Clinical, Pathological, and Molecular Features of Breast Carcinoma Cutaneous Metastasis
Abstract
Simple Summary
Abstract
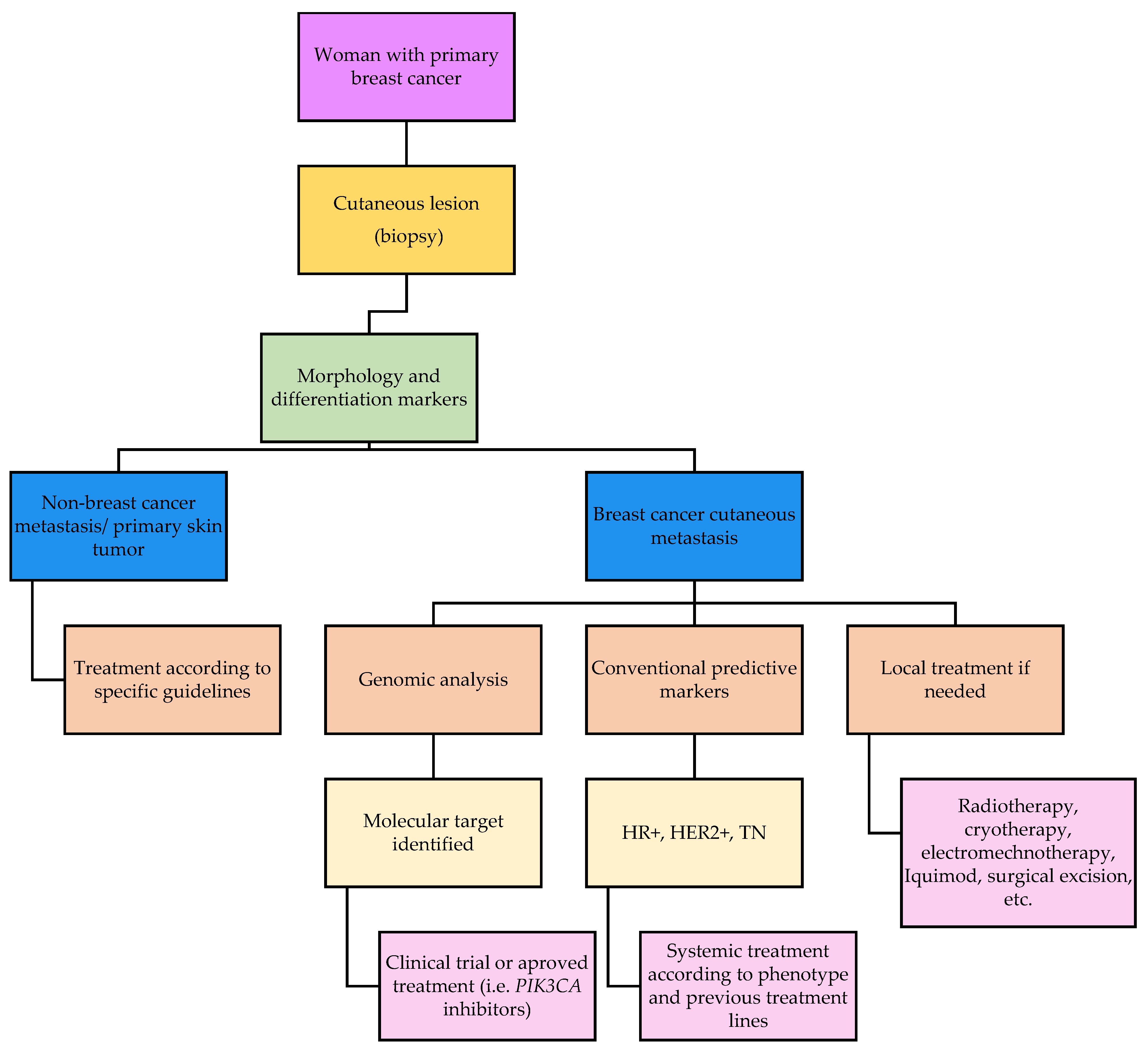
1. Introduction
2. Clinical and Pathological Features of Breast Cancer Cutaneous Metastasis
2.1. Epidemiology, Incidence, and Prognosis of Breast Cancer Cutaneous Metastasis
2.2. Clinical Presentation of Breast Cancer Cutaneous Metastasis
2.3. Histopathology of Breast Cancer Cutaneous Metastasis
Differential Diagnosis of CM of Mammary Origin
2.4. Prognosis and Treatment of Breast Cancer Cutaneous Metastasis
3. Molecular Landscape of BC Metastasis
3.1. Overview of Molecular Alterations in Metastases of Mammary Origin
Differences in Mutational Profile between Primary Tumors and Their Paired Metastases
3.2. Molecular Landscape of Breast Cancer Cutaneous Metastases
3.3. Comparison of Mutational Profile between Cutaneous and Hepatic Metastases
4. Possible Future Directions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Islami, F.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer in Women: Burden and Trends. Cancer Epidemiol. Biomark. Prev. 2017, 26, 444–457. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Chiang, A.C.; Massagué, J. Molecular Basis of Metastasis. N. Engl. J. Med. 2008, 359, 2814–2823. [Google Scholar] [CrossRef]
- Liotta, L.A. Cancer Invasion and Metastases. JAMA 1990, 263, 1123–1126. [Google Scholar] [CrossRef]
- Arozullah, A.M.; Calhoun, E.A.; Wolf, M.; Finley, D.K.; Fitzner, K.A.; Heckinger, E.A.; Gorby, N.S.; Schumock, G.T.; Bennett, C.L. The Financial Burden of Cancer: Estimates from a Study of Insured Women with Breast Cancer. J. Support Oncol. 2004, 2, 271–278. [Google Scholar]
- Coleman, R.E. Skeletal Complications of Malignancy. Cancer 1997, 80, 1588–1594. [Google Scholar] [CrossRef]
- Lipton, A. Bisphosphonates and Metastatic Breast Carcinoma. Cancer 2003, 97, 848–853. [Google Scholar] [CrossRef]
- Richert, M.M.; Welch, D.R. Metastasis of Hormone Receptor Positive Breast Cancer. In Hormone Receptors in Breast Cancer; Fuqua, S.A.W., Ed.; Cancer Treatment and Research; Springer US: Boston, MA, USA, 2009; Volume 147, pp. 1–22. ISBN 978-0-387-09462-5. [Google Scholar]
- Heitz, F.; Harter, P.; Lueck, H.-J.; Fissler-Eckhoff, A.; Lorenz-Salehi, F.; Scheil-Bertram, S.; Traut, A.; du Bois, A. Triple-Negative and HER2-Overexpressing Breast Cancers Exhibit an Elevated Risk and an Earlier Occurrence of Cerebral Metastases. Eur. J. Cancer 2009, 45, 2792–2798. [Google Scholar] [CrossRef] [PubMed]
- Lentzsch, S.; Reichardt, P.; Weber, F.; Budach, V.; Dörken, B. Brain Metastases in Breast Cancer: Prognostic Factors and Management. Eur. J. Cancer 1999, 35, 580–585. [Google Scholar] [CrossRef]
- Bendell, J.C.; Domchek, S.M.; Burstein, H.J.; Harris, L.; Younger, J.; Kuter, I.; Bunnell, C.; Rue, M.; Gelman, R.; Winer, E. Central Nervous System Metastases in Women Who Receive Trastuzumab-Based Therapy for Metastatic Breast Carcinoma. Cancer 2003, 97, 2972–2977. [Google Scholar] [CrossRef]
- Gerratana, L.; Fanotto, V.; Bonotto, M.; Bolzonello, S.; Minisini, A.M.; Fasola, G.; Puglisi, F. Pattern of Metastasis and Outcome in Patients with Breast Cancer. Clin. Exp. Metastasis 2015, 32, 125–133. [Google Scholar] [CrossRef]
- Alcaraz, I.; Cerroni, L.; Rütten, A.; Kutzner, H.; Requena, L. Cutaneous Metastases From Internal Malignancies: A Clinicopathologic and Immunohistochemical Review. Am. J. Dermatopathol. 2012, 34, 347–393. [Google Scholar] [CrossRef]
- Casimiro, L.M. Metástasis cutáneas de neoplasias internas. Med. Cutan. Iber. Lat. Am. 2009, 37, 117–129. [Google Scholar]
- De Giorgi, V.; Grazzini, M.; Alfaioli, B.; Savarese, I.; Corciova, S.A.; Guerriero, G.; Lotti, T. Cutaneous Manifestations of Breast Carcinoma: A Clinical Guide. Dermatol. Ther. 2010, 23, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Guanziroli, E.; Coggi, A.; Venegoni, L.; Fanoni, D.; Ercoli, G.; Boggio, F.; Veraldi, S.; Berti, E.; Gianotti, R.; Ferrero, S.; et al. Cutaneous Metastases of Internal Malignancies: An Experience from a Single Institution. Eur. J. Dermatol. 2017, 27, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.R. Cutaneous Manifestations of Breast Cancer. Semin. Oncol. 2016 43, 331–334. [CrossRef]
- Bittencourt, M.D.J.S.; Carvalho, A.H.; Nascimento, B.A.M.; Freitas, L.K.M.; Parijós, A.M.D. Cutaneous Metastasis of a Breast Cancer Diagnosed 13 Years Before. An. Bras. Dermatol. 2015, 90, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.-S.; Chen, G.-S.; Lu, Y.-W.; Wu, C.-S.; Lan, C.-C. Cutaneous Metastases from Different Internal Malignancies: A Clinical and Prognostic Appraisal. J. Eur. Acad. Dermatol. Venerol. 2008, 22, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, C.; Bachelot, T.; Filleron, T.; Pedrero, M.; Campone, M.; Soria, J.-C.; Massard, C.; Lévy, C.; Arnedos, M.; Lacroix-Triki, M.; et al. Mutational Profile of Metastatic Breast Cancers: A Retrospective Analysis. PLoS Med. 2016, 13, e1002201. [Google Scholar] [CrossRef]
- Yates, L.R.; Knappskog, S.; Wedge, D.; Farmery, J.H.R.; Gonzalez, S.; Martincorena, I.; Alexandrov, L.B.; Van Loo, P.; Haugland, H.K.; Lilleng, P.K.; et al. Genomic Evolution of Breast Cancer Metastasis and Relapse. Cancer Cell 2017, 32, 169–184.e7. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.H.; Park, Y.H.; Kim, J.A.; Kim, J.H.; Yun, J.; Sun, J.M.; Won, Y.W.; Lee, S.; Kim, S.T.; Cho, E.Y.; et al. Patterns of Skin and Soft Tissue Metastases from Breast Cancer According to Subtypes: Relationship between EGFR Overexpression and Skin Manifestations. Oncology 2011, 81, 55–62. [Google Scholar] [CrossRef]
- Luna, A.; Rabassa, M.E.; Isla Larrain, M.; Cabaleiro, P.; Zwenger, A.; Canzoneri, R.; Segal-Eiras, A.; Abba, M.C.; Croce, M.V. Breast Cancer Cutaneous Metastases Are Associated to UMUC1 and Sialyl Lewis x and to Highly Malignant Primary Tumors. Pathol. Res. Pract. 2020, 216, 152859. [Google Scholar] [CrossRef]
- Kelati, A.; Gallouj, S. Dermoscopy of Skin Metastases from Breast Cancer: Two Case Reports. J. Med. Case Rep. 2018, 12, 273. [Google Scholar] [CrossRef]
- Saeed, S.; Keehn, C.A.; Morgan, M.B. Cutaneous Metastasis: A Clinical, Pathological, and Immunohistochemical Appraisal: Cutaneous Metastasis. J. Cutan. Pathol. 2004, 31, 419–430. [Google Scholar] [CrossRef]
- Brownstein, M.H.; Helwig, E.B. Metastatic Tumors of the Skin. Cancer 1972, 29, 1298–1307. [Google Scholar] [CrossRef]
- Spencer, P.S.; Helm, T.N. Skin Metastases in Cancer Patients. Cutis 1987, 39, 119–121. [Google Scholar]
- Nava, G.; Greer, K.; Patterson, J.; Lin, K.Y. Metastatic Cutaneous Breast Carcinoma: A Case Report and Review of the Literature. Can. J. Plast. Surg. 2009, 17, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Pipkin, C.A.; Lio, P.A. Cutaneous Manifestations of Internal Malignancies: An Overview. Dermatol. Clin. 2008, 26, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.B.; Kalb, R.; Zeitouni, N.; Helm, M.; Helm, T. The Presentation, Pathology, and Current Management Strategies of Cutaneous Metastasis. N. Am. J. Med. Sci. 2013, 5, 499. [Google Scholar] [CrossRef] [PubMed]
- Lookingbill, D.P.; Spangler, N.; Helm, K.F. Cutaneous Metastases in Patients with Metastatic Carcinoma: A Retrospective Study of 4020 Patients. J. Am. Acad. Dermatol. 1993, 29, 228–236. [Google Scholar] [CrossRef]
- Marneros, A.G.; Blanco, F.; Husain, S.; Silvers, D.N.; Grossman, M.E. Classification of Cutaneous Intravascular Breast Cancer Metastases Based on Immunolabeling for Blood and Lymph Vessels. J. Am. Acad. Dermatol. 2009, 60, 633–638. [Google Scholar] [CrossRef]
- Lin, J.-H.; Lee, J.-Y.; Chao, S.-C.; Tsao, C.-J. Telangiectatic Metastatic Breast Carcinoma Preceded by En Cuirasse Metastatic Breast Carcinoma. Br. J. Dermatol. 2004, 151, 523–524. [Google Scholar] [CrossRef]
- Pakula, A.S.; Robinson, J.K. Recognizing Malignant Skin Changes Following Breast Cancer. Am. Fam. Physician 1992, 45, 1287–1292. [Google Scholar] [PubMed]
- Maghfour, S. Telangiectatic Cutaneous Metastasis from Breast Carcinoma. Our Dermatol. Online 2021, 12, 47–49. [Google Scholar] [CrossRef]
- Bourke, M.G.; Salwa, S.P.; Sadadcharam, M.; Whelan, M.C.; Forde, P.F.; Larkin, J.O.; Collins, C.G.; O’Reilly, S.; O’Sullivan, G.C.; Clover, A.J.; et al. Effective Treatment of Intractable Cutaneous Metastases of Breast Cancer with Electrochemotherapy: Ten-Year Audit of Single Centre Experience. Breast Cancer Res. Treat. 2017, 161, 289–297. [Google Scholar] [CrossRef]
- Mullinax, K.; Cohen, J.B. Carcinoma En Cuirasse Presenting as Keloids of the Chest. Dermatol. Surg. 2004, 30, 226–228. [Google Scholar] [CrossRef]
- Carlesimo, M.; Rossi, A.; De Marco, G.; Narcisi, A.; Cacchi, C.; Mari, E.; Persechino, F.; Camplone, G. Carcinoma En Cuirasse of the Breast. Eur. J. Dermatol. 2009, 19, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Mallon, E.; Dawber, R.P.R. Alopecia Neoplastica without Alopecia: A Unique Presentation of Breast Carcinoma Scalp Metastasis. J. Am. Acad. Dermatol. 1994, 31, 319–321. [Google Scholar] [CrossRef]
- Scheinfeld, N. Review of Scalp Alopecia Due to a Clinically Unapparent or Minimally Apparent Neoplasm (SACUMAN). Acta Derm. Venereol. 2006, 86, 387–392. [Google Scholar] [CrossRef]
- Mayer, J.E.; Maurer, M.A.; Nguyen, H.T. Diffuse Cutaneous Breast Cancer Metastases Resembling Subcutaneous Nodules with No Surface Changes. Cutis 2018, 101, 219–223. [Google Scholar] [PubMed]
- Conner, K.B.; Cohen, P.R. Cutaneous Metastasis of Breast Carcinoma Presenting as Alopecia Neoplastica. South. Med. J. 2009, 102, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Skafida, E.; Triantafyllopoulou, I.; Flessas, I.; Liontos, M.; Koutsoukos, K.; Zagouri, F.; Dimopoulos, A.-M. Secondary Alopecia Neoplastica Mimicking Alopecia Areata Following Breast Cancer. Case Rep. Oncol. 2020, 13, 627–632. [Google Scholar] [CrossRef]
- Lee, H.; Lim, H.S.; Ki, S.Y.; Lee, J.E.; Lee, J.S.; Park, M.H. Cutaneous Scalp Metastases of Malignant Phyllodes Tumor of the Breast. J. Breast Cancer 2020, 23, 320. [Google Scholar] [CrossRef]
- Schorr, W.F. Alopecia Neoplastica: Hair Loss Resembling Alopecia Areata Caused by Metastatic Breast Cancer. JAMA 1970, 213, 1335. [Google Scholar] [CrossRef]
- Schwartz, R.A. Histopathologic Aspects of Cutaneous Metastatic Disease. J. Am. Acad. Dermatol. 1995, 33, 649–657. [Google Scholar] [CrossRef]
- Azoulay, S.; Adem, C.; Pelletier, F.L.E.; Barete, S.; Frances, C.; Capron, F. Skin Metastases from Unknown Origin: Role of Immunohistochemistry in the Evaluation of Cutaneous Metastases of Carcinoma of Unknown Origin. J. Cutan. Pathol. 2005, 32, 561–566. [Google Scholar] [CrossRef]
- Lewis, G.H.; Subhawong, A.P.; Nassar, H.; Vang, R.; Illei, P.B.; Park, B.H.; Argani, P. Relationship Between Molecular Subtype of Invasive Breast Carcinoma and Expression of Gross Cystic Disease Fluid Protein 15 and Mammaglobin. Am. J. Clin. Pathol. 2011, 135, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Tozbikian, G.H.; Zynger, D.L. A Combination of GATA3 and SOX10 Is Useful for the Diagnosis of Metastatic Triple-Negative Breast Cancer. Hum. Pathol. 2019, 85, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Llamas-Velasco, M.; Pérez-Gónzalez, Y.C.; Daudén, E.; Rütten, A. GATA3 Staining in Primary Cutaneous Apocrine Cribriform Carcinoma: Usefulness to Differentiate It from Breast Cancer Metastasis. J. Cutan. Pathol. 2018, 45, 348–351. [Google Scholar] [CrossRef]
- Valencia-Guerrero, A.; Dresser, K.; Cornejo, K.M. Utility of Immunohistochemistry in Distinguishing Primary Adnexal Carcinoma From Metastatic Breast Carcinoma to Skin and Squamous Cell Carcinoma. Am. J. Dermatopathol. 2018, 40, 389–396. [Google Scholar] [CrossRef]
- Gan, E.Y.; Chio, M.T.-W.; Tan, W.P. A Retrospective Review of Cutaneous Metastases at the National Skin Centre Singapore: Cutaneous Metastases in Asians. Australas. J. Dermatol. 2015, 56, 1–6. [Google Scholar] [CrossRef]
- Kapoor, A.; Singhal, M.; Singh, P.; Kumar, V.; Kumar, H.; Singh, D. Cutaneous Metastasis Involving Face in Breast Cancer: A Series of Three Patients. Clin. Cancer Investig. J. 2014, 3, 545. [Google Scholar] [CrossRef]
- Ferreira, V.A.; Spelta, K.; Diniz, L.M.; Lucas, E.A. Exuberant Case of Cutaneous Metastasis of Breast Cancer. An. Bras. Dermatol. 2018, 93, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Utkan, G.; BüyükçelïK, A.; Okçu, A.H.; Avci, U.; Yalçin, B.; İÇlï, F. Widespread Erythematous Skin Metastasis from Breast Cancer Mimicking Generalized Drug Eruption. Turk. J. Cancer 2009, 39, 66–68. [Google Scholar]
- Krishnasamy, S.R.; Almazan, T.H.; Suero-Abreu, G.A.; Jung, J.Y. Successful Treatment of Cutaneous Metastatic Breast Cancer with Topical Treatments That Potentially Synergize with Systemic Therapy: A Case Series. JAAD Case Rep. 2018, 4, 711–715. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schrijver, W.A.; Selenica, P.; Lee, J.Y.; Ng, C.K.Y.; Burke, K.; Piscuoglio, S.; Berman, S.H.; Reis-Filho, J.S.; Weigelt, B.; Van Diest, P.J.; et al. Mutation Profiling of Key Cancer Genes in Primary Breast Cancers and Their Distant Metastases. Cancer Res. 2018, 78, 3112–3121. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.R.; Pant, D.K.; Shih, N.N.C.; Chen, Y.; Harvey, K.L.; Solomon, A.; Lieberman, D.; Morrissette, J.J.D.; Soucier-Ernst, D.; Goodman, N.G.; et al. Genomic Landscape of Metastatic Breast Cancer Identifies Preferentially Dysregulated Pathways and Targets. J. Clin. Investig. 2020, 130, 4252–4265. [Google Scholar] [CrossRef] [PubMed]
- Roy-Chowdhuri, S.; de Melo Gagliato, D.; Routbort, M.J.; Patel, K.P.; Singh, R.R.; Broaddus, R.; Lazar, A.J.; Sahin, A.; Alvarez, R.H.; Moulder, S.; et al. Multigene Clinical Mutational Profiling of Breast Carcinoma Using Next-Generation Sequencing. Am. J. Clin. Pathol. 2015, 144, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Kjällquist, U.; Erlandsson, R.; Tobin, N.P.; Alkodsi, A.; Ullah, I.; Stålhammar, G.; Karlsson, E.; Hatschek, T.; Hartman, J.; Linnarsson, S.; et al. Exome Sequencing of Primary Breast Cancers with Paired Metastatic Lesions Reveals Metastasis-Enriched Mutations in the A-Kinase Anchoring Protein Family (AKAPs). BMC Cancer 2018, 18, 174. [Google Scholar] [CrossRef]
- Rinaldi, J.; Sokol, E.S.; Hartmaier, R.J.; Trabucco, S.E.; Frampton, G.M.; Goldberg, M.E.; Albacker, L.A.; Daemen, A.; Manning, G. The Genomic Landscape of Metastatic Breast Cancer: Insights from 11,000 Tumors. PLoS ONE 2020, 15, e0231999. [Google Scholar] [CrossRef]
- Tian, C.; Liu, S.; Wang, Y.; Song, X. Prognosis and Genomic Landscape of Liver Metastasis in Patients With Breast Cancer. Front. Oncol. 2021, 11, 588136. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.S.P.; Haberberger, J.; McGregor, K.; Mata, D.A.; Decker, B.; Hiemenz, M.C.; Lechpammer, M.; Danziger, N.; Schiavone, K.; Creeden, J.; et al. Clinicopathologic and Genomic Landscape of Breast Carcinoma Brain Metastases. Oncology 2021, 26, 835–844. [Google Scholar] [CrossRef]
- Goswami, R.S.; Patel, K.P.; Singh, R.R.; Meric-Bernstam, F.; Kopetz, E.S.; Subbiah, V.; Alvarez, R.H.; Davies, M.A.; Jabbar, K.J.; Roy-Chowdhuri, S.; et al. Hotspot Mutation Panel Testing Reveals Clonal Evolution in a Study of 265 Paired Primary and Metastatic Tumors. Clin. Cancer Res. 2015, 21, 2644–2651. [Google Scholar] [CrossRef] [PubMed]
- van Geelen, C.T.; Savas, P.; Teo, Z.L.; Luen, S.J.; Weng, C.-F.; Ko, Y.-A.; Kuykhoven, K.S.; Caramia, F.; Salgado, R.; Francis, P.A.; et al. Clinical Implications of Prospective Genomic Profiling of Metastatic Breast Cancer Patients. Breast Cancer Res. 2020, 22, 91. [Google Scholar] [CrossRef] [PubMed]
- Richard, F.; Majjaj, S.; Venet, D.; Rothé, F.; Pingitore, J.; Boeckx, B.; Marchio, C.; Clatot, F.; Bertucci, F.; Mariani, O.; et al. Characterization of Stromal Tumor-Infiltrating Lymphocytes and Genomic Alterations in Metastatic Lobular Breast Cancer. Clin. Cancer Res. 2020, 26, 6254–6265. [Google Scholar] [CrossRef] [PubMed]
- Moelans, C.B.; van der Groep, P.; Hoefnagel, L.D.C.; van de Vijver, M.J.; Wesseling, P.; Wesseling, J.; van der Wall, E.; van Diest, P.J. Genomic Evolution from Primary Breast Carcinoma to Distant Metastasis: Few Copy Number Changes of Breast Cancer Related Genes. Cancer Lett. 2014, 344, 138–146. [Google Scholar] [CrossRef]
- Curigliano, G.; Bagnardi, V.; Bertolini, F.; Alcalay, M.; Locatelli, M.A.; Fumagalli, L.; Rabascio, C.; Calleri, A.; Adamoli, L.; Criscitiello, C.; et al. Antiangiogenic Therapy in Recurrent Breast Cancer with Lymphangitic Spread to the Chest Wall: A Randomized Phase II Trial of Bevacizumab with Sequential or Concurrent Oral Vinorelbine and Capecitabine. Breast 2015, 24, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Muller, K.E.; Marotti, J.D.; de Abreu, F.B.; Peterson, J.D.; Miller, T.W.; Chamberlin, M.D.; Tsongalis, G.J.; Tafe, L.J. Targeted Next-Generation Sequencing Detects a High Frequency of Potentially Actionable Mutations in Metastatic Breast Cancers. Exp. Mol. Pathol. 2016, 100, 421–425. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Pietenpol, J.A. Clinical Implications of Molecular Heterogeneity in Triple Negative Breast Cancer. Breast 2015, 24, S36–S40. [Google Scholar] [CrossRef]








| References | N | HR-Positive/HER2-Negative N (%) | HER2-Positive N (%) | TN N (%) | Unknown N (%) |
|---|---|---|---|---|---|
| Lefebvre et al. [20] | 28 | 17 (61) | 2 (7) | 7 (25) | 2 (7) |
| Yates et al. [21] | 19 | 9 (47) | 2 (10) | 5 (26) | 3 (16) |
| Kong et al. [22] | 125 | 53 (42.4) | 43 (34.4) | 29 (23.2) | |
| Luna et al. [23] | 26 | 7 (27) | 7 (27) | 10 (39) | 2 (7) |
| References | Total Paired Cases N | Paired Cases of Skin Metastases N | Additional Molecular Alterations in Skin Metastases Not Found in Primary Breast Tumors |
|---|---|---|---|
| Schrijver et al. [57] | 17 | 8 | 33 mutations (ATR, BRCA1, SMAD4, CDH1, ARID1A, ERBB2, IDH1, PIK3R1, RB1, and others) |
| Yates et al. [21] | Cohort 1: 7 | 2 | 4 molecular alterations (amplification of FGFR1/structural variant of TP53, indel of RB1/amplification of TERC) |
| Cohort 2: 51 | 4 | 9 mutations (JAK2, NF1, TP53, AKT1, and ARID1A) | |
| Paul et al. [58] | 28 | 1 | 54 mutations (PIK3CA, TP53, and others) |
| Location | Luminal N (%) | Luminal HER2-Positive N (%) | HER2-Positive N (%) | TN N (%) | ND N (%) |
|---|---|---|---|---|---|
| SKIN | 29 (50) | 2 (3.4) | 6 (10.3) | 15 (25.9) | 6 (10.3) |
| LIVER | 59 (68.6) | 4 (4.6) | 9 (10.5) | 11 (12.8) | 3 (3.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Martínez, S.; Pizarro, D.; Pérez-Mies, B.; Caniego-Casas, T.; Curigliano, G.; Cortés, J.; Palacios, J. Clinical, Pathological, and Molecular Features of Breast Carcinoma Cutaneous Metastasis. Cancers 2021, 13, 5416. https://doi.org/10.3390/cancers13215416
González-Martínez S, Pizarro D, Pérez-Mies B, Caniego-Casas T, Curigliano G, Cortés J, Palacios J. Clinical, Pathological, and Molecular Features of Breast Carcinoma Cutaneous Metastasis. Cancers. 2021; 13(21):5416. https://doi.org/10.3390/cancers13215416
Chicago/Turabian StyleGonzález-Martínez, Silvia, David Pizarro, Belén Pérez-Mies, Tamara Caniego-Casas, Giuseppe Curigliano, Javier Cortés, and José Palacios. 2021. "Clinical, Pathological, and Molecular Features of Breast Carcinoma Cutaneous Metastasis" Cancers 13, no. 21: 5416. https://doi.org/10.3390/cancers13215416
APA StyleGonzález-Martínez, S., Pizarro, D., Pérez-Mies, B., Caniego-Casas, T., Curigliano, G., Cortés, J., & Palacios, J. (2021). Clinical, Pathological, and Molecular Features of Breast Carcinoma Cutaneous Metastasis. Cancers, 13(21), 5416. https://doi.org/10.3390/cancers13215416







