1. Introduction
Adaptive therapy (classic adaptive therapy) [
1,
2,
3,
4,
5,
6] is developed from tumor intra-competition to delay the outgrowth of the resistant subpopulation. It has been well explored in animal experiments and clinical trials, especially in prostate cancer and breast cancer [
2,
3,
4]. The prerequisites for classic adaptive therapy to surpass MTD are clear, especially in the study of Strobl MAR et al. [
7]. That is, strong tumor intra-competition, slow-growing resistant subpopulation, initial low resistant subpopulation, or high turnover tumor cell. These prerequisites ensure the competitive advantage of sensitive subpopulations in the tumor system. However, this means when the tumor has an aggressive resistant subpopulation, the performance of adaptive therapy is not superior to MTD [
7]. An aggressive resistant subpopulation refers to the resistant subpopulation that proliferates rapidly or has a strong competitive ability or high carrying capacity or high initial content. Because in this case, the outcome of MTD is not worse than that of adaptive therapy, indicating that it is worth exploring to integrate the MTD’s principle of killing as many tumor cells as possible into classical adaptive therapy to delay the occurrence of end events.
The classic adaptive therapy regimen consists of repeated treatment cycles, the duration of the treatment cycle generally includes a period of administration and withdrawal [
2,
3,
5]. The indicator of administration and withdrawal is the level of tumor volume or PSA relative to the beginning of adaptive therapy [
2,
3,
5]. This treatment cycle maintains the intensity of tumor intra-competition and appropriate tumor burden. Compared with MTD, classic adaptive therapy takes advantage of the tumor intra-competition to inhibit the proliferation of resistant subpopulations, while MTD mainly benefits from the killing of sensitive subpopulations by high-frequency administration to control tumor burden. However, as the treatment cycle of classic adaptive therapy continues, the proportion of resistant subpopulation gradually increases, and the duration of the treatment cycle continues to decrease until the end event is reached. This is the general end of classic adaptive therapy [
5], which means that the impact of intra-competition is reduced at the later period of classic adaptive therapy.
Hence, it is meaningless to maintain intra-competition in the later period of classic adaptive therapy. At this time, killing a large number of sensitive cells like MTD may increase the outcome of classic adaptive therapy. To maximize the outcome of competitive stress and killing of the sensitive subpopulation, the timing to bridge these two therapies should be chosen wisely. Therefore, the control degree of competitive stress on resistant subpopulations should be quantitatively evaluated. In this way, the timing of treatment switching from the treatment cycle of classic adaptive therapy to high-frequency administration of MTD can be explored effectively. Then system reachability would be introduced to assess the degree of control. In this tumor system, system reachability mainly relies on the proportion of sensitive subpopulation, because it is easily regulated by drugs, while the resistant subpopulation can only be indirectly regulated by sensitive subpopulation. Therefore, an indicator of reachability should be constructed that is associated with the two participants and can predict the duration of each treatment cycle. Based on the purpose of integrating classic adaptive therapy and MTD, the threshold of the reachability indicator would be explored in the study to evaluate the timing of treatment switching, and the feasibility of this reachability-based adaptive therapy would also be verified.
In this research, the fitness cost for the resistant subpopulation of tumor system was avoided, because this property does not always exist in resistant subpopulations, unless the resistant mechanism is mediated by energy-consuming methods, such as multidrug resistance transporters [
8,
9]. Then this study was conducted in EGFR-mutant non-small cell lung cancer (NSCLC). The main resistant mechanism of tyrosine kinase inhibitor (TKI) is the absence of sensitive mutations, so the fitness cost can be avoided. Here we focused on a common TKI resistant mechanism, namely the emergence of KRAS mutation subpopulation [
10]. Therefore, the tumor system was constructed by EGFR-mutant sensitive and KRAS-mutant resistant cell lines, and the modulation drug was osimertinib. To improve the poor performance of classic adaptive therapy in the above cases, this study explored the implementation of reachability-based adaptive therapy. The Lotka-Volterra model was introduced to describe the tumor system quantitatively. Combined with the model, an indicator related to system reachability would be proposed to evaluate the duration of each treatment cycle and the timing of switching to the high-frequency administration. Furthermore, the applicability of the indicator and reachability-based adaptive therapy would be verified.
2. Methods
2.1. Cell Culture
Human lung adenocarcinoma cells (H1975 and A549) were purchased from the National Collection of Authenticated Cell Cultures (Shanghai, China) and confirmed to be mycoplasma-free by Gmyc-PCR kit (Yeasen, Shanghai, China, cat# 40601ES10). All cell lines were cultured in RPMI-1640 (Gibco (Thermo Fisher Scientific), Waltham, MA, USA, cat# 21870076) containing 10% fetal bovine serum (Gibco, cat# 10099141C) and 1% Penicillin-Streptomycin (5000 U/mL) (Gibco, cat# 15070063).
In terms of drug sensitivity, H1975 is sensitive to osimertinib, and A549 is resistant to osimertinib. In addition, H1975 contains EGFR T790M mutation, A549 contains KRAS G12S mutation. H1975/GFP (green fluorescent protein) cells, expressing GFP to track cells, were transfected by eGFP-puromycin resistant lentivirus (Genomeditech, Shanghai, China, cat# GM1002) and selected with 0.5 mg/L puromycin to maintain the expression of label proteins. A549/RFP (mCherry fluorescent protein)/luc (firefly luciferase) cells, expressing RFP and firefly luciferase, were transfected by RFP-Luciferase-puromycin resistant lentivirus (Genomeditech, cat# GM-10619LV) and selected with 1.6 mg/L puromycin (MedChemExpress, Shanghai, China, cat#HY-B1743A) to maintain the expression of label proteins.
2.2. Nutrition Restriction Model Establishment and Growth Curve Acquisition
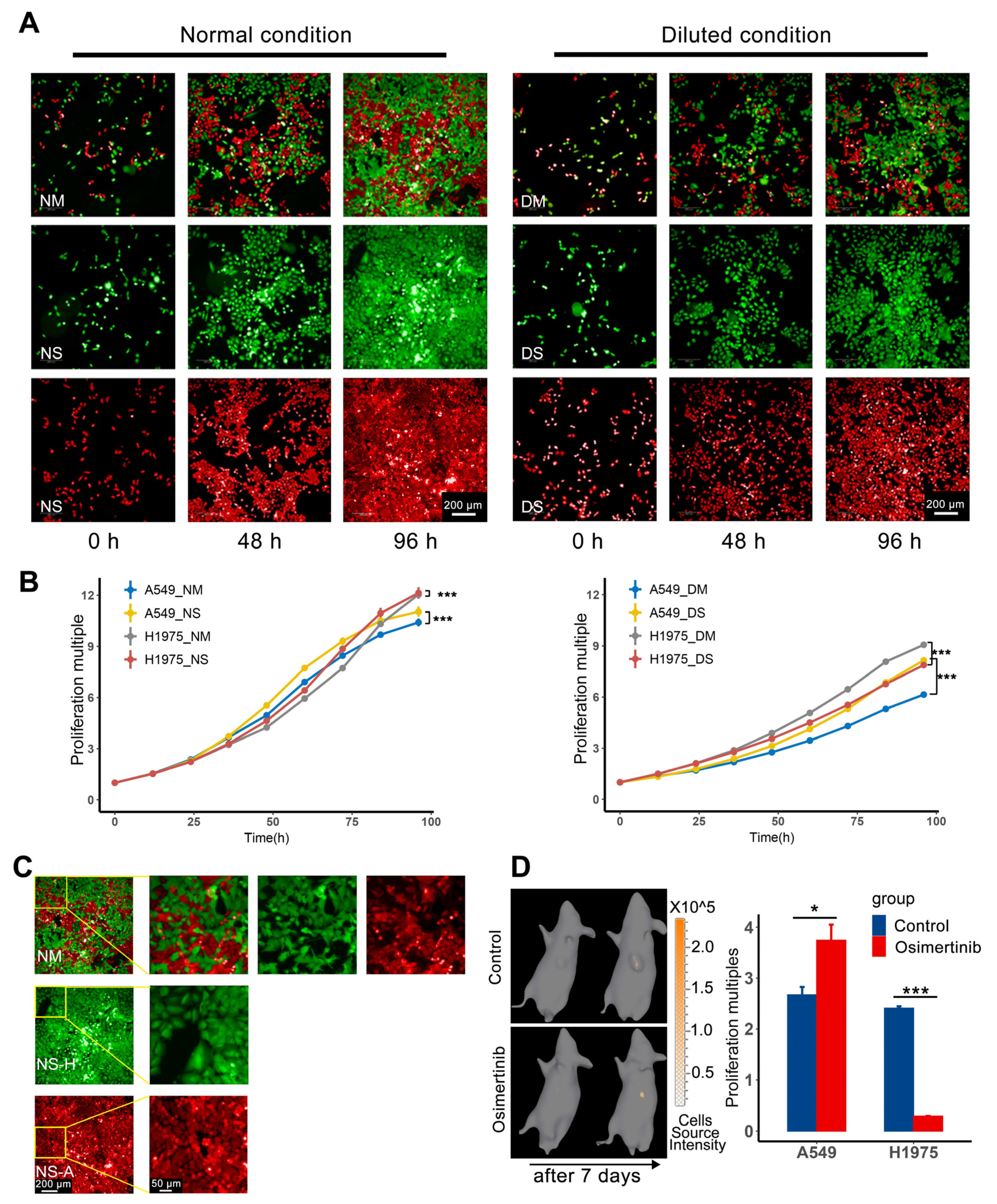
The nutrition restriction model was used to demonstrate one aspect of the competition between subpopulations in vitro. To obtain the growth curve, 24-well plates containing 1:1 mixed H1975/GFP and A549/RFP/luc, the corresponding single cell line as control groups were detected under high-content imaging analysis system Opera Phenix (PerkinElmer, Waltham, MA, USA) from 24 h after cell planting. The number of cells was calculated by the Image Analysis and Evaluation module of high-content analysis software Harmony (version 5.0, PerkinElmer) every 12 h [
11]. As for nutrition restriction, the culture medium was diluted by adding an equal volume of PBS (Gibco, cat#20012027, pH 7.2) directly, and the culture media was replaced after the first time point of imaging. To avoid serious pH deviation and nutrient consumption, the diluted medium was replaced every 12 h.
2.3. Animal
Animal experiments were carried out in compliance with the Chinese law on animal welfare. All animal care and experimental procedures were approved by the animal ethics committee of the Pharmaceutical Animal Experiment Center of China Pharmaceutical University. 5 to 7-week-old female BALB/c nude mice (Charles River, Beijing, China) were allowed to acclimatize (in groups of 3–4 mice per cage) for at least 7 days prior to the experiment and were maintained on a 12-h light:12-h dark cycle with free access to standard rodent chow and water.
2.4. Animal Experiment of Model Fitting
In this experiment, mice were inoculated with different cells to obtain the data for fitting. The cell suspension was inoculated subcutaneously (~5 × 106 cells per mouse) into the left forelimb flank of mice. Firstly, mice were inoculated with H1975 or A549 to fit the tumor growth curve. Then, mice inoculated with H1975 or A549 would be given osimertinib 20 mg/kg/day to fit the parameters of the drug effect. After that, H1975 and A549 were mixed at a ratio of 9:1 and then inoculated to get the competition parameters. The content of H1975 and A549 in the mixed tumor was obtained by In vivo imaging every 3–4 days. Tumor volume was measured by caliper two to three times a week, and calculated according to the formula . For the administration of osimertinib (MedChemExpress, cat# HY-15772), mice were given intragastric osimertinib (20 mg/kg) in normal saline (3% Tween 80). The vehicle control group in the drug effect experiment was given the corresponding solvent without the drug.
2.5. Animal Experiment of AT2 Validation
To compare various therapy protocols, mice were inoculated subcutaneously with mixed tumor cells (~5 × 106 cells per mouse), the mixed ratio of H1975 and A549 was 9:1 before inoculation. Because the sensitive subpopulation should occupy a high proportion at the beginning of therapy, then several rounds of treatment cycles can be carried out. When tumor volume reached 220–300 mm3, mice were randomly grouped according to tumor volume and weight. Tumor volume was measured by caliper two to three times a week and the A549 content in the mixed tumor was collected sparsely. As for the content of H1975, because the duration of in vivo imaging of H1975 for each mouse was more than half an hour, for animal welfare considerations and to ensure the physical condition of the animal, the content of H1975 was not collected at this stage.
2.6. In Vivo Imaging
When tumor volume was about 200 mm
3, IVIS Spectrum (PerkinElmer) was used to evaluate the content of each subpopulation of the mixed tumor through in vivo imaging, and the data was processed using Living Image software (version 4.3.2, PerkinElmer). Serially diluted H1975/GFP or A549/RFP/luc cells were cultured in a 96-well black plate with a transparent bottom. This was used to build a quantification database of bioluminescence and fluorescence intensity with cell numbers. And for luminescence imaging, D-Luciferin potassium salt (Beyotime, Shanghai, China, cat#ST196) should be given to mice 12 min in advance by intraperitoneal injection at a dose of 150 mg/kg body weight. After that, fluorescence imaging tomography or diffuse luminescence imaging tomography was acquired to reconstruct a fluorescent or luminescence source in 3D space and calculate the absolute intensity of that source at depth [
12,
13], then we can get the cell number of the mixed tumors. As for the conversion from cell number to subpopulation volume, using the normal growth data of mixed tumors, the cell number of two subpopulations and the corresponding tumor volume were regressed to get the cell volumes of H1975 and A549. Therefore, based on the reconstruction results of tomography and corresponding quantification database, Living image software can calculate the cell number of H1975 and A549, then the cell number can be converted to subpopulation volume according to the cell volume.
2.7. Model Establishment
Lotka-Volterra model was used to describe the competition of subpopulations [
14]. The tumor cell turnover was included in the intrinsic growth rate and was not considered as a separate item. The efficacy of osimertinib mainly contributed to the death of H1975, and we assumed that its efficacy was positively correlated with an intrinsic growth rate of H1975. Considering the individual variation and the distribution of population parameters, the nonlinear mixed-effects model was selected as an appropriate statistical framework [
15]. This method can reflect the overall changes of the samples, and consider the differences between individuals. The observation described in the model is shown in Equation [
1], which can be divided into structural model f and residual error model g. The structural model f describes the variation of observations to the variables. Residual errors are the difference between model predictions and observations, and the residual error model is set as a proportional error model. Here, i represented individual and j represented corresponding time point. We assumed the set of biological related individual parameters
in this model follows a log-normal distribution, like the distribution of
in equation [
1], vector
indicates the fixed effects, and
indicates the variance of individual random effects. Moreover, the fixed effect is the mean of the population parameters under the pre-assumed distribution and the random effect means the variation of the population parameters between individuals.
are the time points, the residual errors
follows standard normal distribution, and ξ is the regression parameter in the residual error model.
The detailed model of normal growth condition was as following equations [
2,
3], the model of tumor given osimertinib was as following equation [
4].
H and
A represents the content of H1975 and A549,
represents the concentration of osimertinib,
represents the efficacy of osimertinib on H1975. The biological related parameters, intrinsic growth rate,
, carrying capacity,
, competition parameters,
and
were considered interindividual variability, and the subscript (
) of parameter indicated that it belonged to H1975, A549, respectively. The estimation of these biologically related parameters included fixed effects and random effects.
2.8. Parameter Estimation
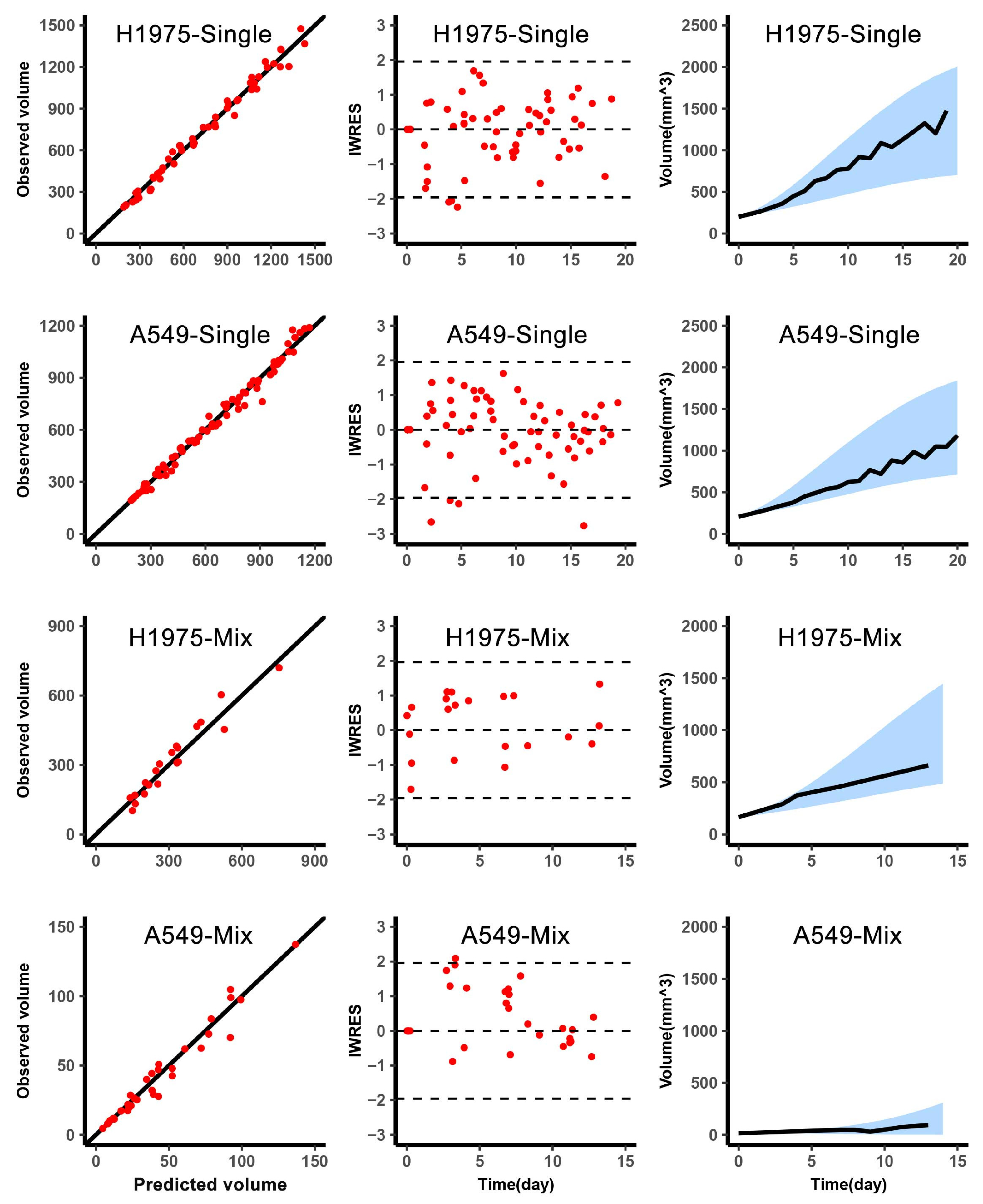
The above model-related parameters were estimated by Monolix 2019R2 (Lixoft SAS, Antony, France, 2019). The fitting results of parameters contained typical value and inter-individual variability. The typical value was the mean of the population parameter and the inter-individual variability was the variance of the parameter distribution. The optimization method of parameters was the maximization of the likelihood function to obtain the appropriate values by stochastic approximation expectation-maximization algorithm [
16]. The model was evaluated based on the goodness-of-fit and individual-weighted residuals (IWRES) to detect misspecifications in the structural and residual error models. And the
-shrinkage was calculated to analyze the degree shrinkage of the individual parameter to the center of the population distribution, which was used to judge the reliability of diagnostic plots [
15,
17].
2.9. Simulation of Therapies
2.9.1. Part1 Estimation Individual Parameters of Experiment Samples
Before the start of the first treatment cycle of classic adaptive therapy and reachability-based adaptive therapy, the observations of tumor volume and single point of A549 content were collected. Based on this data, the individual estimates were obtained from individual empirical Bayes estimates assessed by fitting to the population model.
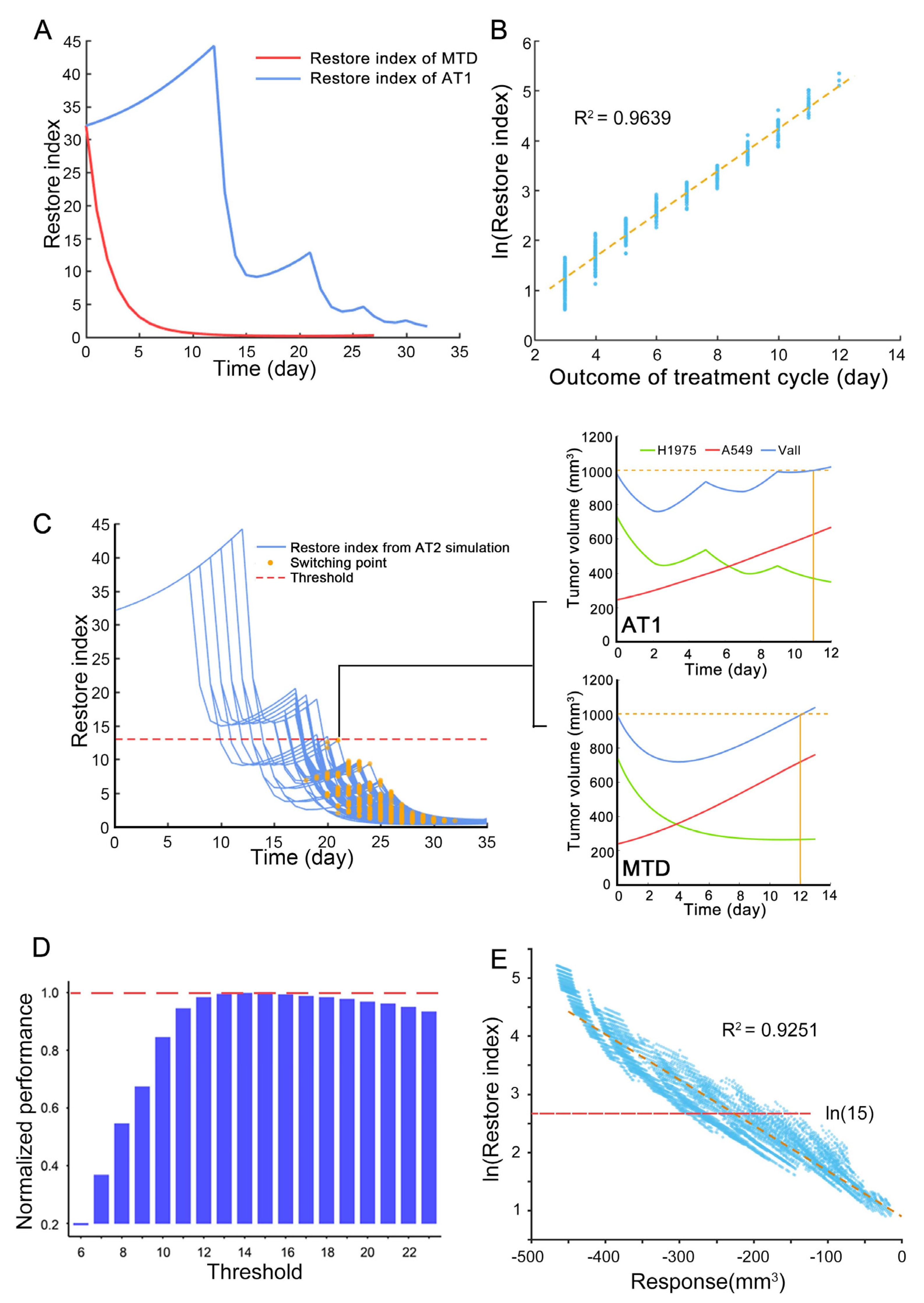
2.9.2. Part2 Estimation the Appropriate Threshold of Restore Index for Reachability-Based Adaptive Therapy
In reachability-based adaptive therapy, there was an indicator of system reachability for evaluating whether to interrupt the treatment cycle. The process of finding the threshold of this interruption point was divided into two steps, the first step was to find the range of the interruption point, and the second step was to find the appropriate value.
The first step was to get the treatment plans of reachability-based adaptive therapy that were not inferior to the classic adaptive therapy through simulation. The regimen of reference classic adaptive therapy was shown in the following Treatment protocols. The tumor composition at the beginning of the simulation was the average value of the mixed tumor samples of the animal experiment of model fitting, and the biological related parameters were the typical value of parameters representing the characteristics of the experimental animals. In the simulation of reachability-based adaptive therapy, the start time of the first treatment cycle was set to different time points, and the treatment cycle was set to 3 times. In each treatment cycle, the duration of drug administration and withdrawal was set at different lengths of time. In each treatment cycle, we can skip the drug withdrawal part and continue to administer it to achieve the purpose of interrupting the treatment cycle in advance. The above simulation treatment plans with the same or better outcomes as the reference classic adaptive therapy were screened out to obtain the range of the threshold.
Then, each integer in the above range was used as a threshold to simulate the outcomes of reachability-based adaptive therapy under different conditions. The different condition means the intrinsic growth rate of resistant subpopulation was defined as 50–150% relative to the typical value of H1975, carrying capacity was defined as 80–120% relative to the typical value of H1975, and competition parameter was defined as 0–200% relative to the typical value of H1975. Finally, the value that maximizes the performance improvement of tumors with aggressive resistant subpopulation and minimizes the similarity to classical adaptive therapy was selected as the threshold.
2.9.3. Part3 Model Validation of Adaptive Therapies in Animal Experiment
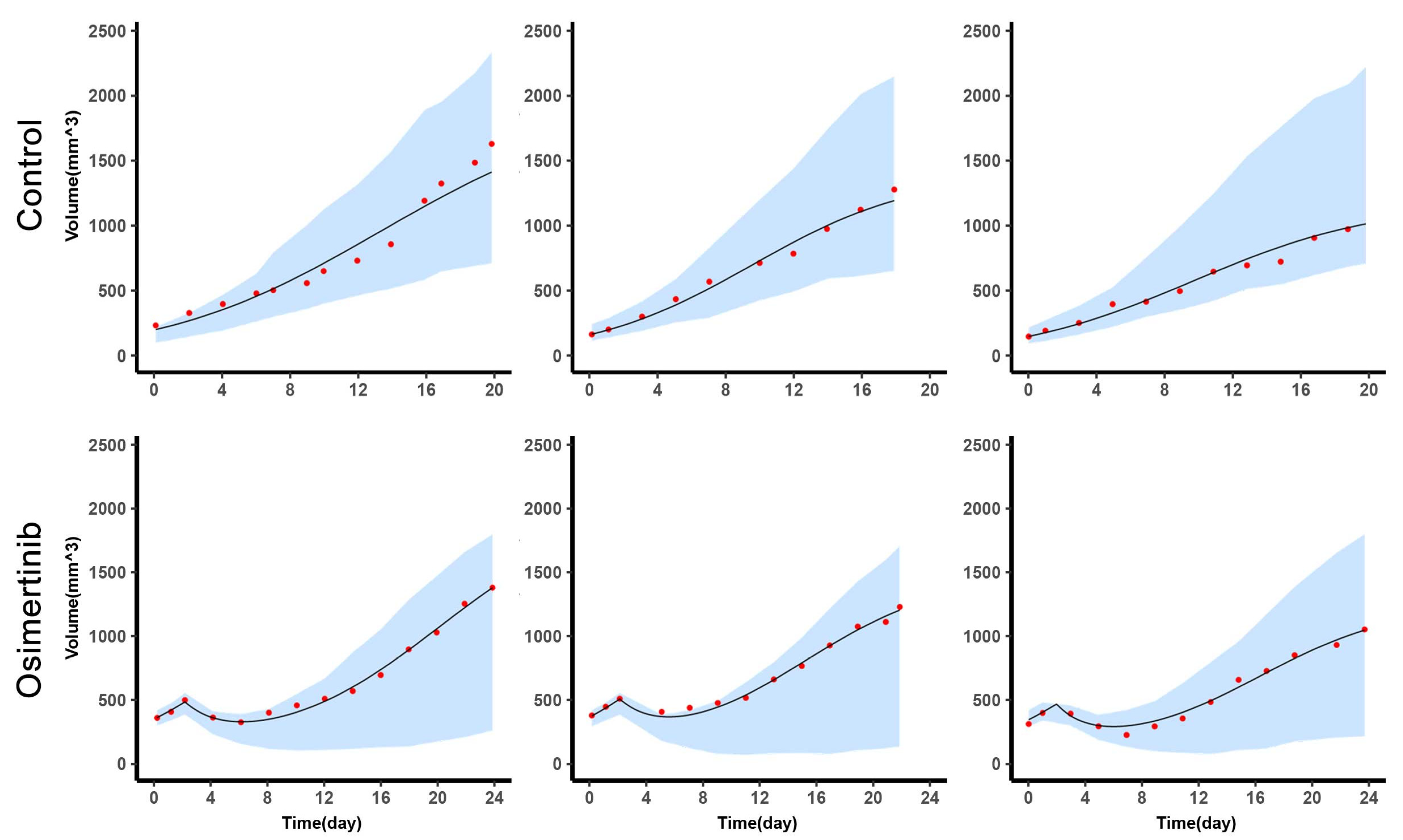
Combined with the administration of each sample in the experiment, GUN Octave (version 6) simulated the changes of tumor volume and A549 content of each sample over time with individual parameters.
2.10. Treatment Protocols
The starting time point of each therapy was calculated from the tumor volume reaching 220–300 mm
3, the end event was set as tumor volume reaching 1000 mm
3 [
2]. In addition, the duration of osimertinib administration was at least two days to reduce the uncertainty of the drug effect of a one-day administration. The general regimen for each therapy in experiment and simulation is as follows:
Control: Normal saline with 3% Tween 80 was given after grouping.
Positive control: Osimertinib was given 20 mg/kg/day when tumor volume was as close as possible to 1000 mm3.
MTD: Osimertinib was given 20 mg/kg/day after grouping. The MTD is aimed at eradicating all cancer cells [
18]. Moreover, MTD cures tumors on the assumption that resistance subpopulation appears after treatment. Therefore, in this study, osimertinib was administered at the maximum dose as soon as possible.
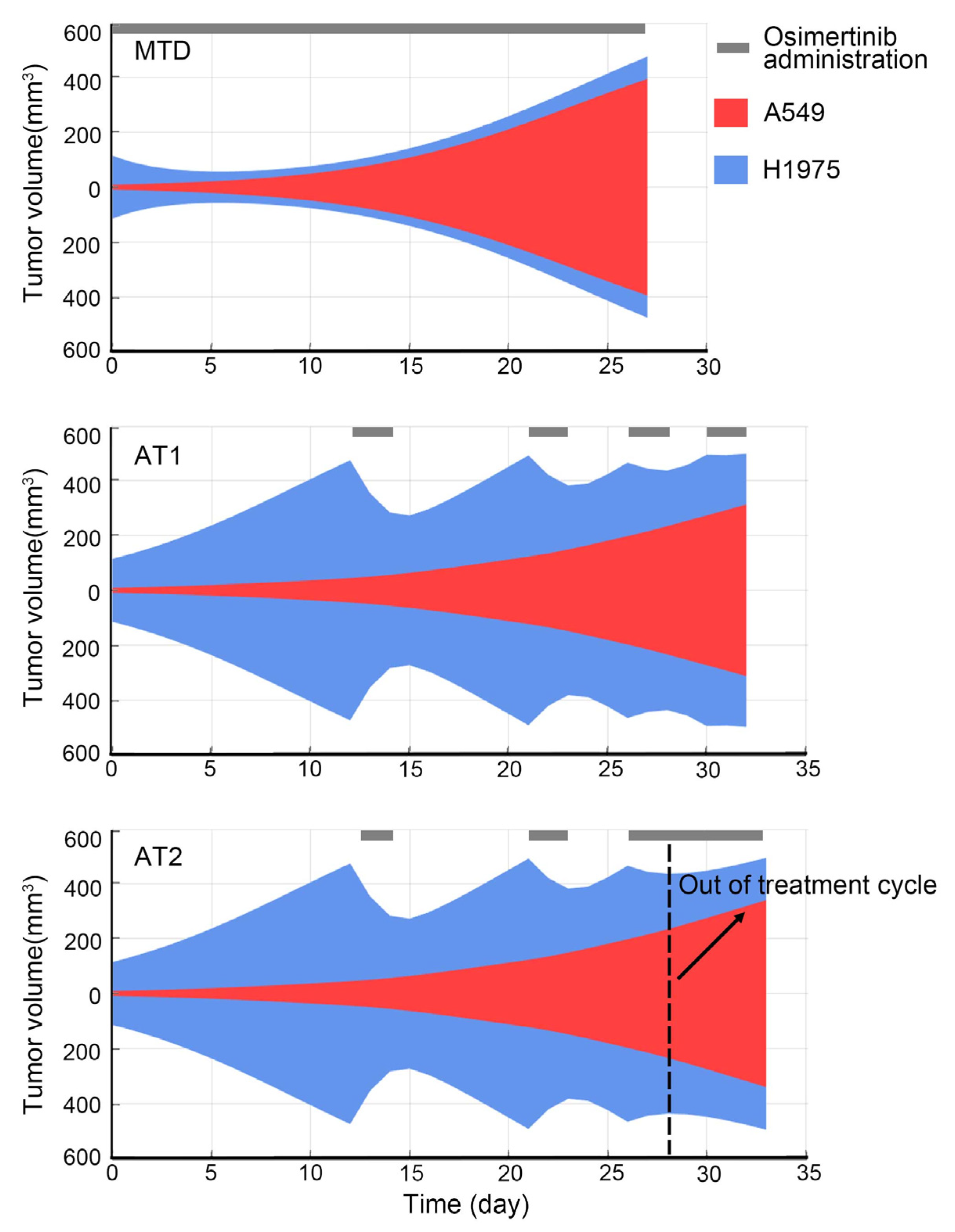
Classic adaptive therapy (AT1): To maximize the use of tumor intra-competition, the first treatment cycle of adaptive therapy started from the tumor volume as close as possible to 1000 mm3. Then the treatment cycle consisted of two days of osimertinib administration and drug withdrawal until the tumor volume was as close as possible to 1000 mm3. If the tumor volume does not decrease with two days of osimertinib administration, osimertinib would not be withdrawn.
Reachability-based adaptive therapy (AT2): The start of the treatment cycle was the same as AT1. However, if the tumor volume reduction was less than 100 mm3 with two days osimertinib administration, the treatment cycle would be interrupted and osimertinib would not be withdrawn. As for the reason for 100 mm3, it was explained in the results.
2.11. Statistical Analysis
In all figures, the data presented were representative of at least 3 independent experiments. Data were expressed as the mean ± SEM and statistically analyzed using the R (3.6.3) and SPSS (version 21.0). The significance analysis of growth curve among different groups used multivariate analysis of variance followed by a post-hoc Bonferroni test, drug effect, and effect of the conditioned medium used analysis of t-test every time point by SPSS and visualized by ggplot2. And survival analysis of different treatments was used pairwise comparisons between group levels with corrections by the survminer package. The acceptable level of significance set at p < 0.05; p-values were shown in figures as *: p < 0.05, **: p < 0.01 and ***: p < 0.005, ****: p < 0.0001, N.S.: not significant.
4. Discussion
It has been studied those factors such as the initial fraction of resistant subpopulations, the proximity of the tumor to carrying capacity, the intrinsic growth rate of a resistant subpopulation, and the rate of cellular turnover affect the time gained of adaptive therapy compared to MTD [
7]. These factors can ensure that a sensitive subpopulation has a higher competitive advantage so that intra-competition can inhibit resistant subpopulations for a long time. But when these prerequisites are not met, the advantage of adaptive therapy over MTD will decrease. In this study, we discussed the influencing factors involving intrinsic growth rate, competition intensity, and carrying capacity. We adjusted classic adaptive therapy by interrupting the treatment cycle in advance and switching to high-frequency administration. Since the outcomes of MTD are not inferior to adaptive therapy in these cases, the benefits of killing sensitive subpopulations should be used wisely in adaptive therapy.
In this research, we introduced a new method that considers the benefits of the treatment cycle and high-frequency administration. To find the switching point of the above two treatments, the restore index that takes into account the response of sensitive subpopulation to the drug and the expansion speed of resistant subpopulation is proposed to measure the ability of the drug to regulate the state of the tumor system at the end of each treatment cycle. This is an approximation of the system reachability of this non-linear tumor system. It is found that the restore index can predict the outcome of each treatment cycle through the simulation. Thus, the restore index can evaluate whether to continue the treatment cycle or switch to high-frequency administration.
The switching point plays an important role in the outcome of reachability-based AT. At the switching point, if the treatment cycle continues, there are not enough sensitive cells that can be killed during the high-frequency administration period, then the benefits of MTD cannot be realized. However, if the treatment cycle is interrupted prematurely, the intra-competition cannot be fully utilized, resulting in reduced outcome of reachability-based AT. Therefore, an appropriate switching point can maximize the benefits of both, and the restore index is an effective tool to find this switching point. In addition, the correlation between restore index and the tumor response to the drug was found through the simulation. This correlation converts the calculation of the potential restore index during the therapy into a direct observation of treatment response. Thus, the treatment would transition to the high-frequency administration when tumor volume response of 2-days osimertinib administration was less than 100 mm3 in the animal experiment of AT2. In addition, when tumors have more than two subpopulations, they can still be divided into sensitive and resistant subpopulations in terms of drug sensitivity. At this time, the reachability-based adaptive therapy can be further explored for its applicable fields.
When we confirmed the in vivo competition of H1975 and A549, mice inoculated with mixed tumors were given osimertinib for 7 days. Based on the experimental results, the comparison between the observed volume of H1975 and the simulated volume without A549 protection was shown in
Supplementary Figure S6. The difference in efficacy indicated that A549 may produce some secretions to support the survival of H1975 during the osimertinib administration, and this phenomenon is also present in other TKIs [
27]. This protective effect is enhanced with the increase in A549 content, and intervention in this protective effect is helpful for tumor control. In this study, the difference in competition parameters of H1975 and A549 also indicates that the resources used by the two subpopulations are not the same. Therefore, through the administration of osimertinib and the interference of the distinct metabolic pathway of A549, periodic oscillations of subpopulations can be realized in this tumor system [
28], so that system reachability can be maintained for a long time.