Anticoagulation with Factor Xa Inhibitors Is Associated with Improved Overall Response and Progression-Free Survival in Patients with Metastatic Malignant Melanoma Receiving Immune Checkpoint Inhibitors—A Retrospective, Real-World Cohort Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Primary Clinical Outcomes
2.3. Bleeding Complications
2.4. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Factors Associated with Disease Progression and Survival upon ICI Therapy
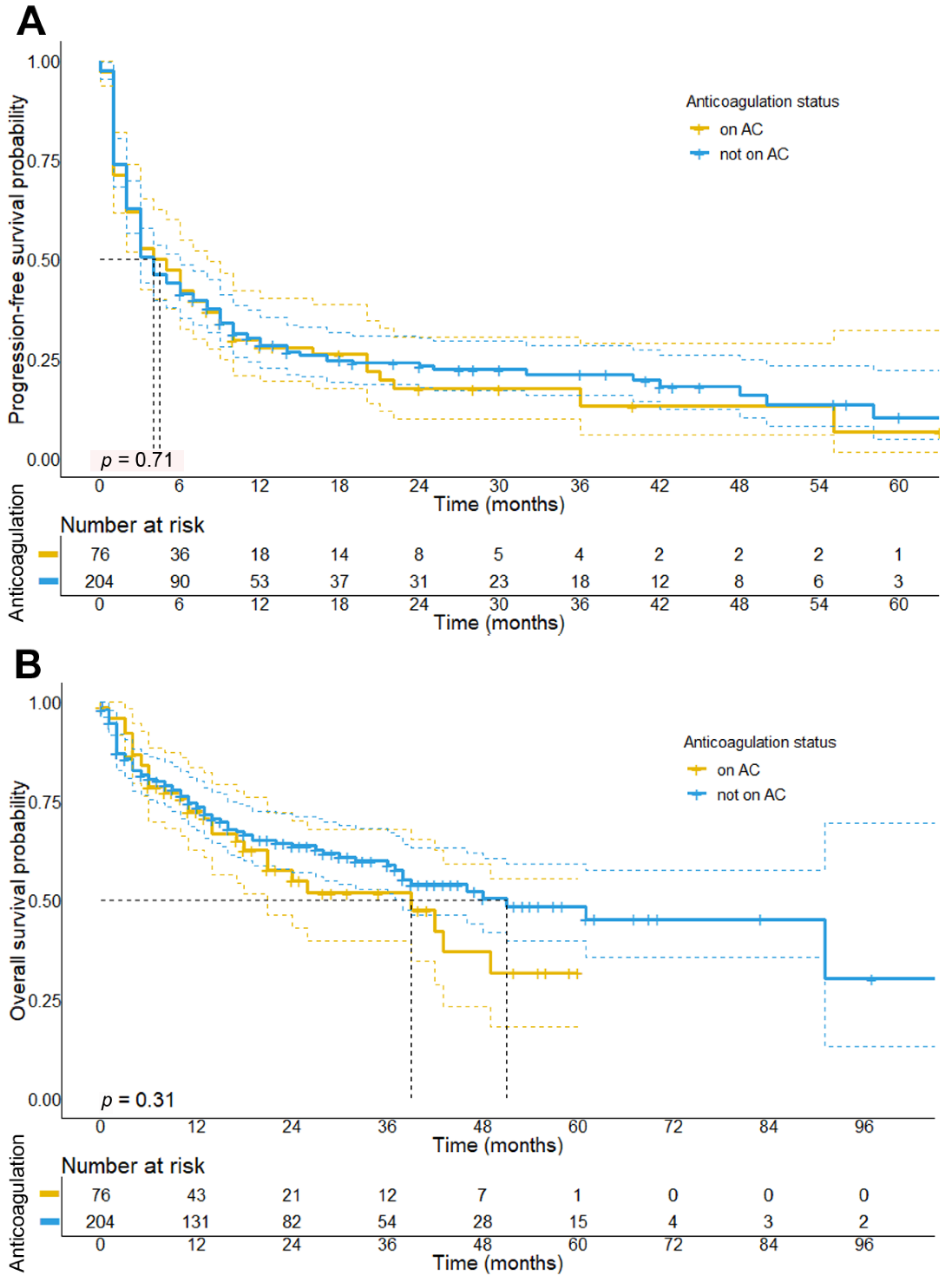
3.3. Impact of Concomitant Anticoagulation Treatment on Survival in Melanoma Patients Receiving ICI
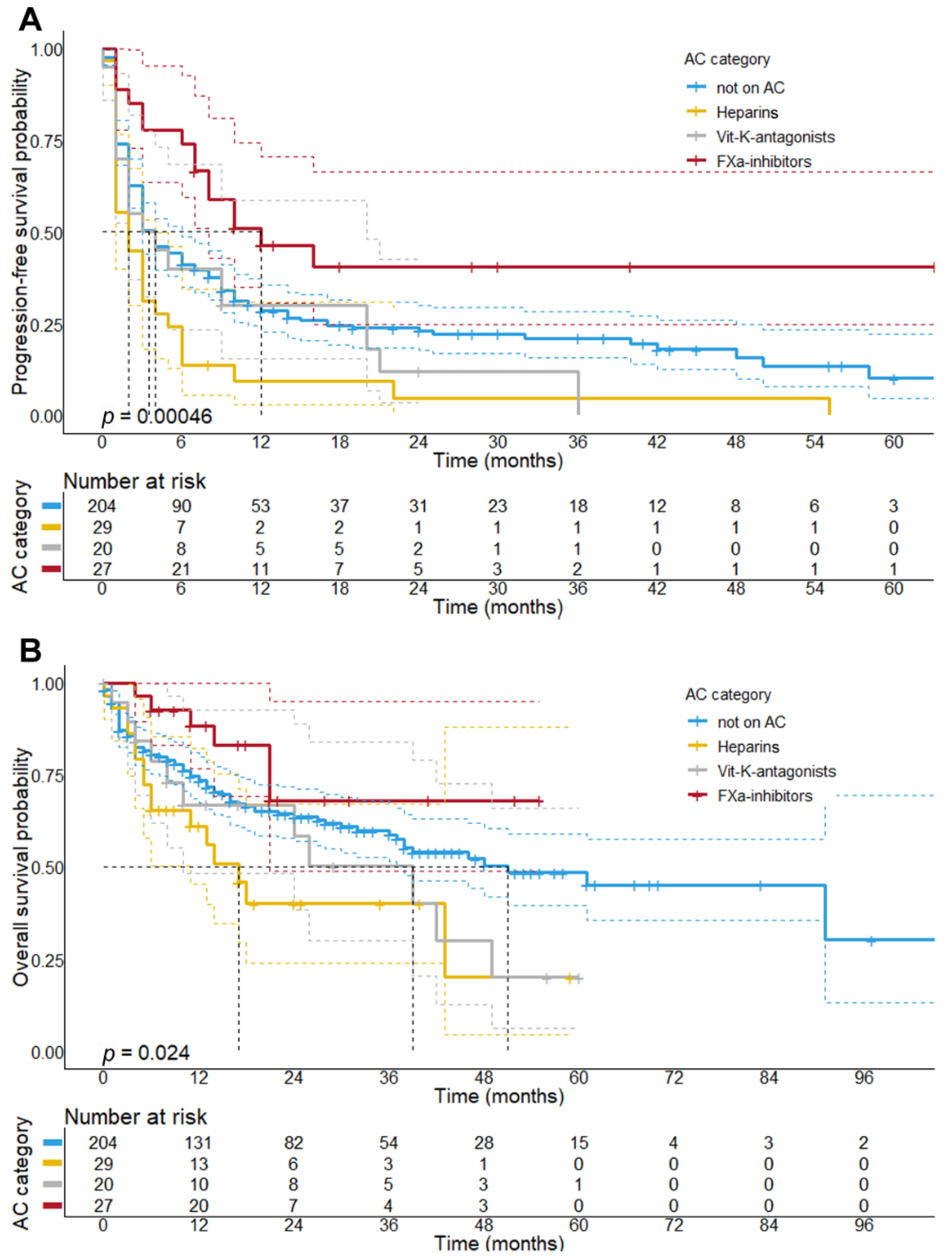
3.4. Concomitant Treatment with Factor-Xa Inhibitors Is Associated with a Better Response and Longer PFS upon Initial ICI Therapy
3.5. Patient Characteristics Receiving Concomitant FXa-Inhibitor Treatment
3.6. Treatment with Anticoagulants Does Not Increase the Event of Bleeding Complications in Melanoma Patients Treated with ICI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | Anticoagulation |
| AE | Adverse event |
| AF | Atrial fibrillation |
| BOR | Best overall response |
| BRAF/MEKi | BRAF/MEK inhibitors |
| CI | Confidence interval |
| cICI | Combined immune checkpoint inhibitor therapy |
| CR | Complete response |
| CTL | Cytotoxic T-lymphocytes |
| DC | Dendritic cells |
| DCR | Disease control rate |
| DOAC | Direct oral anticoagulant(s) |
| DVT | Deep vein thrombosis |
| FXa-i | Factor Xa-inhibitors |
| HR | Hazard ratio |
| ICI | Immune checkpoint inhibitors |
| IFN | Interferon |
| IPI | Ipilimumab |
| LMWH | Low-molecular-weight heparins |
| MBM | Melanoma brain metastases |
| Mφ | Macrophage(s) |
| MR | Mixed response |
| NED | No evidence of disease |
| Nivo | Nivolumab |
| ORR | Objective response rate |
| OS | Overall survival |
| PAR | Protease activated receptors |
| PD | Progressive disease |
| PD-1 | Programmed-cell-death protein 1 |
| PD-L1 | Programmed-cell-death ligand 1 |
| PE | Pulmonary embolism |
| Pembro | Pembrolizumab |
| PFS | Progression-free survival |
| PR | Partial response |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| SD | Stable disease |
| TEE | Thromboembolic events |
| TF | Tissue factor |
| TME | Tumor microenvironment |
| VKA | Vitamin K antagonists |
| VTE | Venous thromboembolic events |
References
- Dougan, M.; Dranoff, G. Immune therapy for cancer. Annu. Rev. Immunol. 2009, 27, 83–117. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Robert, C.; Thomas, L.; Bondarenko, I.; O’Day, S.; Weber, J.; Garbe, C.; Lebbe, C.; Baurain, J.F.; Testori, A.; Grob, J.J.; et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidberger, H.; Rapp, M.; Ebersberger, A.; Hey-Koch, S.; Loquai, C.; Grabbe, S.; Mayer, A. Long-term survival of patients after ipilimumab and hypofractionated brain radiotherapy for brain metastases of malignant melanoma: Sequence matters. Strahlenther. Onkol. 2018, 194, 1144–1151. [Google Scholar] [CrossRef] [Green Version]
- Kluger, H.M.; Chiang, V.; Mahajan, A.; Zito, C.R.; Sznol, M.; Tran, T.; Weiss, S.A.; Cohen, J.V.; Yu, J.; Hegde, U.; et al. Long-Term Survival of Patients With Melanoma With Active Brain Metastases Treated With Pembrolizumab on a Phase II Trial. J. Clin. Oncol. 2019, 37, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Rahman, R.; Cortes, A.; Niemierko, A.; Oh, K.S.; Flaherty, K.T.; Lawrence, D.P.; Sullivan, R.J.; Shih, H.A. The impact of timing of immunotherapy with cranial irradiation in melanoma patients with brain metastases: Intracranial progression, survival and toxicity. J. Neurooncol. 2018, 138, 299–306. [Google Scholar] [CrossRef]
- Harder, N.; Schonmeyer, R.; Nekolla, K.; Meier, A.; Brieu, N.; Vanegas, C.; Madonna, G.; Capone, M.; Botti, G.; Ascierto, P.A.; et al. Automatic discovery of image-based signatures for ipilimumab response prediction in malignant melanoma. Sci. Rep. 2019, 9, 7449. [Google Scholar] [CrossRef]
- Mayer, A.; Haist, M.; Loquai, C.; Grabbe, S.; Rapp, M.; Roth, W.; Vaupel, P.; Schmidberger, H. Role of Hypoxia and the Adenosine System in Immune Evasion and Prognosis of Patients with Brain Metastases of Melanoma: A Multiplex Whole Slide Immunofluorescence Study. Cancers 2020, 12, 3753. [Google Scholar] [CrossRef]
- Chae, Y.K.; Oh, M.S.; Giles, F.J. Molecular Biomarkers of Primary and Acquired Resistance to T-Cell-Mediated Immunotherapy in Cancer: Landscape, Clinical Implications, and Future Directions. Oncologist 2018, 23, 410–421. [Google Scholar] [CrossRef] [Green Version]
- Franklin, C.; Livingstone, E.; Roesch, A.; Schilling, B.; Schadendorf, D. Immunotherapy in melanoma: Recent advances and future directions. Eur. J. Surg. Oncol. 2017, 43, 604–611. [Google Scholar] [CrossRef]
- Metelli, A.; Wu, B.X.; Riesenberg, B.; Guglietta, S.; Huck, J.D.; Mills, C.; Li, A.; Rachidi, S.; Krieg, C.; Rubinstein, M.P.; et al. Thrombin contributes to cancer immune evasion via proteolysis of platelet-bound GARP to activate LTGF-beta. Sci. Transl. Med. 2020, 12, eaay4860. [Google Scholar] [CrossRef]
- Graf, C.; Wilgenbus, P.; Pagel, S.; Pott, J.; Marini, F.; Reyda, S.; Kitano, M.; Macher-Goppinger, S.; Weiler, H.; Ruf, W. Myeloid cell-synthesized coagulation factor X dampens antitumor immunity. Sci. Immunol. 2019, 4, eaaw8405. [Google Scholar] [CrossRef]
- Ruf, W.; Disse, J.; Carneiro-Lobo, T.C.; Yokota, N.; Schaffner, F. Tissue factor and cell signalling in cancer progression and thrombosis. J. Thromb. Haemost. 2011, 9 (Suppl. 1), 306–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haemmerle, M.; Stone, R.L.; Menter, D.G.; Afshar-Kharghan, V.; Sood, A.K. The Platelet Lifeline to Cancer: Challenges and Opportunities. Cancer Cell 2018, 33, 965–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varki, A. Trousseau’s syndrome: Multiple definitions and multiple mechanisms. Blood 2007, 110, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Nichetti, F.; Ligorio, F.; Zattarin, E.; Signorelli, D.; Prelaj, A.; Proto, C.; Galli, G.; Marra, A.; Apollonio, G.; Porcu, L.; et al. Is There an Interplay between Immune Checkpoint Inhibitors, Thromboprophylactic Treatments and Thromboembolic Events? Mechanisms and Impact in Non-Small Cell Lung Cancer Patients. Cancers 2019, 12, 67. [Google Scholar] [CrossRef] [Green Version]
- Ruf, W.; Mueller, B.M. Thrombin generation and the pathogenesis of cancer. Semin. Thromb. Hemost. 2006, 32 (Suppl. 1), 61–68. [Google Scholar] [CrossRef] [PubMed]
- Versteeg, H.H.; Schaffner, F.; Kerver, M.; Petersen, H.H.; Ahamed, J.; Felding-Habermann, B.; Takada, Y.; Mueller, B.M.; Ruf, W. Inhibition of tissue factor signaling suppresses tumor growth. Blood 2008, 111, 190–199. [Google Scholar] [CrossRef] [Green Version]
- Johannet, P.; Sawyers, A.; Gulati, N.; Donnelly, D.; Kozloff, S.; Qian, Y.; Floristan, A.; Hernando, E.; Zhong, J.; Osman, I. Treatment with therapeutic anticoagulation is not associated with immunotherapy response in advanced cancer patients. J. Transl. Med. 2021, 19, 47. [Google Scholar] [CrossRef]
- Zacharski, L.R.; Henderson, W.G.; Rickles, F.R.; Forman, W.B.; Cornell Jr, C.J.; Forcier, A.J.; Edwards, R.L.; Headley, E.; Kim, S.-H.; O’Donnell, J.F.; et al. Effect of warfarin anticoagulation on survival in carcinoma of the lung, colon, head and neck, and prostate: Final Report of VA cooperative study # 75. Cancer 1984, 53, 2046–2052. [Google Scholar] [CrossRef]
- Sussman, T.A.; Li, H.; Hobbs, B.; Funchain, P.; McCrae, K.R.; Khorana, A.A. Incidence of thromboembolism in patients with melanoma on immune checkpoint inhibitor therapy and its adverse association with survival. J. Immunother. Cancer 2021, 9, e001719. [Google Scholar] [CrossRef]
- Johnstone, C.; Rich, S.E. Bleeding in cancer patients and its treatment: A review. Ann. Palliat. Med. 2018, 7, 265–273. [Google Scholar] [CrossRef]
- Alvarado, G.; Noor, R.; Bassett, R.; Papadopoulos, N.E.; Kim, K.B.; Hwu, W.J.; Bedikian, A.; Patel, S.; Hwu, P.; Davies, M.A. Risk of intracranial hemorrhage with anticoagulation therapy in melanoma patients with brain metastases. Melanoma Res. 2012, 22, 310–315. [Google Scholar] [CrossRef]
- Schulman, S.; Kearon, C.; The Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar] [CrossRef]
- Kaatz, S.; Ahmad, D.; Spyropoulos, A.C.; Schulman, S.; Subcommittee on Control of, A. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2015, 13, 2119–2126. [Google Scholar] [CrossRef]
- Couey, M.A.; Bell, R.B.; Patel, A.A.; Romba, M.C.; Crittenden, M.R.; Curti, B.D.; Urba, W.J.; Leidner, R.S. Delayed immune-related events (DIRE) after discontinuation of immunotherapy: Diagnostic hazard of autoimmunity at a distance. J. Immunother. Cancer 2019, 7, 165. [Google Scholar] [CrossRef] [Green Version]
- Moik, F.; Chan, W.E.; Wiedemann, S.; Hoeller, C.; Tuchmann, F.; Aretin, M.B.; Fuereder, T.; Zochbauer-Muller, S.; Preusser, M.; Pabinger, I.; et al. Incidence, risk factors, and outcomes of venous and arterial thromboembolism in immune checkpoint inhibitor therapy. Blood 2021, 137, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agnelli, G.; Gussoni, G.; Bianchini, C.; Verso, M.; Mandala, M.; Cavanna, L.; Barni, S.; Labianca, R.; Buzzi, F.; Scambia, G.; et al. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: A randomised, placebo-controlled, double-blind study. Lancet Oncol. 2009, 10, 943–949. [Google Scholar] [CrossRef]
- Carrier, M.; Abou-Nassar, K.; Mallick, R.; Tagalakis, V.; Shivakumar, S.; Schattner, A.; Kuruvilla, P.; Hill, D.; Spadafora, S.; Marquis, K.; et al. Apixaban to Prevent Venous Thromboembolism in Patients with Cancer. N. Engl. J. Med. 2019, 380, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Soff, G.A.; Kakkar, A.K.; Vadhan-Raj, S.; Riess, H.; Wun, T.; Streiff, M.B.; Garcia, D.A.; Liebman, H.A.; Belani, C.P.; et al. Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer. N. Engl. J. Med. 2019, 380, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Ek, L.; Gezelius, E.; Bergman, B.; Bendahl, P.O.; Anderson, H.; Sundberg, J.; Wallberg, M.; Falkmer, U.; Verma, S.; Belting, M.; et al. Randomized phase III trial of low-molecular-weight heparin enoxaparin in addition to standard treatment in small-cell lung cancer: The RASTEN trial. Ann. Oncol. 2018, 29, 398–404. [Google Scholar] [CrossRef]
- Wun, T.; White, R.H. Epidemiology of cancer-related venous thromboembolism. Best Pract. Res. Clin. Haematol. 2009, 22, 9–23. [Google Scholar] [CrossRef] [Green Version]
- Ibrahimi, S.; Machiorlatti, M.; Vesely, S.K.; Malla, M.; Modhia, F.; Jones, S.A.; Roman, D.; Cherry, M.A. Incidence of Vascular Thromboembolic Events in Patients Receiving Immunotherapy: A Single Institution Experience. Blood 2017, 130, 4864. [Google Scholar] [CrossRef]
- Gutierrez-Sainz, L.; Martinez-Marin, V.; Vinal, D.; Martinez-Perez, D.; Pedregosa, J.; Garcia-Cuesta, J.A.; Villamayor, J.; Zamora, P.; Pinto, A.; Redondo, A.; et al. Incidence of venous thromboembolic events in cancer patients receiving immunotherapy: A single-institution experience. Clin. Transl. Oncol. 2021, 23, 1245–1252. [Google Scholar] [CrossRef]
- Lewis, C.E.; Harney, A.S.; Pollard, J.W. The Multifaceted Role of Perivascular Macrophages in Tumors. Cancer Cell 2016, 30, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Carlson, T.H.; Simon, T.L.; Atencio, A.C. In vivo behavior of human radioiodinated antithrombin III: Distribution among three physiologic pools. Blood 1985, 66, 13–19. [Google Scholar] [CrossRef] [Green Version]

| Clinicopathological Features | Not on Anticoagulation | On Anticoagulation | p-Value |
|---|---|---|---|
| Total number of patients | 204 | 76 | |
| Median age at initiation of ICI | 63.0 | 70.0 | 0.0006 |
| Gender | 0.784 | ||
| Female | 121 (59.3%) | 47 (61.8%) | |
| Male | 83 (40.7%) | 29 (38.2%) | |
| Primary Tumor and Metastases | |||
| Median Breslow thickness (95% CI) 1 | 2.5 (0.7–12.7 mm) | 2.4 (0.7–6.8 mm) | 0.185 |
| Ulceration 2 | 67/132 (50.8%) | 28/54 (51.8%) | 0.511 |
| BRAF status 3 | 88/201 (43.8%) | 28/74 (37.8%) | 0.411 |
| Elevated serum LDH levels (>245 U/L) 4 | 115/173 (66.5%) | 44/65 (67.7%) | 0.269 |
| PTT at initiation of ICI 5 | 28.45 s | 30.89 s | <0.0001 |
Systemic pretreatments prior to ICI
| 86 (42.1%) 48 37 11 5 | 28 (36.8%) 17 12 3 3 | 0.494 |
| Melanoma brain metastases | 65 (32.3%) | 26 (34.6%) | 0.774 |
| Liver metastases | 67 (32.8%) | 25 (33.3%) | 0.523 |
| Treatments | |||
| Initial ICI therapy | 0.020 | ||
| 89 (43.6%) 39 (19.1%) 58 (28.4%) 18 (8.8%) | 20 (26.3%) 16 (21.1%) 35 (46.1%) 5 (6.6%) | |
| Treatment line | 0.883 | ||
| 145 (70.4%) 59 (42.6%) | 55 (72.4%) 21 (36.9%) | |
Median treatment duration (range)
| 3.0 months (0–41) 14 months (7–41) 13 (6.4%) 3 months (1–23) 51 (25.1%) 2 months (0–8) 80 (39.2%) | 4.0 months (0–38) 10 months (7–28) 8 (10.5%) 2 months (1–21) 16 (21.1%) 3 months (0–10) 28 (36.8%) | 0.261 |
| BOR to initial ICI therapy 6 | 0.569 | ||
| 30 (14.7%) 48 (23.5%) 45 (22.1%) 80 (39.2%) 1 | 9 (11.8%) 23 (30.3%) 13 (17.1%) 28 (36.8%) 3 | |
| TP upon initial ICI | 159 (77.9%) | 61 (80.2%) | 0.674 |
| Median progression-free survival (95% CI) | 4.0 months (2.9–5.1) | 4.0 months (1.7–6.2) | 0.705 |
| VTE during initial ICI therapy 7 | 9 (4.4%) | 26 (36.8%) | <0.001 |
| ATE during initial ICI therapy 8 | 6 (2.9%) | 6 (7.9%) | 0.094 |
| Subsequent treatments | |||
|
Patients receiving subsequent therapy Number of post-treatments
| 103 168 78 33 (42.3%) 66 (84.6%) 45 18 (40.0%) 39 (86.6%) | 43 68 29 11 (38.0%) 22 (75.9%) 19 6 (31.5%) 17 (89.5%) | 0.900 0.673 0.587 0.764 0.611 0.614 |
| Follow-up | |||
| Median follow-up upon ICI initiation (95% CI) | 30 months (24.4–35.6) | 22 months (13.6–30.4) | 0.055 |
| Median overall survival (95% CI) | 51 months (30.6–71.4) | 39 months (18.4–59.6) | 0.313 |
| Deceased | 81 (39.8%) | 33 (43.4%) | 0.587 |
| Outcome | Not on Anticoagulation | On Anticoagulation | p-Value |
|---|---|---|---|
| Best overall response—no. (%) | 0.576 | ||
| Complete response (CR) | 30 (14.7%) | 9 (12.3%) | |
| Partial response (PR) | 48 (23.6%) | 23 (31.5%) | |
| Stable disease (SD) | 45 (22.2%) | 13 (17.8%) | |
| Progressive disease (PD) | 80 (39.4%) | 28 (38.4%) | |
| Objective response rate 1 | 0.270 | ||
| No. (%) | 78/203 (38.4%) | 32/73 (43.8%) | |
| 95% CI 1 | 32.0–45.9% | 32.2–55.9% | |
| Disease control rate 2 | 0.946 | ||
| No. (%) | 123/203 (60.5%) | 45/73 (61.6%) | |
| 95% CI 3 | 54.1–68.0% | 49.5–72.8% | |
| Progress during initial ICI | 0.647 | ||
| No. (%) | 159 (77.9%) | 61 (80.2%) | |
| 95% CI 3 | 71.6–83.4% | 69.5–88.5% |
| Outcome | Not on AC | Heparins | VKA | FXa DOAC | Chi-Square Test |
|---|---|---|---|---|---|
| Best overall response—no. (%) | 0.037 | ||||
| Complete response (CR) | 30 (14.7%) | 1 (3.7%) | 1 (5.0%) | 7 (26.9%) | |
| Partial response (PR) | 48 (23.6%) | 5 (18.5%) | 7 (35.0%) | 11 (42.3%) | |
| Stable disease (SD) | 45 (22.2%) | 5 (18.5%) | 4 (20.0%) | 4 (15.4%) | |
| Progressive disease (PD) | 80 (39.4%) | 16 (55.2%) | 8 (40.0%) | 4 (15.4%) | |
| Could not be evaluated | 3 | 2 | 0 | 1 | |
| Objective response rate 1 | 0.005 | ||||
| No. (%) | 78 (38.2%) | 6 (22.2%) | 8 (40.0%) | 18 (69.2%) | |
| 95% CI 3 | 32.0–45.6% | 8.6–42.3% | 18.1–63.9% | 48.2–85.7% | |
| Disease control rate 2 | 0.011 | ||||
| No. (%) | 123 (60.3%) | 11 (40.7%) | 12 (60%) | 22 (84.6%) | |
| 95% CI 3 | 54.1–68.0% | 22.4–61.2% | 36.1–80.9% | 65.1–95.6% | |
| Progress during initial ICI | 0.001 | ||||
| No. (%) | 159 (77.9%) | 28 (96.6%) | 18 (90.0%) | 15 (55.6%) | |
| 95% CI 3 | 71.6–83.4% | 82.2–99.9% | 68.3–98.8% | 35.3–74.5% |
| Secondary Outcome | Not on Anticoagulation | On Anticoagulation | p-Value |
|---|---|---|---|
| Bleeding complications | 0.610 | ||
| No. (%) | 14 (6.9%) | 7 (9.2%) | |
| 95% CI | 3.8–11.2% | 3.8–18.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haist, M.; Stege, H.; Pemler, S.; Heinz, J.; Fleischer, M.I.; Graf, C.; Ruf, W.; Loquai, C.; Grabbe, S. Anticoagulation with Factor Xa Inhibitors Is Associated with Improved Overall Response and Progression-Free Survival in Patients with Metastatic Malignant Melanoma Receiving Immune Checkpoint Inhibitors—A Retrospective, Real-World Cohort Study. Cancers 2021, 13, 5103. https://doi.org/10.3390/cancers13205103
Haist M, Stege H, Pemler S, Heinz J, Fleischer MI, Graf C, Ruf W, Loquai C, Grabbe S. Anticoagulation with Factor Xa Inhibitors Is Associated with Improved Overall Response and Progression-Free Survival in Patients with Metastatic Malignant Melanoma Receiving Immune Checkpoint Inhibitors—A Retrospective, Real-World Cohort Study. Cancers. 2021; 13(20):5103. https://doi.org/10.3390/cancers13205103
Chicago/Turabian StyleHaist, Maximilian, Henner Stege, Saskia Pemler, Jaqueline Heinz, Maria Isabel Fleischer, Claudine Graf, Wolfram Ruf, Carmen Loquai, and Stephan Grabbe. 2021. "Anticoagulation with Factor Xa Inhibitors Is Associated with Improved Overall Response and Progression-Free Survival in Patients with Metastatic Malignant Melanoma Receiving Immune Checkpoint Inhibitors—A Retrospective, Real-World Cohort Study" Cancers 13, no. 20: 5103. https://doi.org/10.3390/cancers13205103
APA StyleHaist, M., Stege, H., Pemler, S., Heinz, J., Fleischer, M. I., Graf, C., Ruf, W., Loquai, C., & Grabbe, S. (2021). Anticoagulation with Factor Xa Inhibitors Is Associated with Improved Overall Response and Progression-Free Survival in Patients with Metastatic Malignant Melanoma Receiving Immune Checkpoint Inhibitors—A Retrospective, Real-World Cohort Study. Cancers, 13(20), 5103. https://doi.org/10.3390/cancers13205103








