clinical Data in a Continuous Mechanical Model. PLoS ONE 2015, 10, e0143032. [Google Scholar] [CrossRef] [Green Version]
- Sotoudeh, H.; Shafaat, O.; Bernstock, J.D.; Brooks, M.D.; Elsayed, G.; Chen, J.A.; Szerip, P.; Chagoya, G.; Gessler, F.; Sotoudeh, E.; et al. Artificial Intelligence in the Management of Glioma: Era of Personalized Medicine. Front. Oncol. 2019, 9, 768. [Google Scholar] [CrossRef]
- European Society of Radiology (ESR). Medical imaging in personalised medicine: A white paper of the research com-mittee of the European Society of Radiology (ESR). Insights Imaging 2015, 6, 141–155. [Google Scholar] [CrossRef]
- Schork, N.J. Artificial Intelligence and Personalized Medicine; Springer: Cham, Switzerland, 2019; Volume 178, pp. 265–283. [Google Scholar] [CrossRef]
- Mcdonald, K.L.; Aw, G.; Kleihues, P. Role of biomarkers in the clinical management of glioblastomas: What are the barriers and how can we overcome them? Front. Neurol. 2013, 3, 188. [Google Scholar] [CrossRef] [Green Version]
- Hottinger, A.F.; Homicsko, K.; Negretti, L.; Lhermitte, B.; Stupp, R. Decision making and management of gliomas: Practical considerations. Ann. Oncol. 2012, 23, x33–x40. [Google Scholar] [CrossRef]
- Staedtke, V.; a Dzaye, O.D.; Holdhoff, M. Actionable Molecular Biomarkers in Primary Brain Tumors. Trends Cancer 2016, 2, 338–349. [Google Scholar] [CrossRef] [Green Version]
- Szopa, W.; Burley, T.A.; Kramer-Marek, G.; Kaspera, W. Diagnostic and Therapeutic Biomarkers in Glioblastoma: Current Status and Future Perspectives. BioMed Res. Int. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; Van Den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radi-otherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Wick, W.; Weller, M.; Bent, M.V.D.; Sanson, M.; Weiler, M.; Von Deimling, A.; Plass, C.; Hegi, M.; Platten, M.; Reifenberger, G. MGMT testing-the challenges for biomarker-based glioma treatment. Nat. Rev. Neurol. 2014, 10, 372–385. [Google Scholar] [CrossRef] [Green Version]
- Mansouri, A.; Hachem, L.D.; Mansouri, S.; Nassiri, F.; Laperriere, N.J.; Xia, D.; Lindeman, N.I.; Wen, P.Y.; Chakravarti, A.; Mehta, M.P.; et al. MGMT promoter methylation status testing to guide therapy for glioblas-toma: Refining the approach based on emerging evidence and current challenges. Neuro-Oncology 2019, 21, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Fuster-Garcia, E.; Estellés, D.L.; Álvarez-Torres, M.D.M.; Juan-Albarracín, J.; Chelebian, E.; Rovira, A.; Acosta, C.A.; Pineda, J.; Oleaga, L.; Mollá-Olmos, E.; et al. MGMT methylation may benefit overall survival in patients with moderately vascularized glioblastomas. Eur. Radiol. 2020, 31, 1738–1747. [Google Scholar] [CrossRef]
- Pineda, E.; Esteve-Codina, A.; Martinez-Garcia, M.; Alameda, F.; Carrato, C.; Arpi, O.; Aldecoa, I.; Menendez, S.; Ribalta, T.; Sarro, N.V.; et al. Glioblastoma gene expression subtypes and correlation with clinical, molecular and immunohistochemical characteristics in a homogenously treated cohort: GLIOCAT project. J. Clin. Oncol. 2019, 37, 2029. [Google Scholar] [CrossRef]
- Juan-Albarracin, J.; Fuster-García, E.; Pérez-Girbes, A.; Aparici-Robles, F.; Alberich-Bayarri, Á.; Revert-Ventura, A.; Marti-Bonmati, L.; Garcia-Gomez, J.M. Glioblastoma: Vascular Habitats Detected at Preoperative Dynamic Susceptibility-weighted Contrast-enhanced Perfusion MR Imaging Predict Survival. Radiology 2018, 287, 944–954. [Google Scholar] [CrossRef]
- Juan-Albarracín, J.; Fuster-Garcia, E.; García-Ferrando, G.A.; García-Gómez, J.M. ONCOhabitats: A system for glioblastoma heterogeneity assessment through MRI. Int. J. Med. Inform. 2019, 128, 53–61. [Google Scholar] [CrossRef]
- Boxerman, J.; Schmainda, K.; Weisskoff, R. Relative Cerebral Blood Volume Maps Corrected for Contrast Agent Extravasation Significantly Correlate with Glioma Tumor Grade, Whereas Uncorrected Maps Do Not. Am. J. Neuroradiol. 2006, 27, 859–867. [Google Scholar]
- Del Mar Álvarez-Torres, M.; Juan-Albarracín, J.; Fuster-Garcia, E.; Bellvís-Bataller, F.; Lorente, D.; Reynés, G.; Font de Mora, J.; Aparici-Robles, F.; Botella, C.; Muñoz-Langa, J.; et al. Robust association between vascular habitats and patient prognosis in glioblastoma: An international multicenter study. J. Magn. Reson. Imaging 2019, 51, 1478–1486. [Google Scholar] [CrossRef]
- Fuster-Garcia, E.; Juan-Albarracín, J.; García-Ferrando, G.A.; Martí-Bonmatí, L.; Aparici-Robles, F.; Garcia-Gomez, J.M. Improving the estimation of prognosis for glioblastoma patients by MR based hemodynamic tissue signatures. NMR Biomed. 2018, 31, e4006. [Google Scholar] [CrossRef] [PubMed]
- Esteller, M.; Hamilton, S.R.; Burger, P.C.; Baylin, S.B.; Herman, J.G. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltrans-ferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res. 1999, 59, 793–797. [Google Scholar] [PubMed]
- Cui, Y.; Ren, S.; Tha, K.K.; Wu, J.; Shirato, H.; Li, R. Volume of high-risk intratumoral subregions at multi-parametric MR imaging predicts overall survival and complements molecular analysis of glioblastoma. Eur. Radiol. 2017, 27, 3583–3592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Li, C.; Gensheimer, M.; Padda, S.; Kato, F.; Shirato, H.; Wei, Y.; Schönlieb, C.-B.; Price, S.J.; Jaffray, D.; et al. Radiological tumour classification across imaging modality and histology. Nat. Mach. Intell. 2021, 3, 787–798. [Google Scholar] [CrossRef]
- Alvarez-Torres, M.; Fuster-Garcia, E.; Juan-Albarracin, J.; Reynes, G.; Aparici-Robles, F.; Ferrer-Lozano, J.; Garcia-Gomez, J.M. Detection of local microvascular proliferation in IDH wild-type Glioblastoma using relative Cerebral Blood Volume. medRxiv 2021. [Google Scholar] [CrossRef]

| Variables | Entire Cohort | Methylated MGMT Population | Unmethylated MGMT Population | p-Values (MW/FE) |
|---|---|---|---|---|
| Number of patients | 123 | 67 | 56 | - |
| Gender | ||||
| -% females | 41.5 | 43.3 | 39.2 | 0.7150 |
| Age at diagnosis (years) | 0.8973 | |||
| -Mean | 60 | 62 | 58 | |
| -Range | (32,80) | (33,80) | (32,77) | |
| Overall Survival (months) | 0.1214 | |||
| -Mean | 20.2 | 22.4 | 17.6 | |
| -Median | 17.1 | 19.3 | 15.5 | |
| -Range | (2.7,72.8) | (2.7,71.6) | (2.7,72.8) | |
| Extent of Resection (#patients) | 0.4524 | |||
| -Complete | 45 | 27 | 18 | |
| -Partial | 78 | 40 | 38 | |
| Concomitant chemotherapy (#patients) | 0.3788 | |||
| -Complete | 110 | 58 | 52 | |
| -Incomplete | 13 | 9 | 4 | |
| Adjuvant chemotherapy (number of cycles) | 0.4435 | |||
| -Mean | 4 | 5 | 4 | |
| -Median | 5 | 5 | 4 | |
| -Range | (0,12) | (0,12) | (0,12) | |
| IDH1 mutation status | 1.0000 | |||
| -Mutated | 2 | 1 | 1 | |
| -Wild type | 93 | 51 | 42 | |
| -Unknown | 28 | 15 | 13 | |
| HAT rCBVmax | 0.4150 | |||
| -Mean | 9.77 | 9.49 | 10.10 | |
| -Median | 9.10 | 9.53 | 8.87 | |
| -Range | (3.39, 21.80) | (3.39, 16.93) | (3.49, 21.8) | |
| Association MGMT Methylation–Overall Survival | Number of Patients | HR [95% CI] | p-Value | |
|---|---|---|---|---|
| Analyzed thresholds | Entire cohort | 123 | 1.58 [1.06, 2.35] | 0.0247 * |
| (I) Th. proposed in [47] = 10.7 | Moderate rCBV | 80 | 1.70 [1.04, 2.79] | 0.0353 * |
| High rCBV | 43 | 1.36 [0.69, 2.67] | 0.3734 | |
| (II) Th. study cohort = 9.1 | Moderate rCBV | 61 | 2.40 [1.34, 4.31] | 0.0032 * |
| High rCBV | 62 | 1.04 [0.60, 1.80] | 0.9008 | |
| (III) Th. combined = 9.8 | Moderate rCBV | 71 | 2.01 [1.19, 3.41] | 0.0095 * |
| High rCBV | 53 | 1.09 [0.59, 2.00] | 0.7894 | |
| Survival Rates According to MGMT Methylation and Tumor Vascularity | Number of Patients | KM Results | |||||
|---|---|---|---|---|---|---|---|
| Kaplan-Meier Analysis | Total | Meth. MGMT | Unmeth. MGMT | Median OS Meth. MGMT | Median os Unmeth. Mgmt | |ΔOS| | p-Value |
| Entire cohort | 123 | 67 | 56 | 578 | 462 | 114 | 0.0220 * |
| Moderate rCBV | 71 | 46 | 34 | 641 | 467 | 174 | 0.0129 * |
| High rCBV | 53 | 21 | 22 | 454 | 461 | 7 | 0.9119 |
| Covariables | HR [95% CI] MGMT | p-Value |
|---|---|---|
| Entire cohort | ||
| MGMT status | 1.53 [0.96, 2.43] | 0.0727 |
| TMZ cycles | 0.78 [0.70, 0.85] | <0.0001 * |
| Moderate rCBV | ||
| MGMT status | 1.75 [1.08, 4.20] | 0.0416 * |
| TMZ cycles | 0.78 [0.66, 0.90] | <0.0001 * |
| High rCBV | ||
| MGMT | 1.03 [0.56, 1.92] | 0.9121 |
| TMZ cycles | 0.77 [0.68, 0.87] | <0.0001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Torres, M.d.M.; Fuster-García, E.; Balaña, C.; Puig, J.; García-Gómez, J.M. Lack of Benefit of Extending Temozolomide Treatment in Patients with High Vascular Glioblastoma with Methylated MGMT. Cancers 2021, 13, 5420. https://doi.org/10.3390/cancers13215420
Álvarez-Torres MdM, Fuster-García E, Balaña C, Puig J, García-Gómez JM. Lack of Benefit of Extending Temozolomide Treatment in Patients with High Vascular Glioblastoma with Methylated MGMT. Cancers. 2021; 13(21):5420. https://doi.org/10.3390/cancers13215420
Chicago/Turabian StyleÁlvarez-Torres, María del Mar, Elies Fuster-García, Carmen Balaña, Josep Puig, and Juan M. García-Gómez. 2021. "Lack of Benefit of Extending Temozolomide Treatment in Patients with High Vascular Glioblastoma with Methylated MGMT" Cancers 13, no. 21: 5420. https://doi.org/10.3390/cancers13215420
APA StyleÁlvarez-Torres, M. d. M., Fuster-García, E., Balaña, C., Puig, J., & García-Gómez, J. M. (2021). Lack of Benefit of Extending Temozolomide Treatment in Patients with High Vascular Glioblastoma with Methylated MGMT. Cancers, 13(21), 5420. https://doi.org/10.3390/cancers13215420








