Opposite Incidence Trends for Differentiated and Medullary Thyroid Cancer in Young Dutch Patients over a 30-Year Time Span
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Definitions
2.3. The Netherlands Cancer Registry
2.4. Statistical Analyses
3. Results
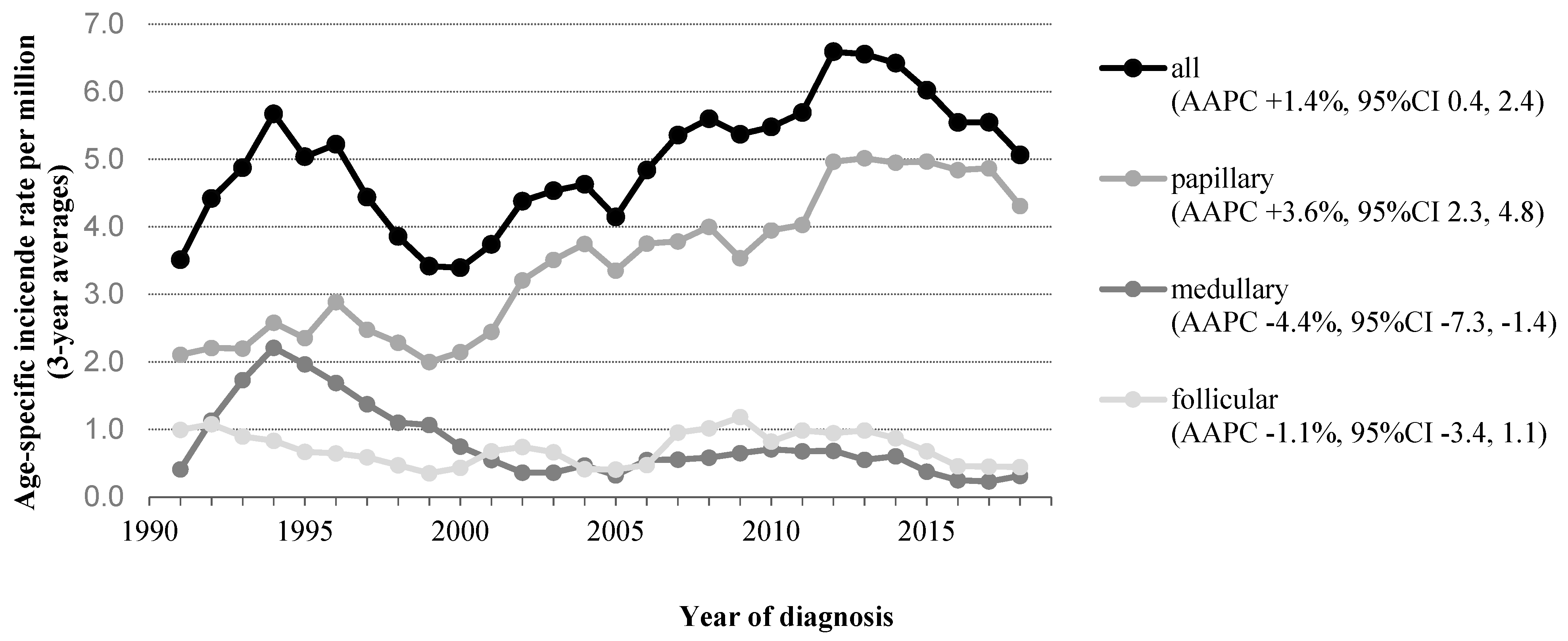
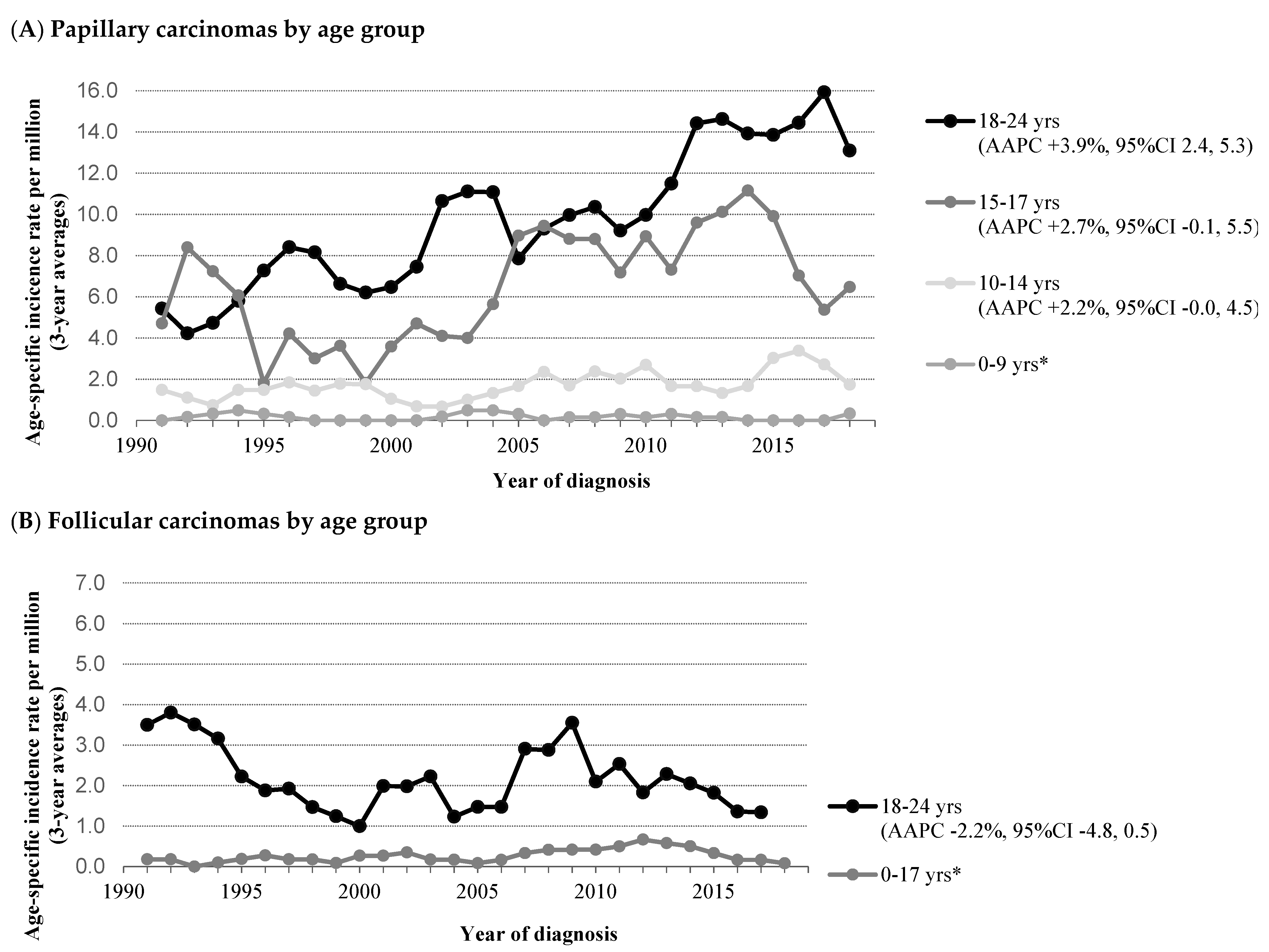
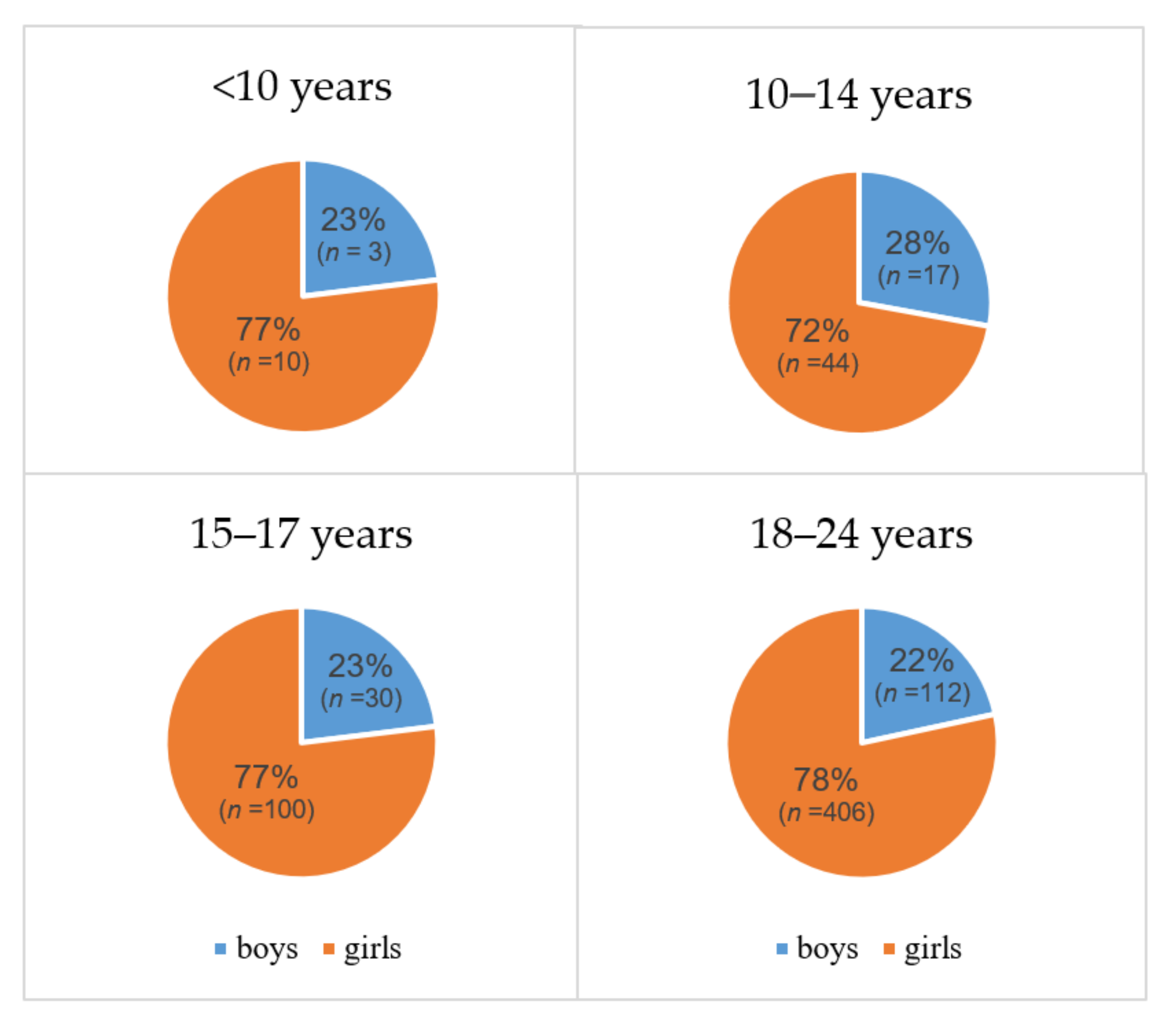
3.1. Differentiated Thyroid Carcinoma (DTC)
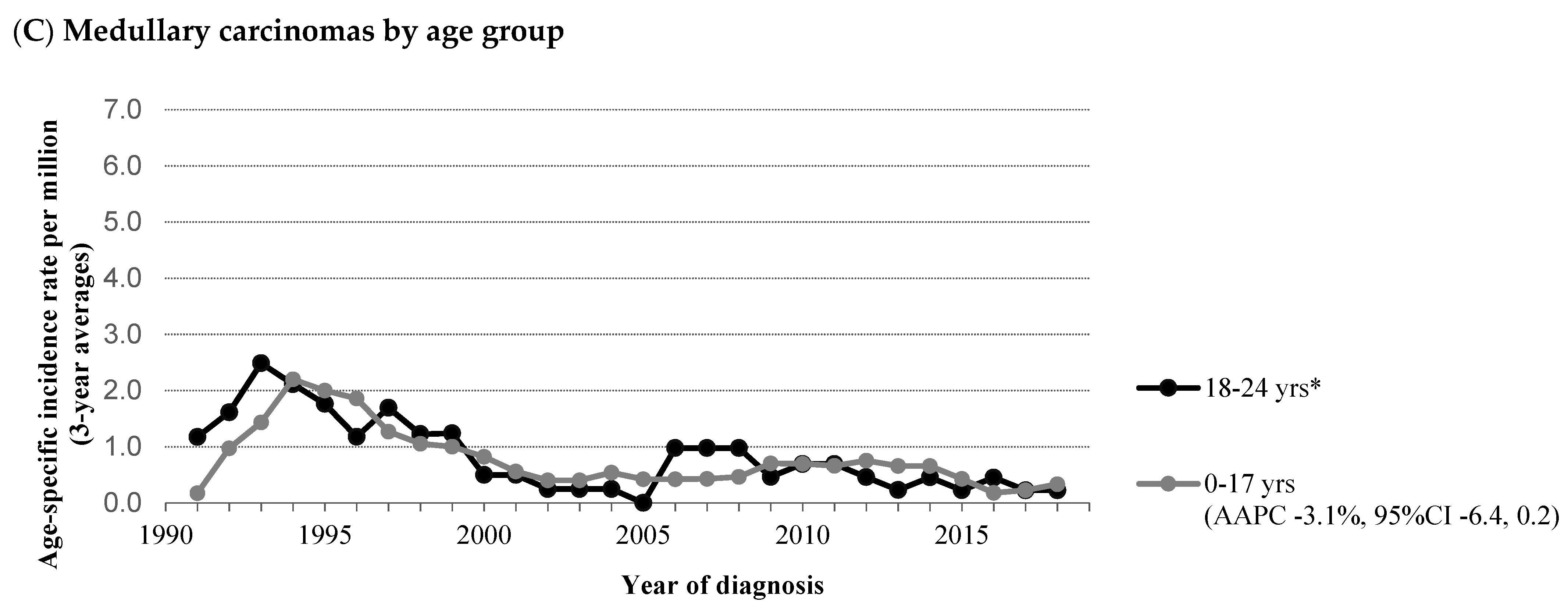
3.2. Medullary Thyroid Carcinoma (MTC)
4. Discussion
4.1. Differentiated Thyroid Carcinoma (DTC)
4.2. Medullary Thyroid Carcinoma (MTC)
4.3. Site of Treatment
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hogan, A.R.; Zhuge, Y.; Perez, E.A.; Koniaris, L.G.; Lew, J.I.; Sola, J.E. Pediatric thyroid carcinoma: Incidence and outcomes in 1753 patients. J. Surg. Res. 2009, 156, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Vergamini, L.B.; Frazier, A.L.; Abrantes, F.L.; Ribeiro, K.B.; Rodriguez-Galindo, C. Increase in the incidence of differentiated thyroid carcinoma in children, adolescents, and young adults: A population-based study. J. Pediatr. 2014, 164, 1481–1485. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.L.; Waguespack, S.G.; Bauer, A.J.; Angelos, P.; Benvenga, S.; Cerutti, J.M.; Dinauer, C.A.; Hamilton, J.; Hay, I.D.; Luster, M.; et al. Management guidelines for children with thyroid nodules and differentiated thyroid cancer the American Thyroid Association Guidelines Task Force on Pediatric Thyroid Cancer. Thyroid 2015, 25, 716–759. [Google Scholar] [CrossRef] [PubMed]
- Reedijk, A.M.J.; Kremer, L.C.; Visser, O.; Lemmens, V.; Pieters, R.; Coebergh, J.W.W.; Karim-Kos, H.E. Increasing incidence of cancer and stage migration towards advanced disease in children and young adolescents in the Netherlands, 1990–2017. Eur. J. Cancer 2020, 134, 115–126. [Google Scholar] [CrossRef]
- Howlader, N.; Noone, A.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D. SEER Cancer Statistics Review, 1975–2016; National Cancer Institute: Bethesda, MD, USA, 2019. [Google Scholar]
- Chan, C.M.; Young, J.; Prager, J.; Travers, S. Pediatric thyroid cancer. Adv. Pediatr. 2017, 64, 171–190. [Google Scholar] [CrossRef]
- Dermody, S.; Walls, A.; Harley, E.H. Pediatric thyroid cancer: An update from the SEER database 2007–2012. Int. J. Pediatr. Otorhinolaryngol. 2016, 89, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.J.; Jin, M.C.; Meister, K.D.; Megwalu, U.C. Pediatric thyroid cancer incidence and mortality trends in the United States, 1973–2013. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 617–623. [Google Scholar] [CrossRef]
- Golpanian, S.; Tashiro, J.; Sola, J.E.; Allen, C.; Lew, J.I.; Hogan, A.R.; Neville, H.L.; Perez, E.A. Surgically treated pediatric nonpapillary thyroid carcinoma. Eur. J. Pediatr. Surg. 2016, 26, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Wells, S.A.; Asa, S.L.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F.; Lee, N.; MacHens, A.; Moley, J.F.; Pacini, F.; et al. Revised American thyroid association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015, 25, 567–610. [Google Scholar] [CrossRef]
- Bernier, M.O.; Withrow, D.R.; Berrington de Gonzalez, A.; Lam, C.J.K.; Linet, M.S.; Kitahara, C.M.; Shiels, M.S. Trends in pediatric thyroid cancer incidence in the United States, 1998–2013. Cancer 2019, 125, 2497–2505. [Google Scholar] [CrossRef]
- Cho, Y.Y.; Jang, H.W.; Joung, J.Y.; Park, S.-M.; Jeong, D.J.; Kim, S.W.; Chung, J.H. Trends in thyroid cancer incidence in Korean children (1999–2012) based on palpation and nonpalpation detection methods. Eur. Thyroid J. 2015, 4, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Schmidt Jensen, J.; Grønhøj, C.; Mirian, C.; Jensen, D.H.; Friborg, J.; Hahn, C.H.; Agander, T.K.; Hjuler, T. Incidence and survival of thyroid cancer in children, adolescents, and young adults in Denmark: A nationwide study from 1980 to 2014. Thyroid 2018, 28, 1128–1133. [Google Scholar] [CrossRef]
- Takano, T. Natural history of thyroid cancer suggests beginning of the overdiagnosis of juvenile thyroid cancer in the United States. Cancer 2019, 125, 4107–4108. [Google Scholar] [CrossRef] [PubMed]
- Bernier, M.O.; Kitahara, C.M.; Shiels, M.S. Reply to natural history of thyroid cancer suggests beginning of the overdiagnosis of juvenile thyroid cancer in the United States and harm of overdiagnosis or extremely early diagnosis behind trends in pediatric thyroid cancer. Cancer 2019, 125, 4109–4110. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, C.M.; Gamborg, M.; De González, A.B.; Sørensen, T.I.A.; Baker, J.L. Childhood height and body mass index were associated with risk of adult thyroid cancer in a large cohort study. Cancer Res. 2014, 74, 235–242. [Google Scholar] [CrossRef]
- Clement, S.C.; Lebbink, C.A.; Klein Hesselink, M.S.; Teepen, J.C.; Links, T.P.; Ronckers, C.M.; van Santen, H.M. Presentation and outcome of subsequent thyroid cancer among childhood cancer survivors compared to sporadic thyroid cancer: A matched national study. Eur. J. Endocrinol. 2020, 183, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Clement, S.C.; Kremer, L.C.M.; Verburg, F.A.; Simmons, J.H.; Goldfarb, M.; Peeters, R.P.; Alexander, E.K.; Bardi, E.; Brignardello, E.; Constine, L.S.; et al. Balancing the benefits and harms of thyroid cancer surveillance in survivors of childhood, adolescent and young adult cancer: Recommendations from the international Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCareSurFup Consortium. Cancer Treat. Rev. 2018, 63, 28–39. [Google Scholar] [PubMed]
- World Health Organization. International Classification of Diseases for Oncology, 3rd ed.; Fritz, A., Percy, C., Jack, A., Shanmugaratnam, K., Sobin, L., Parkin, D.M., Whelan, S., Eds.; World Health Organization: Geneva, Switzerland, 2000; ISBN 9241545348. [Google Scholar]
- Tuttle, R.M.; Haugen, B.; Perrier, N.D. Updated American joint committee on cancer/tumor-node-metastasis staging system for differentiated and anaplastic thyroid cancer (eighth edition): What changed and why? Thyroid 2017, 27, 751–756. [Google Scholar] [CrossRef]
- Van der Sanden, G.A.; Coebergh, J.W.; Schouten, L.J.; Visser, O.; van Leeuwen, F.E. Cancer incidence in The Netherlands in 1989 and 1990: First results of the nationwide Netherlands cancer registry. Coordinating Committee for Regional Cancer Registries. Eur. J. Cancer 1995, 31, 1822–1829. [Google Scholar] [CrossRef]
- Boyle, P.; Parkin, D. Statistical Methods for Registries. Cancer Registration: Principles and Methods; IARC: Lyon, France, 1991. [Google Scholar]
- Clegg, L.X.; Hankey, B.F.; Tiwari, R.; Feuer, E.J.; Edwards, B.K. Estimating average annual per cent change in trend analysis. Stat. Med. 2009, 28, 3670–3682. [Google Scholar] [CrossRef]
- Kim, H.; Fay, M.; Feuer, E.; Midthune, D. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000, 19, 335–351. [Google Scholar] [CrossRef]
- Sankila, R.; Martos Jiménez, M.C.; Miljus, D.; Pritchard-Jones, K.; Steliarova-Foucher, E.; Stiller, C.; Petrovich, S.V.; Budanov, O.; Storm, H.; Christensen, N.; et al. Geographical comparison of cancer survival in European children (1988–1997): Report from the Automated Childhood Cancer Information System project. Eur. J. Cancer 2006, 42, 1972–1980. [Google Scholar] [CrossRef] [PubMed]
- Golpanian, S.; Perez, E.A.; Tashiro, J.; Lew, J.I.; Sola, J.E.; Hogan, A.R. Pediatric papillary thyroid carcinoma: Outcomes and survival predictors in 2504 surgical patients. Pediatr. Surg. Int. 2016, 32, 201–208. [Google Scholar] [CrossRef]
- Derwahl, M.; Nicula, D. Estrogen and its role in thyroid cancer. Endocr. Relat. Cancer 2014, 21, T273–T283. [Google Scholar] [CrossRef]
- Farahati, J.; Bucsky, P.; Parlowsky, T.; Mäder, U.; Reiners, C. Characteristics of differentiated thyroid carcinoma in children and adolescents with respect to age, gender, and histology. Cancer 1997, 80, 2156–2162. [Google Scholar] [CrossRef]
- Lazar, L.; Lebenthal, Y.; Steinmetz, A.; Yackobovitch-Gavan, M.; Phillip, M. Differentiated thyroid carcinoma in pediatric patients: Comparison of presentation and course between pre-pubertal children and adolescents. J. Pediatr. 2009, 154, 708–714. [Google Scholar] [CrossRef]
- Ullmann, T.M.; Gray, K.D.; Limberg, J.; Stefanova, D.; Moore, M.D.; Buicko, J.; Finnerty, B.; Zarnegar, R.; Fahey, T.J.; Beninato, T. Insurance status is associated with extent of treatment for patients with papillary thyroid carcinoma. Thyroid 2019, 29, 1784–1791. [Google Scholar] [CrossRef]
- Van den Broek, M.F.M.; Rijks, E.B.G.; Nikkels, P.G.J.; Wolters, V.M.; van Es, R.J.J.; van Santen, H.M.; van Nesselrooij, B.P.M.; Vriens, M.R.; van Leeuwaarde, R.S.; Valk, G.D.; et al. Timely diagnosis of multiple endocrine neoplasia 2B by identification of intestinal ganglioneuromatosis: A case series. Endocrine 2021, 72, 905–914. [Google Scholar] [CrossRef]
- Mulligan, L.M.; Kwok, J.B.J.; Healey, C.S.; Elsdon, M.J.; Eng, C.; Gardner, E.; Love, D.R.; Molet, S.E.; Moore, J.K.; Papi, L.; et al. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature 1993, 363, 458–460. [Google Scholar] [CrossRef]
- Hofstra, R.M.W.; Landsvater, R.M.; Ceccherini, I.; Stulp, R.P.; Stelwagen, T.; Luo, Y.; Pasini, B.; Höppener, J.W.M.; Kristian, H.; van Amstel, P.; et al. A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature 1994, 367, 375–376. [Google Scholar] [CrossRef] [PubMed]
- Castinetti, F.; Waguespack, S.G.; Machens, A.; Uchino, S.; Hasse-Lazar, K.; Sanso, G.; Else, T.; Dvorakova, S.; Qi, X.P.; Elisei, R.; et al. Natural history, treatment, and long-term follow up of patients with multiple endocrine neoplasia type 2B: An international, multicentre, retrospective study. Lancet Diabetes Endocrinol. 2019, 7, 213–220. [Google Scholar] [CrossRef]
- Baumgarten, H.D.; Bauer, A.J.; Isaza, A.; Mostoufi-Moab, S.; Kazahaya, K.; Adzick, N.S. Surgical management of pediatric thyroid disease: Complication rates after thyroidectomy at the Children’s Hospital of Philadelphia high-volume Pediatric Thyroid Center. J. Pediatr. Surg. 2019, 54, 1969–1975. [Google Scholar] [CrossRef] [PubMed]
| (A) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Total | Average per Year | Period of Diagnosis | |||||||
| 1990–1999 | 2000–2009 | 2010–2019 | ||||||||
| N | % | N | N | % | N | % | N | % | p-value | |
| 722 | 24 | 186 | 26 | 223 | 31 | 313 | 43 | |||
| Age | 0.75 | |||||||||
| 0–9 | 13 | 2 | 0 | 4 | 2 | 5 | 2 | 4 | 1 | |
| 10–14 | 61 | 8 | 2 | 16 | 9 | 18 | 8 | 27 | 9 | |
| 15–17 | 130 | 18 | 4 | 27 | 15 | 46 | 21 | 57 | 18 | |
| 18–24 | 518 | 72 | 17 | 139 | 75 | 154 | 69 | 225 | 72 | |
| Median age (in years, p25–p75) | 20 | (17–23) | 21 | (17–23) | 20 | (17–23) | 20 | (17–23) | 0.23 | |
| Sex | 0.98 | |||||||||
| boys | 162 | 22 | 5 | 42 | 23 | 49 | 22 | 71 | 23 | |
| girls | 560 | 78 | 19 | 144 | 77 | 174 | 78 | 242 | 77 | |
| Histology | 0.002 | |||||||||
| papillary carcinoma | 594 | 82 | 20 | 138 | 74 | 185 | 83 | 271 | 87 | |
| follicular carcinoma | 128 | 18 | 4 | 48 | 26 | 38 | 17 | 42 | 13 | |
| T stage a | <0.001 | |||||||||
| 1 | 198 | 28 | 7 | 25 | 14 | 64 | 31 | 109 | 35 | |
| 2 | 292 | 42 | 10 | 101 | 57 | 82 | 39 | 109 | 35 | |
| 3 | 145 | 21 | 5 | 22 | 13 | 41 | 20 | 82 | 26 | |
| 4 | 62 | 9 | 2 | 28 | 16 | 22 | 11 | 12 | 4 | |
| unknown (3% of total) | 25 | 1 | 10 | 14 | 1 | |||||
| N stage a | 0.02 | |||||||||
| 0 | 379 | 56 | 13 | 98 | 59 | 99 | 48 | 182 | 59 | |
| 1 | 300 | 44 | 10 | 68 | 41 | 108 | 52 | 124 | 41 | |
| unknown (6% of total) | 43 | 1 | 20 | 16 | 7 | |||||
| Metastases a | 0.41 | |||||||||
| no | 606 | 97 | 20 | 140 | 97 | 161 | 95 | 305 | 97 | |
| yes | 20 | 3 | 1 | 4 | 3 | 8 | 5 | 8 | 3 | |
| unknown (13% of total) | 96 | 3 | 42 | 54 | 0 | |||||
| Thyroid carcinoma as second primary cancer | 0.65 | |||||||||
| yes | 18 | 2 | 1 | 6 | 3 | 6 | 3 | 6 | 2 | |
| no | 704 | 98 | 23 | 180 | 97 | 217 | 97 | 307 | 98 | |
| (B) | ||||||||||
| Characteristics | Total | Average per Year | Period of Diagnosis | |||||||
| 1990–1999 | 2000–2009 | 2010–2019 | ||||||||
| N | % | N | N | % | N | % | N | % | p-value | |
| 114 | 4 | 66 | 58 | 25 | 22 | 23 | 20 | |||
| Age | 0.67 | |||||||||
| 0–17 | 78 | 68 | 3 | 43 | 65 | 18 | 72 | 17 | 74 | |
| 18–24 | 36 | 32 | 1 | 23 | 35 | 7 | 28 | 6 | 26 | |
| Median age (in years, p25–p75) | 13 | (6–19) | 13.5 | (8–19) | 11 | (6–18) | 11 | (3–18) | 0.32 | |
| Sex | 0.18 | |||||||||
| boys | 55 | 48 | 2 | 35 | 53 | 8 | 32 | 12 | 52 | |
| girls | 59 | 52 | 2 | 31 | 47 | 17 | 68 | 11 | 48 | |
| T stage a | 0.35 | |||||||||
| 1 | 83 | 78 | 3 | 48 | 79 | 19 | 83 | 16 | 70 | |
| 2 | 12 | 11 | 0 | 7 | 11 | 1 | 4 | 4 | 17 | |
| 3 | 6 | 6 | 0 | 2 | 3 | 1 | 4 | 3 | 13 | |
| 4 | 6 | 6 | 0 | 4 | 7 | 2 | 9 | 0 | 0 | |
| unknown (6% of total) | 7 | 0 | 5 | 2 | 0 | |||||
| N stage a | 0.045 | |||||||||
| 0 | 67 | 72 | 2 | 42 | 82 | 13 | 59 | 12 | 60 | |
| 1 | 26 | 28 | 1 | 9 | 18 | 9 | 41 | 8 | 40 | |
| unknown (18% of total) | 21 | 1 | 15 | 3 | 3 | |||||
| Metastases a | 0.84 | |||||||||
| Yes | 5 | 6 | 0 | 2 | 5 | 1 | 6 | 2 | 9 | |
| no | 75 | 94 | 3 | 37 | 95 | 17 | 94 | 21 | 91 | |
| unknown (30% of total) | 34 | 1 | 27 | 7 | 0 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebbink, C.A.; van den Broek, M.F.M.; Kwast, A.B.G.; Derikx, J.P.M.; Dierselhuis, M.P.; Kruijff, S.; Links, T.P.; van Trotsenburg, A.S.P.; Valk, G.D.; Vriens, M.R.; et al. Opposite Incidence Trends for Differentiated and Medullary Thyroid Cancer in Young Dutch Patients over a 30-Year Time Span. Cancers 2021, 13, 5104. https://doi.org/10.3390/cancers13205104
Lebbink CA, van den Broek MFM, Kwast ABG, Derikx JPM, Dierselhuis MP, Kruijff S, Links TP, van Trotsenburg ASP, Valk GD, Vriens MR, et al. Opposite Incidence Trends for Differentiated and Medullary Thyroid Cancer in Young Dutch Patients over a 30-Year Time Span. Cancers. 2021; 13(20):5104. https://doi.org/10.3390/cancers13205104
Chicago/Turabian StyleLebbink, Chantal A., Medard F. M. van den Broek, Annemiek B. G. Kwast, Joep P. M. Derikx, Miranda P. Dierselhuis, Schelto Kruijff, Thera P. Links, A. S. Paul van Trotsenburg, Gerlof D. Valk, Menno R. Vriens, and et al. 2021. "Opposite Incidence Trends for Differentiated and Medullary Thyroid Cancer in Young Dutch Patients over a 30-Year Time Span" Cancers 13, no. 20: 5104. https://doi.org/10.3390/cancers13205104
APA StyleLebbink, C. A., van den Broek, M. F. M., Kwast, A. B. G., Derikx, J. P. M., Dierselhuis, M. P., Kruijff, S., Links, T. P., van Trotsenburg, A. S. P., Valk, G. D., Vriens, M. R., Verrijn Stuart, A. A., van Santen, H. M., & Karim-Kos, H. E. (2021). Opposite Incidence Trends for Differentiated and Medullary Thyroid Cancer in Young Dutch Patients over a 30-Year Time Span. Cancers, 13(20), 5104. https://doi.org/10.3390/cancers13205104






