Amputation for Extremity Sarcoma: Indications and Outcomes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
Endpoints and Statistics
3. Results
3.1. Patient Characteristics
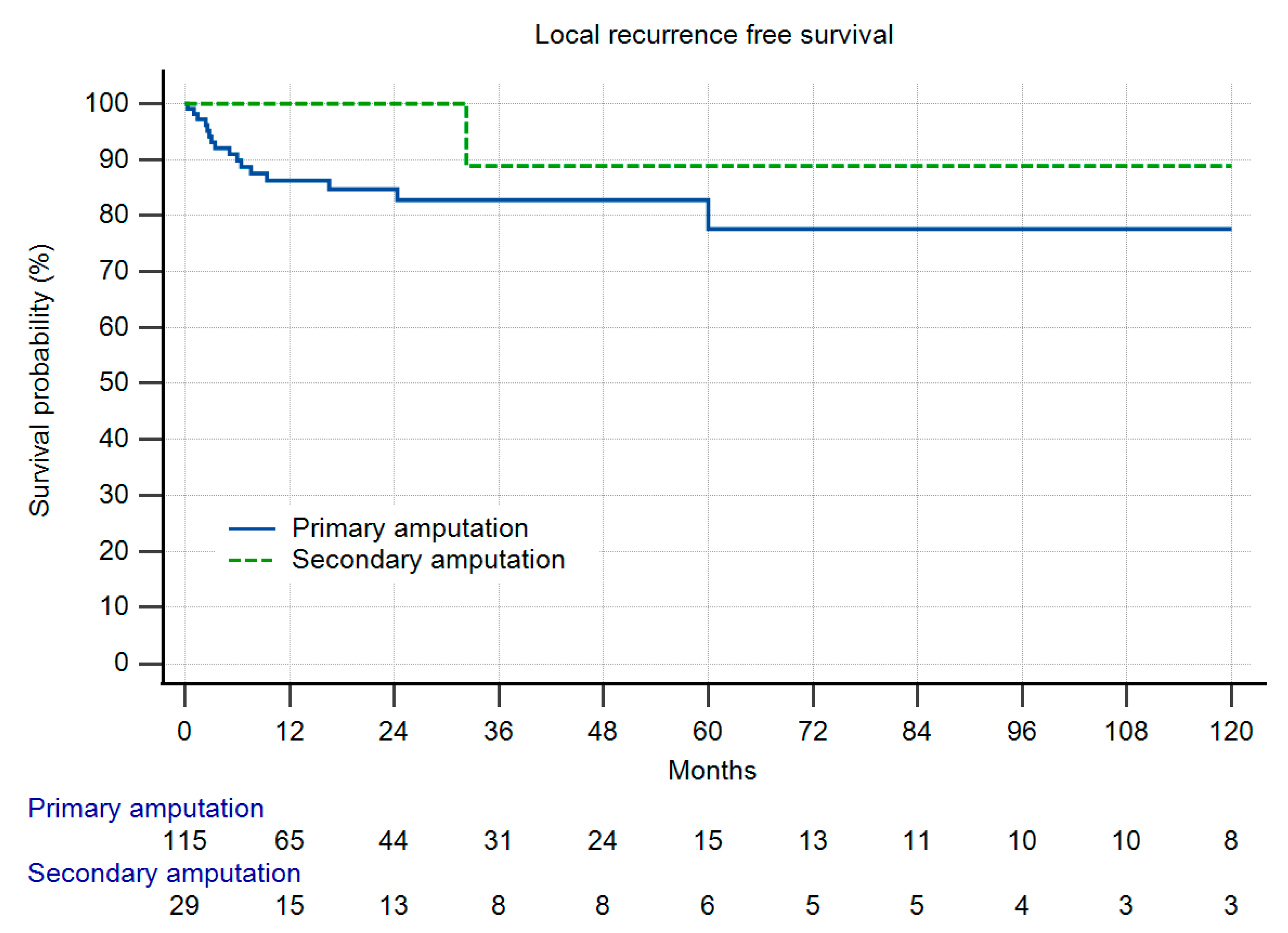
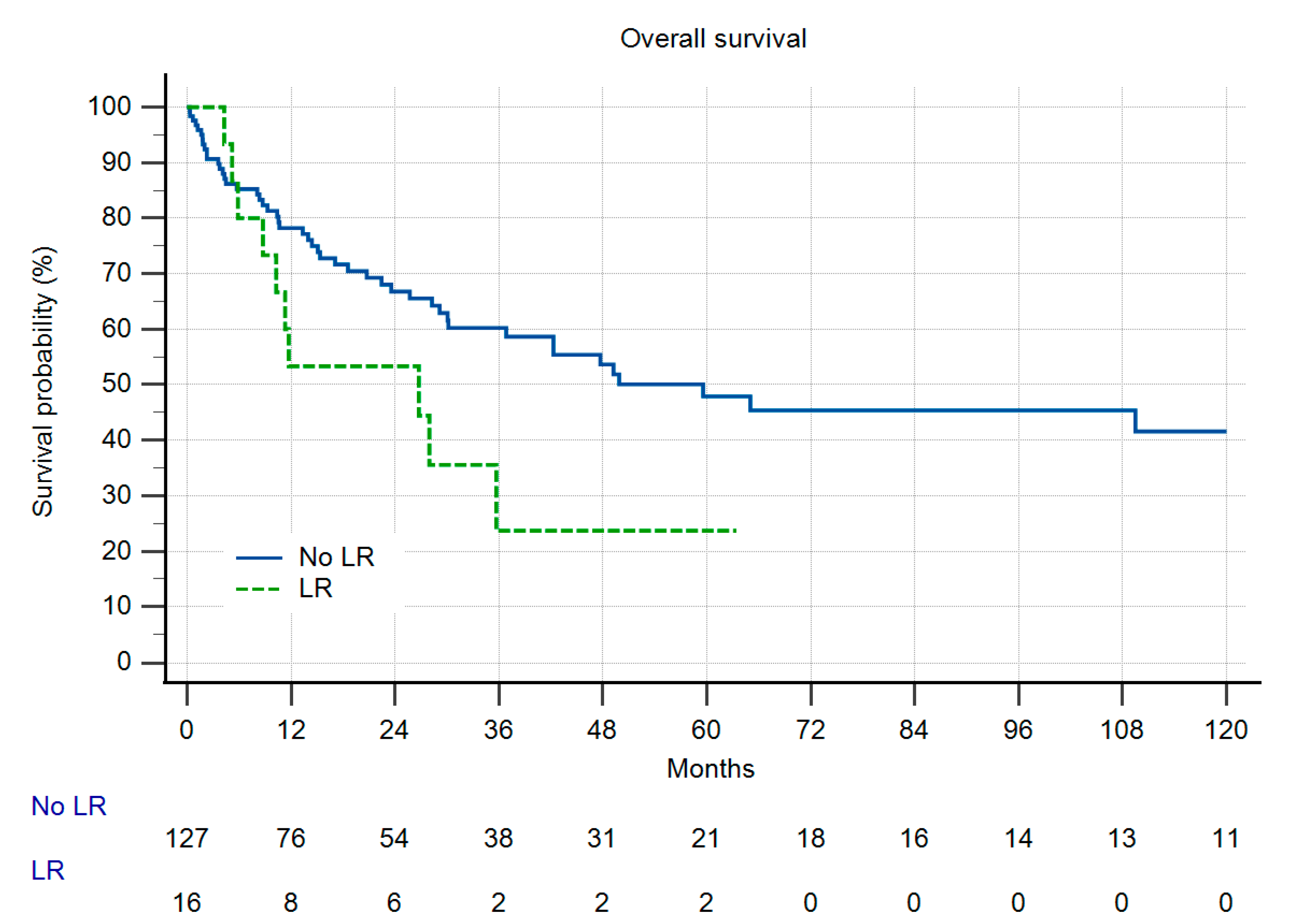
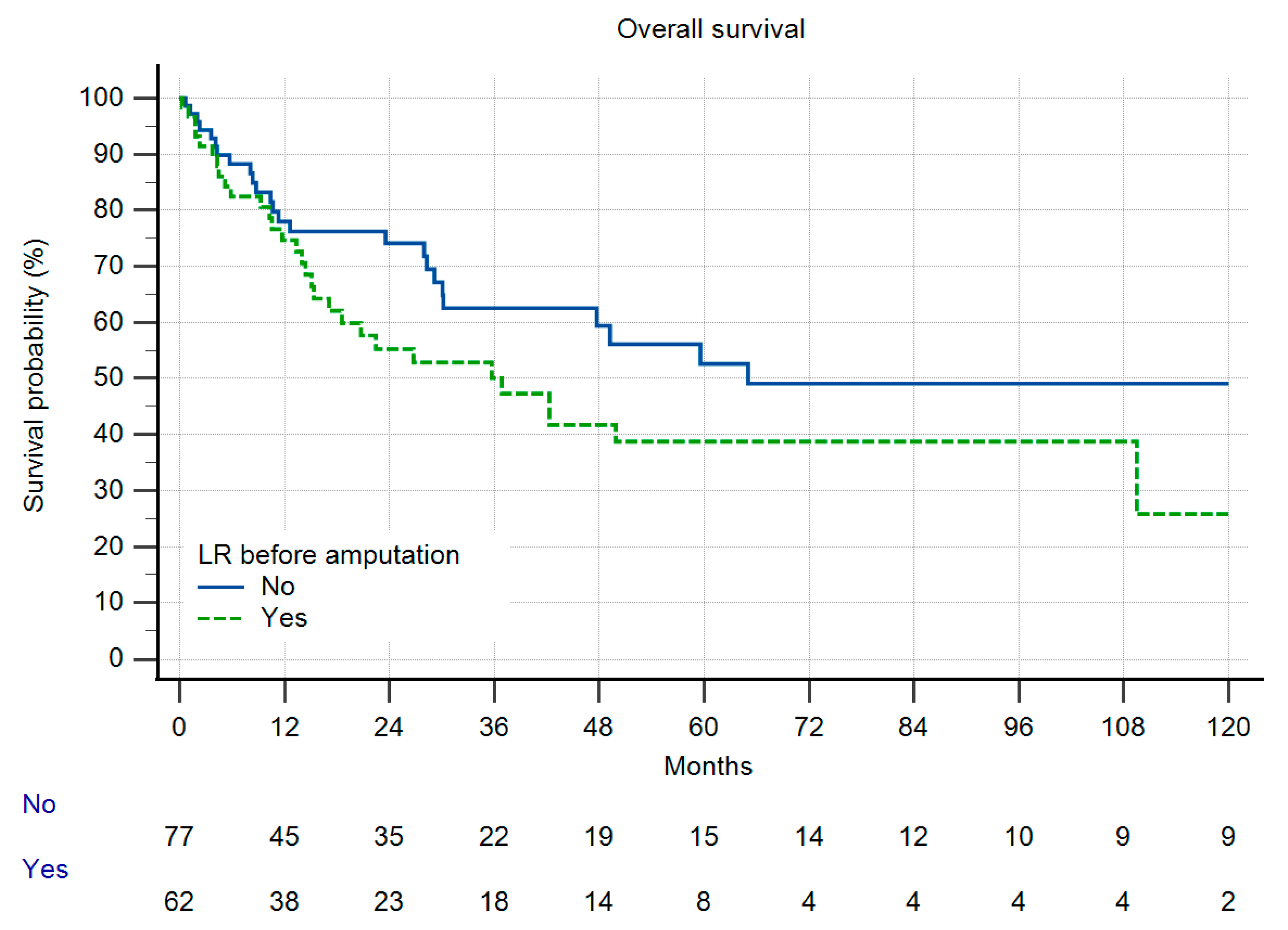
3.2. Metastatic Disease, Local Recurrence-Free Survival and Overall Survival
3.3. Comparison of Both Groups
4. Discussion
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Greenlee, R.T.; Murray, T.; Bolden, S.; Wingo, P.A. Cancer statistics, 2000. CA Cancer J. Clin. 2000, 50, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Morrison, B.A. Soft tissue sarcomas of the extremities. Proc. (Bayl. Univ. Med. Cent.) 2003, 16, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Lahat, G.; Lazar, A.; Lev, D. Sarcoma epidemiology and etiology: Potential environmental and genetic factors. Surg. Clin. N. Am. 2008, 88, 451–481. [Google Scholar] [CrossRef] [PubMed]
- Stiller, C.A.; Trama, A.; Serraino, D.; Rossi, S.; Navarro, C.; Chirlaque, M.D.; Casali, P.G.; Group, R.W. Descriptive epidemiology of sarcomas in Europe: Report from the RARECARE project. Eur. J. Cancer 2013, 49, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, S.A.; Tepper, J.; Glatstein, E.; Costa, J.; Baker, A.; Brennan, M.; DeMoss, E.V.; Seipp, C.; Sindelar, W.F.; Sugarbaker, P.; et al. The treatment of soft-tissue sarcomas of the extremities: Prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann. Surg. 1982, 196, 305–315. [Google Scholar] [CrossRef]
- Williard, W.C.; Collin, C.; Casper, E.S.; Hajdu, S.I.; Brennan, M.F. The changing role of amputation for soft tissue sarcoma of the extremity in adults. Surg. Gynecol. Obstet. 1992, 175, 389–396. [Google Scholar]
- Parsons, C.M.; Pimiento, J.M.; Cheong, D.; Marzban, S.S.; Gonzalez, R.J.; Johnson, D.; Letson, G.D.; Zager, J.S. The role of radical amputations for extremity tumors: A single institution experience and review of the literature. J. Surg. Oncol. 2012, 105, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Reddy, K.I.; Wafa, H.; Gaston, C.L.; Grimer, R.J.; Abudu, A.T.; Jeys, L.M.; Carter, S.R.; Tillman, R.M. Does amputation offer any survival benefit over limb salvage in osteosarcoma patients with poor chemonecrosis and close margins? Bone Jt. J. 2015, 97-b, 115–120. [Google Scholar] [CrossRef]
- Han, G.; Bi, W.Z.; Xu, M.; Jia, J.P.; Wang, Y. Amputation Versus Limb-Salvage Surgery in Patients with Osteosarcoma: A Meta-analysis. World J. Surg. 2016, 40, 2016–2027. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Stamatopoulos, A.; Athanasiadis, D.I.; Kenanidis, E.; Potoupnis, M.; Haidich, A.B.; Tsiridis, E. Limb-salvage surgery offers better five-year survival rate than amputation in patients with limb osteosarcoma treated with neoadjuvant chemotherapy. A systematic review and meta-analysis. J. Bone Oncol. 2020, 25, 100319. [Google Scholar] [CrossRef]
- Mavrogenis, A.F.; Abati, C.N.; Romagnoli, C.; Ruggieri, P. Similar survival but better function for patients after limb salvage versus amputation for distal tibia osteosarcoma. Clin. Orthop. Relat. Res. 2012, 470, 1735–1748. [Google Scholar] [CrossRef] [Green Version]
- Alamanda, V.K.; Crosby, S.N.; Archer, K.R.; Song, Y.; Schwartz, H.S.; Holt, G.E. Amputation for extremity soft tissue sarcoma does not increase overall survival: A retrospective cohort study. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2012, 38, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Erstad, D.J. ASO Author Reflections: Amputation for Extremity Sarcoma. Ann. Surg. Oncol. 2019, 26, 548. [Google Scholar] [CrossRef] [PubMed]
- Erstad, D.J.; Ready, J.; Abraham, J.; Ferrone, M.L.; Bertagnolli, M.M.; Baldini, E.H.; Raut, C.P. Amputation for Extremity Sarcoma: Contemporary Indications and Outcomes. Ann. Surg. Oncol. 2018, 25, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, Y.; Tsoi, K.; Stevenson, J.D.; Parry, M.C.; Fujiwara, T.; Sumathi, V.; Jeys, L.M. Is Microscopic Vascular Invasion in Tumor Specimens Associated with Worse Prognosis in Patients with High-grade Localized Osteosarcoma? Clin. Orthop. Relat. Res. 2020, 478, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Panicek, D.M.; Go, S.D.; Healey, J.H.; Leung, D.H.; Brennan, M.F.; Lewis, J.J. Soft-tissue sarcoma involving bone or neurovascular structures: MR imaging prognostic factors. Radiology 1997, 205, 871–875. [Google Scholar] [CrossRef]
- Ruka, W.; Emrich, L.J.; Driscoll, D.L.; Karakousis, C.P. Prognostic significance of lymph node metastasis and bone, major vessel, or nerve involvement in adults with high-grade soft tissue sarcomas. Cancer 1988, 62, 999–1006. [Google Scholar] [CrossRef]
- Maretty-Nielsen, K. Prognostic factors in soft tissue sarcoma. Dan. Med. J. 2014, 61, B4957. [Google Scholar]
- Kim, C.Y.; Collier, C.D.; Liu, R.W.; Getty, P.J. Are Limb-sparing Surgical Resections Comparable to Amputation for Patients with Pelvic Chondrosarcoma? A Case-control, Propensity Score-matched Analysis of the National Cancer Database. Clin. Orthop. Relat. Res. 2019, 477, 596–605. [Google Scholar] [CrossRef]
- Stevenson, M.G.; Musters, A.H.; Geertzen, J.H.B.; van Leeuwen, B.L.; Hoekstra, H.J.; Been, L.B. Amputations for extremity soft tissue sarcoma in an era of limb salvage treatment: Local control and survival. J. Surg. Oncol. 2018, 117, 434–442. [Google Scholar] [CrossRef]
- Wilke, B.; Cooper, A.; Scarborough, M.; Gibbs, P.; Spiguel, A. A Comparison of Limb Salvage Versus Amputation for Nonmetastatic Sarcomas Using Patient-reported Outcomes Measurement Information System Outcomes. J. Am. Acad. Orthop. Surg. 2019, 27, e381–e389. [Google Scholar] [CrossRef]
- Ghert, M.A.; Abudu, A.; Driver, N.; Davis, A.M.; Griffin, A.M.; Pearce, D.; White, L.; O’Sullivan, B.; Catton, C.N.; Bell, R.S.; et al. The indications for and the prognostic significance of amputation as the primary surgical procedure for localized soft tissue sarcoma of the extremity. Ann. Surg. Oncol. 2005, 12, 10–17. [Google Scholar] [CrossRef]
- Bacci, G.; Ferrari, S.; Lari, S.; Mercuri, M.; Donati, D.; Longhi, A.; Forni, C.; Bertoni, F.; Versari, M.; Pignotti, E. Osteosarcoma of the limb. Amputation or limb salvage in patients treated by neoadjuvant chemotherapy. J. Bone Jt. Surg. Br. Vol. 2002, 84, 88–92. [Google Scholar] [CrossRef]
- Clark, M.A.; Thomas, J.M. Amputation for soft-tissue sarcoma. Lancet. Oncol. 2003, 4, 335–342. [Google Scholar] [CrossRef]
- Bilgeri, A.; Klein, A.; Lindner, L.H.; Nachbichler, S.; Knosel, T.; Birkenmaier, C.; Jansson, V.; Baur-Melnyk, A.; Durr, H.R. The Effect of Resection Margin on Local Recurrence and Survival in High Grade Soft Tissue Sarcoma of the Extremities: How Far Is Far Enough? Cancers 2020, 12, 2560. [Google Scholar] [CrossRef] [PubMed]
- Daigeler, A.; Lehnhardt, M.; Khadra, A.; Hauser, J.; Steinstraesser, L.; Langer, S.; Goertz, O.; Steinau, H.U. Proximal major limb amputations--a retrospective analysis of 45 oncological cases. World J. Surg. Oncol. 2009, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Grimer, R.J.; Aydin, B.K.; Wafa, H.; Carter, S.R.; Jeys, L.; Abudu, A.; Parry, M. Very long-term outcomes after endoprosthetic replacement for malignant tumours of bone. Bone Jt. J. 2016, 98-B, 857–864. [Google Scholar] [CrossRef] [PubMed]
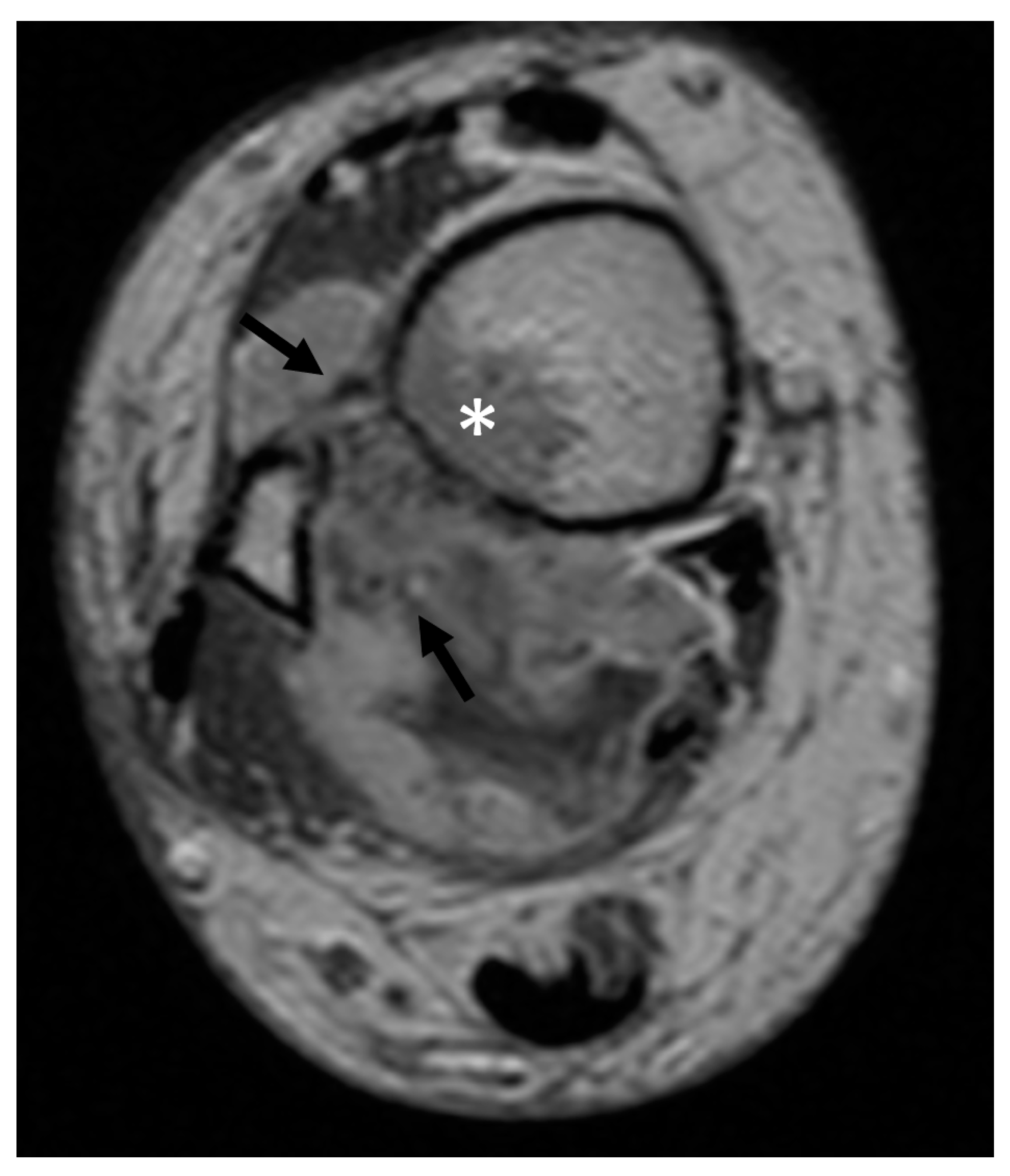
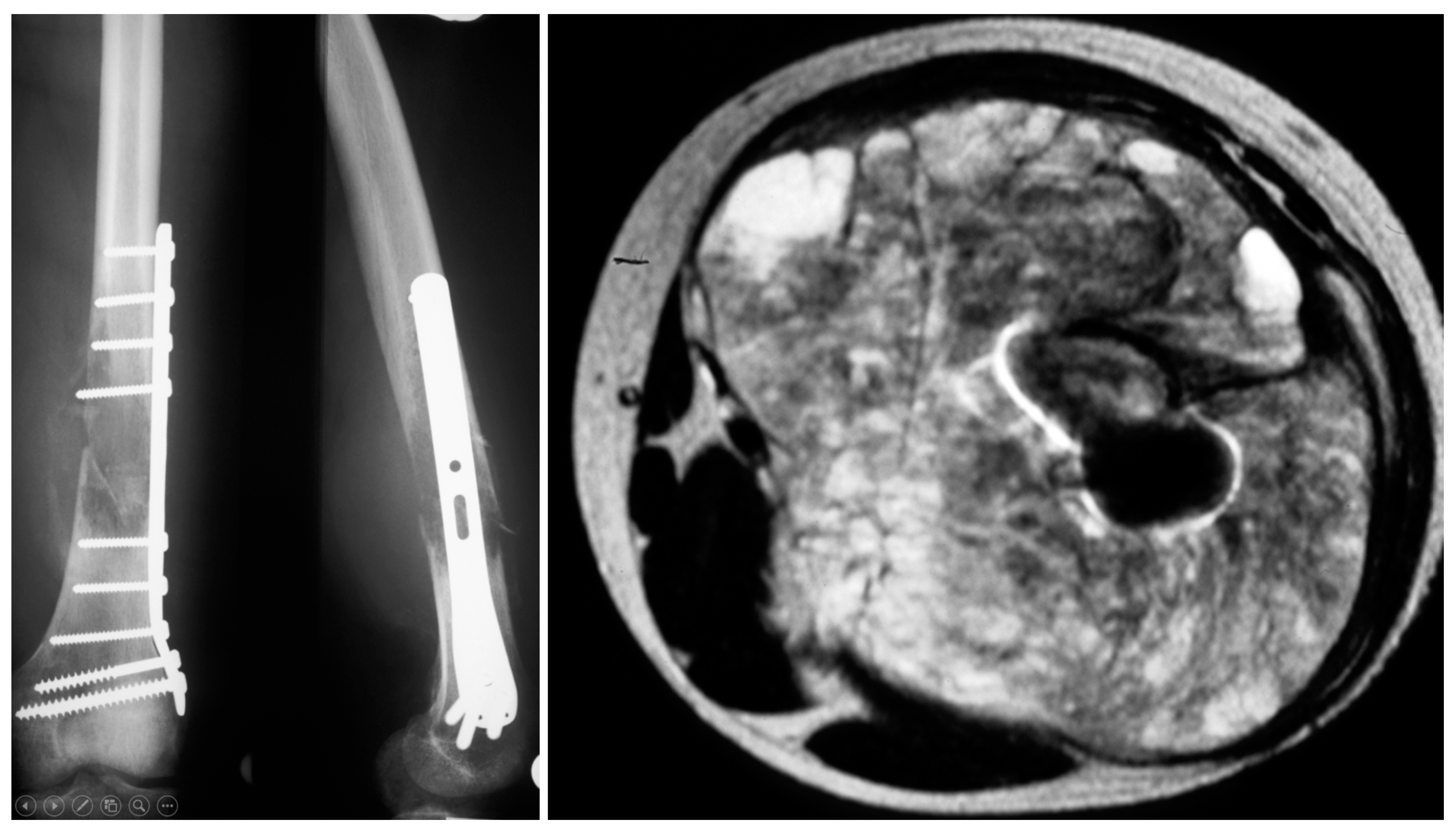
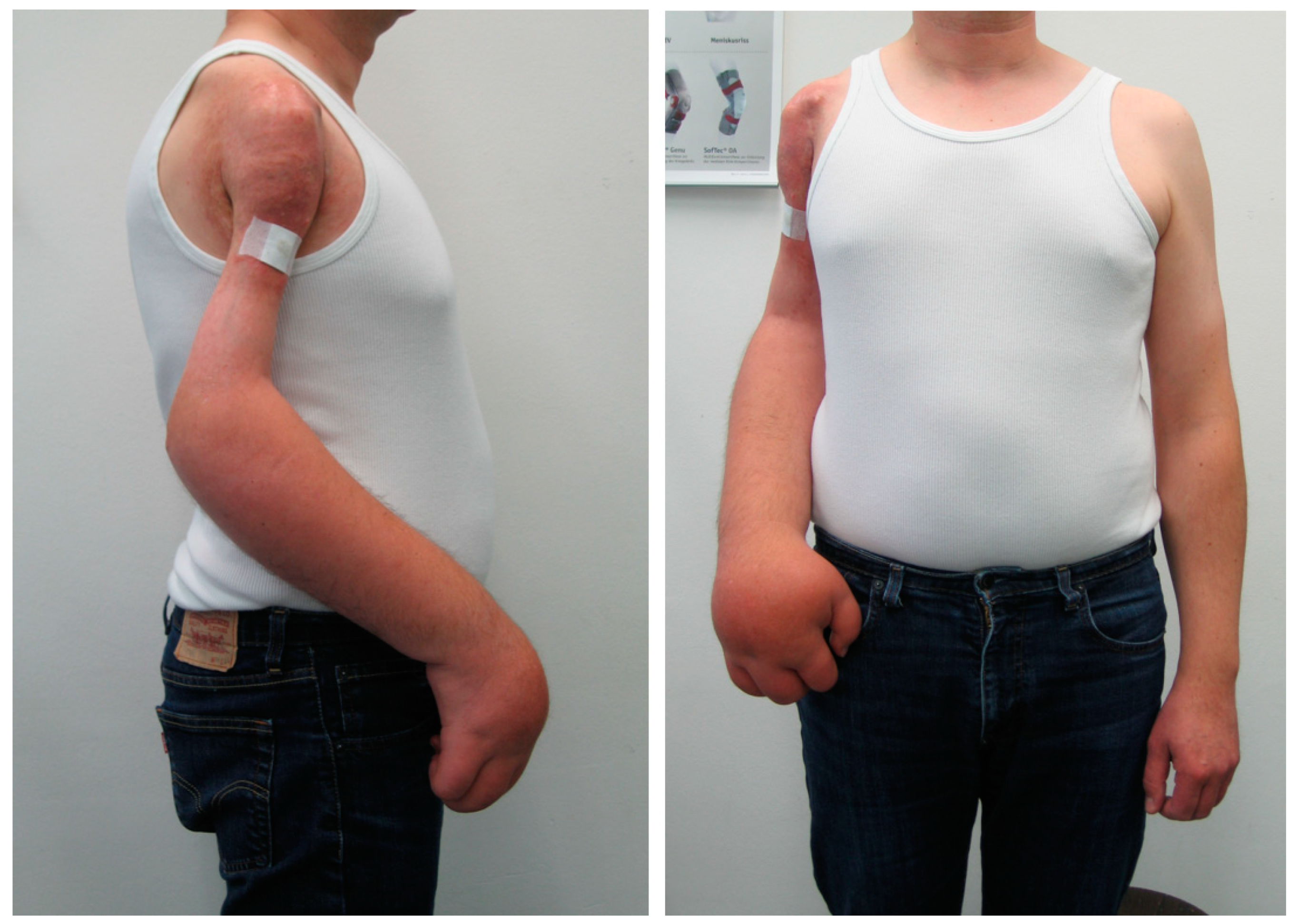

| Total (n = 149) | Group I (n = 120) | Group II (n = 29) | p Value | |
|---|---|---|---|---|
| Indication for primary amputation | ||||
| Multiple compartments involved | 65 (56%) | |||
| Size | 31 (27%) | |||
| Neurovascular involvement | 11 (9%) | |||
| Bone involvement | 7 (6%) | |||
| Combined | 3 (3%) | |||
| Indication for secondary amputation | ||||
| Local recurrence | 4 (14%) | |||
| Contaminated margins after LSS | 8 (28%) | |||
| Infection or ischemia after LSS | 17 (59%) | |||
| Metastatic disease | ||||
| Before amputation | 40 (27%) | 28 (23%) | 12 (41%) | n.s. |
| After amputation | 42 (28%) | 35 (29%) | 7 (24%) | n.s. |
| Margins | ||||
| R0 | 140 (94%) | 114 (95%) | 26 (90%) | |
| R1 | 6 (4%) | 5 (4%) | 1 (3%) | |
| R2 | 3 (2%) | 1 (1%) | 2 (7%) | n.s. |
| Chemotherapy | ||||
| (Neo-)adjuvant | 40 (27%) | 31 (26%) | 9 (31%) | |
| Adjuvant | 18 (12%) | 13 (11%) | 5 (17%) | n.s. |
| Radiotherapy | ||||
| (Neo-)adjuvant | 11 (7%) | 10 (8%) | 1 (3%) | |
| Adjuvant | 8 (5%) | 7 (6%) | 1 (3%) | n.s. |
| Local recurrence | 17 (11%) | 16 (13%) | 1 (3%) | n.s. |
| Total (n = 149) | Group I (n = 120) | Group II (n = 29) | p Value | |
|---|---|---|---|---|
| Median age (years) | 58 (13–89) | 58 (13–89) | 53 (17–79) | n.s. |
| Histological subtype | ||||
| Osteosarcoma | 35 (24%) | 24 (20%) | 11 (38%) | |
| Chondrosarcoma | 18 (12%) | 17 (14%) | 1 (3%) | |
| Undifferentiated sarcoma | 17 (11%) | 12 (19%) | 5 (17%) | |
| Synovial sarcoma | 11 (7%) | 8 (7%) | 3 (10%) | |
| Malignant fibrous histiocytoma | 8 (5%) | 7 (6%) | 1 (3%) | |
| Leiomyosarcoma | 7 (5%) | 4 (3%) | 3 (10%) | |
| Myxofibrosarcoma | 7 (5%) | 7 (6%) | 0 (0%) | |
| Liposarcoma | 6 (4%) | 6 (5%) | 0 (0%) | |
| Others | 40 (27%) | 35 (29%) | 5 (17%) | n.s. |
| Grade (if applicable and recorded) | ||||
| I | 4 (4%) | 4 (5%) | 0 (0%) | |
| II | 32 (33%) | 27 (35%) | 5 (26%) | |
| III | 61 (63%) | 47 (60%) | 14 (74%) | n.s. |
| Size | ||||
| <5 cm | 20 (19%) | 20 (23%) | 0 (0%) | |
| 5–10 cm | 38 (35%) | 31 (35%) | 7 (35%) | |
| >10 cm | 50 (46%) | 37 (42%) | 13 (65%) | p = 0.04 |
| Site | ||||
| Upper extremity | 39 (26%) | 35 (29%) | 4 (14%) | |
| Lower extremity | 103 (69%) | 78 (65%) | 25 (86%) | |
| Pelvis | 7 (5%) | 7 (6%) | 0 (0%) | n.s. |
| Type of amputation | ||||
| Transfemoral | 50 (34%) | 34 (28%) | 16 (55%) | |
| Transtibial | 25 (17%) | 21 (18%) | 4 (14%) | |
| Exarticulation hip | 13 (9%) | 10 (8%) | 3 (10%) | |
| Transhumeral | 13 (9%) | 9 (8%) | 4 (14%) | |
| Lower arm | 8 (5%) | 8 (7%) | 0 (0%) | |
| Pelvis | 8 (5%) | 8 (7%) | 0 (0%) | |
| Exarticulation knee | 8 (5%) | 7 (6%) | 1 (3%) | |
| Exarticulation shoulder | 8 (5%) | 7 (6%) | 1 (3%) | |
| Interscapulothoracic | 7 (5%) | 7 (6%) | 0 (0%) | |
| Partial foot | 5 (3%) | 5 (4%) | 0 (0%) | |
| Partial hand | 4 (3%) | 4 (3%) | 0 (0%) | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirilova, M.; Klein, A.; Lindner, L.H.; Nachbichler, S.; Knösel, T.; Birkenmaier, C.; Baur-Melnyk, A.; Dürr, H.R. Amputation for Extremity Sarcoma: Indications and Outcomes. Cancers 2021, 13, 5125. https://doi.org/10.3390/cancers13205125
Kirilova M, Klein A, Lindner LH, Nachbichler S, Knösel T, Birkenmaier C, Baur-Melnyk A, Dürr HR. Amputation for Extremity Sarcoma: Indications and Outcomes. Cancers. 2021; 13(20):5125. https://doi.org/10.3390/cancers13205125
Chicago/Turabian StyleKirilova, Maya, Alexander Klein, Lars H. Lindner, Silke Nachbichler, Thomas Knösel, Christof Birkenmaier, Andrea Baur-Melnyk, and Hans Roland Dürr. 2021. "Amputation for Extremity Sarcoma: Indications and Outcomes" Cancers 13, no. 20: 5125. https://doi.org/10.3390/cancers13205125
APA StyleKirilova, M., Klein, A., Lindner, L. H., Nachbichler, S., Knösel, T., Birkenmaier, C., Baur-Melnyk, A., & Dürr, H. R. (2021). Amputation for Extremity Sarcoma: Indications and Outcomes. Cancers, 13(20), 5125. https://doi.org/10.3390/cancers13205125






