Dietary Factors and Breast Cancer Prognosis among Breast Cancer Survivors: A Systematic Review and Meta-Analysis of Cohort Studies
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search and Selection Criteria
2.2. Data Extraction
2.3. Quality Assessment
2.4. Data Analysis
2.5. Statistical Analysis
3. Results
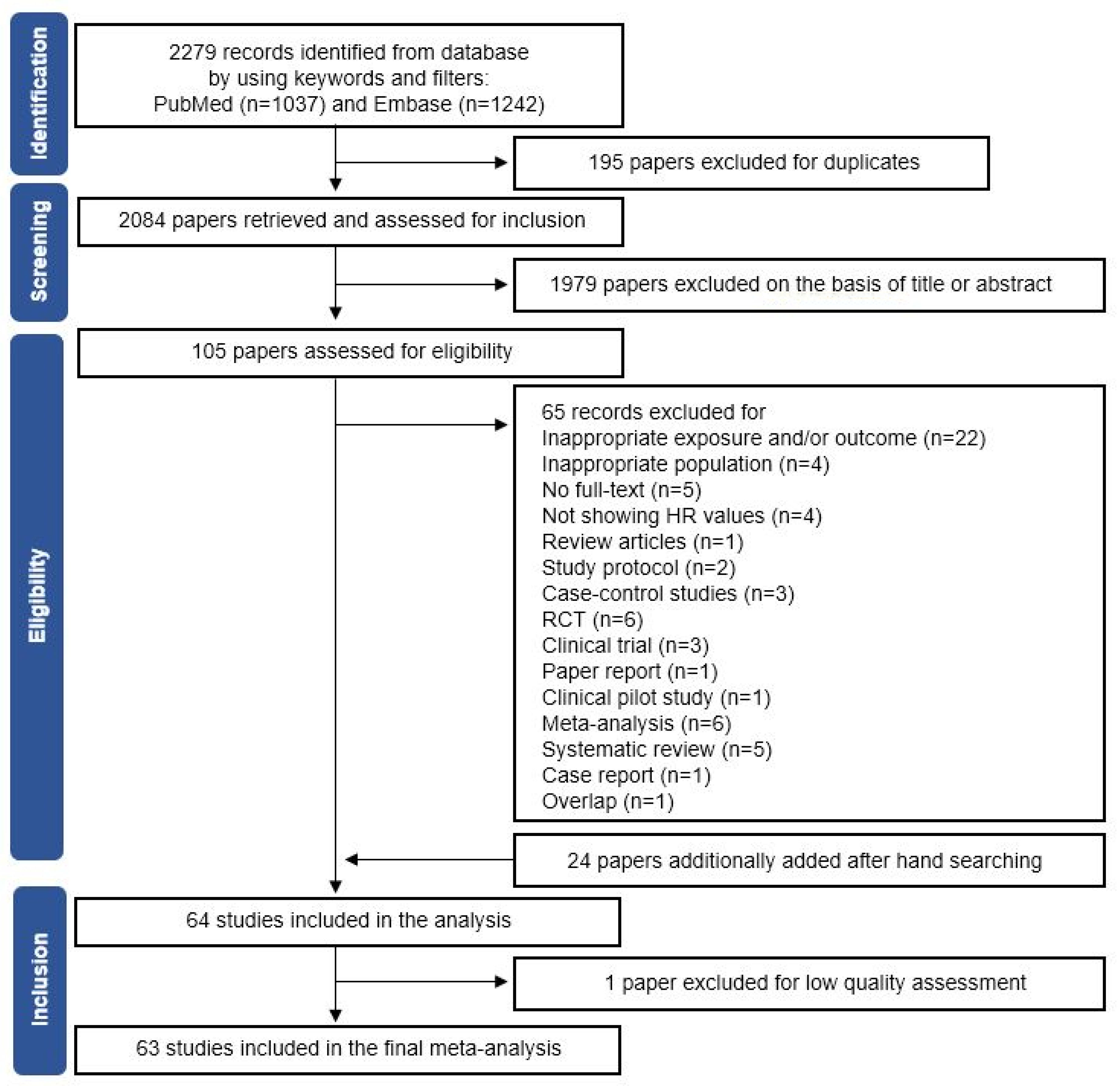
3.1. Selection of Eligible Studies and Quality Assessment
3.2. Study Characteristics
3.3. Main Analysis
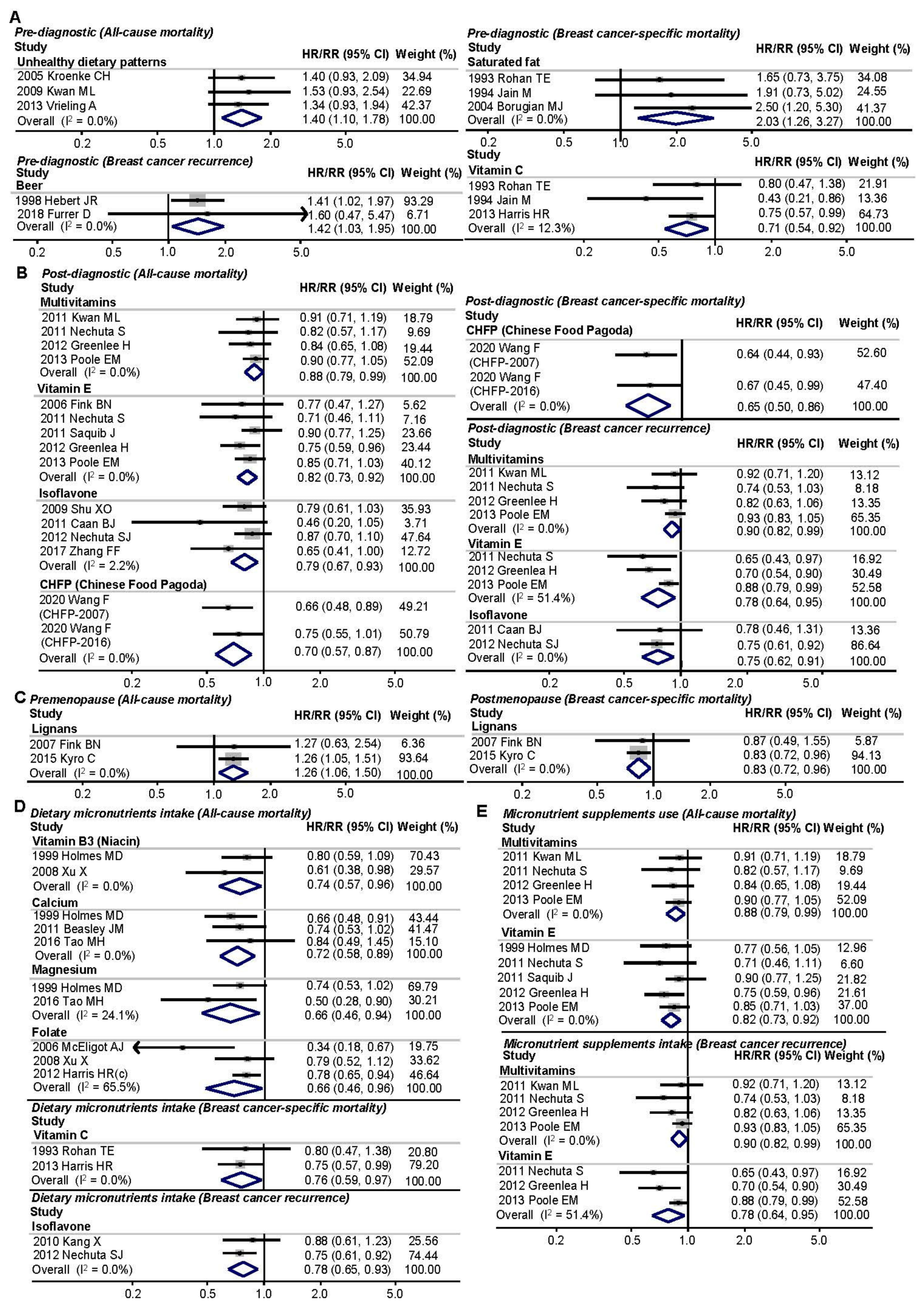
3.4. Subgroup Analyses
3.4.1. Different Intake Time Points of Foods/Nutrients
3.4.2. Menopausal Status
3.4.3. Dietary or Supplementary Micronutrient Intake
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. Continuous Update Project Expert Report 2018; WCRF International: London, UK, 2018. [Google Scholar]
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, Nutrition, Physical Activity and Breast Cancer Survivors; WCRF International: London, UK, 2018. [Google Scholar]
- Buck, K.; Zaineddin, A.K.; Vrieling, A.; Heinz, J.; Linseisen, J.; Flesch-Janys, D.; Chang-Claude, J. Estimated enterolignans, lignan-rich foods, and fibre in relation to survival after postmenopausal breast cancer. Br. J. Cancer 2011, 105, 1151–1157. [Google Scholar] [CrossRef]
- McEligot, A.J.; Largent, J.; Ziogas, A.; Peel, D.; Anton-Culver, H. Dietary fat, fiber, vegetable, and micronutrients are associated with overall survival in postmenopausal women diagnosed with breast cancer. Nutr. Cancer 2006, 55, 132–140. [Google Scholar] [CrossRef]
- Saxe, G.A.; Rock, C.L.; Wicha, M.S.; Schottenfeld, D. Diet and risk for breast cancer recurrence and survival. Breast Cancer Res. Treat. 1999, 53, 241–253. [Google Scholar] [CrossRef]
- Rohan, T.E.; Hiller, J.E.; McMichael, A.J. Dietary factors and survival from breast cancer. Nutr. Cancer 1993, 20, 167–177. [Google Scholar] [CrossRef]
- Holmes, M.D.; Stampfer, M.J.; Colditz, G.A.; Rosner, B.; Hunter, D.J.; Willett, W.C. Dietary factors and the survival of women with breast carcinoma. Cancer 1999, 86, 826–835. [Google Scholar] [CrossRef]
- Belle, F.N.; Kampman, E.; McTiernan, A.; Bernstein, L.; Baumgartner, K.; Baumgartner, R.; Ambs, A.; Ballard-Barbash, R.; Neuhouser, M.L. Dietary fiber, carbohydrates, glycemic index, and glycemic load in relation to breast cancer prognosis in the HEAL cohort. Cancer Epidemiol. Biomark. Prev. 2011, 20, 890–899. [Google Scholar] [CrossRef]
- Beasley, J.M.; Newcomb, P.A.; Trentham-Dietz, A.; Hampton, J.M.; Bersch, A.J.; Passarelli, M.N.; Holick, C.N.; Titus-Ernstoff, L.; Egan, K.M.; Holmes, M.D.; et al. Post-diagnosis dietary factors and survival after invasive breast cancer. Breast Cancer Res. Treat. 2011, 128, 229–236. [Google Scholar] [CrossRef]
- Caan, B.J.; Natarajan, L.; Parker, B.; Gold, E.B.; Thomson, C.; Newman, V.; Rock, C.L.; Pu, M.; Al-Delaimy, W.; Pierce, J.P. Soy food consumption and breast cancer prognosis. Cancer Epidemiol. Biomark. Prev. 2011, 20, 854–858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Kang, H.B.; Li, B.L.; Zhang, R.M. Positive effects of soy isoflavone food on survival of breast cancer patients in China. Asian Pac. J. Cancer Prev. 2012, 13, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.O.; Zheng, Y.; Cai, H.; Gu, K.; Chen, Z.; Zheng, W.; Lu, W. Soy food intake and breast cancer survival. JAMA 2009, 302, 2437–2443. [Google Scholar] [CrossRef]
- Nechuta, S.J.; Caan, B.J.; Chen, W.Y.; Lu, W.; Chen, Z.; Kwan, M.L.; Flatt, S.W.; Zheng, Y.; Zheng, W.; Pierce, J.P.; et al. Soy food intake after diagnosis of breast cancer and survival: An in-depth analysis of combined evidence from cohort studies of US and Chinese women. Am. J. Clin. Nutr. 2012, 96, 123–132. [Google Scholar] [CrossRef]
- Dal Maso, L.; Zucchetto, A.; Talamini, R.; Serraino, D.; Stocco, C.F.; Vercelli, M.; Falcini, F.; Franceschi, S.; Prospective Analysis of Case-Control Studies on Environmental Factors and Health (PACE) Study Group. Effect of obesity and other lifestyle factors on mortality in women with breast cancer. Int. J. Cancer 2008, 123, 2188–2194. [Google Scholar] [CrossRef]
- Irwin, M.L.; Smith, A.W.; McTiernan, A.; Ballard-Barbash, R.; Cronin, K.; Gilliland, F.D.; Baumgartner, R.N.; Baumgartner, K.B.; Bernstein, L. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: The health, eating, activity, and lifestyle study. J. Clin. Oncol. 2008, 26, 3958–3964. [Google Scholar] [CrossRef]
- West-Wright, C.N.; Henderson, K.D.; Sullivan-Halley, J.; Ursin, G.; Deapen, D.; Neuhausen, S.; Reynolds, P.; Chang, E.; Ma, H.; Bernstein, L. Long-term and recent recreational physical activity and survival after breast cancer: The California Teachers Study. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2851–2859. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.L.; McTiernan, A.; Manson, J.E.; Thomson, C.A.; Sternfeld, B.; Stefanick, M.L.; Wactawski-Wende, J.; Craft, L.; Lane, D.; Martin, L.W.; et al. Physical activity and survival in postmenopausal women with breast cancer: Results from the women’s health initiative. Cancer Prev. Res. 2011, 4, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, S.S.; Thygesen, L.C.; Tolstrup, J.S.; Gronbaek, M. Modifiable risk factors and survival in women diagnosed with primary breast cancer: Results from a prospective cohort study. Eur. J. Cancer Prev. 2010, 19, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Emaus, A.; Veierod, M.B.; Tretli, S.; Finstad, S.E.; Selmer, R.; Furberg, A.S.; Bernstein, L.; Schlichting, E.; Thune, I. Metabolic profile, physical activity, and mortality in breast cancer patients. Breast Cancer Res. Treat. 2010, 121, 651–660. [Google Scholar] [CrossRef]
- Cleveland, R.J.; Eng, S.M.; Stevens, J.; Bradshaw, P.T.; Teitelbaum, S.L.; Neugut, A.I.; Gammon, M.D. Influence of prediagnostic recreational physical activity on survival from breast cancer. Eur. J. Cancer Prev. 2012, 21, 46–54. [Google Scholar] [CrossRef]
- Friedenreich, C.M.; Gregory, J.; Kopciuk, K.A.; Mackey, J.R.; Courneya, K.S. Prospective cohort study of lifetime physical activity and breast cancer survival. Int. J. Cancer 2009, 124, 1954–1962. [Google Scholar] [CrossRef]
- Beasley, J.M.; Kwan, M.L.; Chen, W.Y.; Weltzien, E.K.; Kroenke, C.H.; Lu, W.; Nechuta, S.J.; Cadmus-Bertram, L.; Patterson, R.E.; Sternfeld, B.; et al. Meeting the physical activity guidelines and survival after breast cancer: Findings from the after breast cancer pooling project. Breast Cancer Res. Treat. 2012, 131, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, P.J.; Ennis, M.; Pritchard, K.I.; Koo, J.; Trudeau, M.E.; Hood, N. Diet and breast cancer: Evidence that extremes in diet are associated with poor survival. J. Clin. Oncol. 2003, 21, 2500–2507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Folsom, A.R.; Sellers, T.A.; Kushi, L.H.; Potter, J.D. Better breast cancer survival for postmenopausal women who are less overweight and eat less fat. The Iowa Women’s Health Study. Cancer 1995, 76, 275–283. [Google Scholar] [CrossRef]
- Grosso, G.; Godos, J.; Lamuela-Raventos, R.; Ray, S.; Micek, A.; Pajak, A.; Sciacca, S.; D’Orazio, N.; Del Rio, D.; Galvano, F. A comprehensive meta-analysis on dietary flavonoid and lignan intake and cancer risk: Level of evidence and limitations. Mol. Nutr. Food Res. 2017, 61, 1600930. [Google Scholar] [CrossRef]
- Martinez, M.E.; Marshall, J.R.; Giovannucci, E. Diet and cancer prevention: The roles of observation and experimentation. Nat. Rev. Cancer 2008, 8, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Jayedi, A.; Emadi, A.; Khan, T.A.; Abdolshahi, A.; Shab-Bidar, S. Dietary Fiber and Survival in Women with Breast Cancer: A Dose-Response Meta-Analysis of Prospective Cohort Studies. Nutr. Cancer 2020, 73, 1570–1580. [Google Scholar] [CrossRef]
- Qiu, S.; Jiang, C. Soy and isoflavones consumption and breast cancer survival and recurrence: A systematic review and meta-analysis. Eur. J. Nutr. 2019, 58, 3079–3090. [Google Scholar] [CrossRef]
- Nachvak, S.M.; Moradi, S.; Anjom-Shoae, J.; Rahmani, J.; Nasiri, M.; Maleki, V.; Sadeghi, O. Soy, Soy Isoflavones, and Protein Intake in Relation to Mortality from All Causes, Cancers, and Cardiovascular Diseases: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. J. Acad. Nutr. Diet 2019, 119, 1483–1500.e17. [Google Scholar] [CrossRef]
- Brennan, S.F.; Woodside, J.V.; Lunny, P.M.; Cardwell, C.R.; Cantwell, M.M. Dietary fat and breast cancer mortality: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2017, 57, 1999–2008. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2000. [Google Scholar]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Ewertz, M.; Gillanders, S.; Meyer, L.; Zedeler, K. Survival of breast cancer patients in relation to factors which affect the risk of developing breast cancer. Int. J. Cancer 1991, 49, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Miller, A.B.; To, T. Premorbid diet and the prognosis of women with breast cancer. J. Natl. Cancer Inst. 1994, 86, 1390–1397. [Google Scholar] [CrossRef]
- Hebert, J.R.; Hurley, T.G.; Ma, Y. The effect of dietary exposures on recurrence and mortality in early stage breast cancer. Breast Cancer Res. Treat. 1998, 51, 17–28. [Google Scholar] [CrossRef]
- Fleischauer, A.T.; Simonsen, N.; Arab, L. Antioxidant supplements and risk of breast cancer recurrence and breast cancer-related mortality among postmenopausal women. Nutr. Cancer 2003, 46, 15–22. [Google Scholar] [CrossRef]
- Borugian, M.J.; Sheps, S.B.; Kim-Sing, C.; Van Patten, C.; Potter, J.D.; Dunn, B.; Gallagher, R.P.; Hislop, T.G. Insulin, macronutrient intake, and physical activity: Are potential indicators of insulin resistance associated with mortality from breast cancer? Cancer Epidemiol. Biomark. Prev. 2004, 13, 1163–1172. [Google Scholar]
- Boyapati, S.M.; Shu, X.O.; Ruan, Z.X.; Dai, Q.; Cai, Q.; Gao, Y.T.; Zheng, W. Soyfood intake and breast cancer survival: A followup of the Shanghai Breast Cancer Study. Breast Cancer Res. Treat. 2005, 92, 11–17. [Google Scholar] [CrossRef]
- Kroenke, C.H.; Fung, T.T.; Hu, F.B.; Holmes, M.D. Dietary patterns and survival after breast cancer diagnosis. J. Clin. Oncol. 2005, 23, 9295–9303. [Google Scholar] [CrossRef]
- Cui, Y.; Shu, X.O.; Gao, Y.T.; Cai, H.; Tao, M.H.; Zheng, W. Association of ginseng use with survival and quality of life among breast cancer patients. Am. J. Epidemiol. 2006, 163, 645–653. [Google Scholar] [CrossRef]
- Fink, B.N.; Gaudet, M.M.; Britton, J.A.; Abrahamson, P.E.; Teitelbaum, S.L.; Jacobson, J.; Bell, P.; Thomas, J.A.; Kabat, G.C.; Neugut, A.I.; et al. Fruits, vegetables, and micronutrient intake in relation to breast cancer survival. Breast Cancer Res. Treat. 2006, 98, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Fink, B.N.; Steck, S.E.; Wolff, M.S.; Britton, J.A.; Kabat, G.C.; Gaudet, M.M.; Abrahamson, P.E.; Bell, P.; Schroeder, J.C.; Teitelbaum, S.L.; et al. Dietary flavonoid intake and breast cancer survival among women on Long Island. Cancer Epidemiol. Biomark. Prev. 2007, 16, 2285–2292. [Google Scholar] [CrossRef]
- Xu, X.; Gammon, M.D.; Wetmur, J.G.; Bradshaw, P.T.; Teitelbaum, S.L.; Neugut, A.I.; Santella, R.M.; Chen, J. B-vitamin intake, one-carbon metabolism, and survival in a population-based study of women with breast cancer. Cancer Epidemiol. Biomark. Prev. 2008, 17, 2109–2116. [Google Scholar] [CrossRef]
- Guha, N.; Kwan, M.L.; Quesenberry, C.P., Jr.; Weltzien, E.K.; Castillo, A.L.; Caan, B.J. Soy isoflavones and risk of cancer recurrence in a cohort of breast cancer survivors: The Life After Cancer Epidemiology study. Breast Cancer Res. Treat. 2009, 118, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Kwan, M.L.; Weltzien, E.; Kushi, L.H.; Castillo, A.; Slattery, M.L.; Caan, B.J. Dietary patterns and breast cancer recurrence and survival among women with early-stage breast cancer. J. Clin. Oncol. 2009, 27, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Zhang, Q.; Wang, S.; Huang, X.; Jin, S. Effect of soy isoflavones on breast cancer recurrence and death for patients receiving adjuvant endocrine therapy. CMAJ 2010, 182, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
- Kwan, M.L.; Kushi, L.H.; Weltzien, E.; Tam, E.K.; Castillo, A.; Sweeney, C.; Caan, B.J. Alcohol consumption and breast cancer recurrence and survival among women with early-stage breast cancer: The life after cancer epidemiology study. J. Clin. Oncol. 2010, 28, 4410–4416. [Google Scholar] [CrossRef] [PubMed]
- George, S.M.; Irwin, M.L.; Smith, A.W.; Neuhouser, M.L.; Reedy, J.; McTiernan, A.; Alfano, C.M.; Bernstein, L.; Ulrich, C.M.; Baumgartner, K.B.; et al. Postdiagnosis diet quality, the combination of diet quality and recreational physical activity, and prognosis after early-stage breast cancer. Cancer Causes Control 2011, 22, 589–598. [Google Scholar] [CrossRef]
- Kim, E.H.; Willett, W.C.; Fung, T.; Rosner, B.; Holmes, M.D. Diet quality indices and postmenopausal breast cancer survival. Nutr. Cancer 2011, 63, 381–388. [Google Scholar] [CrossRef]
- Kwan, M.L.; Greenlee, H.; Lee, V.S.; Castillo, A.; Gunderson, E.P.; Habel, L.A.; Kushi, L.H.; Sweeney, C.; Tam, E.K.; Caan, B.J. Multivitamin use and breast cancer outcomes in women with early-stage breast cancer: The Life After Cancer Epidemiology study. Breast Cancer Res. Treat. 2011, 130, 195–205. [Google Scholar] [CrossRef]
- Nechuta, S.; Lu, W.; Chen, Z.; Zheng, Y.; Gu, K.; Cai, H.; Zheng, W.; Shu, X.O. Vitamin supplement use during breast cancer treatment and survival: A prospective cohort study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 262–271. [Google Scholar] [CrossRef]
- Saquib, J.; Rock, C.L.; Natarajan, L.; Saquib, N.; Newman, V.A.; Patterson, R.E.; Thomson, C.A.; Al-Delaimy, W.K.; Pierce, J.P. Dietary intake, supplement use, and survival among women diagnosed with early-stage breast cancer. Nutr. Cancer 2011, 63, 327–333. [Google Scholar] [CrossRef]
- Greenlee, H.; Kwan, M.L.; Kushi, L.H.; Song, J.; Castillo, A.; Weltzien, E.; Quesenberry, C.P., Jr.; Caan, B.J. Antioxidant supplement use after breast cancer diagnosis and mortality in the Life After Cancer Epidemiology (LACE) cohort. Cancer 2012, 118, 2048–2058. [Google Scholar] [CrossRef]
- Harris, H.R.; Bergkvist, L.; Wolk, A. Coffee and black tea consumption and breast cancer mortality in a cohort of Swedish women. Br. J. Cancer 2012, 107, 874–878. [Google Scholar] [CrossRef]
- Harris, H.R.; Bergkvist, L.; Wolk, A. Alcohol intake and mortality among women with invasive breast cancer. Br. J. Cancer 2012, 106, 592–595. [Google Scholar] [CrossRef]
- Harris, H.R.; Bergkvist, L.; Wolk, A. Folate intake and breast cancer mortality in a cohort of Swedish women. Breast Cancer Res. Treat. 2012, 132, 243–250. [Google Scholar] [CrossRef]
- Vrieling, A.; Buck, K.; Heinz, J.; Obi, N.; Benner, A.; Flesch-Janys, D.; Chang-Claude, J. Pre-diagnostic alcohol consumption and postmenopausal breast cancer survival: A prospective patient cohort study. Breast Cancer Res. Treat. 2012, 136, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Conroy, S.M.; Maskarinec, G.; Park, S.Y.; Wilkens, L.R.; Henderson, B.E.; Kolonel, L.N. The effects of soy consumption before diagnosis on breast cancer survival: The Multiethnic Cohort Study. Nutr. Cancer 2013, 65, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.R.; Bergkvist, L.; Wolk, A. Vitamin C intake and breast cancer mortality in a cohort of Swedish women. Br. J. Cancer 2013, 109, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Holm, M.; Olsen, A.; Christensen, J.; Kroman, N.T.; Bidstrup, P.E.; Johansen, C.; Overvad, K.; Tjonneland, A. Pre-diagnostic alcohol consumption and breast cancer recurrence and mortality: Results from a prospective cohort with a wide range of variation in alcohol intake. Int. J. Cancer 2013, 132, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Inoue-Choi, M.; Robien, K.; Lazovich, D. Adherence to the WCRF/AICR guidelines for cancer prevention is associated with lower mortality among older female cancer survivors. Cancer Epidemiol. Biomark. Prev. 2013, 22, 792–802. [Google Scholar] [CrossRef]
- Izano, M.A.; Fung, T.T.; Chiuve, S.S.; Hu, F.B.; Holmes, M.D. Are diet quality scores after breast cancer diagnosis associated with improved breast cancer survival? Nutr. Cancer 2013, 65, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, C.H.; Kwan, M.L.; Sweeney, C.; Castillo, A.; Caan, B.J. High- and low-fat dairy intake, recurrence, and mortality after breast cancer diagnosis. J. Natl. Cancer Inst. 2013, 105, 616–623. [Google Scholar] [CrossRef]
- Kwan, M.L.; Chen, W.Y.; Flatt, S.W.; Weltzien, E.K.; Nechuta, S.J.; Poole, E.M.; Holmes, M.D.; Patterson, R.E.; Shu, X.O.; Pierce, J.P.; et al. Postdiagnosis alcohol consumption and breast cancer prognosis in the after breast cancer pooling project. Cancer Epidemiol. Biomark. Prev. 2013, 22, 32–41. [Google Scholar] [CrossRef]
- Newcomb, P.A.; Kampman, E.; Trentham-Dietz, A.; Egan, K.M.; Titus, L.J.; Baron, J.A.; Hampton, J.M.; Passarelli, M.N.; Willett, W.C. Alcohol consumption before and after breast cancer diagnosis: Associations with survival from breast cancer, cardiovascular disease, and other causes. J. Clin. Oncol. 2013, 31, 1939–1946. [Google Scholar] [CrossRef]
- Poole, E.M.; Shu, X.; Caan, B.J.; Flatt, S.W.; Holmes, M.D.; Lu, W.; Kwan, M.L.; Nechuta, S.J.; Pierce, J.P.; Chen, W.Y. Postdiagnosis supplement use and breast cancer prognosis in the After Breast Cancer Pooling Project. Breast Cancer Res. Treat. 2013, 139, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Vrieling, A.; Buck, K.; Seibold, P.; Heinz, J.; Obi, N.; Flesch-Janys, D.; Chang-Claude, J. Dietary patterns and survival in German postmenopausal breast cancer survivors. Br. J. Cancer 2013, 108, 188–192. [Google Scholar] [CrossRef] [PubMed]
- George, S.M.; Ballard-Barbash, R.; Shikany, J.M.; Caan, B.J.; Freudenheim, J.L.; Kroenke, C.H.; Vitolins, M.Z.; Beresford, S.A.; Neuhouser, M.L. Better postdiagnosis diet quality is associated with reduced risk of death among postmenopausal women with invasive breast cancer in the women’s health initiative. Cancer Epidemiol. Biomark. Prev. 2014, 23, 575–583. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bao, P.P.; Zhao, G.M.; Shu, X.O.; Peng, P.; Cai, H.; Lu, W.; Zheng, Y. Modifiable Lifestyle Factors and Triple-negative Breast Cancer Survival: A Population-based Prospective Study. Epidemiology 2015, 26, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Jeffreys, M.; Redaniel, M.T.; Martin, R.M. The effect of pre-diagnostic vitamin D supplementation on cancer survival in women: A cohort study within the UK Clinical Practice Research Datalink. BMC Cancer 2015, 15, 670. [Google Scholar] [CrossRef] [PubMed]
- Kyro, C.; Zamora-Ros, R.; Scalbert, A.; Tjonneland, A.; Dossus, L.; Johansen, C.; Bidstrup, P.E.; Weiderpass, E.; Christensen, J.; Ward, H.; et al. Pre-diagnostic polyphenol intake and breast cancer survival: The European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Breast Cancer Res. Treat. 2015, 154, 389–401. [Google Scholar] [CrossRef]
- McCullough, M.L.; Gapstur, S.M.; Shah, R.; Campbell, P.T.; Wang, Y.; Doyle, C.; Gaudet, M.M. Pre- and postdiagnostic diet in relation to mortality among breast cancer survivors in the CPS-II Nutrition Cohort. Cancer Causes Control 2016, 27, 1303–1314. [Google Scholar] [CrossRef] [PubMed]
- Neuhouser, M.L.; Smith, A.W.; George, S.M.; Gibson, J.T.; Baumgartner, K.B.; Baumgartner, R.; Duggan, C.; Bernstein, L.; McTiernan, A.; Ballard, R. Use of complementary and alternative medicine and breast cancer survival in the Health, Eating, Activity, and Lifestyle Study. Breast Cancer Res. Treat. 2016, 160, 539–546. [Google Scholar] [CrossRef]
- Tao, M.H.; Dai, Q.; Millen, A.E.; Nie, J.; Edge, S.B.; Trevisan, M.; Shields, P.G.; Freudenheim, J.L. Associations of intakes of magnesium and calcium and survival among women with breast cancer: Results from Western New York Exposures and Breast Cancer (WEB) Study. Am. J. Cancer Res. 2016, 6, 105–113. [Google Scholar] [PubMed]
- Holmes, M.D.; Wang, J.; Hankinson, S.E.; Tamimi, R.M.; Chen, W.Y. Protein Intake and Breast Cancer Survival in the Nurses’ Health Study. J. Clin. Oncol. 2017, 35, 325–333. [Google Scholar] [CrossRef]
- Zeinomar, N.; Thai, A.; Cloud, A.J.; McDonald, J.A.; Liao, Y.; Terry, M.B. Alcohol consumption and breast cancer-specific and all-cause mortality in women diagnosed with breast cancer at the New York site of the Breast Cancer Family Registry. PLoS ONE 2017, 12, e0189118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.F.; Haslam, D.E.; Terry, M.B.; Knight, J.A.; Andrulis, I.L.; Daly, M.B.; Buys, S.S.; John, E.M. Dietary isoflavone intake and all-cause mortality in breast cancer survivors: The Breast Cancer Family Registry. Cancer 2017, 123, 2070–2079. [Google Scholar] [CrossRef]
- Zucchetto, A.; Serraino, D.; Shivappa, N.; Hebert, J.R.; Stocco, C.; Puppo, A.; Falcini, F.; Panato, C.; Dal Maso, L.; Polesel, J. Dietary inflammatory index before diagnosis and survival in an Italian cohort of women with breast cancer. Br. J. Nutr. 2017, 117, 1456–1462. [Google Scholar] [CrossRef]
- Furrer, D.; Jacob, S.; Michaud, A.; Provencher, L.; Lemieux, J.; Diorio, C. Association of Tobacco Use, Alcohol Consumption and HER2 Polymorphisms With Response to Trastuzumab in HER2-Positive Breast Cancer Patients. Clin. Breast Cancer 2018, 18, e687–e694. [Google Scholar] [CrossRef]
- Madden, J.M.; Murphy, L.; Zgaga, L.; Bennett, K. De novo vitamin D supplement use post-diagnosis is associated with breast cancer survival. Breast Cancer Res. Treat. 2018, 172, 179–190. [Google Scholar] [CrossRef]
- Minami, Y.; Kanemura, S.; Kawai, M.; Nishino, Y.; Tada, H.; Miyashita, M.; Ishida, T.; Kakugawa, Y. Alcohol consumption and survival after breast cancer diagnosis in Japanese women: A prospective patient cohort study. PLoS ONE 2019, 14, e0224797. [Google Scholar] [CrossRef]
- Andersen, J.L.M.; Hansen, L.; Thomsen, B.L.R.; Christiansen, L.R.; Dragsted, L.O.; Olsen, A. Pre- and post-diagnostic intake of whole grain and dairy products and breast cancer prognosis: The Danish Diet, Cancer and Health cohort. Breast Cancer Res. Treat. 2020, 179, 743–753. [Google Scholar] [CrossRef]
- Madden, J.M.; Leacy, F.P.; Zgaga, L.; Bennett, K. Fitting Marginal Structural and G-Estimation Models Under Complex Treatment Patterns: Investigating the Association Between De Novo Vitamin D Supplement Use After Breast Cancer Diagnosis and All-Cause Mortality Using Linked Pharmacy Claim and Registry Data. Am. J. Epidemiol. 2020, 189, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cai, H.; Gu, K.; Shi, L.; Yu, D.; Zhang, M.; Zheng, W.; Zheng, Y.; Bao, P.; Shu, X.O. Adherence to Dietary Recommendations among Long-Term Breast Cancer Survivors and Cancer Outcome Associations. Cancer Epidemiol. Biomark. Prev. 2020, 29, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Sun, J.Z.; Wu, Q.X.; Li, Z.Y.; Li, D.X.; Xiong, Y.F.; Zhong, G.C.; Shi, Y.; Li, Q.; Zheng, J.; et al. Long-term anti-inflammatory diet in relation to improved breast cancer prognosis: A prospective cohort study. NPJ Breast Cancer 2020, 6, 36. [Google Scholar] [CrossRef]
- Wu, T.; Hsu, F.C.; Pierce, J.P. Increased Acid-Producing Diet and Past Smoking Intensity Are Associated with Worse Prognoses Among Breast Cancer Survivors: A Prospective Cohort Study. J. Clin. Med. 2020, 9, 1817. [Google Scholar] [CrossRef]
- Dong, J.Y.; Qin, L.Q. Soy isoflavones consumption and risk of breast cancer incidence or recurrence: A meta-analysis of prospective studies. Breast Cancer Res. Treat. 2011, 125, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Chi, F.; Wu, R.; Zeng, Y.C.; Xing, R.; Liu, Y.; Xu, Z.G. Post-diagnosis soy food intake and breast cancer survival: A meta-analysis of cohort studies. Asian Pac. J. Cancer Prev. 2013, 14, 2407–2412. [Google Scholar] [CrossRef]
- Taylor, C.K.; Levy, R.M.; Elliott, J.C.; Burnett, B.P. The effect of genistein aglycone on cancer and cancer risk: A review of in vitro, preclinical, and clinical studies. Nutr. Rev. 2009, 67, 398–415. [Google Scholar] [CrossRef] [PubMed]
- Helferich, W.G.; Andrade, J.E.; Hoagland, M.S. Phytoestrogens and breast cancer: A complex story. Inflammopharmacology 2008, 16, 219–226. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Santell, R.C.; Haslam, S.Z.; Helferich, W.G. Estrogenic effects of genistein on the growth of estrogen receptor-positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Res. 1998, 58, 3833–3838. [Google Scholar] [PubMed]
- Hwang, C.S.; Kwak, H.S.; Lim, H.J.; Lee, S.H.; Kang, Y.S.; Choe, T.B.; Hur, H.G.; Han, K.O. Isoflavone metabolites and their in vitro dual functions: They can act as an estrogenic agonist or antagonist depending on the estrogen concentration. J. Steroid Biochem. Mol. Biol. 2006, 101, 246–253. [Google Scholar] [CrossRef]
- Anjom-Shoae, J.; Sadeghi, O.; Larijani, B.; Esmaillzadeh, A. Dietary intake and serum levels of trans fatty acids and risk of breast cancer: A systematic review and dose-response meta-analysis of prospective studies. Clin. Nutr. 2020, 39, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Hou, L.; Wang, W. Dietary total fat and fatty acids intake, serum fatty acids and risk of breast cancer: A meta-analysis of prospective cohort studies. Int. J. Cancer 2016, 138, 1894–1904. [Google Scholar] [CrossRef]
- Wu, A.H.; Pike, M.C.; Stram, D.O. Meta-analysis: Dietary fat intake, serum estrogen levels, and the risk of breast cancer. J. Natl. Cancer Inst. 1999, 91, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Lof, M.; Sandin, S.; Lagiou, P.; Hilakivi-Clarke, L.; Trichopoulos, D.; Adami, H.O.; Weiderpass, E. Dietary fat and breast cancer risk in the Swedish women’s lifestyle and health cohort. Br. J. Cancer 2007, 97, 1570–1576. [Google Scholar] [CrossRef]
- Le Guevel, R.; Pakdel, F. Assessment of oestrogenic potency of chemicals used as growth promoter by in-vitro methods. Hum. Reprod. 2001, 16, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Sohn, K.H.; Rhee, S.H.; Hwang, D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J. Biol. Chem. 2001, 276, 16683–16689. [Google Scholar] [CrossRef]
- Sun, Q.; Xie, W.; Wang, Y.; Chong, F.; Song, M.; Li, T.; Xu, L.; Song, C. Alcohol Consumption by Beverage Type and Risk of Breast Cancer: A Dose-Response Meta-Analysis of Prospective Cohort Studies. Alcohol. Alcohol. 2020, 55, 246–253. [Google Scholar] [CrossRef]
- Chen, J.Y.; Zhu, H.C.; Guo, Q.; Shu, Z.; Bao, X.H.; Sun, F.; Qin, Q.; Yang, X.; Zhang, C.; Cheng, H.Y.; et al. Dose-Dependent Associations between Wine Drinking and Breast Cancer Risk—Meta-Analysis Findings. Asian Pac. J. Cancer Prev. 2016, 17, 1221–1233. [Google Scholar] [CrossRef]
- Ma, H.; Malone, K.E.; McDonald, J.A.; Marchbanks, P.A.; Ursin, G.; Strom, B.L.; Simon, M.S.; Sullivan-Halley, J.; Bernstein, L.; Lu, Y. Pre-diagnosis alcohol consumption and mortality risk among black women and white women with invasive breast cancer. BMC Cancer 2019, 19, 800. [Google Scholar] [CrossRef]
- Wong, A.W.; Paulson, Q.X.; Hong, J.; Stubbins, R.E.; Poh, K.; Schrader, E.; Nunez, N.P. Alcohol promotes breast cancer cell invasion by regulating the Nm23-ITGA5 pathway. J. Exp. Clin. Cancer Res. 2011, 30, 75. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, Y.; Zhong, S. Alcohol Intake and Abnormal Expression of Brf1 in Breast Cancer. Oxid. Med. Cell Longev. 2019, 2019, 4818106. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, M.; Ke, Z.J.; Luo, J. Cellular and molecular mechanisms underlying alcohol-induced aggressiveness of breast cancer. Pharmacol. Res. 2017, 115, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, K.; Chen, G.-C.; Zhang, R.; Du, X.; Zou, S.-Y.; Shi, B.-M.; Qin, L.-Q. Calcium intake and breast cancer risk: Meta-analysis of prospective cohort studies. Br. J. Nutr. 2016, 116, 158–166. [Google Scholar] [CrossRef]
- Chen, P.; Hu, P.; Xie, D.; Qin, Y.; Wang, F.; Wang, H. Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res. Treat. 2010, 121, 469–477. [Google Scholar]
- Lipkin, M.; Newmark, H.L. Vitamin D, calcium and prevention of breast cancer: A review. J. Am. Coll. Nutr. 1999, 18, 392S–397S. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.H.; Holmes, M.D.; Hankinson, S.E.; Wu, K.; Colditz, G.A.; Willett, W.C. Intake of dairy products, calcium, and vitamin d and risk of breast cancer. J. Natl. Cancer Inst. 2002, 94, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, Y.; Wang, L.; Zhang, C.X. Folate intake and breast cancer prognosis: A meta-analysis of prospective observational studies. Eur. J. Cancer Prev. 2015, 24, 113–121. [Google Scholar] [CrossRef]
- Kim, Y.I. Does a high folate intake increase the risk of breast cancer? Nutr. Rev. 2006, 64, 468–475. [Google Scholar] [CrossRef]
- Stolzenberg-Solomon, R.Z.; Chang, S.C.; Leitzmann, M.F.; Johnson, K.A.; Johnson, C.; Buys, S.S.; Hoover, R.N.; Ziegler, R.G. Folate intake, alcohol use, and postmenopausal breast cancer risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am. J. Clin. Nutr. 2006, 83, 895–904. [Google Scholar] [CrossRef]
- Velentzis, L.S.; Cantwell, M.M.; Cardwell, C.; Keshtgar, M.R.; Leathem, A.J.; Woodside, J.V. Lignans and breast cancer risk in pre- and post-menopausal women: Meta-analyses of observational studies. Br. J. Cancer 2009, 100, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Touillaud, M.S.; Thiebaut, A.C.; Fournier, A.; Niravong, M.; Boutron-Ruault, M.C.; Clavel-Chapelon, F. Dietary lignan intake and postmenopausal breast cancer risk by estrogen and progesterone receptor status. J. Natl. Cancer Inst. 2007, 99, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Buck, K.; Zaineddin, A.K.; Vrieling, A.; Linseisen, J.; Chang-Claude, J. Meta-analyses of lignans and enterolignans in relation to breast cancer risk. Am. J. Clin. Nutr. 2010, 92, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Adlercreutz, H. Lignans and human health. Crit. Rev. Clin. Lab. Sci. 2007, 44, 483–525. [Google Scholar] [CrossRef]
- Sonestedt, E.; Wirfalt, E. Enterolactone and breast cancer: Methodological issues may contribute to conflicting results in observational studies. Nutr. Res. 2010, 30, 667–677. [Google Scholar] [CrossRef]
- Liu, Z.; Fei, Y.J.; Cao, X.H.; Xu, D.; Tang, W.J.; Yang, K.; Xu, W.X.; Tang, J.H. Lignans intake and enterolactone concentration and prognosis of breast cancer: A systematic review and meta-analysis. J. Cancer 2021, 12, 2787–2796. [Google Scholar] [CrossRef]
- Rock, C.L.; Flatt, S.W.; Thomson, C.A.; Stefanick, M.L.; Newman, V.A.; Jones, L.A.; Natarajan, L.; Ritenbaugh, C.; Hollenbach, K.A.; Pierce, J.P.; et al. Effects of a high-fiber, low-fat diet intervention on serum concentrations of reproductive steroid hormones in women with a history of breast cancer. J. Clin. Oncol. 2004, 22, 2379–2387. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.A.; Zhao, Z.; Zang, E.A.; Wynn, T.T.; Simi, B.; Rivenson, A. Wheat bran and psyllium diets: Effects on N-methylnitrosourea-induced mammary tumorigenesis in F344 rats. J. Natl. Cancer Inst. 1996, 88, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Nangia-Makker, P.; Hogan, V.; Honjo, Y.; Baccarini, S.; Tait, L.; Bresalier, R.; Raz, A. Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. J. Natl. Cancer Inst. 2002, 94, 1854–1862. [Google Scholar] [CrossRef]
- Park, Y.; Brinton, L.A.; Subar, A.F.; Hollenbeck, A.; Schatzkin, A. Dietary fiber intake and risk of breast cancer in postmenopausal women: The National Institutes of Health-AARP Diet and Health Study. Am. J. Clin. Nutr. 2009, 90, 664–671. [Google Scholar] [CrossRef]
- Monroe, K.R.; Murphy, S.P.; Henderson, B.E.; Kolonel, L.N.; Stanczyk, F.Z.; Adlercreutz, H.; Pike, M.C. Dietary fiber intake and endogenous serum hormone levels in naturally postmenopausal Mexican American women: The Multiethnic Cohort Study. Nutr. Cancer 2007, 58, 127–135. [Google Scholar] [CrossRef]
- King, D.E.; Egan, B.M.; Woolson, R.F.; Mainous, A.G., 3rd; Al-Solaiman, Y.; Jesri, A. Effect of a high-fiber diet vs a fiber-supplemented diet on C-reactive protein level. Arch. Intern. Med. 2007, 167, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Al-Lahham, S.; Roelofsen, H.; Rezaee, F.; Weening, D.; Hoek, A.; Vonk, R.; Venema, K. Propionic acid affects immune status and metabolism in adipose tissue from overweight subjects. Eur. J. Clin. Investig. 2012, 42, 357–364. [Google Scholar] [CrossRef]
- Clausen, M.R.; Bonnen, H.; Mortensen, P.B. Colonic fermentation of dietary fibre to short chain fatty acids in patients with adenomatous polyps and colonic cancer. Gut 1991, 32, 923–928. [Google Scholar] [CrossRef]
- Lawenda, B.D.; Kelly, K.M.; Ladas, E.J.; Sagar, S.M.; Vickers, A.; Blumberg, J.B. Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy? J. Natl. Cancer Inst. 2008, 100, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Norman, H.A.; Butrum, R.R.; Feldman, E.; Heber, D.; Nixon, D.; Picciano, M.F.; Rivlin, R.; Simopoulos, A.; Wargovich, M.J.; Weisburger, E.K.; et al. The role of dietary supplements during cancer therapy. J. Nutr. 2003, 133, 3794S–3799S. [Google Scholar] [CrossRef]
- Ambrosone, C.B.; Zirpoli, G.R.; Hutson, A.D.; McCann, W.E.; McCann, S.E.; Barlow, W.E.; Kelly, K.M.; Cannioto, R.; Sucheston-Campbell, L.E.; Hershman, D.L.; et al. Dietary Supplement Use During Chemotherapy and Survival Outcomes of Patients With Breast Cancer Enrolled in a Cooperative Group Clinical Trial (SWOG S0221). J. Clin. Oncol. 2020, 38, 804–814. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, X.; Xiang, Y.B.; Yang, G.; Li, H.; Gao, Y.T.; Zheng, W.; Shu, X.O. Adherence to dietary guidelines and mortality: A report from prospective cohort studies of 134,000 Chinese adults in urban Shanghai. Am. J. Clin. Nutr. 2014, 100, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Lay, S.; Yu, H.N.; Shen, S.R. Dietary Guidelines for Chinese Residents (2016): Comments and comparisons. J. Zhejiang Univ. Sci. B 2016, 17, 649–656. [Google Scholar] [CrossRef]
- Han, M.A.; Zeraatkar, D.; Guyatt, G.H.; Vernooij, R.W.M.; El Dib, R.; Zhang, Y.; Algarni, A.; Leung, G.; Storman, D.; Valli, C.; et al. Reduction of Red and Processed Meat Intake and Cancer Mortality and Incidence: A Systematic Review and Meta-analysis of Cohort Studies. Ann. Intern. Med. 2019, 171, 711–720. [Google Scholar] [CrossRef]
- Xiao, Y.; Xia, J.; Li, L.; Ke, Y.; Cheng, J.; Xie, Y.; Chu, W.; Cheung, P.; Kim, J.H.; Colditz, G.A.; et al. Associations between dietary patterns and the risk of breast cancer: A systematic review and meta-analysis of observational studies. Breast Cancer Res. 2019, 21, 16. [Google Scholar] [CrossRef]
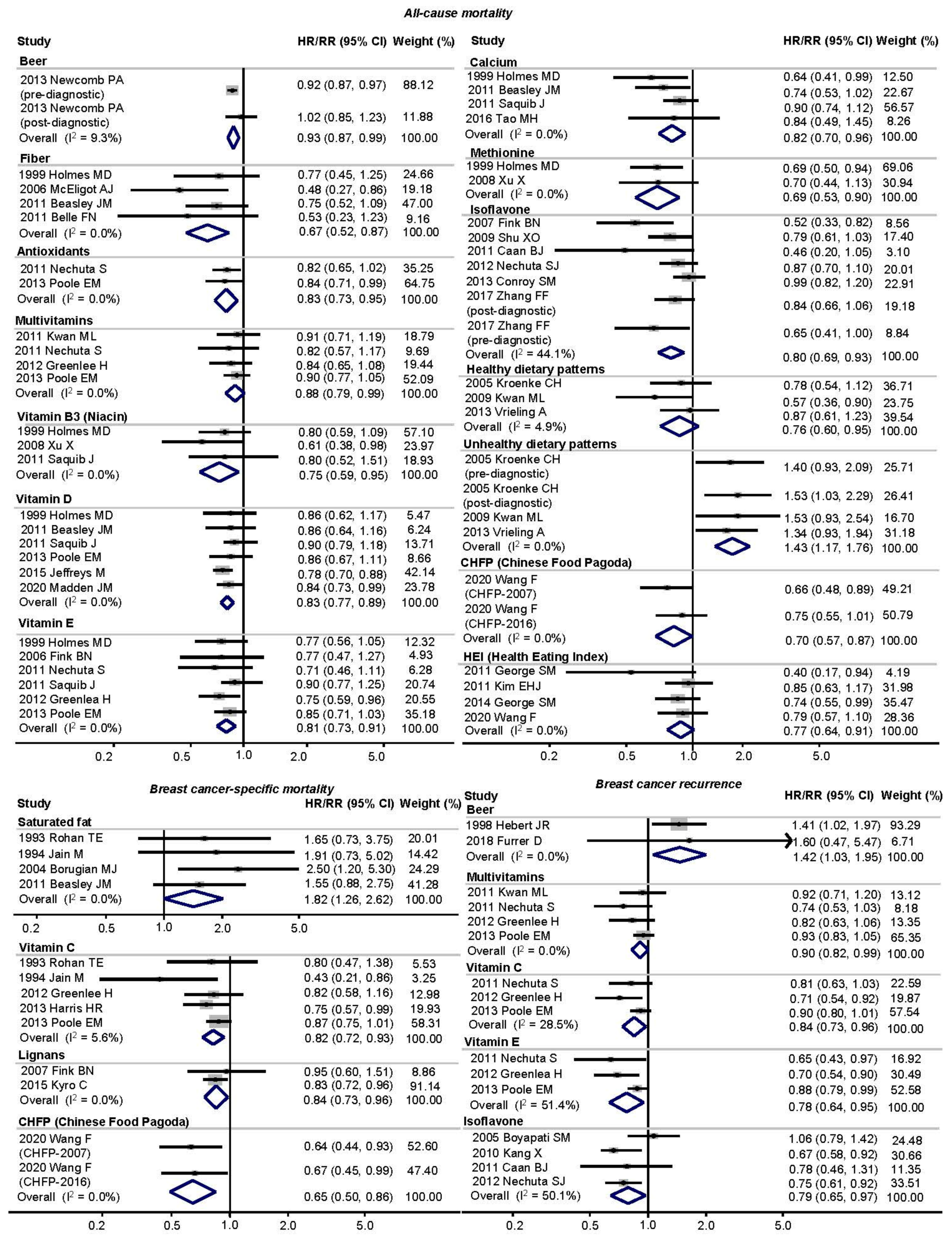
| All-Cause Mortality | Breast-Cancer-Specific Mortality | Breast Cancer Recurrence | ||||
|---|---|---|---|---|---|---|
| Overall | N (I2) | RR/HR (95% CI) | N (I2) | RR/HR (95% CI) | N (I2) | RR/HR (95% CI) |
| Single food items | ||||||
| Alcohol | 10 (46.6%) | 0.92 (0.83 to 1.02) | 13 (55.9%) | 1.12 (0.96 to 1.31) | 4 (26.2%) | 1.20 (0.99 to 1.44) |
| Beer | 2 (9.3%) | 0.93 (0.87 to 0.99) | 3 (67.5%) | 1.14 (0.84 to 1.56) | 2 (0.0%) | 1.42 (1.03 to 1.95) |
| Wine | 3 (0.0%) | 0.88 (0.84 to 0.93) | 3 (5.9%) | 1.00 (0.90 to 1.10) | 2 (84.5%) | 0.80 (0.26 to 2.45) |
| Spirits | 2 (0.0%) | 0.90 (0.86 to 0.95) | 2 (35.8%) | 0.88 (0.74 to 1.05) | ||
| Coffee | 1 (NA) | 1.12 (0.84 to 1.51) | 1 (NA) | 1.14 (0.71 to 1.83) | ||
| Tea | 2 (38.4%) | 0.82 (0.54 to 1.26) | 1 (NA) | 1.02 (0.67 to 1.55) | ||
| 1 (NA) | 0.60 (0.29 to 1.27) | |||||
| Whole grain products | 5 (0.0%) | 1.01 (0.96 to 1.06) | 4 (0.0%) | 1.04 (0.98 to 1.10) | 4 (14.1%) | 1.01 (0.94 to 1.08) |
| Grains | 2 (0.0%) | 1.08 (0.94 to 1.25) | 2 (0.0%) | 1.13 (0.88 to 1.44) | ||
| Oatmeal/muesli | 2 (0.0%) | 0.78 (0.63 to 0.97) | 2 (0.0%) | 0.92 (0.72 to 1.18) | 2 (0.0%) | 0.91 (0.71 to 1.16) |
| Rye bread | 2 (0.0%) | 1.13 (1.02 to 1.25) | 2 (43.0%) | 1.19 (1.00 to 1.42) | 2 (38.4%) | 1.11 (0.92 to 1.34) |
| Any fruits, fruit juices, and vegetables | 1 (NA) | 0.68 (0.42 to 1.09) | ||||
| Fruits and vegetables | 3 (72.8%) | 0.88 (0.60 to 1.30) | 3 (0.7%) | 1.09 (0.81 to 1.47) | 1 (NA) | 0.58 (0.25 to 1.36) |
| Fruits | 3 (62.0%) | 1.00 (0.67 to 1.48) | 1 (NA) | 1.39 (0.64 to 2.99) | ||
| Fruits and fruit juices | 1 (NA) | 0.87 (0.57 to 1.35) | ||||
| Citrus fruits | 1 (NA) | 0.93 (0.61 to 1.42) | ||||
| Vegetables | 4 (60.4%) | 0.98 (0.68 to 1.40) | 1 (NA) | 0.96 (0.38 to 2.45) | ||
| Cruciferous vegetables | 2 (0.0%) | 1.03 (0.83 to 1.28) | 1 (NA) | 0.95 (0.59 to 1.54) | ||
| Leafy vegetables | 1 (NA) | 0.72 (0.41 to 1.24) | ||||
| Yellow vegetables | 1 (NA) | 0.90 (0.58 to 1.40) | ||||
| Dairy products | 5 (40.6%) | 1.04 (0.95 to 1.13) | 4 (0.0%) | 0.99 (0.93 to 1.05) | 3 (0.0%) | 0.98 (0.91 to 1.04) |
| High-fat dairy products | 1 (NA) | 1.09 (0.88 to 1.35) | ||||
| Low-fat dairy products | 1 (NA) | 0.84 (0.69 to 1.04) | ||||
| Butter/margarine/lard | 1 (NA) | 1.16 (0.86 to 1.58) | 1 (NA) | 1.30 (1.03 to 1.64) | ||
| Cheese | 2 (0.0%) | 1.16 (1.06 to 1.27) | 2 (0.0%) | 1.11 (0.92 to 1.35) | 2 (0.0%) | 1.18 (0.98 to 1.43) |
| Milk | 2 (0.0%) | 1.02 (0.96 to 1.08) | 2 (0.0%) | 1.00 (0.93 to 1.07) | 2 (0.0%) | 0.95 (0.88 to 1.02) |
| Yogurt | 2 (0.0%) | 0.91 (0.79 to 1.05) | 2 (0.0%) | 0.85 (0.71 to 1.03) | 2 (0.0%) | 0.99 (0.83 to 1.18) |
| Soy products | 1 (NA) | 1.03 (0.81 to 1.33) | 1 (NA) | 1.03 (0.71 to 1.50) | ||
| Fish | 1 (NA) | 0.94 (0.62 to 1.43) | 1 (NA) | 0.93 (0.76 to 1.15) | ||
| Poultry | 1 (NA) | 0.60 (0.39 to 0.92) | 1 (NA) | 0.85 (0.69 to 1.05) | ||
| Red and processed meat | 4 (66.8%) | 0.89 (0.71 to 1.13) | 4 (0.0%) | 1.05 (0.83 to 1.31) | 2 (0.0%) | 1.04 (0.85 to 1.28) |
| Natural products | 1 (NA) | 0.95 (0.67 to 1.35) | 1 (NA) | 1.15 (0.69 to 1.94) | ||
| Ginseng | 1 (NA) | 0.71 (0.52 to 0.98) | 1 (NA) | 0.70 (0.53 to 0.93) | ||
| Macronutrients | ||||||
| Carbohydrates | 2 (0.0%) | 0.97 (0.73 to 1.29) | 4 (0.0%) | 1.01 (0.72 to 1.43) | 1 (NA) | 0.77 (0.27 to 2.19) |
| E-Carb | 1 (NA) | 1.70 (0.70 to 3.80) | ||||
| Fat | 3 (83.1%) | 1.52 (0.85 to 2.74) | 4 (0.0%) | 1.02 (0.83 to 1.25) | ||
| Trans fat | 1 (NA) | 1.78 (1.35 to 2.32) | 1 (NA) | 1.42 (0.80 to 2.52) | ||
| Saturated fat | 2 (89.3%) | 2.40 (0.78 to 7.38) | 4 (0.0%) | 1.82 (1.26 to 2.62) | ||
| Saturated fat/total fat | 1 (NA) | 1.93 (1.00 to 3.74) | ||||
| Monounsaturated fat | 1 (NA) | 1.14 (0.86 to 1.52) | 2 (0.0%) | 1.01 (0.62 to 1.65) | ||
| Polyunsaturated fat | 1 (NA) | 0.91 (0.70 to 1.19) | 2 (0.0%) | 1.19 (0.66 to 2.12) | ||
| Omega-3 fatty acids | 1 (NA) | 1.00 (0.62 to 1.60) | ||||
| Linoleic fatty acids | 1 (NA) | 2.39 (1.21 to 4.69) | ||||
| Oleic fatty acids | 1 (NA) | 3.56 (1.67 to 7.59) | ||||
| 18:2 trans fatty acid | 1 (NA) | 1.58 (1.03 to 2.43) | ||||
| Protein | 2 (38.5%) | 0.86 (0.62 to 1.19) | 3 (63.8%) | 0.72 (0.37 to 1.39) | 1 (NA) | 0.84 (0.69 to 1.03) |
| Soy protein | 1 (NA) | 0.71 (0.54 to 0.92) | 1 (NA) | 0.99 (0.73 to 1.33) | ||
| 1 (NA) | 0.68 (0.54 to 0.87) | |||||
| Animal protein | 1 (NA) | 0.74 (0.61 to 0.91) | ||||
| Vegetable protein | 1 (NA) | 1.29 (1.05 to 1.59) | ||||
| Essential amino acids | 1 (NA) | 0.86 (0.71 to 1.05) | ||||
| Branched-chain amino acids | 1 (NA) | 0.82 (0.68 to 1.00) | ||||
| Pantothenic acid | 1 (NA) | 0.92 (0.67 to 1.29) | ||||
| Tryptophan | 1 (NA) | 0.63 (0.46 to 0.87) | ||||
| Fiber | 4 (0.0%) | 0.67 (0.52 to 0.87) | 4 (0.0%) | 0.75 (0.54 to 1.04) | 1 (NA) | 0.68 (0.27 to 1.70) |
| Micronutrients | ||||||
| Multivitamins | 4 (0.0%) | 0.88 (0.79 to 0.99) | 3 (0.0%) | 0.89 (0.79 to 1.02) | 4 (0.0%) | 0.90 (0.82 to 0.99) |
| Antioxidants | 2 (0.0%) | 0.83 (0.73 to 0.95) | 1 (NA) | 0.86 (0.73 to 1.02) | 2 (57.3%) | 0.87 (0.73 to 1.04) |
| 1 (NA) | 0.54 (0.27 to 1.04) | |||||
| Vitamin A | 3 (36.4%) | 0.97 (0.80 to 1.18) | 3 (35.0%) | 0.91 (0.64 to 1.30) | 1 (NA) | 1.07 (0.72 to 1.60) |
| Retinol | 2 (36.9%) | 0.96 (0.76 to 1.21) | 1 (NA) | 1.05 (0.56 to 1.95) | ||
| Vitamin B | 1 (NA) | 0.87 (0.74 to 1.02) | 1 (NA) | 0.90 (0.74 to 1.10) | 1 (NA) | 0.88 (0.75 to 1.03) |
| Thiamin | 2 (58.0%) | 0.68 (0.45 to 1.02) | 2 (0.0%) | 0.48 (0.30 to 0.78) | ||
| Riboflavin | 2 (0.0%) | 0.85 (0.65 to 1.11) | 1 (NA) | 0.72 (0.41 to 1.29) | ||
| Niacin | 3 (0.0%) | 0.75 (0.59 to 0.95) | 1 (NA) | 0.61 (0.34 to 1.09) | ||
| Vitamin B6 | 3 (0.0%) | 0.91 (0.74 to 1.11) | 1 (NA) | 0.77 (0.44 to 1.36) | ||
| Vitamin B12 | 1 (NA) | 1.20 (0.80 to 1.81) | 1 (NA) | 1.10 (0.65 to 1.85) | ||
| Vitamin C | 8 (57.1%) | 0.88 (0.76 to 1.01) | 5 (5.6%) | 0.82 (0.72 to 0.93) | 3 (28.5%) | 0.84 (0.73 to 0.96) |
| 1 (NA) | 0.64 (0.32 to 1.27) | |||||
| Vitamin D | 6 (0.0%) | 0.83 (0.77 to 0.89) | 3 (0.0%) | 0.85 (0.72 to 1.01) | 1 (NA) | 0.82 (0.60 to 1.12) |
| Vitamin E | 6 (0.0%) | 0.81 (0.73 to 0.91) | 3 (0.0%) | 0.84 (0.70 to 1.00) | 3 (51.4%) | 0.78 (0.64 to 0.95) |
| 1 (NA) | 0.55 (0.28 to 1.08) | |||||
| Carotenoids | 1 (NA) | 1.63 (1.06 to 2.50) | 1 (NA) | 1.93 (1.14 to 3.28) | 1 (NA) | 1.23 (0.76 to 1.96) |
| Carotenes | 1 (NA) | 0.96 (0.70 to 1.31) | ||||
| Alpha-Carotene | 4 (0.0%) | 1.01 (0.85 to 1.21) | 1 (NA) | 0.98 (0.59 to 1.64) | ||
| Beta-Carotene | 5 (51.7%) | 0.96 (0.74 to 1.24) | 4 (33.2%) | 0.92 (0.63 to 1.36) | 1 (NA) | 0.89 (0.50 to 1.60) |
| Provitamin A | 1 (NA) | 0.58 (0.34 to 0.99) | ||||
| Beta-Cryptoxanthin | 4 (61.2%) | 0.88 (0.64 to 1.20) | 1 (NA) | 0.81 (0.45 to 1.45) | ||
| Lutein/zeaxanthin | 4 (54.4%) | 0.77 (0.56 to 1.07) | 1 (NA) | 1.16 (0.62 to 2.19) | ||
| Lycopene | 5 (0.0%) | 0.91 (0.76 to 1.09) | 2 (0.0%) | 1.51 (0.90 to 2.55) | 1 (NA) | 1.17 (0.35 to 3.89) |
| Selenium | 3 (0.0%) | 0.88 (0.68 to 1.14) | 1 (NA) | 0.90 (0.45 to 1.79) | 1 (NA) | 0.89 (0.53 to 1.49) |
| Betaine | 1 (NA) | 0.81 (0.54 to 1.20) | 1 (NA) | 0.72 (0.44 to 1.17) | ||
| Caffeine | 1 (NA) | 0.77 (0.55 to 1.07) | ||||
| Calcium | 4 (0.0%) | 0.82 (0.70 to 0.96) | 3 (31.4%) | 0.77 (0.48 to 1.24) | ||
| Magnesium | 3 (50.1%) | 0.76 (0.53 to 1.07) | 1 (NA) | 0.70 (0.33 to 1.49) | ||
| Iron | 2 (70.7%) | 1.12 (0.61 to 2.03) | ||||
| Iodine | 1 (NA) | 0.90 (0.67 to 1.21) | ||||
| Potassium | 1 (NA) | 0.98 (0.69 to 1.38) | ||||
| Sodium | 1 (NA) | 0.79 (0.57 to 1.09) | ||||
| Zinc | 3 (24.8%) | 0.92 (0.73 to 1.15) | 1 (NA) | 0.82 (0.44 to 1.53) | 1 (NA) | 0.79 (0.49 to 1.28) |
| Methionine | 2 (0.0%) | 0.69 (0.53 to 0.90) | 1 (NA) | 0.70 (0.39 to 1.28) | ||
| Flavonoids | 2 (0.0%) | 1.02 (0.95 to 1.09) | 2 (0.0%) | 1.03 (0.93 to 1.14) | ||
| Flavan-3-ols | 1 (NA) | 1.01 (0.70 to 1.46) | 1 (NA) | 0.89 (0.55 to 1.43) | ||
| Flavones | 1 (NA) | 0.63 (0.41 to 0.96) | 1 (NA) | 0.48 (0.27 to 0.84) | ||
| Flavonols | 1 (NA) | 1.12 (0.78 to 1.62) | 1 (NA) | 1.20 (0.77 to 1.87) | ||
| Flavonones | 1 (NA) | 1.03 (0.72 to 1.48) | 1 (NA) | 0.98 (0.62 to 1.56) | ||
| Phenolic acids | 1 (NA) | 0.97 (0.91 to 1.05) | 1 (NA) | 0.99 (0.89 to 1.10) | ||
| Stilebenes | 1 (NA) | 0.97 (0.95 to 1.00) | 1 (NA) | 0.97 (0.94 to 1.00) | ||
| Anthocyanidins | 1 (NA) | 1.42 (1.01 to 2.00) | 1 (NA) | 0.68 (0.41 to 1.13) | ||
| Folate | 5 (71.5%) | 0.84 (0.65 to 1.07) | 2 (68.0%) | 0.96 (0.61 to 1.50) | ||
| Isoflavones | 7 (44.1%) | 0.80 (0.69 to 0.93) | 4 (0.0%) | 0.89 (0.75 to 1.05) | 4 (50.1%) | 0.79 (0.65 to 0.97) |
| 1 (NA) | 0.77 (0.60 to 0.98) | |||||
| Daidzein | 1 (NA) | 0.96 (0.52 to 1.76) | ||||
| Genistein | 1 (NA) | 0.95 (0.52 to 1.75) | ||||
| Glycetin | 1 (NA) | 0.80 (0.42 to 1.50) | ||||
| Lignans | 2 (5.1%) | 0.96 (0.86 to 1.06) | 2 (0.0%) | 0.84 (0.73 to 0.96) | ||
| Dietary patterns | ||||||
| Healthy diet pattern | 3 (4.90%) | 0.76 (0.60 to 0.95) | 3 (0.0%) | 0.92 (0.70 to 1.22) | 2 (0.30%) | 0.82 (0.61 to 1.09) |
| Unhealthy diet pattern | 4 (0.0%) | 1.43 (1.17 to 1.76) | 4 (0.0%) | 1.03 (0.79 to 1.33) | 2 (0.0%) | 0.94 (0.70 to 1.27) |
| Dietary scores/indexes | ||||||
| ACS guidelines | 2 (0.0%) | 0.98 (0.85 to 1.12) | 2 (0.0%) | 1.17 (0.88 to 1.54) | ||
| CHFP | 2 (0.0%) | 0.70 (0.57 to 0.87) | 2 (0.0%) | 0.65 (0.50 to 0.86) | ||
| DII | 2 (55.5%) | 1.14 (0.86 to 1.52) | 2 (50.5%) | 1.13 (0.76 to 1.67) | ||
| HEI | 4 (0.0%) | 0.77 (0.64 to 0.91) | 5 (53.3%) | 1.01 (0.74 to 1.38) | ||
| DASH | 1 (NA) | 0.66 (0.49 to 0.91) | 2 (40.8%) | 0.73 (0.52 to 1.02) | ||
| aMED | 1 (NA) | 0.87 (0.64 to 1.17) | 1 (NA) | 1.15 (0.74 to 1.77) | ||
| DQIR | 1 (NA) | 0.78 (0.58 to 1.07) | 1 (NA) | 0.81 (0.53 to 1.24) | ||
| Glycemic index | 1 (NA) | 1.40 (0.78 to 2.50) | 1 (NA) | 1.60 (0.80 to 3.21) | 1 (NA) | 1.56 (0.77 to 3.31) |
| Glycemic load | 1 (NA) | 1.23 (0.46 to 3.31) | 1 (NA) | 1.11 (0.37 to 3.34) | 1.14 (0.38 to 3.44) | |
| NEAP | 1 (NA) | 1.54 (1.04 to 2.29) | 1 (NA) | 1.52 (1.01 to 2.32) | 1 (NA) | 1.15 (0.88 to 1.50) |
| PRAL | 1 (NA) | 1.30 (0.87 to 1.94) | 1 (NA) | 1.27 (0.83 to 1.94) | 1 (NA) | 1.09 (0.83 to 1.43) |
| RFS | 1 (NA) | 1.03 (0.74 to 1.42) | 1 (NA) | 1.54 (0.95 to 2.47) | ||
| WCRF/AICR adherence score | 1 (NA) | 0.61 (0.39 to 0.96) | 1 (NA) | 0.88 (0.41 to 1.91) | ||
| All-cause mortality | Breast-cancer-specific mortality | Breast cancer recurrence | ||||
| Prediagnostic | N (I2) | RR/HR (95% CI) | N (I2) | RR/HR (95% CI) | N (I2) | RR/HR (95% CI) |
| Single food items | ||||||
| Alcohol | 6 (56.5%) | 0.98 (0.82 to 1.17) | 9 (60.1%) | 1.17 (0.95 to 1.43) | 2 (44.8%) | 1.30 (0.86 to 1.97) |
| Beer | 1 (NA) | 0.92 (0.87 to 0.97) | 2 (74.2%) | 1.14 (0.70 to 1.87) | 2 (0.0%) | 1.42 (1.03 to 1.95) |
| Wine | 1 (NA) | 0.88 (0.84 to 0.93) | 1 (NA) | 0.98 (0.91 to 1.06) | 1 (NA) | 0.42 (0.18 to 0.95) |
| Spirits | 1 (NA) | 0.91 (0.87 to 0.96) | 1 (NA) | 0.92 (0.85 to 1.00) | ||
| Tea | 1 (NA) | 0.94 (0.72 to 1.23) | 1 (NA) | 1.02 (0.67 to 1.55) | ||
| Coffee | 1 (NA) | 1.12 (0.84 to 1.51) | 1 (NA) | 1.14 (0.71 to 1.83) | ||
| Grains | 1 (NA) | 1.08 (0.91 to 1.29) | 1 (NA) | 1.07 (0.79 to 1.46) | ||
| Whole grain products | 2 (0.0%) | 1.02 (0.96 to 1.08) | 2 (0.0%) | 1.05 (0.98 to 1.12) | 2 (0.0%) | 1.03 (0.97 to 1.11) |
| Oatmeal/muesli | 1 (NA) | 0.80 (0.61 to 1.05) | 1 (NA) | 0.99 (0.73 to 1.35) | 1 (NA) | 1.03 (0.83 to 1.29) |
| Rye bread | 1 (NA) | 1.10 (0.98 to 1.25) | 1 (NA) | 1.11 (0.96 to 1.29) | 1 (NA) | 1.04 (0.89 to 1.20) |
| Dairy products | 2 (29.6%) | 0.96 (0.75 to 1.25) | 1 (NA) | 0.98 (0.91 to 1.06) | 1 (NA) | 0.98 (0.91 to 1.06) |
| High-fat dairy products | 1 (NA) | 1.09 (0.88 to 1.35) | ||||
| Low-fat dairy products | 1 (NA) | 0.84 (0.69 to 1.04) | ||||
| Milk | 1 (NA) | 1.03 (0.96 to 1.10) | 1 (NA) | 0.99 (0.91 to 1.08) | 1 (NA) | 0.96 (0.89 to 1.05) |
| Cheese | 1 (NA) | 1.16 (0.97 to 1.17) | 1 (NA) | 1.18 (0.94 to 1.47) | 1 (NA) | 1.17 (0.94 to 1.45) |
| Butter/margarine/lard | 1 (NA) | 1.16 (0.86 to 1.58) | 1 (NA) | 1.30 (1.03 to 1.64) | ||
| Yogurt | 1 (NA) | 0.90 (0.75 to 1.07) | 1 (NA) | 0.86 (0.69 to 1.08) | 1 (NA) | 1.02 (0.83 to 1.27) |
| Soy products | 1 (NA) | 1.03 (0.81 to 1.33) | 1 (NA) | 1.03 (0.71 to 1.50) | ||
| Fruits and vegetables | 1 (NA) | 1.06 (0.85 to 1.33) | 1 (NA) | 1.00 (0.66 to 1.50) | ||
| Vegetables | 1 (NA) | 0.98 (0.62 to 1.53) | ||||
| Fish | 1 (NA) | 0.94 (0.62 to 1.43) | 1 (NA) | 0.93 (0.76 to 1.15) | ||
| Poultry | 1 (NA) | 0.60 (0.39 to 0.92) | 1 (NA) | 0.85 (0.69 to 1.05) | ||
| Red and processed meat | 1 (NA) | 0.88 (0.73 to 1.06) | 2 (0.0%) | 1.16 (0.87 to 1.54) | 2 (0.0%) | 1.04 (0.85 to 1.28) |
| Ginseng | 1 (NA) | 0.71 (0.52 to 0.98) | 1 (NA) | 0.70 (0.53 to 0.93) | ||
| Macronutrients | ||||||
| Carbohydrates | 2 (0.0%) | 1.15 (0.71 to 1.87) | ||||
| E-Carb | 1 (NA) | 1.70 (0.70 to 3.80) | ||||
| Fat | 1 (NA) | 1.21 (0.78 to 1.90) | 3 (25.1%) | 1.12 (0.80 to 1.59) | ||
| Saturated fat | 3 (0.0%) | 2.03 (1.26 to 3.27) | ||||
| Saturated fat/total fat | 1 (NA) | 1.93 (1.00 to 3.74) | ||||
| Monounsaturated fat | 1 (NA) | 1.33 (0.56 to 3.13) | ||||
| Polyunsaturated fat | 1 (NA) | 0.90 (0.52 to 1.55) | ||||
| 18:2 trans fatty acid | 1 (NA) | 1.58 (1.03 to 2.43) | ||||
| Omega-3 fatty acids | 1 (NA) | 1.00 (0.62 to 1.60) | ||||
| Protein | 1 (NA) | 0.70 (0.46 to 1.08) | 2 (23.7%) | 0.53 (0.29 to 0.96) | 1 (NA) | 0.84 (0.69 to 1.03) |
| Soy protein | 1 (NA) | 0.99 (0.73 to 1.33) | ||||
| Essential amino acids | 1 (NA) | 0.86 (0.71 to 1.05) | ||||
| Animal protein | 1 (NA) | 0.74 (0.61 to 0.91) | ||||
| Vegetable protein | 1 (NA) | 1.29 (1.05 to 1.59) | ||||
| Branched-chain amino acids | 1 (NA) | 0.82 (0.68 to 1.00) | ||||
| Fiber | 1 (NA) | 0.77 (0.45 to 1.25) | 1 (NA) | 0.70 (0.40 to 1.30) | ||
| Micronutrients | ||||||
| Vitamin A | 1 (NA) | 0.56 (0.28 to 1.09) | ||||
| Retinol | 1 (NA) | 1.05 (0.56 to 1.95) | ||||
| Vitamin B | 1 (NA) | 0.87 (0.74 to 1.02) | ||||
| Thiamin | 1 (NA) | 0.54 (0.38 to 0.88) | 2 (0.0%) | 0.48 (0.30 to 0.78) | ||
| Riboflavin | 1 (NA) | 0.92 (0.58 to 1.44) | 1 (NA) | 0.72 (0.41 to 1.29) | ||
| Niacin | 1 (NA) | 0.61 (0.38 to 0.98) | 1 (NA) | 0.61 (0.34 to 1.09) | ||
| Vitamin B6 | 1 (NA) | 0.95 (0.61 to 1.48) | 1 (NA) | 0.77 (0.44 to 1.36) | ||
| Vitamin B12 | 1 (NA) | 1.20 (0.80 to 1.81) | 1 (NA) | 1.10 (0.65 to 1.85) | ||
| Vitamin C | 1 (NA) | 0.84 (0.71 to 1.00) | 3 (12.3%) | 0.71 (0.54 to 0.92) | ||
| Vitamin D | 1 (NA) | 0.78 (0.70 to 0.88) | ||||
| Vitamin E | 1 (NA) | 0.55 (0.26 to 1.17) | ||||
| Beta-Carotene | 2 (52.2%) | 0.70 (0.36 to 1.38) | ||||
| Provitamin A | 1 (NA) | 0.58 (0.34 to 0.99) | ||||
| Lutein/zeaxanthin | 1 (NA) | 0.85 (0.53 to 1.38) | ||||
| Calcium | 2 (0.0%) | 0.71 (0.51 to 1.00) | 2 (43.5%) | 0.92 (0.47 to 1.79) | ||
| Magnesium | 1 (NA) | 0.50 (0.28 to 0.90) | 1 (NA) | 0.70 (0.33 to 1.49) | ||
| Betaine | 1 (NA) | 0.81 (0.54 to 1.20) | 1 (NA) | 0.72 (0.44 to 1.17) | ||
| Methionine | 1 (NA) | 0.70 (0.44 to 1.13) | 1 (NA) | 0.70 (0.39 to 1.28) | ||
| Flavonoids | 2 (0.0%) | 1.02 (0.95 to 1.09) | 2 (0.0%) | 1.03 (0.93 to 1.14) | ||
| Flavan-3-ols | 1 (NA) | 1.01 (0.70 to 1.46) | 1 (NA) | 0.89 (0.55 to 1.43) | ||
| Flavonols | 1 (NA) | 1.12 (0.78 to 1.62) | 1 (NA) | 1.20 (0.77 to 1.87) | ||
| Flavonones | 1 (NA) | 1.03 (0.72 to 1.48) | 1 (NA) | 0.98 (0.62 to 1.56) | ||
| Flavones | 1 (NA) | 0.63 (0.41 to 0.96) | 1 (NA) | 0.48 (0.27 to 0.84) | ||
| Phenolic acids | 1 (NA) | 0.99 (0.89 to 1.10) | ||||
| Stilebenes | 1 (NA) | 0.97 (0.94 to 1.00) | ||||
| Anthocyanidins | 1 (NA) | 1.42 (1.01 to 2.00) | 1 (NA) | 0.68 (0.41 to 1.13) | ||
| Folate | 2 (18.3%) | 0.83 (0.68 to 1.00) | 2 (68.0%) | 0.96 (0.61 to 1.50) | ||
| Isoflavone | 3 (70.4%) | 0.81 (0.60 to 1.08) | 3 (0.0%) | 0.93 (0.74 to 1.17) | 2 (82.8%) | 0.83 (0.53 to 1.31) |
| Daidzein | 1 (NA) | 0.96 (0.52 to 1.76) | ||||
| Genistein | 1 (NA) | 0.95 (0.52 to 1.75) | ||||
| Glycetin | 1 (NA) | 0.80 (0.42 to 1.50) | ||||
| Lignans | 2 (0.0%) | 0.84 (0.73 to 0.96) | ||||
| Dietary patterns | ||||||
| Healthy diet pattern | 2 (51.5%) | 0.72 (0.48 to 1.09) | 2 (0.0%) | 0.86 (0.61 to 1.20) | 2 (0.30%) | 0.82 (0.61 to 1.09) |
| Unhealthy diet pattern | 3 (0.0%) | 1.40 (1.10 to 1.78) | 3 (0.0%) | 1.04 (0.77 to 1.40) | 2 (0.0%) | 0.94 (0.70 to 1.27) |
| Dietary scores/indexes | ||||||
| ACS guidelines | 1 (NA) | 1.00 (0.84 to 1.18) | 1 (NA) | 1.06 (0.79 to 1.42) | ||
| DII | 1 (NA) | 1.00 (0.78 to 1.28) | 1 (NA) | 0.97 (0.73 to 1.27) | ||
| All-cause mortality | Breast-cancer-specific mortality | Breast cancer recurrence | ||||
| Postdiagnostic | N (I2) | RR/HR (95% CI) | N (I2) | RR/HR (95% CI) | N (I2) | RR/HR (95% CI) |
| Single food items | ||||||
| Alcohol | 4 (43.3%) | 0.88 (0.75 to 1.03) | 4 (57.9%) | 1.04 (0.77 to 1.41) | 2 (46.2%) | 1.16 (0.90 to 1.49) |
| Beer | 1 (NA) | 1.02 (0.85 to 1.23) | 1 (NA) | 1.26 (0.88 to 1.79) | ||
| Wine | 2 (0.0%) | 0.93 (0.80 to 1.09) | 2 (15.3%) | 1.14 (0.84 to 1.54) | 1 (NA) | 1.33 (0.97 to 1.81) |
| Spirits | 1 (NA) | 0.84 (0.70 to 1.00) | 1 (NA) | 0.74 (0.53 to 1.03) | ||
| Tea | 1 (NA) | 0.58 (0.29 to 1.16) | 1 (NA) | 0.60 (0.29 to 1.27) | ||
| Grains | 1 (NA) | 1.09 (0.86 to 1.38) | 1 (NA) | 1.24 (0.81 to 1.88) | ||
| Whole grain products | 2 (0.0%) | 0.97 (0.89 to 1.07) | 2 (0.0%) | 1.02 (0.91 to 1.13) | 2 (26.2%) | 0.92 (0.78 to 1.08) |
| Oatmeal/muesli | 1 (NA) | 0.75 (0.53 to 1.07) | 1 (NA) | 0.82 (0.55 to 1.22) | 1 (NA) | 0.93 (0.62 to 1.40) |
| Rye bread | 1 (NA) | 1.21 (0.98 to 1.47) | 1 (NA) | 1.34 (1.06 to 1.70) | 1 (NA) | 1.27 (0.97 to 1.66) |
| Dairy products | 2 (31.3%) | 1.03 (0.89 to 1.19) | 2 (0.0%) | 0.99 (0.87 to 1.12) | 1 (NA) | 0.93 (0.80 to 1.07) |
| Milk | 1 (NA) | 1.00 (0.90 to 1.12) | 1 (NA) | 1.02 (0.89 to 1.17) | 1 (NA) | 0.92 (0.78 to 1.08) |
| Cheese | 1 (NA) | 1.09 (0.70 to 1.47) | 1 (NA) | 0.95 (0.66 to 1.37) | 1 (NA) | 1.23 (0.85 to 1.78) |
| Yogurt | 1 (NA) | 0.93 (0.74 to 1.16) | 1 (NA) | 0.84 (0.60 to 1.18) | 1 (NA) | 0.92 (0.67 to 1.25) |
| Any fruits, fruit juices, and vegetables | 1 (NA) | 0.68 (0.42 to 1.09) | ||||
| Fruits and vegetables | 1 (NA) | 1.03 (0.80 to 1.33) | 1 (NA) | 1.31 (0.83 to 2.06) | ||
| Fruits | 2 (80.4%) | 0.94 (0.44 to 2.23) | 1 (NA) | 1.39 (0.64 to 2.99) | ||
| Fruits and fruit juices | 1 (NA) | 0.87 (0.57 to 1.35) | ||||
| Citrus fruits | 1 (NA) | 0.93 (0.61 to 1.42) | ||||
| Vegetables | 3 (73.6%) | 0.97 (0.58 to 1.63) | 1 (NA) | 0.96 (0.38 to 2.45) | ||
| Cruciferous vegetables | 2 (0.0%) | 1.03 (0.83 to 1.28) | 1 (NA) | 0.95 (0.59 to 1.54) | ||
| Leafy vegetables | 1 (NA) | 0.72 (0.41 to 1.24) | ||||
| Yellow vegetables | 1 (NA) | 0.90 (0.58 to 1.40) | ||||
| Red and processed meat | 2 (86.5%) | 0.84 (0.49 to 1.46) | 2 (0.0%) | 0.88 (0.61 to 1.28) | ||
| Natural products | 1 (NA) | 0.95 (0.67 to 1.35) | 1 (NA) | 1.15 (0.69 to 1.94) | ||
| Macronutrients | ||||||
| Carbohydrates | 2 (0.0%) | 0.97 (0.73 to 1.29) | 2 (0.0%) | 0.89 (0.55 to 1.45) | 1 (NA) | 0.77 (0.27 to 2.19) |
| Fat | 2 (91.5%) | 1.76 (0.61 to 5.11) | 1 (NA) | 0.92 (0.53 to 1.60) | ||
| Trans fat | 1 (NA) | 1.78 (1.35 to 2.32) | 1 (NA) | 1.42 (0.80 to 2.52) | ||
| Saturated fat | 2 (89.3%) | 2.40 (0.78 to 7.38) | 1 (NA) | 1.55 (0.88 to 2.75) | ||
| Monounsaturated fat | 1 (NA) | 1.14 (0.86 to 1.52) | 1 (NA) | 0.89 (0.49 to 1.60) | ||
| Polyunsaturated fat | 1 (NA) | 0.91 (0.70 to 1.19) | 1 (NA) | 1.63 (0.87 to 3.03) | ||
| Linoleic fatty acids | 1 (NA) | 2.39 (1.21 to 4.69) | ||||
| Oleic fatty acids | 1 (NA) | 3.56 (1.67 to 7.59) | ||||
| Protein | 1 (NA) | 0.98 (0.73 to 1.31) | 1 (NA) | 1.19 (0.66 to 2.14) | ||
| Soy protein | 1 (NA) | 0.71 (0.54 to 0.92) | 1 (NA) | 0.68 (0.54 to 0.87) | ||
| Fiber | 3 (0.0%) | 0.64 (0.48 to 0.86) | 3 (0.0%) | 0.77 (0.52 to 1.15) | 1 (NA) | 0.68 (0.27 to 1.70) |
| Micronutrients | ||||||
| Antioxidants | 2 (0.0%) | 0.83 (0.73 to 0.95) | 1 (NA) | 0.86 (0.73 to 1.02) | 2 (57.3%) | 0.87 (0.73 to 1.04) |
| 1 (NA) | 0.54 (0.27 to 1.04) | |||||
| Multivitamins | 4 (0.0%) | 0.88 (0.79 to 0.99) | 3 (0.0%) | 0.89 (0.79 to 1.02) | 4 (0.0%) | 0.90 (0.82 to 0.99) |
| Vitamin A | 2 (0.0%) | 1.05 (0.89 to 1.26) | 2 (0.0%) | 1.01 (0.77 to 1.32) | 1 (NA) | 1.07 (0.72 to 1.60) |
| Retinol | 1 (NA) | 1.05 (0.86 to 1.30) | ||||
| Vitamin B | 1 (NA) | 0.90 (0.74 to 1.10) | 1 (NA) | 0.88 (0.75 to 1.03) | ||
| Niacin | 1 (NA) | 0.80 (0.52 to 1.51) | ||||
| Vitamin B6 | 1 (NA) | 1.02 (0.74 to 1.42) | ||||
| Vitamin C | 6 (58.9%) | 0.85 (0.71 to 1.02) | 2 (0.0%) | 0.86 (0.75 to 1.01) | 3 (28.5%) | 0.84 (0.73 to 0.96) |
| 1 (NA) | 0.64 (0.32 to 1.27) | |||||
| Vitamin D | 4 (0.0%) | 0.86 (0.78 to 0.95) | 3 (0.0%) | 0.85 (0.72 to 1.01) | 1 (NA) | 0.82 (0.60 to 1.12) |
| Vitamin E | 5 (0.0%) | 0.82 (0.73 to 0.92) | 2 (0.0%) | 0.86 (0.71 to 1.03) | 3 (51.4%) | 0.78 (0.64 to 0.95) |
| 1 (NA) | 0.55 (0.28 to 1.08) | |||||
| Carotenoids | 1 (NA) | 1.63 (1.06 to 2.50) | 1 (NA) | 1.93 (1.14 to 3.28) | 1 (NA) | 1.23 (0.76 to 1.96) |
| Alpha-Carotene | 3 (0.0%) | 1.05 (0.85 to 1.30) | 1 (NA) | 0.98 (0.59 to 1.64) | ||
| Beta-Carotene | 4 (62.4%) | 0.96 (0.68 to 1.36) | 2 (0.0%) | 1.16 (0.76 to 1.78) | 1 (NA) | 0.89 (0.50 to 1.60) |
| Beta-Cryptoxanthin | 3 (72.5%) | 0.86 (0.54 to 1.38) | 1 (NA) | 0.81 (0.45 to 1.45) | ||
| Lutein/zeaxanthin | 3(69.5%) | 0.74 (0.47 to 1.16) | 1 (NA) | 1.16 (0.62 to 2.19) | ||
| Lycopene | 4 (0.0%) | 0.98 (0.78 to 1.22) | 2 (0.0%) | 1.51 (0.90 to 2.55) | 1 (NA) | 1.17 (0.35 to 3.89) |
| Selenium | 2 (0.0%) | 0.93 (0.59 to 1.46) | 1 (NA) | 0.90 (0.45 to 1.79) | 1 (NA) | 0.89 (0.53 to 1.49) |
| Calcium | 2 (0.0%) | 0.85 (0.71 to 1.01) | 1 (NA) | 0.59 (0.32 to 1.08) | ||
| Magnesium | 1 (NA) | 1.02 (0.68 to 1.53) | ||||
| Zinc | 2 (44.7%) | 0.96 (0.67 to 1.37) | 1 (NA) | 0.82 (0.44 to 1.53) | 1 (NA) | 0.79 (0.49 to 1.28) |
| Folate | 2 (91.0%) | 0.64 (0.20 to 2.01) | ||||
| Iron | 1 (NA) | 1.60 (0.91 to 2.90) | ||||
| Isoflavone | 4 (2.2%) | 0.79 (0.67 to 0.93) | 1 (NA) | 1.03 (0.46 to 2.28) | 2 (0.0%) | 0.75 (0.62 to 0.91) |
| 1 (NA) | 0.77 (0.60 to 0.98) | |||||
| Dietary patterns | ||||||
| Healthy diet pattern | 1 (NA) | 0.78 (0.54 to 1.12) | 1 (NA) | 1.07 (0.66 to 1.73) | ||
| Unhealthy diet pattern | 1 (NA) | 1.53 (1.03 to 2.29) | 1 (NA) | 1.01 (0.60 to 1.70) | ||
| Dietary scores/indexes | ||||||
| ACS guidelines | 1 (NA) | 0.93 (0.73 to 1.18) | 1 (NA) | 1.44 (0.90 to 2.30) | ||
| CHFP | 2 (0.0%) | 0.70 (0.57 to 0.87) | 2 (0.0%) | 0.65 (0.50 to 0.86) | ||
| DII | 1 (NA) | 1.34 (1.01 to 1.81) | 1 (NA) | 1.47 (0.89 to 2.43) | ||
| HEI | 3 (24.3%) | 0.77 (0.59 to 1.00) | 3 (75.3%) | 0.90 (0.43 to 1.88) | ||
| DASH | 1 (NA) | 0.66 (0.49 to 0.91) | 1 (NA) | 0.85 (0.61 to 1.19) | ||
| aMED | 1 (NA) | 0.87 (0.64 to 1.17) | 1 (NA) | 1.15 (0.74 to 1.77) | ||
| DQIR | 1 (NA) | 0.78 (0.58 to 1.07) | 1 (NA) | 0.81 (0.53 to 1.24) | ||
| Glycemic index | 1 (NA) | 1.40 (0.78 to 2.50) | 1 (NA) | 1.60 (0.80 to 3.21) | 1 (NA) | 1.56 (0.77 to 3.31) |
| Glycemic load | 1 (NA) | 1.23 (0.46 to 3.31) | 1 (NA) | 1.11 (0.37 to 3.34) | 1 (NA) | 1.14 (0.38 to 3.44) |
| RFS | 1 (NA) | 1.03 (0.74 to 1.42) | ||||
| All-Cause Mortality | Breast-Cancer-Specific Mortality | Breast Cancer Recurrence | ||||
|---|---|---|---|---|---|---|
| Premenopause | N (I2) | RR/HR (95% CI) | N (I2) | RR/HR (95% CI) | N (I2) | RR/HR (95% CI) |
| Single food items | ||||||
| Alcohol | 1 (NA) | 0.23 (0.03 to 1.54) | ||||
| Beer | 1 (NA) | 2.33 (1.35 to 4.00) | 1 (NA) | 1.58 (1.15 to 2.17) | ||
| Butter/margarine/lard | 1 (NA) | 1.03 (0.61 to 1.76) | 1 (NA) | 1.67 (1.17 to 2.39) | ||
| Any fruits, fruit juices, and vegetables | 1 (NA) | 1.38 (0.65 to 2.91) | ||||
| Fruits and fruit juices | 1 (NA) | 1.10 (0.48 to 2.52) | ||||
| Citrus fruits | 1 (NA) | 1.70 (0.75 to 3.89) | ||||
| Vegetables | 1 (NA) | 1.40 (0.71 to 2.76) | ||||
| Cruciferous vegetables | 1 (NA) | 0.72 (0.34 to 1.54) | ||||
| Leafy vegetables | 1 (NA) | 0.85 (0.39 to 1.85) | ||||
| Yellow vegetables | 1 (NA) | 1.09 (0.52 to 2.28) | ||||
| Meat/liver/bacon | 1 (NA) | 2.60 (0.96 to 7.03) | 1 (NA) | 1.93 (0.89 to 4.15) | ||
| Macronutrients | ||||||
| Carbohydrates | 1 (NA) | 1.30 (0.30 to 5.10) | ||||
| E-Carb | 1 (NA) | 2.10 (0.50 to 8.60) | ||||
| Fat | 1 (NA) | 4.80 (1.30 to 18.10) | ||||
| Saturated fat | 2 (78.8%) | 2.09 (0.51 to 8.54) | ||||
| Saturated fat/total fat | 1 (NA) | 1.25 (0.47 to 3.31) | ||||
| Protein | 1 (NA) | 0.20 (0.10 to 0.90) | ||||
| Soy protein | 1 (NA) | 1.09 (0.74 to 1.60) | ||||
| Fiber | 1 (NA) | 0.70 (0.20 to 1.60) | ||||
| Micronutrients | ||||||
| Vitamin A | 1 (NA) | 0.78 (0.54 to 1.17) | ||||
| Thiamin | 1 (NA) | 1.20 (1.07 to 3.66) | ||||
| Vitamin C | 1 (NA) | 0.90 (0.42 to 1.94) | 1 (NA) | 0.54 (0.30 to 0.98) | ||
| Vitamin E | 1 (NA) | 0.96 (0.44 to 2.09) | 1 (NA) | 0.64 (0.33 to 1.26) | ||
| Alpha-Carotene | 1 (NA) | 0.76 (0.35 to 1.67) | ||||
| Beta-Carotene | 1 (NA) | 0.82 (0.37 to 1.82) | 1 (NA) | 0.87 (0.65 to 1.18) | ||
| Beta-Cryptoxanthin | 1 (NA) | 1.13 (0.53 to 2.41) | ||||
| Lutein/zeaxanthin | 1 (NA) | 1.71 (0.89 to 3.29) | ||||
| Lycopene | 1 (NA) | 0.61 (0.29 to 1.29) | ||||
| Flavonoids | 2 (69.4%) | 1.18 (0.65 to 2.15) | 2 (45.0%) | 1.18 (0.74 to 1.87) | ||
| Flavan-3-ols | 1 (NA) | 1.76 (0.91 to 3.42) | 1 (NA) | 1.75 (0.83 to 3.69) | ||
| Flavones | 1 (NA) | 0.69 (0.32 to 1.47) | 1 (NA) | 0.45 (0.17 to 1.19) | ||
| Flavonols | 1 (NA) | 1.64 (0.84 to 3.17) | 1 (NA) | 1.64 (0.78 to 3.46) | ||
| Flavonones | 1 (NA) | 1.08 (0.48 to 2.43) | 1 (NA) | 0.61 (0.21 to 1.81) | ||
| Phenolic acids | 1 (NA) | 1.04 (0.90 to 1.20) | 1 (NA) | 1.00 (0.83 to 1.21) | ||
| Stilebenes | 1 (NA) | 0.99 (0.92 to 1.06) | 1 (NA) | 0.99 (0.91 to 1.08) | ||
| Anthocyanidins | 1 (NA) | 0.62 (0.27 to 1.40) | 1 (NA) | 0.81 (0.35 to 1.89) | ||
| Calcium | 1 (NA) | 0.93 (0.58 to 1.50) | ||||
| Isoflavone | 1 (NA) | 0.71 (0.34 to 1.48) | 2 (0.0%) | 0.96 (0.69 to 1.34) | 1 (NA) | 0.88 (0.61 to 1.23) |
| Daidzein | 1 (NA) | 1.74 (0.63 to 4.76) | ||||
| Genistein | 1 (NA) | 1.75 (0.65 to 4.76) | ||||
| Glycetin | 1 (NA) | 1.60 (0.54 to 4.72) | ||||
| Lignans | 2 (0.0%) | 1.26 (1.06 to 1.50) | 2 (0.0%) | 1.23 (0.98 to 1.55) | ||
| Dietary scores/indexes | ||||||
| DII | 1 (NA) | 0.84 (0.52 to 1.34) | 1 (NA) | 0.76 (0.46 to 1.26) | ||
| All-Cause Mortality | Breast-Cancer-Specific Mortality | Breast Cancer Recurrence | ||||
| Postmenopause | N (I2) | RR/HR (95% CI) | N (I2) | RR/HR (95% CI) | N (I2) | RR/HR (95% CI) |
| Single food items | ||||||
| Alcohol | 2 (9.2%) | 1.14 (0.85 to 1.54) | 1 (NA) | 1.74 (1.13 to 2.67) | 1 (NA) | 1.08 (0.73 to 1.58) |
| Whole grain products | 4 (0.0%) | 1.01 (0.96 to 1.06) | 4 (0.0%) | 1.04 (0.98 to 1.10) | 4 (14.0%) | 1.01 (0.94 to 1.08) |
| Rye bread | 2 (0.0%) | 1.13 (1.02 to 1.25) | 2 (43.0%) | 1.19 (0.10 to 1.42) | 2 (38.4%) | 1.11 (0.92 to 1.34) |
| Oatmeal/muesli | 2 (0.0%) | 0.78 (0.63 to 0.97) | 2 (0.0%) | 0.92 (0.72 to 1.18) | 2 (0.0%) | 0.91 (0.71 to 1.16) |
| Dairy products | 2 (0.0%) | 1.01 (0.96 to 1.08) | 2 (0.0%) | 0.98 (0.92 to 1.05) | 2 (0.0%) | 0.97 (0.91 to 1.04) |
| Cheese | 2 (0.0%) | 1.16 (1.05 to 1.27) | 2 (0.0%) | 1.11 (0.92 to 1.35) | 2 (0.0%) | 1.18 (0.98 to 1.43) |
| Milk | 2 (0.0%) | 1.02 (0.96 to 1.08) | 2 (0.0%) | 1.00 (0.93 to 1.07) | 2 (0.0%) | 0.95 (0.88 to 1.02) |
| Yogurt | 2 (0.0%) | 0.91 (0.79 to 1.05) | 2 (0.0%) | 0.85 (0.71 to 1.03) | 2 (0.0%) | 0.99 (0.83 to 1.18) |
| Soy products | 1 (NA) | 1.03 (0.81 to 1.33) | 1 (NA) | 1.03 (0.71 to 1.50) | ||
| Any fruits, fruit juices, and vegetables | 1 (NA) | 0.68 (0.42 to 1.09) | ||||
| Fruits | 1 (NA) | 0.63 (0.38 to 1.05) | ||||
| Fruits and fruit juices | 1 (NA) | 0.87 (0.57 to 1.35) | ||||
| Citrus fruits | 1 (NA) | 0.93 (0.61 to 1.42) | ||||
| Vegetables | 2 (73.7%) | 0.80 (0.42 to 1.51) | ||||
| Cruciferous vegetables | 1 (NA) | 1.07 (0.67 to 1.72) | ||||
| Leafy vegetables | 1 (NA) | 0.72 (0.41 to 1.24) | ||||
| Yellow vegetables | 1 (NA) | 0.90 (0.58 to 1.40) | ||||
| Macronutrients | ||||||
| Carbohydrates | 1 (NA) | 2.00 (0.70 to 5.70) | ||||
| E-Carb | 1 (NA) | 1.70 (0.60 to 4.90) | ||||
| Fat | 1 (NA) | 3.12 (1.79 to 5.44) | 1 (NA) | 0.70 (0.20 to 2.20) | ||
| Saturated fat | 1 (NA) | 4.45 (2.26 to 8.78) | 2 (0.0%) | 1.29 (0.96 to 1.72) | ||
| Saturated fat/total fat | 1 (NA) | 2.53 (1.20 to 5.33) | ||||
| Linoleic fatty acids | 1 (NA) | 2.39 (1.21 to 4.69) | ||||
| Oleic fatty acids | 1 (NA) | 3.56 (1.67 to 7.59) | ||||
| Fiber | 1 (NA) | 0.48 (0.27 to 0.86) | 1 (NA) | 0.80 (0.30 to 1.80) | ||
| Protein | 1 (NA) | 0.60 (0.20 to 1.60) | ||||
| Soy protein | 1 (NA) | 0.79 (0.49 to 1.28) | ||||
| Micronutrients | ||||||
| Antioxidants | 1 (NA) | 0.54 (0.27 to 1.04) | ||||
| Vitamin A | 1 (NA) | 0.84 (0.67 to 1.06) | ||||
| Thiamin | 1 (NA) | 0.62 (0.36 to 1.05) | ||||
| Vitamin C | 2 (82.7%) | 0.71 (0.30 to 1.68) | 1 (NA) | 0.74 (0.50 to 1.11) | ||
| 1 (NA) | 0.64 (0.32 to 1.27) | |||||
| Vitamin D | 1 (NA) | 0.78 (0.70 to 0.88) | ||||
| Vitamin E | 1 (NA) | 0.77 (0.47 to 1.27) | 1 (NA) | 0.76 (0.51 to 1.13) | ||
| 1 (NA) | 0.55 (0.28 to 1.08) | |||||
| Alpha-Carotene | 2 (40.1%) | 0.99 (0.65 to 1.53) | ||||
| Beta-Carotene | 2 (78.8%) | 0.74 (0.35 to 1.57) | 1 (NA) | 0.84 (0.68 to 1.03) | ||
| Beta-Cryptoxanthin | 2 (20.5%) | 0.70 (0.47 to 1.04) | ||||
| Provitamin A | 1 (NA) | 0.58 (0.34 to 0.99) | ||||
| Lutein/zeaxanthin | 2 (0.0%) | 0.59 (0.41 to 0.84) | ||||
| Lycopene | 2 (0.0%) | 0.78 (0.54 to 1.11) | ||||
| Flavonoids | 2 (18.7%) | 0.99 (0.84 to 1.17) | 2 (60.5%) | 0.88 (0.55 to 1.41) | ||
| Flavan-3-ols | 1 (NA) | 0.84 (0.53 to 1.32) | 1 (NA) | 0.63 (0.34 to 1.18) | ||
| Flavones | 1 (NA) | 0.59 (0.35 to 0.99) | 1 (NA) | 0.49 (0.24 to 0.99) | ||
| Flavonols | 1 (NA) | 0.98 (0.62 to 1.53) | 1 (NA) | 1.02 (0.59 to 1.79) | ||
| Flavonones | 1 (NA) | 0.99 (0.66 to 1.49) | 1 (NA) | 1.09 (0.65 to 1.82) | ||
| Phenolic acids | 1 (NA) | 0.97 (0.91 to 1.05) | 1 (NA) | 0.99 (0.89 to 1.10) | ||
| Stilebenes | 1 (NA) | 0.97 (0.95 to 1.00) | 1 (NA) | 0.97 (0.94 to 1.00) | ||
| Anthocyanidins | 1 (NA) | 0.66 (0.40 to 1.08) | 1 (NA) | 0.62 (0.33 to 1.18) | ||
| Isoflavone | 2 (83.9%) | 0.70 (0.32 to 1.53) | 3 (0.0%) | 0.91 (0.74 to 1.13) | 1 (NA) | 0.67 (0.58 to 0.92) |
| Daidzein | 1 (NA) | 0.70 (0.27 to 1.77) | ||||
| Genistein | 1 (NA) | 0.69 (0.27 to 1.75) | ||||
| Glycetin | 1 (NA) | 0.51 (0.18 to 1.38) | ||||
| Lignans | 2 (0.0%) | 0.94 (0.86 to 1.03) | 2 (0.0%) | 0.83 (0.72 to 0.96) | ||
| Calcium | 1 (NA) | 0.71 (0.49 to 1.05) | ||||
| Folate | 1 (NA) | 0.34 (0.18 to 0.67) | ||||
| Dietary patterns | ||||||
| Healthy diet pattern | 1 (NA) | 0.87 (0.61 to 1.23) | 1 (NA) | 0.89 (0.59 to 1.35) | 1 (NA) | 0.71 (0.48 to 1.06) |
| Unhealthy diet pattern | 1 (NA) | 1.34 (0.93 to 1.94) | 1 (NA) | 0.99 (0.64 to 1.52) | 1 (NA) | 0.91 (0.61 to 1.36) |
| Dietary scores/indexes | ||||||
| DII | 2 (25.3%) | 1.19 (0.93 to 1.51) | 2 (5.6%) | 1.18 (0.89 to 1.58) | ||
| HEI | 1 (NA) | 0.74 (0.55 to 0.99) | 1 (NA) | 0.91 (0.60 to 1.40) | ||
| WCRF/AICR adherence score | 1 (NA) | 0.61 (0.39 to 0.96) | 1 (NA) | 0.88 (0.41 to 1.91) | ||
| All-Cause Mortality | Breast-Cancer-Specific Mortality | Breast Cancer Recurrence | ||||
|---|---|---|---|---|---|---|
| Dietary | N (I2) | RR/HR (95% CI) | N (I2) | RR/HR (95% CI) | N (I2) | RR/HR (95% CI) |
| Vitamin A | 2 (65.2%) | 0.94 (0.66 to 1.34) | 1 (NA) | 1.24 (0.68 to 2.24) | ||
| Retinol | 1 (NA) | 1.05 (0.56 to 1.95) | ||||
| Thiamin | 1 (NA) | 0.54 (0.38 to 0.88) | 1 (NA) | 0.44 (0.24 to 0.81) | ||
| Riboflavin | 1 (NA) | 0.92 (0.58 to 1.44) | 1 (NA) | 0.72 (0.41 to 1.29) | ||
| Niacin | 2 (0.0%) | 0.74 (0.57 to 0.96) | 1 (NA) | 0.61 (0.34 to 1.09) | ||
| Vitamin B6 | 1 (NA) | 0.95 (0.61 to 1.48) | 1 (NA) | 0.77 (0.44 to 1.36) | ||
| Vitamin B12 | 1 (NA) | 1.20 (0.80 to 1.81) | 1 (NA) | 1.10 (0.65 to 1.85) | ||
| Vitamin C | 3 (66.1%) | 0.78 (0.54 to 1.14) | 2 (0.0%) | 0.76 (0.59 to 0.97) | ||
| Vitamin D | 1 (NA) | 0.86 (0.64 to 1.16) | 1 (NA) | 1.02 (0.58 to 1.79) | ||
| Vitamin E | 1 (NA) | 0.77 ().47 to 1.27) | ||||
| Carotenes | 1 (NA) | 0.96 (0.70 to 1.31) | ||||
| Alpha-Carotene | 4 (0.0%) | 1.01 (0.85 to 1.21) | 1 (NA) | 0.98 (0.59 to 1.64) | ||
| Beta-Carotene | 4 (61.4%) | 0.92 (0.68 to 1.24) | 3 (34.5%) | 0.82 (0.53 to 1.29) | ||
| Beta-Cryptoxanthin | 4 (61.2%) | 0.88 (0.64 to 1.20) | 1 (NA) | 0.81 (0.45 to 1.45) | ||
| Lutein/zeaxanthin | 4 (54.9%) | 0.79 (0.58 to 1.06) | 1 (NA) | 1.16 (0.62 to 2.19) | ||
| Lycopene | 4 (11.7%) | 0.90 (0.74 to 1.09) | 1 (NA) | 1.42 (0.80 to 2.50) | ||
| Selenium | 1 (NA) | 0.86 (0.63 to 1.19) | ||||
| Betaine | 1 (NA) | 0.81 (0.54 to 1.20) | 1 (NA) | 0.72 (0.44 to 1.17) | ||
| Calcium | 3 (0.0%) | 0.72 (0.58 to 0.89) | 2 (62.5%) | 0.85 (0.39 to 1.86) | ||
| Magnesium | 2 (24.1%) | 0.66 (0.46 to 0.94) | 1 (NA) | 0.70 (0.33 to 1.49) | ||
| Methionine | 2 (0.0%) | 0.69 (0.53 to 0.90) | 1 (NA) | 0.70 (0.39 to 1.28) | ||
| Iodine | 1 (NA) | 0.90 (0.67 to 1.21) | ||||
| Potassium | 1 (NA) | 0.98 (0.69 to 1.38) | ||||
| Sodium | 1 (NA) | 0.79 (0.57 to 1.09) | ||||
| Flavonoids | 1 (NA) | 1.02 (0.95 to 1.10) | 1 (NA) | 1.04 (0.93 to 1.15) | ||
| Phenolic acids | 1 (NA) | 0.97 (0.91 to 1.05) | 1 (NA) | 0.99 (0.89 to 1.10) | ||
| Stilebenes | 1 (NA) | 0.97 (0.95 to 1.00) | 1 (NA) | 0.97 (0.94 to 1.00) | ||
| Folate | 3 (65.5%) | 0.66 (0.46 to 0.96) | 2 (0.0%) | 0.79 (0.61 to 1.01) | ||
| Isoflavone | 3 (0.0%) | 0.90 (0.79 to 1.02) | 3 (0.0%) | 0.91 (0.77 to 1.08) | 2 (0.0%) | 0.78 (0.65 to 0.93) |
| 1 (NA) | 0.77 (0.60 to 0.98) | |||||
| Daidzein | 1 (NA) | 0.96 (0.52 to 1.76) | ||||
| Genistein | 1 (NA) | 0.95 (0.52 to 1.75) | ||||
| Glycetin | 1 (NA) | 0.80 (0.42 to 1.50) | ||||
| Lignans | 1 (NA) | 0.94 (0.86 to 1.04) | 1 (NA) | 0.83 (0.72 to 0.96) | ||
| Caffeine | 1 (NA) | 0.77 (0.55 to 1.07) | ||||
| All-cause mortality | Breast-cancer-specific mortality | Breast cancer recurrence | ||||
| Supplementary | N (I2) | RR/HR (95% CI) | N (I2) | RR/HR (95% CI) | N (I2) | RR/HR (95% CI) |
| Antioxidants | 2 (0.0%) | 0.83 (0.73 to 0.95) | 1 (NA) | 0.86 (0.73 to 1.02) | 2 (57.3%) | 0.87 (0.73 to 1.04) |
| 1 (NA) | 0.54 (0.27 to 1.04) | |||||
| Multivitamins | 4 (0.0%) | 0.88 (0.79 to 0.99) | 3 (0.0%) | 0.89 (0.79 to 1.02) | 4 (0.0%) | 0.90 (0.82 to 0.99) |
| Vitamin A | 1 (NA) | 1.02 (0.82 to 1.27) | 2 (50.4%) | 0.80 (0.49 to 1.32) | 1 (NA) | 1.07 (0.72 to 1.60) |
| Retinol | 2 (36.9%) | 0.96 (0.76 to 1.21) | ||||
| Vitamin B | 1 (NA) | 0.87 (0.74 to 1.02) | 1 (NA) | 0.90 (0.74 to 1.10) | 1 (NA) | 0.88 (0.75 to 1.03) |
| Thiamin | 1 (NA) | 0.82 (0.59 to 1.13) | 1 (NA) | 0.57 (0.26 to 1.25) | ||
| Riboflavin | 1 (NA) | 0.81 (0.58 to 1.13) | ||||
| Niacin | 1 (NA) | 0.80 (0.52 to 1.51) | ||||
| Vitamin B6 | 2 (21.5%) | 0.89 (0.69 to 1.16) | ||||
| Vitamin C | 6 (57.5%) | 0.89 (0.75 to 1.06) | 3 (45.9%) | 0.79 (0.61 to 1.02) | 3 (28.5%) | 0.84 (0.73 to 0.96) |
| 1 (NA) | 0.64 (0.32 to 1.27) | |||||
| Vitamin D | 5 (0.0%) | 0.82 (0.76 to 0.89) | 2 (0.0%) | 0.84 (0.70 to 1.00) | 1 (NA) | 0.82 (0.60 to 1.12) |
| Vitamin E | 5 (0.0%) | 0.82 (0.73 to 0.92) | 3 (0.0%) | 0.84 (0.70 to 1.00) | 3 (51.4%) | 0.78 (0.64 to 0.95) |
| 1 (NA) | 0.55 (0.28 to 1.08) | |||||
| Carotenoids | 1 (NA) | 1.63 (1.06 to 2.50) | 1 (NA) | 1.93 (1.14 to 3.28) | 1 (NA) | 1.23 (0.76 to 1.96) |
| Beta-Carotene | 2 (49.2%) | 0.91 (0.54 to 1.54) | 1 (NA) | 1.33 (0.69 to 2.55) | 1 (NA) | 0.89 (0.50 to 1.60) |
| Provitamin A | 1 (NA) | 0.58 (0.34 to 0.99) | ||||
| Lycopene | 1 (NA) | 1.38 (0.41 to 4.61) | 1 (NA) | 2.09 (0.59 to 7.43) | 1 (NA) | 1.17 (0.35 to 3.89) |
| Selenium | 2 (0.0%) | 0.93 (0.59 to 1.46) | 1 (NA) | 0.90 (0.45 to 1.79) | 1 (NA) | 0.89 (0.53 to 1.49) |
| Calcium | 1 (NA) | 0.90 (0.74 to 1.12) | 1 (NA) | 0.66 (0.33 to 1.31) | ||
| Magnesium | 1 (NA) | 1.02 (0.68 to 1.53) | ||||
| Folate | 3 (0.0%) | 1.03 (0.86 to 1.22) | ||||
| Iron | 2 (70.7%) | 1.12 (0.61 to 2.03) | ||||
| Zinc | 3 (24.8%) | 0.92 (0.73 to 1.15) | 1 (NA) | 0.82 (0.44 to 1.53) | 1 (NA) | 0.79 (0.49 to 1.28) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-H.; Hoang, T.; Kim, J. Dietary Factors and Breast Cancer Prognosis among Breast Cancer Survivors: A Systematic Review and Meta-Analysis of Cohort Studies. Cancers 2021, 13, 5329. https://doi.org/10.3390/cancers13215329
Park S-H, Hoang T, Kim J. Dietary Factors and Breast Cancer Prognosis among Breast Cancer Survivors: A Systematic Review and Meta-Analysis of Cohort Studies. Cancers. 2021; 13(21):5329. https://doi.org/10.3390/cancers13215329
Chicago/Turabian StylePark, Sin-Hye, Tung Hoang, and Jeongseon Kim. 2021. "Dietary Factors and Breast Cancer Prognosis among Breast Cancer Survivors: A Systematic Review and Meta-Analysis of Cohort Studies" Cancers 13, no. 21: 5329. https://doi.org/10.3390/cancers13215329
APA StylePark, S.-H., Hoang, T., & Kim, J. (2021). Dietary Factors and Breast Cancer Prognosis among Breast Cancer Survivors: A Systematic Review and Meta-Analysis of Cohort Studies. Cancers, 13(21), 5329. https://doi.org/10.3390/cancers13215329






