Survival Is Related to Estrogen Signal Transduction Pathway Activity in Postmenopausal Women Diagnosed with High-Grade Serous Ovarian Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Sample Preparation, mRNA Extraction and RT-qPCR Analysis
2.4. OncoSignal Pathway Assay
2.5. Statistical Analysis
2.6. Ethical Approval
3. Results
3.1. Study Population
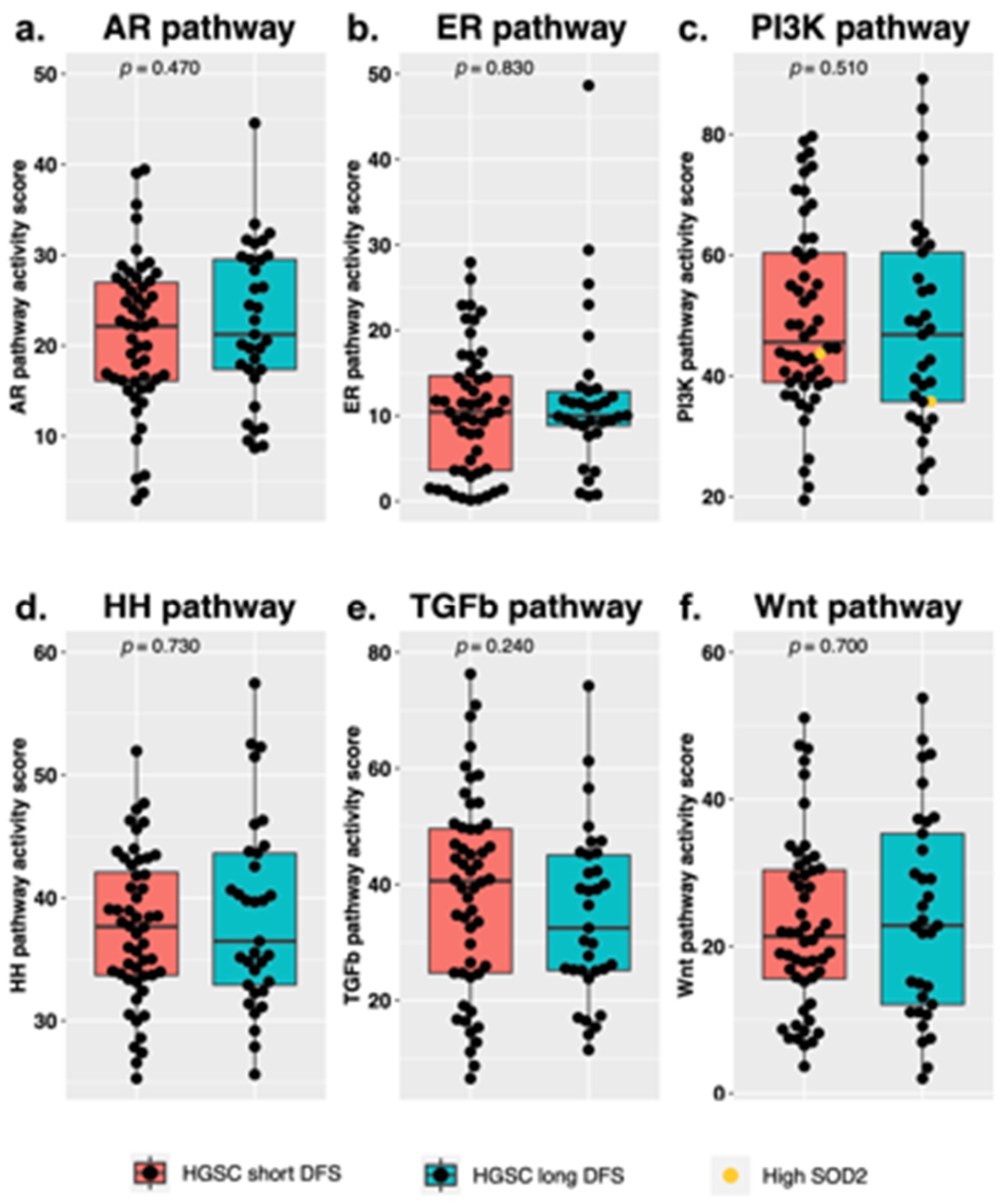
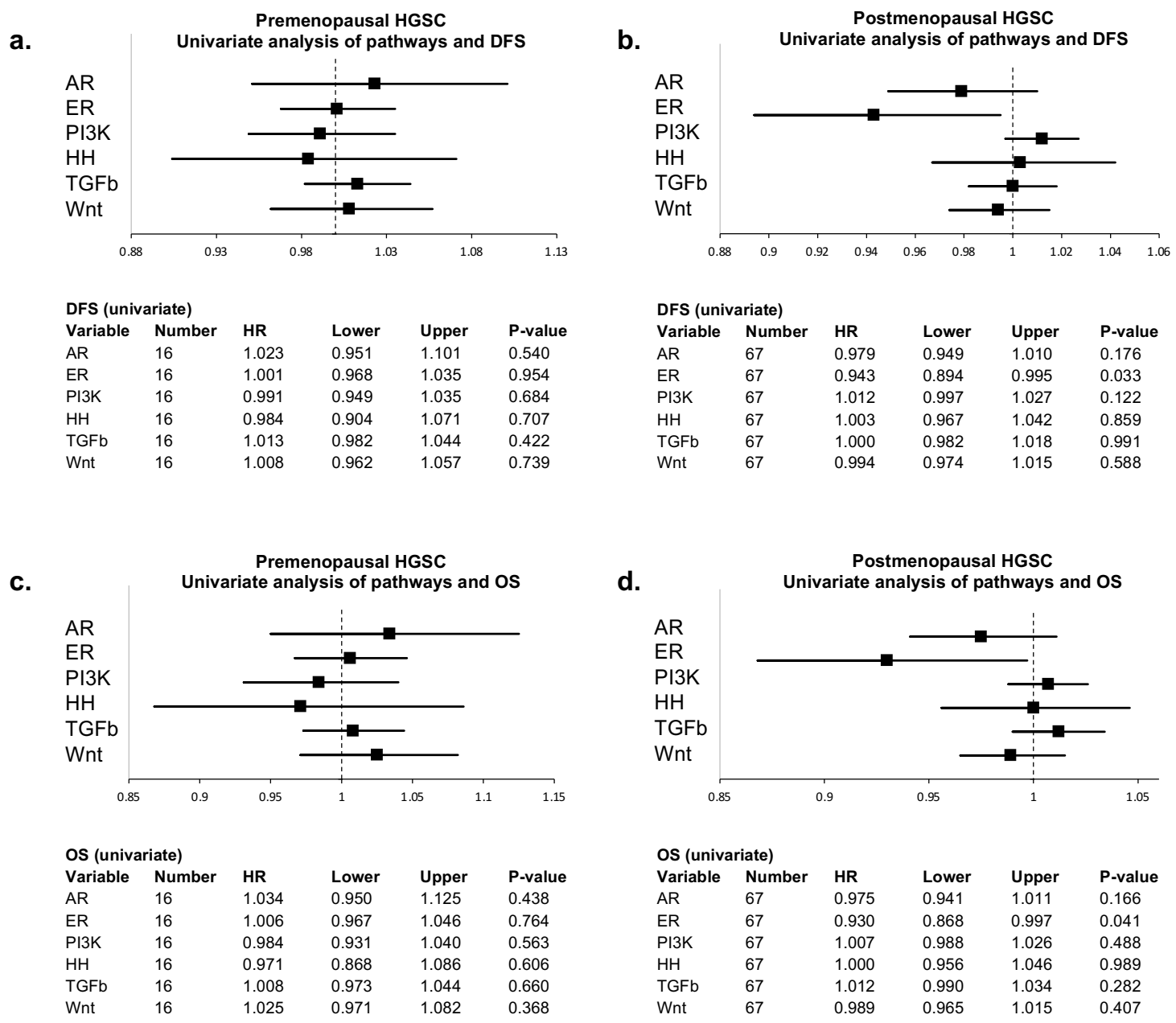
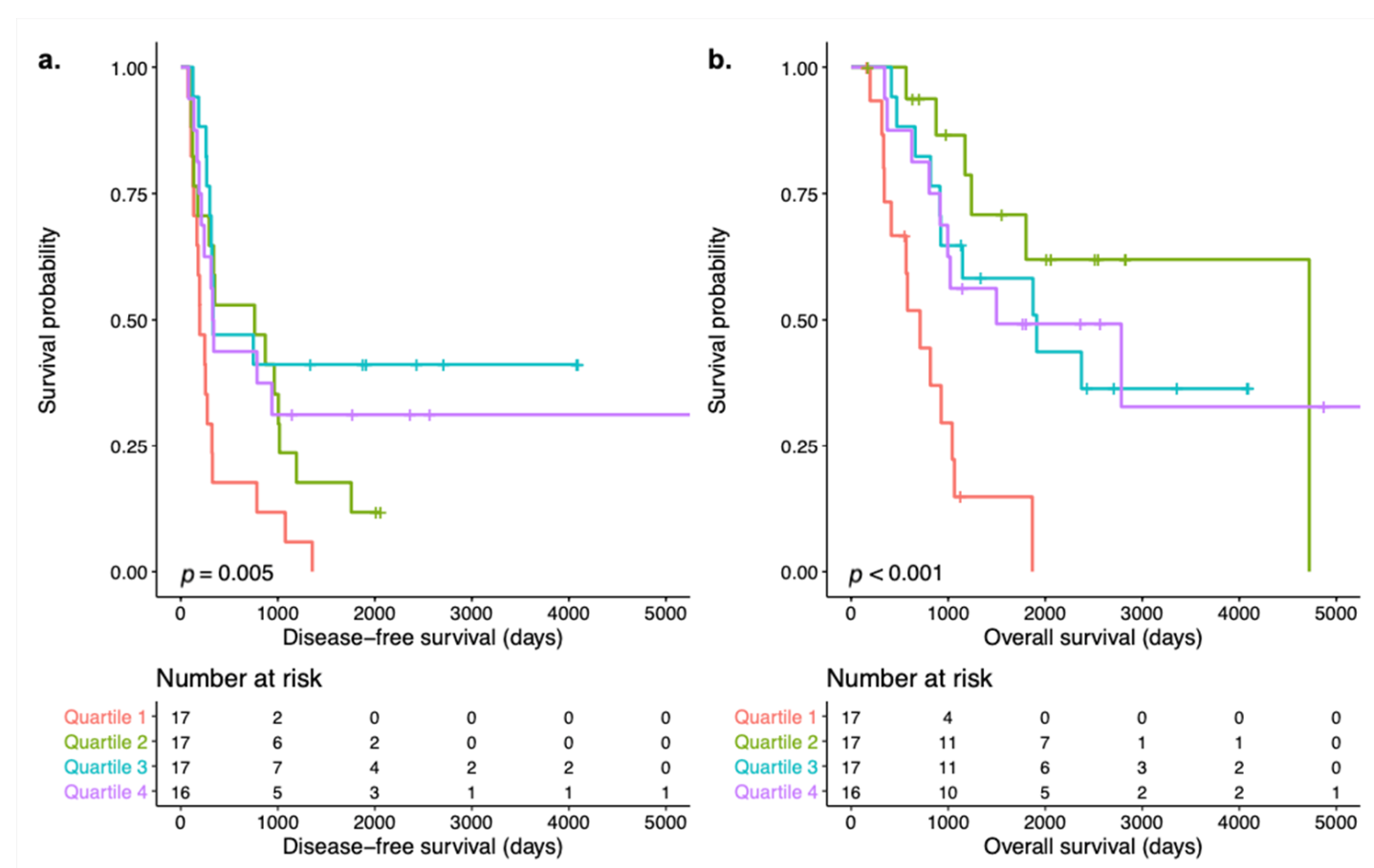
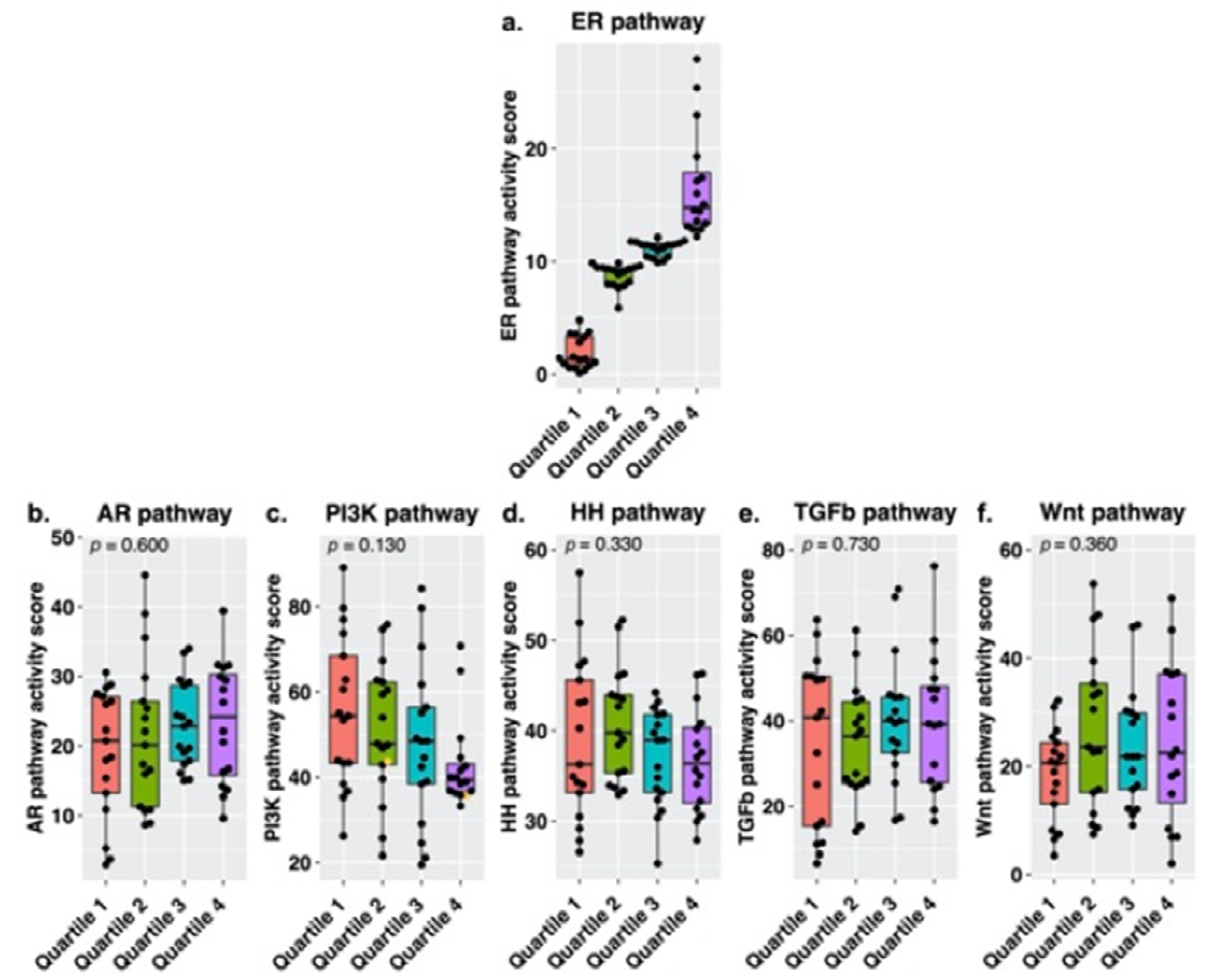
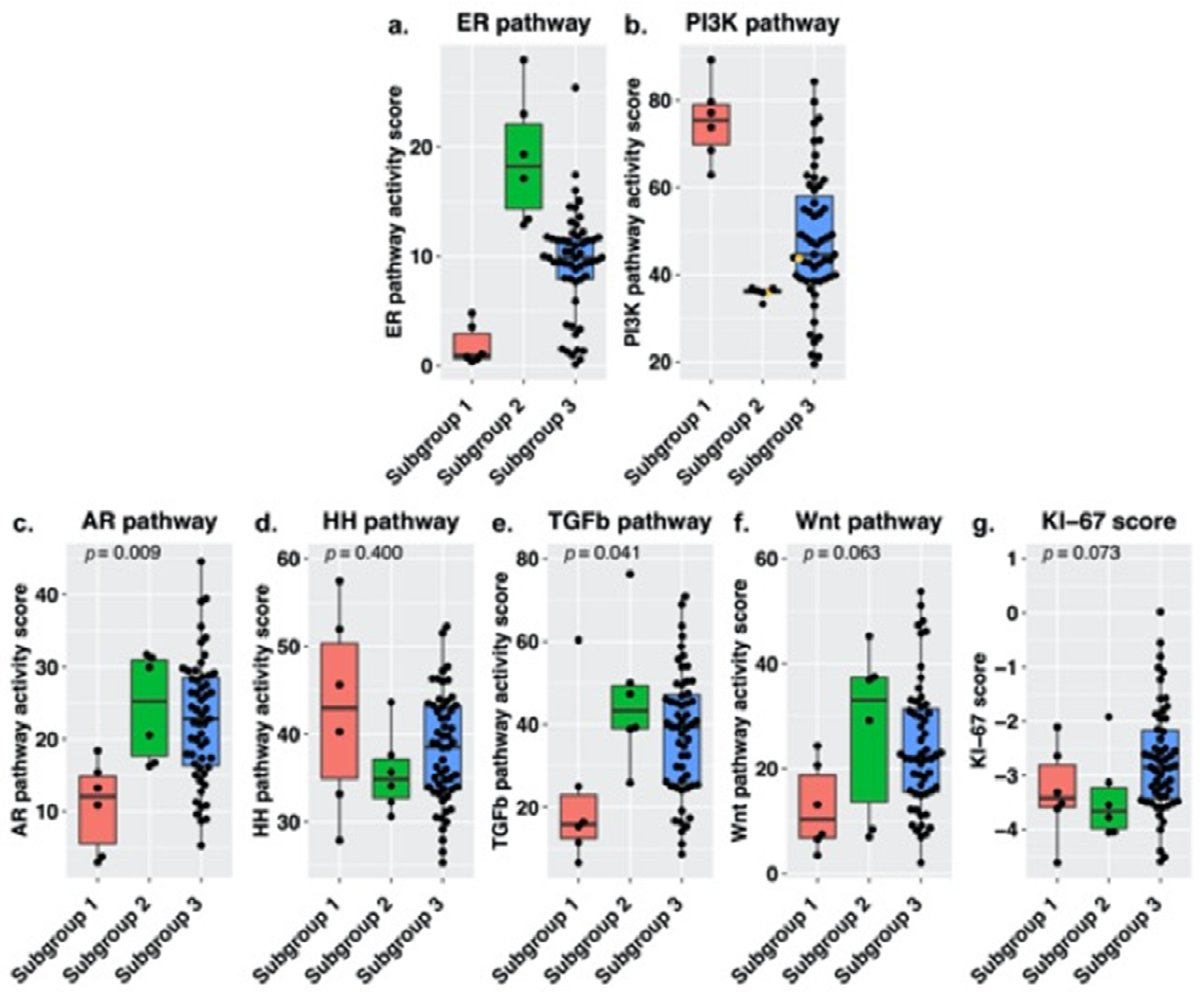
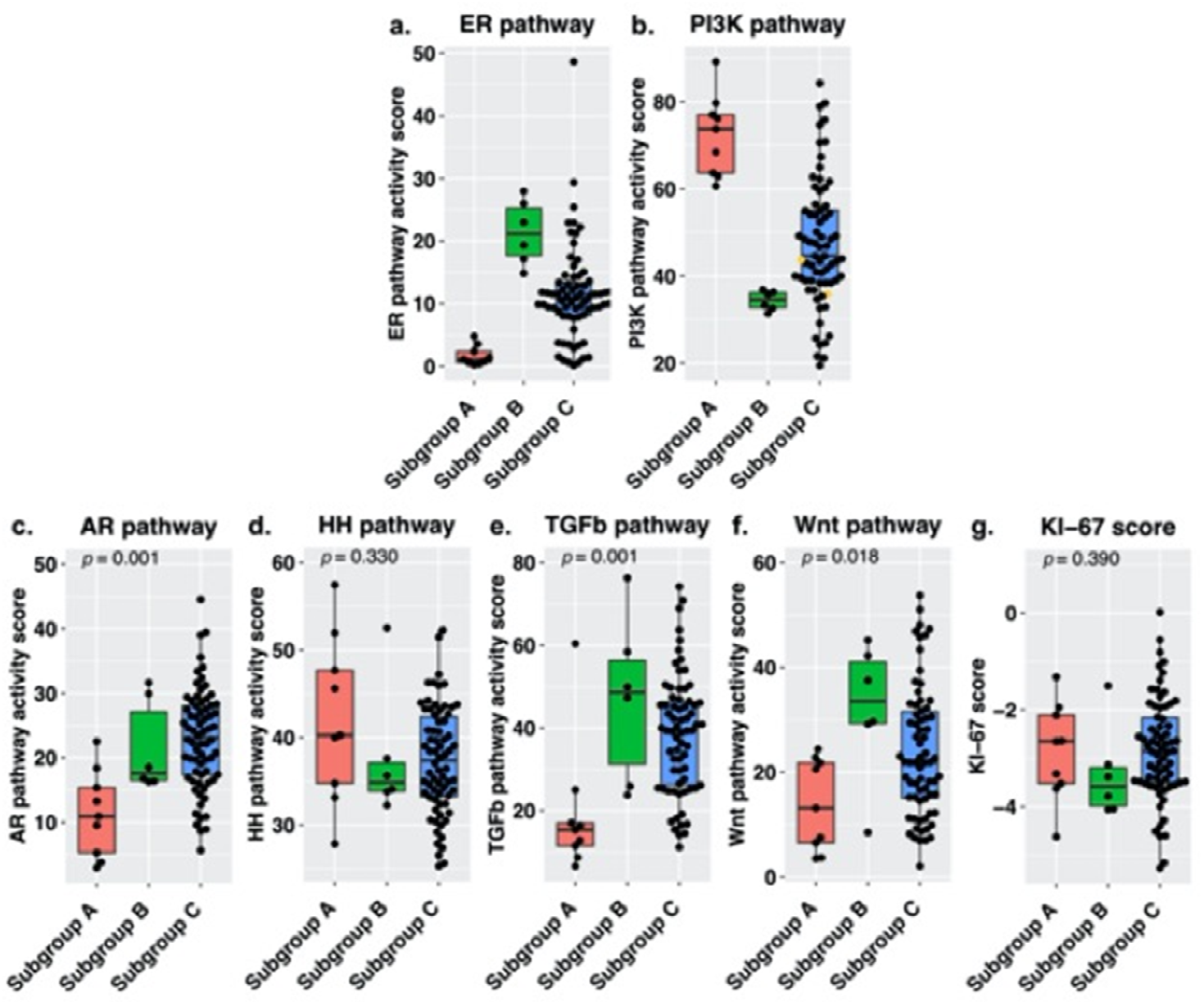
3.2. Signal Transduction Pathway Activity in the Short and Long DFS Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA. Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA. Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Prat, J.; Mutch, D.G. Pathology of cancers of the female genital tract including molecular pathology. Int. J. Gynecol. Obstet. 2018, 143, 93–108. [Google Scholar] [CrossRef] [PubMed]
- IKNL NKR Cijfers. Available online: https://www.iknl.nl/nkr-cijfers?fs%7Cepidemiologie_id=7&fs%7Ctumor_id=305&fs%7Coverlevingssoort_id=75&fs%7Cperiode_van_diagnose_id=113%2C114%2C115%2C116%2C117%2C118&fs%7Cjaren_na_diagnose_id=16%2C17%2C18%2C19%2C20%2C21%2C22%2C23%2C24%2C25%2C26%2C27&cs%7Ct (accessed on 26 October 2020).
- Dao, F.; Schlappe, B.A.; Tseng, J.; Lester, J.; Nick, A.M.; Lutgendorf, S.K.; Mcmeekin, S.; Coleman, R.L.; Moore, K.N.; Karlan, B.Y.; et al. Characteristics of 10-year survivors of high-grade serous ovarian carcinoma. Gynecol. Oncol. 2016, 141, 260–263. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, C.A.; Miller, A.; Casablanca, Y.; Horowitz, N.S.; Rungruang, B.; Krivak, T.C.; Richard, S.D.; Rodriguez, N.; Birrer, M.J.; Backes, F.J.; et al. Clinicopathologic characteristics associated with long-term survival in advanced epithelial ovarian cancer: An NRG Oncology/Gynecologic Oncology Group ancillary data study. Gynecol. Oncol. 2018, 148, 275–280. [Google Scholar] [CrossRef] [Green Version]
- Hoppenot, C.; Eckert, M.A.; Tienda, S.M.; Lengyel, E. Who are the long-term survivors of high grade serous ovarian cancer? Gynecol. Oncol. 2018, 148, 204–212. [Google Scholar] [CrossRef]
- Slamon, D.J.; Godolphin, W.; Jones, L.A.; Holt, J.A.; Wong, S.G.; Keith, D.E.; Levin, W.J.; Stuart, S.G.; Udove, J.; Ullrich, A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989, 244, 707–712. [Google Scholar] [CrossRef]
- Owens, M.A.; Horten, B.C.; Da Silva, M.M. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin. Breast Cancer 2004, 5, 63–69. [Google Scholar] [CrossRef]
- Verhaegh, W.; Van Ooijen, H.; Inda, M.A.; Hatzis, P.; Versteeg, R.; Smid, M.; Martens, J.; Foekens, J.; Van De Wiel, P.; Clevers, H.; et al. Selection of personalized patient therapy through the use of knowledge-based computational models that identify tumor-driving signal transduction pathways. Cancer Res. 2014, 74, 2936–2945. [Google Scholar] [CrossRef] [Green Version]
- Verhaegh, W.; van de Stolpe, A. Knowledge-based computational models. Oncotarget 2014, 5, 5196–5197. [Google Scholar] [CrossRef]
- van de Stolpe, A.; Holtzer, L.; van Ooijen, H.; de Inda, M.A.; Verhaegh, W. Enabling precision medicine by unravelling disease pathophysiology: Quantifying signal transduction pathway activity across cell and tissue types. Sci. Rep. 2019, 9, 1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Ooijen, H.; Hornsveld, M.; Dam-de Veen, C.; Velter, R.; Dou, M.; Verhaegh, W.; Burgering, B.; van de Stolpe, A. Assessment of Functional Phosphatidylinositol 3-Kinase Pathway Activity in Cancer Tissue Using Forkhead Box-O Target Gene Expression in a Knowledge-Based Computational Model. Am. J. Pathol. 2018, 188, 1956–1972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inda, M.A.; Blok, E.J.; Kuppen, P.J.K.; Charehbili, A.; Den Biezen, E.C.; Van Brussel, A.; Fruytier, S.E.; Kranenbarg, E.M. Estrogen Receptor pathway activity score to predict clinical response or resistance to neo—Adjuvant endocrine therapy in primary breast cancer. Mol. Cancer Ther. 2019, 19, 680–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Weelden, W.J.; van der Putten, L.J.M.; Inda, M.A.; van Brussel, A.; Snijders, M.P.L.M.; Schriever, L.M.M.; Bulten, J.; Massuger, L.F.A.G.; van de Stolpe, A.; Pijnenborg, J.M.A. Oestrogen receptor pathway activity is associated with outcome in endometrial cancer. Br. J. Cancer 2020, 123, 785–792. [Google Scholar] [CrossRef]
- Ghirardi, V.; Moruzzi, M.C.; Bizzarri, N.; Vargiu, V.; D’Indinosante, M.; Garganese, G.; Pasciuto, T.; Loverro, M.; Scambia, G.; Fagotti, A. Minimal residual disease at primary debulking surgery versus complete tumor resection at interval debulking surgery in advanced epithelial ovarian cancer: A survival analysis. Gynecol. Oncol. 2020, 157, 209–213. [Google Scholar] [CrossRef]
- Karagol, H.; Saip, P.; Eralp, Y.; Topuz, S.; Berkman, S.; Ilhan, R.; Topuz, E. Factors related to recurrence after pathological complete response to postoperative chemotherapy in patients with epithelial ovarian cancer. Tumori 2009, 95, 207–211. [Google Scholar] [CrossRef]
- Manning-Geist, B.L.; Hicks-Courant, K.; Gockley, A.A.; Clark, R.M.; Del Carmen, M.G.; Growdon, W.B.; Horowitz, N.S.; Berkowitz, R.S.; Muto, M.G.; Worley, M.J. A novel classification of residual disease after interval debulking surgery for advanced-stage ovarian cancer to better distinguish oncologic outcome. Am. J. Obstet. Gynecol. 2019, 221, 326.e1–326.e7. [Google Scholar] [CrossRef]
- van de Stolpe, A.; Verhaegh, W.; Blay, J.Y.; Ma, C.X.; Pauwels, P.; Pegram, M.; Prenen, H.; De Ruysscher, D.; Saba, N.F.; Slovin, S.F.; et al. RNA Based Approaches to Profile Oncogenic Pathways From Low Quantity Samples to Drive Precision Oncology Strategies. Front. Genet. 2021, 11, 598118. [Google Scholar] [CrossRef]
- van de Stolpe, A. Quantitative Measurement of Functional Activity of the PI3K Signaling Pathway in Cancer. Cancers 2019, 11, 293. [Google Scholar] [CrossRef] [Green Version]
- van der Ploeg, P.; Bucks, K.; Bekkers, R.; de Hullu, J.; van de Stolpe, A.; van der Wurff, A.; Vos, M.; Piek, J. EP1014 Signal Transduction Pathway Activity in Normal Fallopian Tube Epithelium and High-Grade Serous Carcinoma. Int. J. Gynecol. Obstet. 2019, 29, A534. [Google Scholar]
- Lazennec, G. Estrogen receptor beta, a possible tumor suppressor involved in ovarian carcinogenesis. Cancer Lett. 2006, 231, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Mal, R.; Magner, A.; David, J.; Datta, J.; Vallabhaneni, M.; Kassem, M.; Manouchehri, J.; Willingham, N.; Stover, D.; Vandeusen, J.; et al. Estrogen Receptor Beta (ERβ): A Ligand Activated Tumor Suppressor. Front. Oncol. 2020, 10, 2151. [Google Scholar] [CrossRef]
- Sieh, W.; Köbel, M.; Longacre, T.A.; Bowtell, D.D.; de Fazio, A.; Goodman, M.T.; Høgdall, E.; Deen, S.; Wentzensen, N.; Moysich, K.B.; et al. Hormone-receptor expression and ovarian cancer survival: An Ovarian Tumor Tissue Analysis consortium study. Lancet. Oncol. 2013, 14, 853–862. [Google Scholar] [CrossRef] [Green Version]
- Van Kruchten, M.; Van Der Marel, P.; De Munck, L.; Hollema, H.; Arts, H.; Timmer-Bosscha, H.; De Vries, E.; Hospers, G.; Reyners, A. Hormone receptors as a marker of poor survival in epithelial ovarian cancer. Gynecol. Oncol. 2015, 138, 634–639. [Google Scholar] [CrossRef]
- Liu, J.F.; Hirsch, M.S.; Lee, H.; Matulonis, U.A. Prognosis and hormone receptor status in older and younger patients with advanced-stage papillary serous ovarian carcinoma. Gynecol. Oncol. 2009, 115, 401–406. [Google Scholar] [CrossRef]
- Shen, Z.; Luo, H.; Li, S.; Sheng, B.; Zhao, M.; Zhu, H.; Zhu, X. Correlation between estrogen receptor expression and prognosis in epithelial ovarian cancer: A meta-analysis. Oncotarget 2017, 8, 62400–62413. [Google Scholar] [CrossRef] [Green Version]
- van der Ploeg, P.; van Lieshout, L.A.M.; van de Stolpe, A.; Bosch, S.L.; Lentjes-Beer, M.H.F.M.; Bekkers, R.L.M.; Piek, J.M.J. Functional estrogen receptor signaling pathway activity in high-grade serous ovarian carcinoma as compared to estrogen receptor protein expression by immunohistochemistry. Cell. Oncol. 2021, 44, 951–957. [Google Scholar] [CrossRef]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar] [CrossRef]
- Mungenast, F.; Aust, S.; Vergote, I.; Vanderstichele, A.; Sehouli, J.; Braicu, E.; Mahner, S.; Castillo-Tong, D.C.; Zeillinger, R.; Thalhammer, T. Clinical significance of the estrogen-modifying enzymes steroid sulfatase and estrogen sulfotransferase in epithelial ovarian cancer. Oncol. Lett. 2017, 13, 4047–4054. [Google Scholar] [CrossRef] [Green Version]
- Mungenast, F.; Thalhammer, T. Estrogen biosynthesis and action in ovarian cancer. Front. Endocrinol. 2014, 5, 192. [Google Scholar] [CrossRef] [Green Version]
- Martins, F.C.; Couturier, D.-L.; Paterson, A.; Karnezis, A.N.; Chow, C.; Nazeran, T.M.; Odunsi, A.; Gentry-Maharaj, A.; Vrvilo, A.; Hein, A.; et al. Clinical and pathological associations of PTEN expression in ovarian cancer: A multicentre study from the Ovarian Tumour Tissue Analysis Consortium. Br. J. Cancer 2020, 123, 793–802. [Google Scholar] [CrossRef]
- Carver, B.S.; Chapinski, C.; Wongvipat, J.; Hieronymus, H.; Chen, Y.; Chandarlapaty, S.; Arora, V.K.; Le, C.; Koutcher, J.; Scher, H.; et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 2011, 19, 575–586. [Google Scholar] [CrossRef] [Green Version]
- Hill, A.; Cristea, M.; He, M.; Frankel, P.; Phd, S.N.; Pal, S.K.; Jones, J.O.; Jones, J. Androgen Receptor and PI3K Pathway Activity in Ovarian Cancer. J. Cancer Res. Therap. Oncol. 2019, 7, 103. [Google Scholar]
- Roberts, C.M.; Cardenas, C.; Tedja, R. The Role of Intra-Tumoral Heterogeneity and Its Clinical Relevance in Epithelial Ovarian Cancer Recurrence and Metastasis. Cancers 2019, 11, 1083. [Google Scholar] [CrossRef] [Green Version]
- Labbé, E.; Lock, L.; Letamendia, A.; Gorska, A.E.; Gryfe, R.; Gallinger, S.; Moses, H.L.; Attisano, L. Transcriptional cooperation between the transforming growth factor-beta and Wnt pathways in mammary and intestinal tumorigenesis. Cancer Res. 2007, 67, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Yin, J.; Wang, H.; Jiang, G.; Deng, M.; Zhang, G.; Bu, X.; Cai, S.; Du, J.; He, Z. FOXO3a modulates WNT/β-catenin signaling and suppresses epithelial-to-mesenchymal transition in prostate cancer cells. Cell. Signal. 2015, 27, 510–518. [Google Scholar] [CrossRef]
- van Lieshout, L.; van de Stolpe, A.; van der Ploeg, P.; Bowtell, D.; de Hullu, J.; Piek, J. Signal Transduction Pathway Activity in High-Grade, Serous Ovarian Carcinoma Reveals a More Favorable Prognosis in Tumors with Low PI3K and High NF-κB Pathway Activity: A Novel Approach to a Long-Standing Enigma. Cancers 2020, 12, 2660. [Google Scholar] [CrossRef]
- Tothill, R.W.; Tinker, A.V.; George, J.; Brown, R.; Fox, S.B.; Lade, S.; Johnson, D.S.; Trivett, M.K.; Etemadmoghadam, D.; Locandro, B.; et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin. Cancer Res. 2008, 14, 5198–5208. [Google Scholar] [CrossRef] [Green Version]
| Variation | Short DFS n = 52 (%) | Long DFS n = 33 (%) | p-Value * |
|---|---|---|---|
| Age at diagnosis | 0.856 | ||
| Mean (SD) | 62 (12) | 61 (12) | |
| Parity | 0.477 | ||
| 0 | 7 (13) | 7 (21) | |
| 1–2 | 20 (38) | 16 (48) | |
| ≥3 | 17 (33) | 8 (24) | |
| Missing | 8 (15) | 2 (6) | |
| Menopausal status | 1.000 | ||
| Premenopausal | 10 (19) | 6 (18) | |
| Postmenopausal | 40 (77) | 27 (82) | |
| Missing | 2 (4) | 0 (0) | |
| FIGO stage | 0.758 | ||
| IIIC | 43 (83) | 29 (88) | |
| IV | 9 (17) | 4 (12) | |
| CA125 concentration at diagnosis | 0.003 | ||
| Median (IQR) | 657 (258–2125) | 244 (120–415) | |
| Missing | 2 | 2 | |
| CA125 concentration after treatment | 0.027 | ||
| Median (IQR) | 13 (9–17) | 10 (6–14) | |
| Missing | 12 | 3 | |
| Debulking type | 0.007 | ||
| Primary | 21 (40) | 24 (73) | |
| Interval | 26 (50) | 9 (27) | |
| Other ** | 5 (10) | 0 (0) | |
| Debulking outcome | 0.033 | ||
| Complete (no macroscopic residue) | 30 (58) | 27 (82) | |
| Optimal (residue <1cm) | 9 (17) | 5 (15) | |
| Incomplete (residue >1cm) | 12 (23) | 1 (3) | |
| Missing | 1 (2) | 0 (0) | |
| Number of recurrences | <0.001 | ||
| No recurrence | 0 (0) | 16 (49) | |
| 1 | 40 (76) | 14 (42) | |
| 2 | 6 (12) | 1 (3) | |
| ≥3 | 6 (12) | 2 (6) | |
| Disease-free survival (days) | <0.001 | ||
| Median (IQR) | 195 (128–297) | 1192 (952–2210) | |
| Overall survival (days) | <0.001 | ||
| Median (IQR) | 704 (425–991) | 2058 (1618–2804) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Lieshout, L.; van der Ploeg, P.; Wesseling-Rozendaal, Y.; van de Stolpe, A.; Bosch, S.; Lentjes-Beer, M.; Ottenheijm, M.; Meriaan, A.; Vos, C.; de Hullu, J.; et al. Survival Is Related to Estrogen Signal Transduction Pathway Activity in Postmenopausal Women Diagnosed with High-Grade Serous Ovarian Carcinoma. Cancers 2021, 13, 5101. https://doi.org/10.3390/cancers13205101
van Lieshout L, van der Ploeg P, Wesseling-Rozendaal Y, van de Stolpe A, Bosch S, Lentjes-Beer M, Ottenheijm M, Meriaan A, Vos C, de Hullu J, et al. Survival Is Related to Estrogen Signal Transduction Pathway Activity in Postmenopausal Women Diagnosed with High-Grade Serous Ovarian Carcinoma. Cancers. 2021; 13(20):5101. https://doi.org/10.3390/cancers13205101
Chicago/Turabian Stylevan Lieshout, Laura, Phyllis van der Ploeg, Yvonne Wesseling-Rozendaal, Anja van de Stolpe, Steven Bosch, Marjolein Lentjes-Beer, Meggy Ottenheijm, Annelen Meriaan, Caroline Vos, Joanne de Hullu, and et al. 2021. "Survival Is Related to Estrogen Signal Transduction Pathway Activity in Postmenopausal Women Diagnosed with High-Grade Serous Ovarian Carcinoma" Cancers 13, no. 20: 5101. https://doi.org/10.3390/cancers13205101
APA Stylevan Lieshout, L., van der Ploeg, P., Wesseling-Rozendaal, Y., van de Stolpe, A., Bosch, S., Lentjes-Beer, M., Ottenheijm, M., Meriaan, A., Vos, C., de Hullu, J., Massuger, L., Bekkers, R., & Piek, J. (2021). Survival Is Related to Estrogen Signal Transduction Pathway Activity in Postmenopausal Women Diagnosed with High-Grade Serous Ovarian Carcinoma. Cancers, 13(20), 5101. https://doi.org/10.3390/cancers13205101








