Simple Summary
Pancreatic cancer is one of the deadliest cancers with the lowest survival rate. Little progress has been achieved in prolonging the survival for patients with pancreatic adenocarcinoma. Hence, special attention should be paid to pre-cancerous lesions, for instance, an intraductal papillary mucinous neoplasm (IPMN). Here, we reviewed its genetic characteristics and the mouse models involving mutations in specific pathways, and updated our current perception of how this lesion develops into a precursor of invasive cancer.
Abstract
The intraductal papillary mucinous neoplasm (IPMN) is attracting research attention because of its increasing incidence and proven potential to progress into invasive pancreatic ductal adenocarcinoma (PDAC). In this review, we summarized the key signaling pathways or protein complexes (GPCR, TGF, SWI/SNF, WNT, and PI3K) that appear to be involved in IPMN pathogenesis. In addition, we collected information regarding all the genetic mouse models that mimic the human IPMN phenotype with specific immunohistochemistry techniques. The mouse models enable us to gain insight into the complex mechanism of the origin of IPMN, revealing that it can be developed from both acinar cells and duct cells according to different models. Furthermore, recent genomic studies describe the potential mechanism by which heterogeneous IPMN gives rise to malignant carcinoma through sequential, branch-off, or de novo approaches. The most intractable problem is that the risk of malignancy persists to some extent even if the primary IPMN is excised with a perfect margin, calling for the re-evaluation and improvement of diagnostic, pre-emptive, and therapeutic measures.
1. Introduction
Pancreatic cysts are being identified with increasing frequency as a result of the increasing use of cross-sectional imaging [1], the enhanced quality of imaging-workup, and the aging population. The incidence of pancreatic cysts increases with age, with a prevalence of 0.5% in those younger than 40 and 25% in those in their 70s [2,3]. However, these incidences are likely to be underestimated because pancreatic cysts often present with no clinical symptoms and the lesion is frequently detected serendipitously using abdominal imaging performed for another condition or during regular check-ups.
Pancreatic cystic tumors are a large family consisting of the following major groups: serous tumors (including serous cyst adenoma and cystadenocarcinoma); solid pseudopapillary neoplasms (SPNs); pancreatic pseudocysts; and cystic neuroendocrine tumors (cNETs) and mucinous tumors, including mucinous cystic neoplasia (MCNs) and intraductal papillary mucinous neoplasia (IPMNs) [4]. Among them, IPMN is the most common, accounting for about 20–40% of all pancreatic cystic tumors and for 1–3% of all exocrine pancreatic neoplasms [5]. The mean age of the diagnosis of IPMN is at the mid-60s equally in men and women [6,7]. IPMNs have been reported to be more common in patients who smoke cigarettes [8], have diabetes, have Peutz–Jeghers syndrome, have familial adenomatous polyposis syndrome [9], or familial pancreatic carcinoma, or who have a family history of pancreatic ductal adenocarcinoma [10]. IPMN has become a research hotspot because of its precursor role in invasive pancreatic ductal adenocarcinoma (PDAC) [11], together with two other precursors, namely pancreatic intraepithelial neoplasia (PanINs) and MCN [12].
IPMNs are defined as potentially malignant intraductal epithelial neoplasms that are grossly visible [13] (classical standard of > 10 mm, with a lower cutoff of 5 mm as acknowledged recently [14,15,16]), which consist of mucin-producing columnar cells. IPMNs can be classified morphologically as the main duct (MD), branch duct (BD), or both, according to their location [13,17]. The molecular mechanism of PanIN is relatively well understood, whereas that of IPMN is not. In this review, we summarize the recent advances in the study of IPMN pathogenesis, with particular emphasis on genomic profiling and on the use of genetically engineered mouse models (GEMMs).
2. Diagnosis and Clinical Pathology
The diagnosis of pancreatic cystic neoplasms typically starts with cross-sectional imaging, i.e., computed tomography (CT) and magnetic resonance cholangio-pancreatography (MRCP). Evaluation of the communication between the dilated branch ducts and the main pancreatic duct is important for distinguishing IPMNs from other cystic lesions [11]. For patients who need additional evaluation, endoscopic ultrasound with fine-needle aspiration (EUS-FNA) can provide high-quality imaging of the pancreas and the opportunity to sample pancreatic lesions for both cytology and cyst fluid analysis (amylase, carcinoembryonic antigen [CEA] level) [18]. If there is still concern about possible malignancy after EUS-FNA or if the extent of the IPMN is unclear, additional testing may be done. Testing may include endoscopic retrograde cholangio-pancreatography (ERCP), with aspiration of the pancreatic duct contents or brushing of the pancreatic duct, pancreatoscopy, intraductal ultrasonography, positron emission tomography (PET), or assessment of serum tumor markers.
The reported risk of malignancy for patients with MD-IPMN ranges from 38% to 68%, but is much lower for BD-IPMN, which ranges from 11% to 30% in patients who received resection [15,19,20].
The 2015 American Gastroenterological Association (AGA) guidelines do not recommend resection for main duct dilatation alone, unless it involves the presence of a nodule or is cytologically positive for malignancy [21]. However, the 2017 International Association of Pancreatology (IAP) [22] and 2018 European [23] guidelines are more radical. Patients with the involvement of the main pancreatic duct (≥10 mm) are advised to undergo surgical resection.
However, for BD-IPMN, the situation is more complicated. According to the 2018 European guidelines, the absolute indications for resection (high-risk stigmata) include: the presence of obstructive jaundice, an enhanced mural nodule (≥5 mm) or solid mass, and cytology positive for high-grade dysplasia or cancer [23]. In addition, conservative treatment seems to be appropriate, with a high 5-year-disease-specific survival (DSS) of 96% in elderly people who only have worrisome features [24]. Notably, current clinical criteria are not precise enough to recognize the risk and molecular markers are urgently required [25].
Histologically, BD-IPMN almost always corresponds to a gastric type and MD-IPMN can be further divided into intestinal, oncocytic, and pancreatobiliary types. These four subtypes can also be distinguished through immunohistochemical staining of mucin 1 (MUC1); MUC2; mucin 5AC, oligomeric mucus/gel-forming (MUC5AC); and caudal type homeobox 2 (CDX2; Table 1) [26].

Table 1.
Description of four historical types of IPMN.
The gastric type is the most common, with a favorable prognosis and lower malignant risk [30]. The oncocytic type shows the same MUC levels as the pancreatobiliary type but the lining cells reveal strong eosinophilic cytoplasm [28]. This histological classification is widely leveraged among the construction of GEMMs because each subtype might correspond to a certain mechanism.
3. Genetic Signatures and Their Clinical Application
Although IPMN shares some common genetic mutations with pancreatic infiltrating ductal adenocarcinoma (e.g., KRAS proto-oncogene, GTPase (KRAS), and tumor protein P53 (TP53) and cyclin-dependent kinase inhibitor 2A (CDKN2A)), it additionally comprises unique mutations in the guanine nucleotide-binding protein-stimulating α subunit (GNAS; 41–79%) [31,32,33] and ubiquitin E3 ligase ring finger 43 (RNF43; 38%) [34,35], which are putatively related to the pathogenesis of IPMN. Basturk et al. performed comprehensive molecular sequencing to identify the mutation frequency of other genes, namely chromatin-remodeling genes (32%), PI3K (encoding phosphatidylinositol-4,5 -bisphosphate 3-kinase) (27%), and FGFR2 (encoding fibroblast growth factor receptor 2) (18%) [35].
GNAS [36] serves as a key oncogene in IPMN. The oncogenic mutations of GNAS (primarily R201C and R201H) markedly activate adenylyl cyclase, leading to the intensified formation of cyclic AMP, a second messenger that stimulates multiple downstream effectors [37]. GNAS mutations seem to be specific for IPMNs [38] and are most prevalent in intestinal-type IPMNs, being found in 78–100% of these neoplasms; however, Gnas mutations only mimic gastric or pancreatobiliary type IPMN in GEMMs, which remains a puzzle [39,40].
RNF43 is acknowledged as a tumor suppressor and a negative regulator of the Wnt pathway by reducing the membrane level of the Frizzled receptor [41]. Unlike Gnas mutations, the knockout or mutation of Rnf43 only accelerates Kras-driven tumorigenesis [42] and has not been verified to simulate IPMN lesions in mouse models. These mutation features might be utilized in molecular-targeted therapy, especially for RNF43, where antibodies targeting the Wnt pathway have already been applied.
Recently, several studies showed distinct genetic features in different stages of IPMN. Hotspot mutation of KLF4 (encoding Kruppel such as factor 4) in tissue samples was recently revealed using multiregion whole-exome sequencing, which has been proven to be enriched especially in low-grade dysplasia [43]. In addition, MUC5AC expression in circulating extracellular vesicles was significantly higher (sensitivity of 82%, specificity of 100%) in high-grade lesions [44]. Genetic heterogeneity of early driver genes is significantly more prevalent in low-grade IPMNs [45].
According to the genomic characteristics of IPMN, many emerging diagnostic tools have been tested using advanced sequencing technology for the early detection and surveillance of invasive progression. The basic logic is to detect the unique early mutation of GNAS and TP53/SMAD4 (encoding SMAD family member 4) mutations, which are related to high-grade dysplasia [33].
Pancreatic cyst fluid obtained through EUS-FNA [46] is a reliable sample for IPMN diagnosis (sensitivity, 89%; specificity, 100%) [47], with a low risk of complications (2–3%) [23]. Moreover, the monoclonal antibody Das-1 in the cyst fluid can be analyzed to define the risk of malignancy (88% sensitivity, 99% specificity) [48]. A study also used secretin-stimulated pancreatic fluid to identify IPMN through GNAS mutations (64.1% sensitivity, 100% specificity) [49]. In addition, DNA methylation can also be analyzed for molecular diagnosis (sensitivity, 83%; specificity, 86%) [50]. Targeted genotyping (GNAS/KRAS) of cell-free DNA (cfDNA) from blood samples can also distinguish IPMN from control cases (sensitivity, 81%; specificity, 84.2%) and the amount of cfDNA detected is much higher in metastatic pancreatic ductal adenocarcinoma (PDAC) than in IPMN for differential diagnosis [51]. Considering the higher abundance of circulating tumor cells (CTCs) in portal vein blood compared to in peripheral blood, researchers have discovered that the count of mesenchymal-CTCs and vimentin+ CTCs correlates with a poorer differentiated tumor and a shorter survival [52,53].
For malignancy detection, SMAD4/TP53 mutation detected in pancreatic fluid can distinguish PDAC from IPMN cases, with a 32.4% sensitivity and 100% specificity [54]. Another study reported a new method to detect circulating pancreas epithelial cells in blood samples before tumor formation [55] because epithelial denudation exists widely in pre-cancerous cases [56].
4. The Progression of IPMN into PDAC
It used to be taken for granted that different IPMNs found in a patient have monoclonal origins [57] and are direct precursors to pancreatic cancer [58]; however, more recent studies indicate the opposite. Single-cell sequencing [59] and microdissection, followed by capture-based targeted sequencing [45], were performed on IPMN samples, which suggested polyclonal precursors and revealed distinct mutations in driver genes.
Invasive adenocarcinoma found in a patient with concurrent IPMN does not always originate from the cystic lesion. Felsenstein et al. collected samples comprising IPMN and adenocarcinoma simultaneously, and isolated them for targeted next generation-sequencing, which revealed a striking result: 18% of adenocarcinomas were actually independent of the concurrent IPMN [60]. Further studies proposed three pathways of cancerization, classified as sequential, branch-off, and de novo, with a frequency of about 1/3 each [38,61]. This recent genomic evidence suggests that IPMN should no longer be considered as a single locoregional disease but rather is a complex lesion with genetic heterogeneity, which induces the carcinogenesis of the whole pancreas.
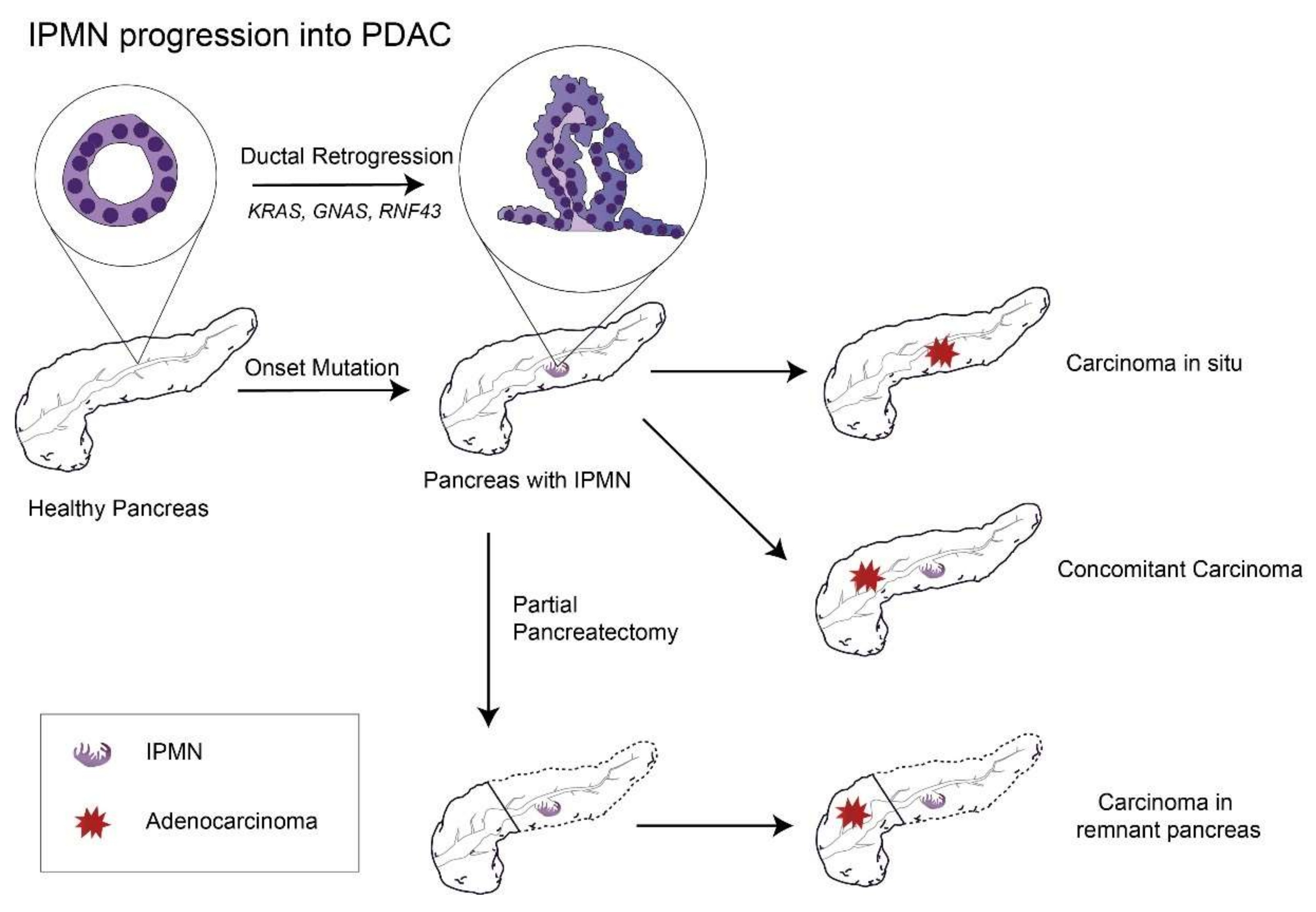
One meta study, which included both MD-IPMN and BD-IPMN cases followed without surgery, analyzed the cumulative incidence of pancreatic cancer and found that the 10-year chance of developing pancreatic cancer was 25% for MD-IPMN [62]. Although the risk of cancer might decrease greatly after partial pancreatectomy of MD-IPMN with a negative margin, it still exists within the remnant pancreas [63] (with a 10-year incidence of pancreatic cancer of 38.3% for high-grade dysplasia, 3.0% for low-grade dysplasia, and 21.2% in total [64]), suggesting that IPMN is a sign that the whole pancreas is undergoing an irreversible process of carcinogenesis (Figure 1).

Figure 1.
The remaining risks of PDAC progression in the remnant pancreas after partial pancreatectomy to remove the primary IPMN lesion. Abbreviations: IPMN, intraductal papillary mucinous neoplasm; PDAC, pancreatic ductal adenocarcinoma; GNAS, guanine nucleotide-binding protein-stimulating α subunit; and RNF43: ubiquitin E3 ligase ring finger 43.
For BD-IPMN, retrospective studies discovered that the overall cumulative incidence rates of pancreatic carcinoma were 1.1% at 5 years, 3.5% at 10 years, and 12.0% at 15 years, and notably, the incidence of IPMN-derived carcinoma and concomitant PDAC was almost equal [65,66]. Therefore, current guidelines for BD-IPMN are rather conservative [20]. The risk factors of malignancy at follow-up involves cyst size (≥30 mm), increased serum level of carbohydrate antigen 19-9 (CA19-9), and thickened cyst walls [22]. In addition, a growth rate of ≥2.5 mm/y is recommended as an independent predictor of pancreatic cancer during surveillance.
To date, surgery is the only effective method to eradicate IPMN. The choices include total pancreatectomy, pancreaticoduodenectomy, distal pancreatectomy, and segmental resection of the tumor, which is determined by the location of the tumor and the extent of involvement of the gland [20].
There are alternatives that are still in the experimental phase. EUS-guided pancreatic cyst ablation using ethanol and/or paclitaxel [67], and SB-IPMN enucleation using a combination of blunt dissection, bipolar cautery, small clips, and/or fine sutures [68] are two novel approaches that have been tested in several clinical trials. Both modalities enable a shorter operative time and in-hospital stay, and, importantly, a better reserved endocrine and exocrine function [69]. However, long-term safety issues have not been well investigated. Additionally the ablation is reported to have a 2–10% rate of complications, presumably linked to the use of ethanol [70,71].
Considering that the rate of postoperative severe complications was determined as 14.0% [72], we still need more biomarkers to better predict the malignant risks for further decisions concerning surgery.
New therapies involving molecular treatment are also urgently required. However, a selection bias of the patients cannot be ignored. For example, patients left unresected when diagnosed with MD-IPMN (pancreatic duct size of ≥ 10 mm) is against the current IAP and European guidelines, unless there are concerns related to the patient’s poor physical condition.
5. Genetically Engineered Mouse Models of IPMN
Over the past three decades, with the increasing understanding of the genetic mutations underlying tumorigenesis, researchers have developed various GEMMs that reproduce the genetic events in in vivo settings, allowing for de novo tumor formation in a native immune-proficient microenvironment [73]. GEMMs have provided a platform to define genotype–phenotype relationships in IPMN pathobiology and have greatly increased our understanding of its pathological progress. Almost every PDAC harbors oncogenic mutations in the KRAS gene [74], the protein product of which mediates a wide variety of cellular functions, including proliferation, differentiation, and survival. A widely used classical mouse model to elicit mPanIN that can further progress to PDAC (latency of >1 y) [75] is based on the KRAS mutation, which strongly stimulates its intrinsically inefficient activity [76].
Although the sole mutation of KRAS is not strong enough to induce IPMN lesions in mice, artificially generating another alteration of a certain gene in the meantime has proven to be valid. Therefore, there is a general strategy to create IPMN models by using the Lox-stop-lox KrasG12D (LSL-KrasG12D) allele [77], which undergoes Cre-mediated excision of the stop codon concomitant with another mutation, deletion, or overexpression.
By manipulating different promoters that express in different cell types or at different time points (prenatal or postnatal), researchers have attributed the pivotal role in the formation of mouse IPMN to pancreatic duct cells, as is often verified in human IPMN. The main mechanism is “ductal retrogression” at the onset of IPMN, in which the mature duct cells downregulate ductal markers such as SRY box 9 (SOX9) and present a morphology more similar to progenitor cells [78].
We have collected all the genetic mouse models of IPMN published since 2006 (when the first model was created by Bardeesy et al.) as well as those that provide an adequate description, including the histological types, invasion rates, targeted cell type (P48 is another name for Ptf1a, a promoter targeting multipotent progenitors of pancreatic ducts and of both exocrine and endocrine cells during embryologic development, which is activated at E9.5 and retained in acinar cells [79,80,81]), and metastasis (if available). We summarized the involved signal pathways of the potential mechanisms (Table 2). The existing models provide us with inspirations and insights to build more mouse models concentrating on other genes involved in these pathways or complexes, i.e., G protein-coupled receptors (GPCR) and transforming growth factors (TGFs), as well as the SWI/SNF (SWItch/sucrose non-fermentable), WNT, and PI3K pathways.
- (1)
- Concerning the GPCR pathway: GNAS is a component of GPCR-regulated adenylyl cyclase signal transduction pathways. Gnas mutations have been applied in mouse models, with mutations R201H or R201C being feasible for the formation of IPMN [82,83]. However, there remains some discrepancies between human IPMN and these established mouse models. The transgenic mouse models needed to have synergistic mutations of Gnas and Kras to develop a cystic tumor, while in human cases, IPMNs can develop with mutations in either GNAS or KRAS. In addition, the cystic tumor developed in the Tg-GnasR201H:KrasG12D mice always showed a gastric or pancreatobiliary phenotype.
- (2)
- Concerning the TGF-β pathway: TGF-β is a secreted polypeptide that can bind to its receptors and trigger phosphorylation of SMAD2 and SMAD3. Phosphorylated SMAD2 and SMAD3 then interact with SMAD4. The SMAD2/3/4 complex accumulates within the nucleus and acts as a potent inhibitor of epithelial cell growth and survival via modulation of the expression of cell cycle regulators and the activation of apoptosis [84]. Paradoxically, TGF-β is known to be a growth suppressor in the non-neoplastic epithelium but acts as a metastatic tumor promoter in advanced cancers [85,86], thus it might play a key role in regulating epithelium identity.
- (3)
- Since Bardeesy et al. discovered that disturbance of TGF-β/SMAD4-signaling induces the formation of IPMN and progression of PDAC, other targets in this superfamily have been associated with cyst formation. The deletion of the genes such as Acvr1b (encoding activin A receptor type 1B), Tif1g (encoding transcription intermediary factor 1-gamma, which regulates SMAD4), and Tff2 (encoding trefoil Factor 2, an upstream element of SMAD4) in pancreas progenitor cells, and their cooperation with KrasG12D have been proven to induce IPMNs.
- (4)
- Concerning the SWI/SNF complex: Mutations of the SWI/SNF complex subunit genes have been found in 12–23% of human PDAC cases and reduced or lost expression of BRG1 (encoding Brahma protein-like 1) was observed in human IPMN [87]. Among the chromatin-remodeling complexes, homozygous deletion of Brg1 or Arid1a (encoding AT-rich interaction domain 1A) has been proved to elicit IPMN lesions in mouse models. A recent study showed that these two genes cooperate to inhibit the dedifferentiation of duct cells and the subsequent IPMN formation through the regulation of genes that sustain pancreatic duct cell identity, including Sox9 [78].
Unlike other genes that have similar consequences in the development of PanIN and IPMN, SWI/SNF subunit genes show complex expression in different cell types and at different time points [88,89]. Brg1 or Arid1a deletion inhibits KRAS-dependent acinar-to-ductal metaplasia (ADM) and PanIN development of acinar cells, but promotes the preneoplastic transformation in duct cells. Spontaneous PanIN formation is drastically attenuated in the Brg1 model [90] but can still be seen in the Arid1a model [91,92]. In addition, the malignant risk is higher and the cancer progresses faster in the Brg1 (3/7 at 9 w) knockout model than in the Arid1a knockout model (3/15 at 48 w), suggesting a more important role of Brg1 than Arid1a in invasive IPMN.
Furthermore, Ptf1a-CreERT2 (in which Ptf1a encodes pancreas-associated transcription factor 1a and CreERT2 is Cre recombinase (Cre) fused to a mutant estrogen ligand-binding domain), KrasG12D, and Arid1af/f mice treated with tamoxifen (a postnatal Acini-targeting model) developed PanINs but not IPMNs, while Hnf1b-CreERT2, KrasG12D, and Arid1af/f (a postnatal ductal cell-targeting model, in which Hnf1b encodes hepatocyte nuclear factor 1 beta) mice developed cystic lesions whose mucin expression pattern was similar to the IPMN in Ptf1a-Cre, KrasG12D, and Arid1af/f mice. Therefore, unlike the Tff2−/− model, in which the pancreatic duct gland cells are believed to be the cellular origin, mouse Arid1a or Brg1-deficient IPMNs have been proven to originate from the ductal compartment rather than from the acinar compartment [91].
- (5)
- Concerning the PI3K pathway: Loss of PTEN (encoding phosphatase and the tensin homolog, also known as phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase) expression occurs in human PDAC and is associated with poor prognosis of IPMN [93]. Combined Pten deletion and expression of oncogenic Kras in embryonic pancreatic precursor cells with Pdx1-Cre (in which Pdx1 encodes pancreatic and duodenal homeobox 1) failed to induce IPMN [94,95]. However, Kopp et al. found that IPMN only formed in response to postnatal ductal cell-specific, but not acinar cell-specific, Pten deletion [1]. This postnatal model can better mimic human IPMNs, which are usually solitary, and the lesions tend to occur in mature and highly differentiated cells rather than in progenitor cells. The postnatal homozygous deletion of Pten alone is able to generate IPMN, with faster progression to PDAC when combined with Kras mutations.
- (6)
- Concerning others: STK11 is a tumor suppressor gene that encodes serine–threonine kinase 11 (also known as liver kinase B1 (LKB1)), which is central to the control of cellular energy metabolism. Patients with heterozygous germline LKB1 mutations (i.e., patients with Peutz–Jeghers syndrome) show an elevated incidence of IPMN [96]. Collet et al. generated a new model driving IPMN formation from well-identified postnatal duct cells, termed tamoxifen-induced Sox9-CreER, LSL-KrasG12D, and Lkb1f/f [97], in which the loss of the LKB1 function was proven to suppress Wnt-signaling to generate IPMNs [98].
Another IPMN mouse model comprising the transgenic overexpression of Tgfα under the control of the pancreatic Elastase promoter (Ela-Tgfα), combined with the KRAS G12D mutation, is highly metastatic, with the rate of 50% at 6–8 months, predominantly in liver, lung, peritoneum, and lymph nodes [26]. This might suggest that the downstream epidermal growth factor receptor (EGFR)/signal transducer and activator of transcription 3 (STAT3)-signaling is critical for IPMN and disseminated metastases.

Table 2.
Genetically engineered mouse models of IPMN.
Table 2.
Genetically engineered mouse models of IPMN.
| GEM Models | Histological Type | Latency | Invasive | Targeted Cell Type [99] | Function | Metastases | References |
|---|---|---|---|---|---|---|---|
| Ptf1a-cre; LSL-KrasG12D; and CAG-LSL-GnasR201H; | Gastric and pancreatobiliary | 4–5 w | NA | Acinar, duct, and endocrine | GPCR | Die at 5–6 w | Taki et al., 2016 [82] |
| P48-Cre;LSL-KrasG12D and Rosa26R-LSL-rtTA- Tet-OGnasR201C | Pancreatobiliary | 10 w | 29% at 43 w | Acinar, duct, and endocrine | GPCR | 20% | Ideno et al., 2018 [83] |
| Ptf1a-Cre; LSL-KrasG12D; and Smad4f/f | Gastric | 8 w | 16.7% | Acinar, duct, and endocrine | TGFβ | NA | Bardeesy et al., 2006 [100] |
| Pdx1-Cre; LSL-KrasG12D; and Tif1γf/f | NA | 7 w | 0 at 13 w | Acinar, duct, and endocrine | TGFβ | NA | Vincent et al., 2009 [101] Vincent et al., 2012 [102] |
| Pdx1-Cre; LSL-KrasG12D; and Acvr1bf/f | NA | 12 w | 72% at 3–9 m | Acinar, duct, and endocrine | TGFβ | 9% | Qiu et al., 2016 [103] |
| Pdx1-Cre; LSL-KrasG12D; and Tff2−/− | Gastric | 6 w | 16.7% | Acinar, duct, and endocrine | TGFβ | 16.7% | Yamaguchi et al., 2016 [104] |
| Ptf1a-Cre; LSL-KrasG12D; and Brg1f/f | Pancreatobiliary | 9 w | 43% at 9 w, 71% at 18 w | Acinar, duct, and endocrine | SWI/SNF | NA | Von Figura et al., 2014 [105] |
| Ptf1a-Cre; KrasG12D; and Arid1af/f | Gastric pancreatobiliary and oncocytic | 12 w | 20% at 48 w | Acinar, duct, and endocrine | SWI/SNF | 3/19 | Wenjia Wang et al., 2019 [92] Kimura et al., 2018 [91] |
| Sox9-CreERT2 and Ptenf/f | Pancreatobiliary and oncocytic | 6–14 m | 31.5% | Duct | PI3K pathway | NA | Kopp et al., 2018 [1] |
| Sox9-CreERT2; LSL-KrasG12D; and Ptenf/+ | Mainly pancreatobiliary | 4–8 m | 70% | Duct | PI3K pathway | NA | Kopp et al., 2018 [1] |
| Sox9-CreER; LSL-KrasG12D; and Lkb1f/f | Gastric | 8 w | Yes | Duct | WNT/β-cat | NA | Collet et. al, 2019 [97] |
| P48-Cre; LSL- KrasG12D; and Ela-Tgfa | Pancreatobiliary | 12 w | Died at 7 m | Acinar, duct, and endocrine | TGFa/EGFR | 50% | Siveke et al., 2007 [26] |
Abbreviations: GEM, genetically engineered mouse models; N/A, not available; GPCR, G protein-coupled receptors; TGFs, transforming growth factors; SWI/SNF, SWItch/sucrose non-fermentable; w, weeks; Lkb1, liver kinase B1; PTEN, phosphatase and tensin homolog; EGFR, epidermal growth factor receptor; SOX9, SRY box 9; LSL, Lox-stop-lox; Arid1a, AT-rich interaction domain 1A; Brg1, encoding Brahma protein-like 1; Tff2, trefoil Factor 2; Acvr1b, activin A receptor type 1B; Tif1g, transcription intermediary factor 1-gamma; Ela, elastase; and Gnas, guanine nucleotide-binding protein-stimulating α subunit.
6. Conclusions and Future Perspectives
PDAC is the fourth leading cause of cancer-related death in the United States, with an approximately 9% five-year survival rate [106]. Little progress has been achieved in prolonging the survival for patients with pancreatic adenocarcinoma. Hence, special attention should be paid to pre-cancerous lesions, for instance, IPMNs.
We summarized key signal pathways or complexes (GPCR, TGF, SWI/SNF, WNT, and PI3K) in IPMN pathogenesis, which are able to elicit IPMN lesions in genetic mouse models. Many other effector genes involved in these pathways might have the potential to generate similar IPMN lesions in mice, which needs to be further tested in the future. Several recent mouse models targeting postnatal duct cells with the induction of tamoxifen provided evidence for the ductal origin of IPMN. These various murine models can serve as a preclinical platform to address prevailing questions, from the characterization and dissection of both histopathological and molecular features to the response to novel therapies.
However, pre-clinical mouse models have some intrinsic limitations. The genetic models are almost all based on bacteriophage-derived Cre recombinase, which means the oncogene activation and target ablation occur at the same time and in the same cells [99]. In addition, human IPMNs often display a dozen altered signal pathways, which cannot be attributed to one single gene. Among all the mouse models, the intestinal type of IPMN, which specifically expresses MUC2 and CDX2 [64], has not been successfully mimicked, representing a knowledge gap regarding the pathogenesis of the intestinal pathway. Future studies may have to focus on the exploration of mutation signatures in the intestinal type to identify the pivotal driver genes. It is important to note that this study only includes the mouse models based on the technology of the Cre recombinase system and there are other strategies, such as oncogenes [107] or the CRISPR/Cas9 [108,109] delivery-based system in the context of in vivo electroporation technology, to induce murine pancreatic neoplasms. This preclinical model is promising in developing more IPMN mouse models in the near future.
Although pancreatic surgery is the only effective method to treat IPMN, the malignant risk only decreases to a slight extent, leaving the remnant pancreas at high risk. Recent molecular findings based on next generation-sequencing indicated that IPMN is often heterogeneous and complex, and might generate adenocarcinoma inside or from a distant position that seems to be intact. Therefore, we propose a new concept: IPMN is not only a traditional lesion in situ but also represents dispersive damage, including changes to immune cells, cytokines, and stroma cells [110,111], which favor carcinogenesis whether the original IPMN is excised or not. Consequently, traditional therapy, such as surgery, is not sufficient when confronted with this intractable disease and more molecular therapies need to be developed to supplement surgery, given that adjuvant therapy for invasive IPMN has proven to be efficient in improving overall survival [112].
In conclusion, we reviewed the existing literature about IPMN, summarized its genetic characteristics and the mouse models involving mutations in specific pathways, and updated our current perception of how IPMN develops into a precursor of PDAC.
Author Contributions
Conceptualization, J.L. and T.L.; Writing—original draft preparation, J.L.; Writing—review and editing, J.L. and T.W.; Visualization, J.L., J.Z. and T.L.; Supervision, J.Z. and T.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Zhejiang Provincial Public Welfare Project (LGF20H160027), the National Natural Science Foundation of China (81802359, 81801640), the National Natural Science Foundation of China (U20A20378), the Zhejiang Provincial Key Research and Development Program (2019C03019), and Natural Science Foundation of Zhejiang Province (grant numbers LY21H100003).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kopp, J.L.; Dubois, C.L.; Schaeffer, D.F.; Samani, A.; Taghizadeh, F.; Cowan, R.W.; Rhim, A.D.; Stiles, B.L.; Valasek, M.; Sander, M. Loss of Pten and Activation of Kras Synergistically Induce Formation of Intraductal Papillary Mucinous Neoplasia From Pancreatic Ductal Cells in Mice. Gastroenterology 2018, 154, 1509–1523.e5. [Google Scholar] [CrossRef]
- de Jong, K.; Nio, C.Y.; Hermans, J.J.; Dijkgraaf, M.G.; Gouma, D.J.; van Eijck, C.H.; van Heel, E.; Klass, G.; Fockens, P.; Bruno, M.J. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin. Gastroenterol. Hepatol. 2010, 8, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Sekhar, A.; Rofsky, N.M.; Pedrosa, I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am. J. Gastroenterol. 2010, 105, 2079–2084. [Google Scholar] [CrossRef]
- Kosmahl, M.; Pauser, U.; Anlauf, M.; Sipos, B.; Peters, K.; Lüttges, J.; Klöppel, G. Cystic pancreas tumors and their classification: Features old and new. Der Pathol. 2005, 26, 22–30. [Google Scholar]
- Gnoni, A.; Licchetta, A.; Scarpa, A.; Azzariti, A.; Brunetti, A.E.; Simone, G.; Nardulli, P.; Santini, D.; Aieta, M.; Delcuratolo, S.; et al. Carcinogenesis of pancreatic adenocarcinoma: Precursor lesions. Int. J. Mol. Sci. 2013, 14, 19731–19762. [Google Scholar] [CrossRef]
- Scheiman, J.M.; Hwang, J.H.; Moayyedi, P. American gastroenterological association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015, 148, 824–848.e22. [Google Scholar] [CrossRef]
- Chandwani, R.; Allen, P.J. Cystic Neoplasms of the Pancreas. Annu. Rev. Med. 2016, 67, 45–57. [Google Scholar] [CrossRef]
- Hruban, R.H.; Maitra, A.; Kern, S.E.; Goggins, M. Precursors to pancreatic cancer. Gastroenterol. Clin. N. Am. 2007, 36, 831–849. [Google Scholar] [CrossRef]
- Maire, F.; Hammel, P.; Terris, B.; Olschwang, S.; O’Toole, D.; Sauvanet, A.; Palazzo, L.; Ponsot, P.; Laplane, B.; Lévy, P.; et al. Intraductal papillary and mucinous pancreatic tumour: A new extracolonic tumour in familial adenomatous polyposis. Gut 2002, 51, 446–449. [Google Scholar] [CrossRef]
- Capurso, G.; Boccia, S.; Salvia, R.; Del Chiaro, M.; Frulloni, L.; Arcidiacono, P.G.; Zerbi, A.; Manta, R.; Fabbri, C.; Ventrucci, M.; et al. Risk factors for intraductal papillary mucinous neoplasm (IPMN) of the pancreas: A multicentre case-control study. Am. J. Gastroenterol. 2013, 108, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Grützmann, R.; Niedergethmann, M.; Pilarsky, C.; Klöppel, G.; Saeger, H.D. Intraductal papillary mucinous tumors of the pancreas: Biology, diagnosis, and treatment. Oncologist 2010, 15, 1294–1309. [Google Scholar] [CrossRef]
- Brugge, W.R.; Lauwers, G.Y.; Sahani, D.; Fernandez-del Castillo, C.; Warshaw, A.L. Cystic neoplasms of the pancreas. N. Engl. J. Med. 2004, 351, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Hruban, R.H.; Takaori, K.; Klimstra, D.S.; Adsay, N.V.; Albores-Saavedra, J.; Biankin, A.V.; Biankin, S.A.; Compton, C.; Fukushima, N.; Furukawa, T.; et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am. J. Surg. Pathol. 2004, 28, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Bournet, B.; Kirzin, S.; Carrère, N.; Portier, G.; Otal, P.; Selves, J.; Musso, C.; Suc, B.; Moreau, J.; Fourtanier, G.; et al. Clinical fate of branch duct and mixed forms of intraductal papillary mucinous neoplasia of the pancreas. J. Gastroenterol. Hepatol. 2009, 24, 1211–1217. [Google Scholar] [CrossRef]
- Crippa, S.; Fernández-Del Castillo, C.; Salvia, R.; Finkelstein, D.; Bassi, C.; Domínguez, I.; Muzikansky, A.; Thayer, S.P.; Falconi, M.; Mino-Kenudson, M.; et al. Mucin-producing neoplasms of the pancreas: An analysis of distinguishing clinical and epidemiologic characteristics. Clin. Gastroenterol. Hepatol. 2010, 8, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.W.; Jang, J.Y.; Lee, S.E.; Lim, C.S.; Lee, K.U.; Kim, S.W. Clinicopathologic analysis of surgically proven intraductal papillary mucinous neoplasms of the pancreas in SNUH: A 15-year experience at a single academic institution. Langenbeck’s Arch. Surg. 2012, 397, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Fernández-del Castillo, C.; Adsay, V.; Chari, S.; Falconi, M.; Jang, J.Y.; Kimura, W.; Levy, P.; Pitman, M.B.; Schmidt, C.M.; et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012, 12, 183–197. [Google Scholar] [CrossRef]
- Nakagawa, A.; Yamaguchi, T.; Ohtsuka, M.; Ishihara, T.; Sudo, K.; Nakamura, K.; Hara, T.; Denda, T.; Miyazaki, M. Usefulness of multidetector computed tomography for detecting protruding lesions in intraductal papillary mucinous neoplasm of the pancreas in comparison with single-detector computed tomography and endoscopic ultrasonography. Pancreas 2009, 38, 131–136. [Google Scholar] [CrossRef]
- Stark, A.; Donahue, T.R.; Reber, H.A.; Hines, O.J. Pancreatic Cyst Disease: A Review. JAMA 2016, 315, 1882–1893. [Google Scholar] [CrossRef]
- van Huijgevoort, N.C.M.; Del Chiaro, M.; Wolfgang, C.L.; van Hooft, J.E.; Besselink, M.G. Diagnosis and management of pancreatic cystic neoplasms: Current evidence and guidelines. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Vege, S.S.; Ziring, B.; Jain, R.; Moayyedi, P. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015, 148, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Fernández-Del Castillo, C.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.L. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017, 17, 738–753. [Google Scholar] [CrossRef]
- European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018, 67, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Crippa, S.; Bassi, C.; Salvia, R.; Malleo, G.; Marchegiani, G.; Rebours, V.; Levy, P.; Partelli, S.; Suleiman, S.L.; Banks, P.A.; et al. Low progression of intraductal papillary mucinous neoplasms with worrisome features and high-risk stigmata undergoing non-operative management: A mid-term follow-up analysis. Gut 2017, 66, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Skaro, M.; Nanda, N.; Gauthier, C.; Felsenstein, M.; Jiang, Z.; Qiu, M.; Shindo, K.; Yu, J.; Hutchings, D.; Javed, A.A.; et al. Prevalence of Germline Mutations Associated With Cancer Risk in Patients With Intraductal Papillary Mucinous Neoplasms. Gastroenterology 2019, 156, 1905–1913. [Google Scholar] [CrossRef] [PubMed]
- Siveke, J.T.; Einwächter, H.; Sipos, B.; Lubeseder-Martellato, C.; Klöppel, G.; Schmid, R.M. Concomitant pancreatic activation of Kras(G12D) and Tgfa results in cystic papillary neoplasms reminiscent of human IPMN. Cancer Cell 2007, 12, 266–279. [Google Scholar] [CrossRef]
- Furukawa, T.; Klöppel, G.; Volkan Adsay, N.; Albores-Saavedra, J.; Fukushima, N.; Horii, A.; Hruban, R.H.; Kato, Y.; Klimstra, D.S.; Longnecker, D.S.; et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: A consensus study. Virchows Arch. Int. J. Pathol. 2005, 447, 794–799. [Google Scholar] [CrossRef]
- Furukawa, T.; Hatori, T.; Fujita, I.; Yamamoto, M.; Kobayashi, M.; Ohike, N.; Morohoshi, T.; Egawa, S.; Unno, M.; Takao, S.; et al. Prognostic relevance of morphological types of intraductal papillary mucinous neoplasms of the pancreas. Gut 2011, 60, 509–516. [Google Scholar] [CrossRef]
- Xiao, H.D.; Yamaguchi, H.; Dias-Santagata, D.; Kuboki, Y.; Akhavanfard, S.; Hatori, T.; Yamamoto, M.; Shiratori, K.; Kobayashi, M.; Shimizu, M.; et al. Molecular characteristics and biological behaviours of the oncocytic and pancreatobiliary subtypes of intraductal papillary mucinous neoplasms. J. Pathol. 2011, 224, 508–516. [Google Scholar] [CrossRef]
- Ban, S.; Naitoh, Y.; Mino-Kenudson, M.; Sakurai, T.; Kuroda, M.; Koyama, I.; Lauwers, G.Y.; Shimizu, M. Intraductal papillary mucinous neoplasm (IPMN) of the pancreas: Its histopathologic difference between 2 major types. Am. J. Surg. Pathol. 2006, 30, 1561–1569. [Google Scholar] [CrossRef]
- Wu, J.; Matthaei, H.; Maitra, A.; Dal Molin, M.; Wood, L.D.; Eshleman, J.R.; Goggins, M.; Canto, M.I.; Schulick, R.D.; Edil, B.H.; et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci. Transl. Med. 2011, 3, 92ra66. [Google Scholar] [CrossRef]
- Furukawa, T.; Kuboki, Y.; Tanji, E.; Yoshida, S.; Hatori, T.; Yamamoto, M.; Shibata, N.; Shimizu, K.; Kamatani, N.; Shiratori, K. Whole-exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci. Rep. 2011, 1, 161. [Google Scholar] [CrossRef]
- Amato, E.; Molin, M.D.; Mafficini, A.; Yu, J.; Malleo, G.; Rusev, B.; Fassan, M.; Antonello, D.; Sadakari, Y.; Castelli, P.; et al. Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas. J. Pathol. 2014, 233, 217–227. [Google Scholar] [CrossRef]
- Springer, S.; Wang, Y.; Dal Molin, M.; Masica, D.L.; Jiao, Y.; Kinde, I.; Blackford, A.; Raman, S.P.; Wolfgang, C.L.; Tomita, T.; et al. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology 2015, 149, 1501–1510. [Google Scholar] [CrossRef]
- Basturk, O.; Berger, M.F.; Yamaguchi, H.; Adsay, V.; Askan, G.; Bhanot, U.K.; Zehir, A.; Carneiro, F.; Hong, S.M.; Zamboni, G.; et al. Pancreatic intraductal tubulopapillary neoplasm is genetically distinct from intraductal papillary mucinous neoplasm and ductal adenocarcinoma. Mod. Pathol. 2017, 30, 1760–1772. [Google Scholar] [CrossRef] [PubMed]
- O’Hayre, M.; Vázquez-Prado, J.; Kufareva, I.; Stawiski, E.W.; Handel, T.M.; Seshagiri, S.; Gutkind, J.S. The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat. Rev. Cancer 2013, 13, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Patra, K.C.; Bardeesy, N.; Mizukami, Y. Diversity of Precursor Lesions For Pancreatic Cancer: The Genetics and Biology of Intraductal Papillary Mucinous Neoplasm. Clin. Transl. Gastroenterol. 2017, 8, e86. [Google Scholar] [CrossRef] [PubMed]
- Omori, Y.; Ono, Y.; Tanino, M.; Karasaki, H.; Yamaguchi, H.; Furukawa, T.; Enomoto, K.; Ueda, J.; Sumi, A.; Katayama, J.; et al. Pathways of Progression From Intraductal Papillary Mucinous Neoplasm to Pancreatic Ductal Adenocarcinoma Based on Molecular Features. Gastroenterology 2019, 156, 647–661.e2. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, W.; Sasaki, E.; Murakami, Y.; Yamao, K.; Shimizu, Y.; Yatabe, Y. GNAS mutation is a frequent event in pancreatic intraductal papillary mucinous neoplasms and associated adenocarcinomas. Virchows Arch. Int. J. Pathol. 2015, 466, 665–674. [Google Scholar] [CrossRef]
- Molin, M.D.; Matthaei, H.; Wu, J.; Blackford, A.; Debeljak, M.; Rezaee, N.; Wolfgang, C.L.; Butturini, G.; Salvia, R.; Bassi, C.; et al. Clinicopathological correlates of activating GNAS mutations in intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann. Surg. Oncol. 2013, 20, 3802–3808. [Google Scholar] [CrossRef]
- Jiang, X.; Hao, H.X.; Growney, J.D.; Woolfenden, S.; Bottiglio, C.; Ng, N.; Lu, B.; Hsieh, M.H.; Bagdasarian, L.; Meyer, R.; et al. Inactivating mutations of RNF43 confer Wnt dependency in pancreatic ductal adenocarcinoma. Proc. Natl. Acad. Sci. USA 2013, 110, 12649–12654. [Google Scholar] [CrossRef]
- Mishra, A.; Emamgholi, F.; Erlangga, Z.; Hartleben, B.; Unger, K.; Wolff, K.; Teichmann, U.; Kessel, M.; Woller, N.; Kühnel, F.; et al. Generation of focal mutations and large genomic deletions in the pancreas using inducible in vivo genome editing. Carcinogenesis 2020, 41, 334–344. [Google Scholar] [CrossRef]
- Fujikura, K.; Hosoda, W.; Felsenstein, M.; Song, Q.; Reiter, J.G.; Zheng, L.; Beleva Guthrie, V.; Rincon, N.; Dal Molin, M.; Dudley, J.; et al. Multiregion whole-exome sequencing of intraductal papillary mucinous neoplasms reveals frequent somatic mutations predominantly in low-grade regions. Gut 2021, 70, 928–939. [Google Scholar] [CrossRef]
- Yang, K.S.; Ciprani, D.; O’Shea, A.; Liss, A.S.; Yang, R.; Fletcher-Mercaldo, S.; Mino-Kenudson, M.; Fernández-Del Castillo, C.; Weissleder, R. Extracellular Vesicle Analysis Allows for Identification of Invasive IPMN. Gastroenterology 2021, 160, 1345–1358. [Google Scholar] [CrossRef]
- Fischer, C.G.; Beleva Guthrie, V.; Braxton, A.M.; Zheng, L.; Wang, P.; Song, Q.; Griffin, J.F.; Chianchiano, P.E.; Hosoda, W.; Niknafs, N.; et al. Intraductal Papillary Mucinous Neoplasms Arise From Multiple Independent Clones, Each With Distinct Mutations. Gastroenterology 2019, 157, 1123–1137. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.J.; Iacobuzio-Donahue, C.A.; Klimstra, D.S. Cyst Fluid Analysis in Pancreatic Intraductal Papillary Mucinous Neoplasms. Clin. Cancer Res. 2016, 22, 4966–4967. [Google Scholar] [CrossRef][Green Version]
- Singhi, A.D.; McGrath, K.; Brand, R.E.; Khalid, A.; Zeh, H.J.; Chennat, J.S.; Fasanella, K.E.; Papachristou, G.I.; Slivka, A.; Bartlett, D.L.; et al. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut 2018, 67, 2131–2141. [Google Scholar] [CrossRef] [PubMed]
- Das, K.K.; Geng, X.; Brown, J.W.; Morales-Oyarvide, V.; Huynh, T.; Pergolini, I.; Pitman, M.B.; Ferrone, C.; Al Efishat, M.; Haviland, D.; et al. Cross Validation of the Monoclonal Antibody Das-1 in Identification of High-Risk Mucinous Pancreatic Cystic Lesions. Gastroenterology 2019, 157, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Kanda, M.; Knight, S.; Topazian, M.; Syngal, S.; Farrell, J.; Lee, J.; Kamel, I.; Lennon, A.M.; Borges, M.; Young, A.; et al. Mutant GNAS detected in duodenal collections of secretin-stimulated pancreatic juice indicates the presence or emergence of pancreatic cysts. Gut 2013, 62, 1024–1033. [Google Scholar] [CrossRef][Green Version]
- Majumder, S.; Raimondo, M.; Taylor, W.R.; Yab, T.C.; Berger, C.K.; Dukek, B.A.; Cao, X.; Foote, P.H.; Wu, C.W.; Devens, M.E.; et al. Methylated DNA in Pancreatic Juice Distinguishes Patients With Pancreatic Cancer From Controls. Clin. Gastroenterol. Hepatol. 2019, 18, 676–683. [Google Scholar] [CrossRef]
- Berger, A.W.; Schwerdel, D.; Costa, I.G.; Hackert, T.; Strobel, O.; Lam, S.; Barth, T.F.; Schröppel, B.; Meining, A.; Büchler, M.W.; et al. Detection of Hot-Spot Mutations in Circulating Cell-Free DNA From Patients With Intraductal Papillary Mucinous Neoplasms of the Pancreas. Gastroenterology 2016, 151, 267–270. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, X.; Zhang, Q.; Yang, J.; Chen, Q.; Wang, J.; Li, X.; Chen, J.; Ma, T.; Li, G.; et al. Vimentin-positive circulating tumor cells as a biomarker for diagnosis and treatment monitoring in patients with pancreatic cancer. Cancer Lett. 2019, 452, 237–243. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, H.; Wang, H.; Xu, C.; Zhou, S.; Zhao, J.; Shen, S.; Xu, G.; Wang, L.; Zou, X.; et al. Endoscopic Ultrasound-Guided Acquisition of Portal Venous Circulating Tumor Cells as a Potential Diagnostic and Prognostic Tool for Pancreatic Cancer. Cancer Manag. Res. 2021, 13, 7649–7661. [Google Scholar] [CrossRef]
- Yu, J.; Sadakari, Y.; Shindo, K.; Suenaga, M.; Brant, A.; Almario, J.A.N.; Borges, M.; Barkley, T.; Fesharakizadeh, S.; Ford, M.; et al. Digital next-generation sequencing identifies low-abundance mutations in pancreatic juice samples collected from the duodenum of patients with pancreatic cancer and intraductal papillary mucinous neoplasms. Gut 2017, 66, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Rhim, A.D.; Thege, F.I.; Santana, S.M.; Lannin, T.B.; Saha, T.N.; Tsai, S.; Maggs, L.R.; Kochman, M.L.; Ginsberg, G.G.; Lieb, J.G.; et al. Detection of circulating pancreas epithelial cells in patients with pancreatic cystic lesions. Gastroenterology 2014, 146, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Gómez, V.; Majumder, S.; Smyrk, T.C.; Topazian, M.D.; Chari, S.T.; Gleeson, F.C.; Harmsen, W.S.; Enders, F.T.; Abu Dayyeh, B.K.; Iyer, P.G.; et al. Pancreatic cyst epithelial denudation: A natural phenomenon in the absence of treatment. Gastrointest. Endosc. 2016, 84, 788–793. [Google Scholar] [CrossRef]
- Date, K.; Ohtsuka, T.; Fujimoto, T.; Tamura, K.; Kimura, H.; Matsunaga, T.; Mochidome, N.; Miyazaki, T.; Mori, Y.; Oda, Y.; et al. Molecular Evidence for Monoclonal Skip Progression in Main Duct Intraductal Papillary Mucinous Neoplasms of the Pancreas. Ann. Surg. 2017, 265, 969–977. [Google Scholar] [CrossRef]
- Noë, M.; Niknafs, N.; Fischer, C.G.; Hackeng, W.M.; Beleva Guthrie, V.; Hosoda, W.; Debeljak, M.; Papp, E.; Adleff, V.; White, J.R.; et al. Genomic characterization of malignant progression in neoplastic pancreatic cysts. Nat. Commun. 2020, 11, 4085. [Google Scholar] [CrossRef]
- Kuboki, Y.; Fischer, C.G.; Beleva Guthrie, V.; Huang, W.; Yu, J.; Chianchiano, P.; Hosoda, W.; Zhang, H.; Zheng, L.; Shao, X.; et al. Single-cell sequencing defines genetic heterogeneity in pancreatic cancer precursor lesions. J. Pathol. 2019, 247, 347–356. [Google Scholar] [CrossRef]
- Felsenstein, M.; Noë, M.; Masica, D.L.; Hosoda, W.; Chianchiano, P.; Fischer, C.G.; Lionheart, G.; Brosens, L.A.A.; Pea, A.; Yu, J.; et al. IPMNs with co-occurring invasive cancers: Neighbours but not always relatives. Gut 2018, 67, 1652–1662. [Google Scholar] [CrossRef]
- Pea, A.; Yu, J.; Rezaee, N.; Luchini, C.; He, J.; Dal Molin, M.; Griffin, J.F.; Fedor, H.; Fesharakizadeh, S.; Salvia, R.; et al. Targeted DNA Sequencing Reveals Patterns of Local Progression in the Pancreatic Remnant Following Resection of Intraductal Papillary Mucinous Neoplasm (IPMN) of the Pancreas. Ann. Surg. 2017, 266, 133–141. [Google Scholar] [CrossRef]
- Choi, S.H.; Park, S.H.; Kim, K.W.; Lee, J.Y.; Lee, S.S. Progression of Unresected Intraductal Papillary Mucinous Neoplasms of the Pancreas to Cancer: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2017, 15, 1509–1520.e4. [Google Scholar] [CrossRef]
- Marchegiani, G.; Mino-Kenudson, M.; Ferrone, C.R.; Morales-Oyarvide, V.; Warshaw, A.L.; Lillemoe, K.D.; Castillo, C.F. Patterns of Recurrence After Resection of IPMN: Who, When, and How? Ann. Surg. 2015, 262, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Adsay, N.V.; Merati, K.; Basturk, O.; Iacobuzio-Donahue, C.; Levi, E.; Cheng, J.D.; Sarkar, F.H.; Hruban, R.H.; Klimstra, D.S. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: Delineation of an “intestinal” pathway of carcinogenesis in the pancreas. Am. J. Surg. Pathol. 2004, 28, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Oyama, H.; Tada, M.; Takagi, K.; Tateishi, K.; Hamada, T.; Nakai, Y.; Hakuta, R.; Ijichi, H.; Ishigaki, K.; Kanai, S.; et al. Long-term Risk of Malignancy in Branch-Duct Intraductal Papillary Mucinous Neoplasms. Gastroenterology 2020, 158, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Marchegiani, G.; Andrianello, S.; Pollini, T.; Caravati, A.; Biancotto, M.; Secchettin, E.; Bonamini, D.; Malleo, G.; Bassi, C.; Salvia, R. “Trivial” Cysts Redefine the Risk of Cancer in Presumed Branch-Duct Intraductal Papillary Mucinous Neoplasms of the Pancreas: A Potential Target for Follow-Up Discontinuation? Am. J. Gastroenterol. 2019, 114, 1678–1684. [Google Scholar] [CrossRef]
- Barthet, M.; Giovannini, M.; Lesavre, N.; Boustiere, C.; Napoleon, B.; Koch, S.; Gasmi, M.; Vanbiervliet, G.; Gonzalez, J.-M. Endoscopic ultrasound-guided radiofrequency ablation for pancreatic neuroendocrine tumors and pancreatic cystic neoplasms: A prospective multicenter study. Endoscopy 2019, 51, 836–842. [Google Scholar] [CrossRef]
- Ratnayake, C.B.; Biela, C.; Windsor, J.A.; Pandanaboyana, S. Enucleation for branch duct intraductal papillary mucinous neoplasms: A systematic review and meta-analysis. HPB (Oxf.) 2019, 21, 1593–1602. [Google Scholar] [CrossRef]
- Choi, J.-H.; Seo, D.W.; Song, T.J.; Park, D.H.; Lee, S.S.; Lee, S.K.; Kim, M.-H. Long-term outcomes after endoscopic ultrasound-guided ablation of pancreatic cysts. Endoscopy 2017, 49, 866–873. [Google Scholar] [CrossRef]
- Kaiser, J.; Fritz, S.; Klauss, M.; Bergmann, F.; Hinz, U.; Strobel, O.; Schneider, L.; Büchler, M.W.; Hackert, T. Enucleation: A treatment alternative for branch duct intraductal papillary mucinous neoplasms. Surgery 2017, 161, 602–610. [Google Scholar] [CrossRef]
- Oh, H.-C.; Seo, D.W. Endoscopic ultrasonography-guided pancreatic cyst ablation (with video). J. Hepatobiliary Pancreat. Sci. 2015, 22, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Marchegiani, G.; Pollini, T.; Andrianello, S.; Tomasoni, G.; Biancotto, M.; Javed, A.A.; Kinny-Köster, B.; Amini, N.; Han, Y.; Kim, H.; et al. Progression vs Cyst Stability of Branch-Duct Intraductal Papillary Mucinous Neoplasms After Observation and Surgery. JAMA Surg. 2021. [Google Scholar] [CrossRef]
- Gengenbacher, N.; Singhal, M.; Augustin, H.G. Preclinical mouse solid tumour models: Status quo, challenges and perspectives. Nat. Rev. Cancer 2017, 17, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Carpenter, E.S.; Takeuchi, K.K.; Halbrook, C.J.; Peverley, L.V.; Bien, H.; Hall, J.C.; DelGiorno, K.E.; Pal, D.; Song, Y.; et al. PI3K regulation of RAC1 is required for KRAS-induced pancreatic tumorigenesis in mice. Gastroenterology 2014, 147, 1405–1416.e7. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A.J.; Bardeesy, N.; Sinha, M.; Lopez, L.; Tuveson, D.A.; Horner, J.; Redston, M.S.; DePinho, R.A. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003, 17, 3112–3126. [Google Scholar] [CrossRef]
- Hezel, A.F.; Kimmelman, A.C.; Stanger, B.Z.; Bardeesy, N.; Depinho, R.A. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006, 20, 1218–1249. [Google Scholar] [CrossRef] [PubMed]
- Jackson, E.L.; Willis, N.; Mercer, K.; Bronson, R.T.; Crowley, D.; Montoya, R.; Jacks, T.; Tuveson, D.A. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001, 15, 3243–3248. [Google Scholar] [CrossRef]
- Roy, N.; Malik, S.; Villanueva, K.E.; Urano, A.; Lu, X.; Von Figura, G.; Seeley, E.S.; Dawson, D.W.; Collisson, E.A.; Hebrok, M. Brg1 promotes both tumor-suppressive and oncogenic activities at distinct stages of pancreatic cancer formation. Genes Dev. 2015, 29, 658–671. [Google Scholar] [CrossRef]
- Lee, A.Y.L.; Dubois, C.L.; Sarai, K.; Zarei, S.; Schaeffer, D.F.; Sander, M.; Kopp, J.L. Cell of origin affects tumour development and phenotype in pancreatic ductal adenocarcinoma. Gut 2018, 68, gutjnl-2017-314426. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, Q.V.; Lee, K.; Rosen, B.P.; González, F.; Soh, C.L.; Huangfu, D. Genome Editing of Lineage Determinants in Human Pluripotent Stem Cells Reveals Mechanisms of Pancreatic Development and Diabetes. Cell Stem Cell 2016, 18, 755–768. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Cooper, B.; Gannon, M.; Ray, M.; MacDonald, R.J.; Wright, C.V.E. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet 2002, 32, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Taki, K.; Ohmuraya, M.; Tanji, E.; Komatsu, H.; Hashimoto, D.; Semba, K.; Araki, K.; Kawaguchi, Y.; Baba, H.; Furukawa, T. GNAS(R201H) and Kras(G12D) cooperate to promote murine pancreatic tumorigenesis recapitulating human intraductal papillary mucinous neoplasm. Oncogene 2016, 35, 2407–2412. [Google Scholar] [CrossRef] [PubMed]
- Ideno, N.; Yamaguchi, H.; Ghosh, B.; Gupta, S.; Okumura, T.; Steffen, D.J.; Fisher, C.G.; Wood, L.D.; Singhi, A.D.; Nakamura, M.; et al. GNAS Induces Pancreatic Cystic Neoplasms in Mice That Express Activated KRAS by Inhibiting YAP1 Signaling. Gastroenterology 2018, 155, 1593–1607.e12. [Google Scholar] [CrossRef] [PubMed]
- Bierie, B.; Moses, H.L. Tumour microenvironment: TGFbeta: The molecular Jekyll and Hyde of cancer. Nat. Rev. Cancer 2006, 6, 506–520. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Maitra, A. EMT: Matter of Life or Death? Cell 2016, 164, 840–842. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Dal Molin, M.; Hong, S.-M.; Hebbar, S.; Sharma, R.; Scrimieri, F.; de Wilde, R.F.; Mayo, S.C.; Goggins, M.; Wolfgang, C.L.; Schulick, R.D.; et al. Loss of expression of the SWI/SNF chromatin remodeling subunit BRG1/SMARCA4 is frequently observed in intraductal papillary mucinous neoplasms of the pancreas. Hum. Pathol. 2012, 43, 585–591. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Livshits, G.; Alonso-Curbelo, D.; Morris, J.P.; Koche, R.; Saborowski, M.; Wilkinson, J.E.; Lowe, S.W. Arid1a restrains Kras-dependent changes in acinar cell identity. Elife 2018, 7, e35216. [Google Scholar] [CrossRef]
- Wang, S.C.; Nassour, I.; Xiao, S.; Zhang, S.; Luo, X.; Lee, J.; Li, L.; Sun, X.; Nguyen, L.H.; Chuang, J.-C.; et al. SWI/SNF component restrains pancreatic neoplasia formation. Gut 2019, 68, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Fukuda, A.; Roy, N.; Hiramatsu, Y.; Leonhardt, L.; Kakiuchi, N.; Hoyer, K.; Ogawa, S.; Goto, N.; Ikuta, K.; et al. The BRG1/SOX9 axis is critical for acinar cell-derived pancreatic tumorigenesis. J. Clin. Investig. 2018, 128, 3475–3489. [Google Scholar] [CrossRef]
- Kimura, Y.; Fukuda, A.; Ogawa, S.; Maruno, T.; Takada, Y.; Tsuda, M.; Hiramatsu, Y.; Araki, O.; Nagao, M.; Yoshikawa, T.; et al. ARID1A Maintains Differentiation of Pancreatic Ductal Cells and Inhibits Development of Pancreatic Ductal Adenocarcinoma in Mice. Gastroenterology 2018, 155, 194–209.e2. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Friedland, S.C.; Guo, B.; O’Dell, M.R.; Alexander, W.B.; Whitney-Miller, C.L.; Agostini-Vulaj, D.; Huber, A.R.; Myers, J.R.; Ashton, J.M.; et al. ARID1A, a SWI/SNF subunit, is critical to acinar cell homeostasis and regeneration and is a barrier to transformation and epithelial-mesenchymal transition in the pancreas. Gut 2019, 68, 1245–1258. [Google Scholar] [CrossRef]
- Garcia-Carracedo, D.; Turk, A.T.; Fine, S.A.; Akhavan, N.; Tweel, B.C.; Parsons, R.; Chabot, J.A.; Allendorf, J.D.; Genkinger, J.M.; Remotti, H.E.; et al. Loss of PTEN expression is associated with poor prognosis in patients with intraductal papillary mucinous neoplasms of the pancreas. Clin. Cancer Res. 2013, 19, 6830–6841. [Google Scholar] [CrossRef]
- Ying, H.; Elpek, K.G.; Vinjamoori, A.; Zimmerman, S.M.; Chu, G.C.; Yan, H.; Fletcher-Sananikone, E.; Zhang, H.; Liu, Y.; Wang, W.; et al. PTEN is a major tumor suppressor in pancreatic ductal adenocarcinoma and regulates an NF-κB-cytokine network. Cancer Discov. 2011, 1, 158–169. [Google Scholar] [CrossRef]
- Hill, R.; Calvopina, J.H.; Kim, C.; Wang, Y.; Dawson, D.W.; Donahue, T.R.; Dry, S.; Wu, H. PTEN loss accelerates KrasG12D-induced pancreatic cancer development. Cancer Res. 2010, 70, 7114–7124. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Rosty, C.; Jansen, M.; Fukushima, N.; Ueki, T.; Yeo, C.J.; Cameron, J.L.; Iacobuzio-Donahue, C.A.; Hruban, R.H.; Goggins, M. STK11/LKB1 Peutz-Jeghers gene inactivation in intraductal papillary-mucinous neoplasms of the pancreas. Am. J. Pathol. 2001, 159, 2017–2022. [Google Scholar] [CrossRef]
- Collet, L.; Ghurburrun, E.; Meyers, N.; Assi, M.; Pirlot, B.; Leclercq, I.A.; Couvelard, A.; Komuta, M.; Cros, J.; Demetter, P.; et al. Kras and Lkb1 mutations synergistically induce intraductal papillary mucinous neoplasm derived from pancreatic duct cells. Gut 2020, 69, 704–714. [Google Scholar] [CrossRef]
- Jacob, L.S.; Wu, X.; Dodge, M.E.; Fan, C.W.; Kulak, O.; Chen, B.; Tang, W.; Wang, B.; Amatruda, J.F.; Lum, L. Genome-wide RNAi screen reveals disease-associated genes that are common to Hedgehog and Wnt signaling. Sci. Signal. 2011, 4, ra4. [Google Scholar] [CrossRef]
- Guerra, C.; Barbacid, M. Genetically engineered mouse models of pancreatic adenocarcinoma. Mol. Oncol. 2013, 7, 232–247. [Google Scholar] [CrossRef] [PubMed]
- Bardeesy, N.; Cheng, K.H.; Berger, J.H.; Chu, G.C.; Pahler, J.; Olson, P.; Hezel, A.F.; Horner, J.; Lauwers, G.Y.; Hanahan, D.; et al. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 2006, 20, 3130–3146. [Google Scholar] [CrossRef]
- Vincent, D.F.; Yan, K.P.; Treilleux, I.; Gay, F.; Arfi, V.; Kaniewski, B.; Marie, J.C.; Lepinasse, F.; Martel, S.; Goddard-Leon, S.; et al. Inactivation of TIF1gamma cooperates with Kras to induce cystic tumors of the pancreas. PLoS Genet. 2009, 5, e1000575. [Google Scholar] [CrossRef]
- Vincent, D.F.; Gout, J.; Chuvin, N.; Arfi, V.; Pommier, R.M.; Bertolino, P.; Jonckheere, N.; Ripoche, D.; Kaniewski, B.; Martel, S.; et al. Tif1γ suppresses murine pancreatic tumoral transformation by a Smad4-independent pathway. Am. J. Pathol. 2012, 180, 2214–2221. [Google Scholar] [CrossRef]
- Qiu, W.; Tang, S.M.; Lee, S.; Turk, A.T.; Sireci, A.N.; Qiu, A.; Rose, C.; Xie, C.; Kitajewski, J.; Wen, H.J.; et al. Loss of Activin Receptor Type 1B Accelerates Development of Intraductal Papillary Mucinous Neoplasms in Mice With Activated KRAS. Gastroenterology 2016, 150, 218–228.e12. [Google Scholar] [CrossRef]
- Yamaguchi, J.; Mino-Kenudson, M.; Liss, A.S.; Chowdhury, S.; Wang, T.C.; Fernández-Del Castillo, C.; Lillemoe, K.D.; Warshaw, A.L.; Thayer, S.P. Loss of Trefoil Factor 2 From Pancreatic Duct Glands Promotes Formation of Intraductal Papillary Mucinous Neoplasms in Mice. Gastroenterology 2016, 151, 1232–1244.e10. [Google Scholar] [CrossRef]
- von Figura, G.; Fukuda, A.; Roy, N.; Liku, M.E.; Morris Iv, J.P.; Kim, G.E.; Russ, H.A.; Firpo, M.A.; Mulvihill, S.J.; Dawson, D.W.; et al. The chromatin regulator Brg1 suppresses formation of intraductal papillary mucinous neoplasm and pancreatic ductal adenocarcinoma. Nat. Cell Biol. 2014, 16, 255–267. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA A Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Lim, K.-M.; Park, S.G.; Jung, S.Y.; Choi, H.-J.; Lee, D.H.; Kim, W.-J.; Hong, S.-M.; Yu, E.-S.; Son, W.-C. Pancreatic cancer induced by in vivo electroporation-enhanced sleeping beauty transposon gene delivery system in mouse. Pancreas 2014, 43, 614–618. [Google Scholar] [CrossRef]
- Maresch, R.; Mueller, S.; Veltkamp, C.; Öllinger, R.; Friedrich, M.; Heid, I.; Steiger, K.; Weber, J.; Engleitner, T.; Barenboim, M.; et al. Multiplexed pancreatic genome engineering and cancer induction by transfection-based CRISPR/Cas9 delivery in mice. Nat. Commun. 2016, 7, 10770. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, C.; Tong, F.; Deng, J.; Huang, G.; Sang, Y. Review of applications of CRISPR-Cas9 gene-editing technology in cancer research. Biol Proced. Online 2021, 23, 14. [Google Scholar] [CrossRef]
- Hernandez-Barco, Y.G.; Bardeesy, N.; Ting, D.T. No Cell Left Unturned: Intraductal Papillary Mucinous Neoplasm Heterogeneity. Clin. Cancer Res. 2019, 25, 2027–2029. [Google Scholar] [CrossRef] [PubMed]
- Bernard, V.; Semaan, A.; Huang, J.; San Lucas, F.A.; Mulu, F.C.; Stephens, B.M.; Guerrero, P.A.; Huang, Y.; Zhao, J.; Kamyabi, N.; et al. Single-Cell Transcriptomics of Pancreatic Cancer Precursors Demonstrates Epithelial and Microenvironmental Heterogeneity as an Early Event in Neoplastic Progression. Clin. Cancer Res. 2019, 25, 2194–2205. [Google Scholar] [CrossRef] [PubMed]
- McMillan, M.T.; Lewis, R.S.; Drebin, J.A.; Teitelbaum, U.R.; Lee, M.K.; Roses, R.E.; Fraker, D.L.; Vollmer, C.M. The efficacy of adjuvant therapy for pancreatic invasive intraductal papillary mucinous neoplasm (IPMN). Cancer 2016, 122, 521–533. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).