European Multicenter Study on Degradable Starch Microsphere TACE: The Digestible Way to Conquer HCC in Patients with High Tumor Burden
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Treatment and Therapeutic Concept
2.3. Assessment of Hepatic Tumor Response and Survival
2.4. Safety Analysis
2.5. Statistics
3. Results
3.1. Demographics
3.2. Treatment Characteristics
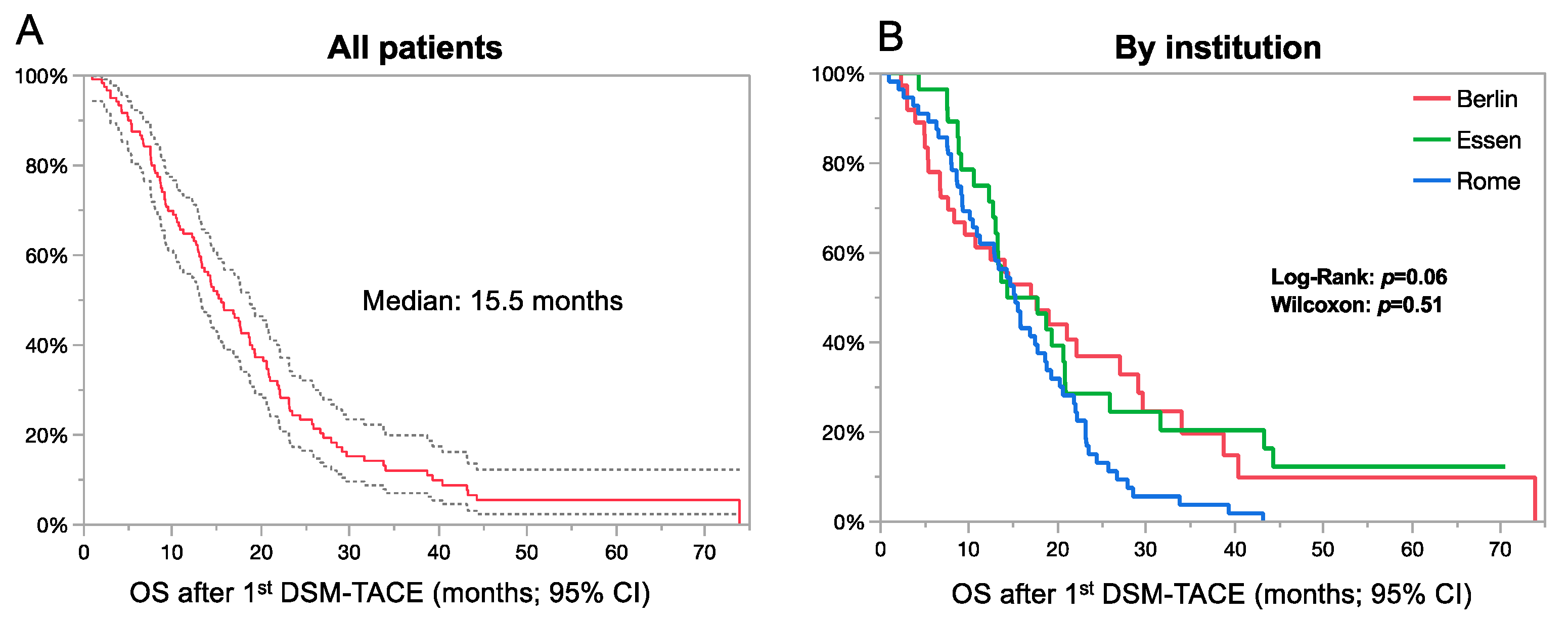
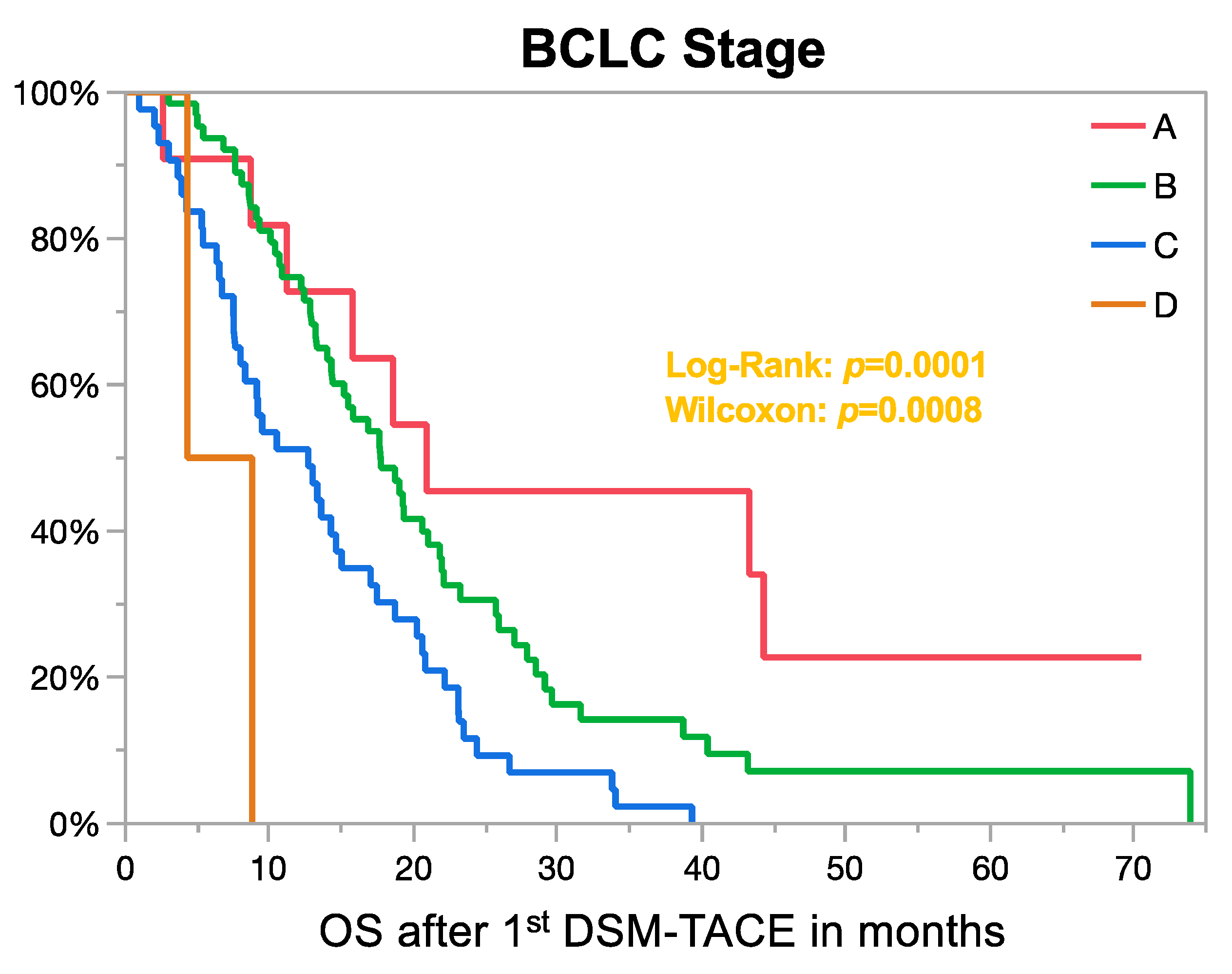
3.3. Survival Analysis
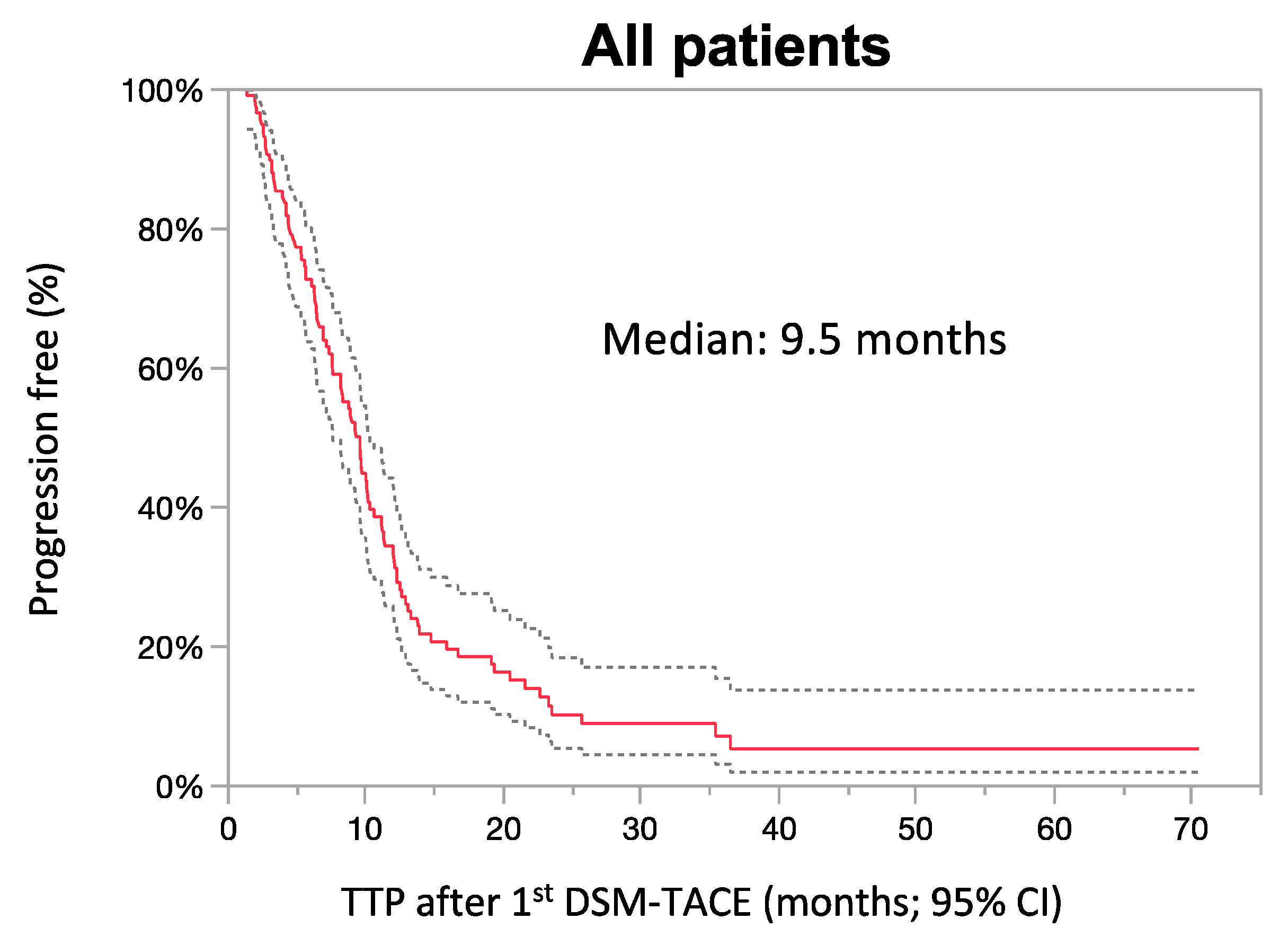
3.4. Response Analysis
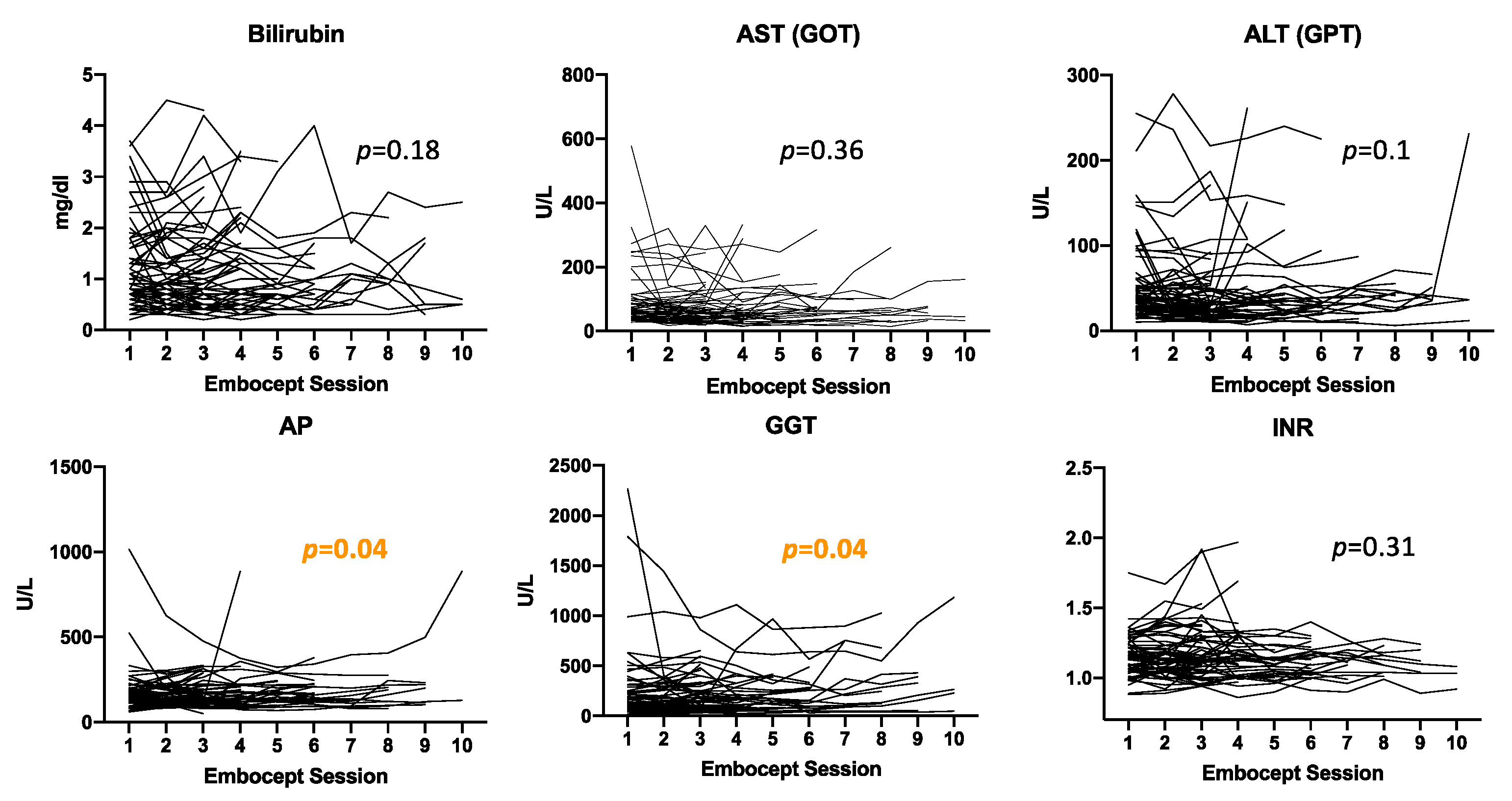
3.5. Safety Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [CrossRef] [PubMed]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.M.; Piscaglia, F.; Raoul, J.-L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raoul, J.-L.; Forner, A.; Bolondi, L.; Cheung, T.T.; Kloeckner, R.; de Baere, T. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence. Cancer Treat. Rev. 2018, 72, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Niessen, C.; Unterpaintner, E.; Goessmann, H.; Schlitt, H.J.; Mueller-Schilling, M.; Wohlgemuth, W.A.; Stroszczynski, C.; Wiggermann, P. Degradable Starch Microspheres versus Ethiodol and Doxorubicin in Transarterial Chemoembolization of Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2014, 25, 240–247. [Google Scholar] [CrossRef]
- Lencioni, R.; De Baere, T.; Soulen, M.C.; Rilling, W.S.; Geschwind, J.-F.H. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology 2016, 64, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Orlacchio, A.; Chegai, F.; Roma, S.; Merolla, S.; Bosa, A.; Francioso, S. Degradable starch microspheres transarterial chemoembolization (DSMs-TACE) in patients with unresectable hepatocellular carcinoma (HCC): Long-term results from a single-center 137-patient cohort prospective study. La Radiol. Med. 2019, 125, 98–106. [Google Scholar] [CrossRef]
- Haubold, J.; Reinboldt, M.P.; Wetter, A.; Li, Y.; Ludwig, J.M.; Lange, C.; Wedemeyer, H.; Schotten, C.; Umutlu, L.; Theysohn, J. DSM-TACE of HCC: Evaluation of Tumor Response in Patients Ineligible for Other Systemic or Loco-Regional Therapies. Rofo 2020, 192, 862–869. [Google Scholar] [CrossRef] [Green Version]
- Iezzi, R.; Pompili, M.; Rinninella, E.; Annicchiarico, E.; Garcovich, M.; Cerrito, L.; Ponziani, F.; De Gaetano, A.; Siciliano, M.; Basso, M.; et al. TACE with degradable starch microspheres (DSM-TACE) as second-line treatment in HCC patients dismissing or ineligible for sorafenib. Eur. Radiol. 2018, 29, 1285–1292. [Google Scholar] [CrossRef]
- Gross, A.; Albrecht, T. Transarterial Chemoembolisation (TACE) with Degradable Starch Microspheres (DSM) and Anthracycline in Patients with Locally Extensive Hepatocellular Carcinoma (HCC): Safety and Efficacy. Cardiovasc. Interv. Radiol. 2019, 43, 402–410. [Google Scholar] [CrossRef]
- Schicho, A.; Pereira, P.L.; Haimerl, M.; Niessen, C.; Michalik, K.; Beyer, L.P.; Stroszczynski, C.; Wiggermann, P. Transarterial chemoembolization (TACE) with degradable starch microspheres (DSM) in hepatocellular carcinoma (HCC): Multi-center results on safety and efficacy. Oncotarget 2017, 8, 72613–72620. [Google Scholar] [CrossRef] [Green Version]
- Pieper, C.C.; Meyer, C.; Vollmar, B.; Hauenstein, K.; Schild, H.H.; Wilhelm, K.E. Temporary Arterial Embolization of Liver Parenchyma with Degradable Starch Microspheres (EmboCept®S) in a Swine Model. Cardiovasc. Interv. Radiol. 2014, 38, 435–441. [Google Scholar] [CrossRef]
- Ikai, I.; Kudo, M.; Arii, S.; Omata, M.; Kojiro, M.; Sakamoto, M.; Takayasu, K.; Hayashi, N.; Makuuchi, M.; Matsuyama, Y.; et al. Report of the 18th follow-up survey of primary liver cancer in Japan. Hepatol. Res. 2010, 40, 1043–1059. [Google Scholar] [CrossRef]
- Kudo, M.; Izumi, N.; Kokudo, N.; Matsui, O.; Sakamoto, M.; Nakashima, O.; Kojiro, M.; Makuuchi, M. Management of Hepatocellular Carcinoma in Japan: Consensus-Based Clinical Practice Guidelines Proposed by the Japan Society of Hepatology (JSH) 2010 Updated Version. Dig. Dis. 2011, 29, 339–364. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) Assessment for Hepatocellular Carcinoma. Semin. Liver Dis. 2010, 30, 052–060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filippiadis, D.K.; Binkert, C.; Pellerin, O.; Hoffmann, R.T.; Krajina, A.; Pereira, P.L. Cirse Quality Assurance Document and Standards for Classification of Complications: The Cirse Classification System. Cardiovasc. Interv. Radiol. 2017, 40, 1141–1146. [Google Scholar] [CrossRef]
- Orlacchio, A.; Chegai, F.; Merolla, S.; Francioso, S.; Del Giudice, C.; Angelico, M.; Tisone, G.; Simonetti, G. Downstaging disease in patients with hepatocellular carcinoma outside up-to-seven criteria: Strategies using degradable starch microspheres transcatheter arterial chemo-embolization. World J. Hepatol. 2015, 7, 1694–1700. [Google Scholar] [CrossRef] [Green Version]
- Lingiah, V.A.; Niazi, M.; Olivo, R.; Paterno, F.; Guarrera, J.V.; Pyrsopoulos, N.T. Liver Transplantation Beyond Milan Criteria. J. Clin. Transl. Hepatol. 2020, 8, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitale, A.; Lai, Q.; Farinati, F.; Bucci, L.; Giannini, E.G.; Napoli, L.; Ciccarese, F.; Rapaccini, G.L.; Di Marco, M.; Caturelli, E.; et al. Utility of Tumor Burden Score to Stratify Prognosis of Patients with Hepatocellular Cancer: Results of 4759 Cases from ITA.LI.CA Study Group. J. Gastrointest. Surg. 2018, 22, 859–871. [Google Scholar] [CrossRef]
- Giannini, E.G.; Farinati, F.; Ciccarese, F.; Pecorelli, A.; Rapaccini, G.L.; Di Marco, M.; Benvegnù, L.; Caturelli, E.; Zoli, M.; Borzio, F.; et al. Prognosis of untreated hepatocellular carcinoma. Hepatology 2014, 61, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.-L.; Kang, Y.-K.; Chen, Z.; Tsao, C.-J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.-S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2008, 10, 25–34. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.M.; Hilgard, P.; Gane, E.; Blanc, J.-F.; De Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Blanc, J.F.; Khemissa, F.; Bronowicki, J.P.; Monterymard, C.; Perarnau, J.M.; Bourgeois, V.; Obled, S.; Abdelghani, M.B.; Mabile-Archambeaud, I.; Faroux, R.; et al. Phase 2 trial comparing sorafenib, pravastatin, their combination or supportive care in HCC with Child–Pugh B cirrhosis. Hepatol. Int. 2021, 15, 93–104. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.-L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.-Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.-W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- De Lorenzo, S.; Tovoli, F.; Barbera, M.A.; Garuti, F.; Palloni, A.; Frega, G.; Garajovà, I.; Rizzo, A.; Trevisani, F.; Brandi, G. Metronomic capecitabine vs. best supportive care in Child-Pugh B hepatocellular carcinoma: A proof of concept. Sci. Rep. 2018, 8, 9997. [Google Scholar] [CrossRef]
- Vilgrain, V.; Pereira, H.; Assenat, E.; Guiu, B.; Ilonca, A.D.; Pageaux, G.-P.; Sibert, A.; Bouattour, M.; Lebtahi, R.; Allaham, W.; et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): An open-label randomised controlled phase 3 trial. Lancet Oncol. 2017, 18, 1624–1636. [Google Scholar] [CrossRef]
- Chow, P.K.; Gandhi, M.; Tan, S.-B.; Khin, M.W.; Khasbazar, A.; Ong, J.; Choo, S.P.; Cheow, P.C.; Chotipanich, C.; Lim, K.; et al. SIRveNIB: Selective Internal Radiation Therapy Versus Sorafenib in Asia-Pacific Patients With Hepatocellular Carcinoma. J. Clin. Oncol. 2018, 36, 1913–1921. [Google Scholar] [CrossRef]
- Miksad, R.A.; Ogasawara, S.; Xia, F.; Fellous, M.; Piscaglia, F. Liver function changes after transarterial chemoembolization in US hepatocellular carcinoma patients: The LiverT study. BMC Cancer 2019, 19, 759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, M.; Kudo, M.; Aikata, H.; Nagamatsu, H.; Ishii, H.; Yokosuka, O.; Torimura, T.; Morimoto, M.; Ikeda, K.; Kumada, H.; et al. Transarterial chemoembolization with miriplatin vs. epirubicin for unresectable hepatocellular carcinoma: A phase III randomized trial. J. Gastroenterol. 2017, 53, 281–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marelli, L.; Stigliano, R.; Triantos, C.; Senzolo, M.; Cholongitas, E.; Davies, N.; Tibballs, J.; Meyer, T.; Patch, D.W.; Burroughs, A.K. Transarterial Therapy for Hepatocellular Carcinoma: Which Technique Is More Effective? A Systematic Review of Cohort and Randomized Studies. Cardiovasc. Interv. Radiol. 2006, 30, 6–25. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Martinelli, E.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.; Meyer, T.; Nault, J.-C.; Neumann, U.; Ricke, J.; et al. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann. Oncol. 2021, 32, 801–805. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristic | Number of Patients (%) |
|---|---|
| Cirrhosis | 109 (90%) |
| Etiology of cirrhosis | |
| Alcohol | 33 (30.3%) |
| Viral | 41 (37.6%) |
| Mixed | 15 (13.8%) |
| Other | 18 (16.5%) |
| Unknown | 2 (1.8%) |
| Ascites | |
| None | 77 (64%) |
| Mild | 15 (12%) |
| Moderate to severe | 29 (24%) |
| Encephalopathy | |
| None | 119 (98.3%) |
| Grade I–II | 2 (1.7%) |
| Disease extent | |
| Bilobar | 77 (63.6%) |
| Unilobar | 44 (36.4%) |
| Number of lesions | |
| Uninodular | 17 (14%) |
| 2–3 nodules | 30 (24.8%) |
| Multinodular (>3 nodules) | 74 (61.2%) |
| Largest lesion (standard deviation; range) | 4 cm (±4.3; 0.8–24.7 cm) |
| Vascular invasion | 32 (26.4%) |
| No | 89 (73.6%) |
| PVTT (vp1–3) | 28 (23.1%) |
| PVTT (vp1–3) + HVTT (vv3) | 1 (0.83%) |
| PVTT (vp4) | 1 (0.83%) |
| PVTT (vp4) + HVTT (vv2) | 1 (0.83%) |
| HVTT (vv2) | 1 (0.83%) |
| Limited extrahepatic metastases | 27 (22.3%) |
| Child–Pugh-class | |
| A | 79 (65.3%) |
| B | 37 (30.6) |
| C | 5 (4.1%) |
| BCLC stage | |
| A | 11 (9.1%) |
| B | 64 (53.9%) |
| C | 43 (35.6%) |
| D | 3 (2.5%) |
| ECOG | |
| 0 | 55 (46%) |
| 1 | 31 (25.6%) |
| 2 | 7 (5.8%) |
| 3 | 2 (1.7%) |
| Unknown | 26 (21.5%) |
| Pretreatment | 61 (50.4%) |
| Resection | 19 |
| Ablation | 21 |
| DEB-TACE a | 21 |
| cTACE b | 3 |
| Radioembolization c | 19 |
| Sorafenib | 20 |
| Liver transplantation | 1 |
| Radiation therapy | 1 |
| Systemic chemotherapy | 1 |
| Laboratory Value | Normal Range | ULN > 1 to 2 | ULN > 2 |
|---|---|---|---|
| INR (0.8–1.2) | 62.8% | 37.2% | - |
| Bilirubin (0.1–1.2 mg/dL) | 56.2% | 25% | 18.8% |
| Creatinine (0.8–1.2 mg/dL) | 78.1% | 21% | 0.9% |
| AST (5–40 IU/L) | 32.7% | 38.5% | 28.9% |
| ALT (7–56 IU/L) | 71.4% | 18.5% | 10% |
| AP (44–147 IU/L) | 61.2% | 32.8% | 6.1% |
| GGT (8–38 IU/L) | 5.1% | 30.3% | 64.7% |
| AFP (10–20 ng/mL) | 45.6% | 10.1% | 44.3% |
| Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Subgroups | Number of Patients | Median OS in Months (95% CI) | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Gender | Female | 23 | 20.8 (10.4–33.8) | 0.62 (0.38–1.03) | 0.06 | - | - |
| Male | 98 | 14.4 (12.8–17.6) | 1 | - | |||
| Ascites | No | 77 | 17.4 (14.3–20.6) | 0.7 (0.47–1.03) | 0.07 | - | - |
| Yes | 44 | 13 (8.7–19) | 1 | - | |||
| Number of nodules | Uninodular | 30 | 16.9 (9.1–27.9) | 1.47 (0.8–2.7) | 0.39 | - | - |
| 2–3 nodules | 17 | 13.3 (10.1–26.7) | 1.2 (0.77–1.9) | - | |||
| Multinodular | 74 | 17.4 (12.7–19) | 1 | - | |||
| Largest liver lesion | ≤5 cm | 72 | 15.5 (12.8–19) | 0.82 (0.55–1.22) | 0.33 | - | - |
| >5 cm | 49 | 14.3 (7.6–18.7) | 1 | - | |||
| Largest liver lesion | ≤7 cm | 93 | 16.9 (13.3–20.3) | 0.69 (0.44–1.08) | 0.1 | - | |
| >7 cm | 28 | 12.7 (7.5–17.7) | 1 | ||||
| Largest liver lesion | ≤10 cm | 109 | 16.9 (13.3–19.3) | 0.38 (0.2–0.7) | 0.006 | 0.34 (0.17–0.68) | 0.002 |
| >10 cm | 12 | 11.5 (3–17) | 1 | 1 | |||
| Lobar involvement | Unilobar | 44 | 19 (14–22.2) | 0.52 (0.33–0.78) | 0.0022 | 0.63 (0.4–0.98) | 0.042 |
| Bilobar | 77 | 13.6 (10.9–17.4) | 1 | 1 | |||
| Vascular invasion | No | 89 | 16.9 (13.3–19.3) | 0.57 (0.38–0.88) | 0.013 | 0.96 (0.48–1.38) | 0.9 |
| Yes | 32 | 13.8 (7.6–18.7) | 1 | 1 | |||
| Child–Pugh class a | A | 79 | 17 (13.2–20.6) | 0.23 (0.08–0.67) | 0.021 | 0.3 (0.07–1.34) | 0.17 |
| B | 37 | 15.2 (10.4–19) | 0.34 (0.12–1.003) | 0.42 (0.1–1.9) | |||
| C | 5 | 8.95 (4.3–10.9) | 1 | 1 | |||
| BCLC stage b | A | 11 | 20.9 (8.9–.) | 0.1 (0.02–0.5) | 0.0005 | 0.24 (0.03–2.1) | 0.43 |
| B | 64 | 17.7 (14.3–21.8) | 0.2 (0.04–0.7) | 0.3 (0.05–2.6) | |||
| C | 43 | 12.7 (7.7–15.2) | 0.3 (0.07–1.32) | 0.48 (0.05–4.1) | |||
| D | 3 | 6.6 (4.3–8.8) | 1 | 1 | |||
| Prior therapy | No | 60 | 14.6 (12.4–20.8) | 1.3 (0.89–1.9) | 0.19 | - | - |
| Yes | 61 | 15.8 (11.2–19.3) | 1 | - | |||
| Extrahepatic metastases | No | 94 | 17.7 (14–20.2) | 0.46 (0.29–0.73) | 0.002 | 0.6 (0.35–1.03) | 0.07 |
| Yes | 27 | 11.2 (8–14.3) | 1 | 1 | |||
| Univariate Analysis | |||||
|---|---|---|---|---|---|
| Subgroups | Number of Patients | Median OS in Months (95% CI) | HR (95% CI) | p-Value | |
| Chemotherapeutic drug a | Epirubicin | 43 | 17.7 (13.3–21) | 0.91 (0.62–1.4) | 0.34 |
| Doxorubicin | 75 | 13.6 (11.2–17.6) | 1 | ||
| Doxorubicin + Mitomycin C | 3 | 19.3 (17.7–.) | 0.43 (0.11–1.7) | ||
| Embolization pattern a | Selective | 49 | 15.5 (11.2–19.25) | 1 | 0.12 |
| Unilobar | 39 | 17.6 (9.1–23.3) | 0.7 (0.43–1.1) | ||
| Bilobar | 33 | 14.3 (9.5–20.6) | 1.12 (0.71–1.78) | ||
| Lipiodol added b | No | 89 | 15.8 (13–18.7) | 1 | 0.64 |
| Yes | 32 | 14.2 (7.6–21) | 1.1 (0.71–1.75) | ||
| Parameter | Grade | All Patients | Bilobar | Lobar | Selective | Pearson Correlation (p-Value) |
|---|---|---|---|---|---|---|
| Bilirubin (n = 266) | 0 | 129 (48.5%) | 47 (46.5%) | 62 (55.4%) | 20 (61%) | p = 0.28 |
| 1 | 69 (25.9%) | 32 (31.6%) | 32 (28.6%) | 5 (15.2%) | ||
| 2 | 64 (32%) | 19 (18.8%) | 18 (16.1%) | 7 (21.2%) | ||
| 3 | 4 (1.5%) | 3 (3%) | - | 1 (3%) | ||
| 4 | - | - | - | - | ||
| AST (n = 282) | 0 | 131 (46.5%) | 40 (40%) | 69 (49.6%) | 22 (50%) | p = 0.18 |
| 1 | 98 (34.8%) | 32 (32%) | 54 (28.8%) | 12 (27%) | ||
| 2 | 31 (11%) | 16 (16%) | 9 (6.5%) | 6 (13.6%) | ||
| 3 | 20 (7.1%) | 10 (10%) | 6 (4.3%) | 4 (9.1%) | ||
| 4 | 2 (0.71%) | 1 (1%) | 1 (0.7%) | - | ||
| ALT (n = 242) | 0 | 159 (65.7%) | 68 (68.7%) | 77 (70%) | 14 (42.4%) | p = 0.006 |
| 1 | 70 (28.9%) | 24 (24.2%) | 29 (26.4%) | 17 (51.2%) | ||
| 2 | 7 (2.9%) | 6 (6.1%) | 0 (0%) | 1 (3%) | ||
| 3 | 6 (2.4%) | 1 (1%) | 4 (3.6%) | 1 (3%) | ||
| 4 | - | - | - | - | ||
| GGT (n = 244) | 0 | 236 (96.7%) | 93 (92.1%) | 111 (100%) | 32 (100%) | p = 0.003 |
| 1 | 8 (3.3%) | 8 (7.9%) | - | - | ||
| 2 | - | - | - | - | ||
| 3 | - | - | - | - | ||
| 4 | - | - | - | - | ||
| AP (n = 238) | 0 | 227 (95.4%) | 97 (97%) | 99 (93.4%) | 31 (96.9%) | p = 0.43 |
| 1 | 11 (4.6%) | 3 (3%) | 7 (6.6%) | 1 (3.1% | ||
| 2 | - | - | - | - | ||
| 3 | - | - | - | - | ||
| 4 | - | - | - | - | ||
| INR (n = 240) | 0 | 230 (97%) | 95 (96.9%) | 103 (97.2%) | 32 (97%) | p = 0.99 |
| 1 | 7 (3%) | 3 (3.1%) | 3 (2.8%) | 1 (3%) | ||
| 2 | - | - | - | - | ||
| 3 | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ludwig, J.M.; Iezzi, R.; Theysohn, J.M.; Albrecht, T.; Posa, A.; Gross, A. European Multicenter Study on Degradable Starch Microsphere TACE: The Digestible Way to Conquer HCC in Patients with High Tumor Burden. Cancers 2021, 13, 5122. https://doi.org/10.3390/cancers13205122
Ludwig JM, Iezzi R, Theysohn JM, Albrecht T, Posa A, Gross A. European Multicenter Study on Degradable Starch Microsphere TACE: The Digestible Way to Conquer HCC in Patients with High Tumor Burden. Cancers. 2021; 13(20):5122. https://doi.org/10.3390/cancers13205122
Chicago/Turabian StyleLudwig, Johannes M., Roberto Iezzi, Jens M. Theysohn, Thomas Albrecht, Alessandro Posa, and Alexander Gross. 2021. "European Multicenter Study on Degradable Starch Microsphere TACE: The Digestible Way to Conquer HCC in Patients with High Tumor Burden" Cancers 13, no. 20: 5122. https://doi.org/10.3390/cancers13205122
APA StyleLudwig, J. M., Iezzi, R., Theysohn, J. M., Albrecht, T., Posa, A., & Gross, A. (2021). European Multicenter Study on Degradable Starch Microsphere TACE: The Digestible Way to Conquer HCC in Patients with High Tumor Burden. Cancers, 13(20), 5122. https://doi.org/10.3390/cancers13205122








