The Effect of a Histone Deacetylase Inhibitor (AR-42) and Zoledronic Acid on Adult T-Cell Leukemia/Lymphoma Osteolytic Bone Tumors
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Reagents
2.2. Transduction of MT-2 Cells with Luciferase
2.3. Bioluminescent Imaging
2.4. Cell Viability Assays
2.5. Treatment Study
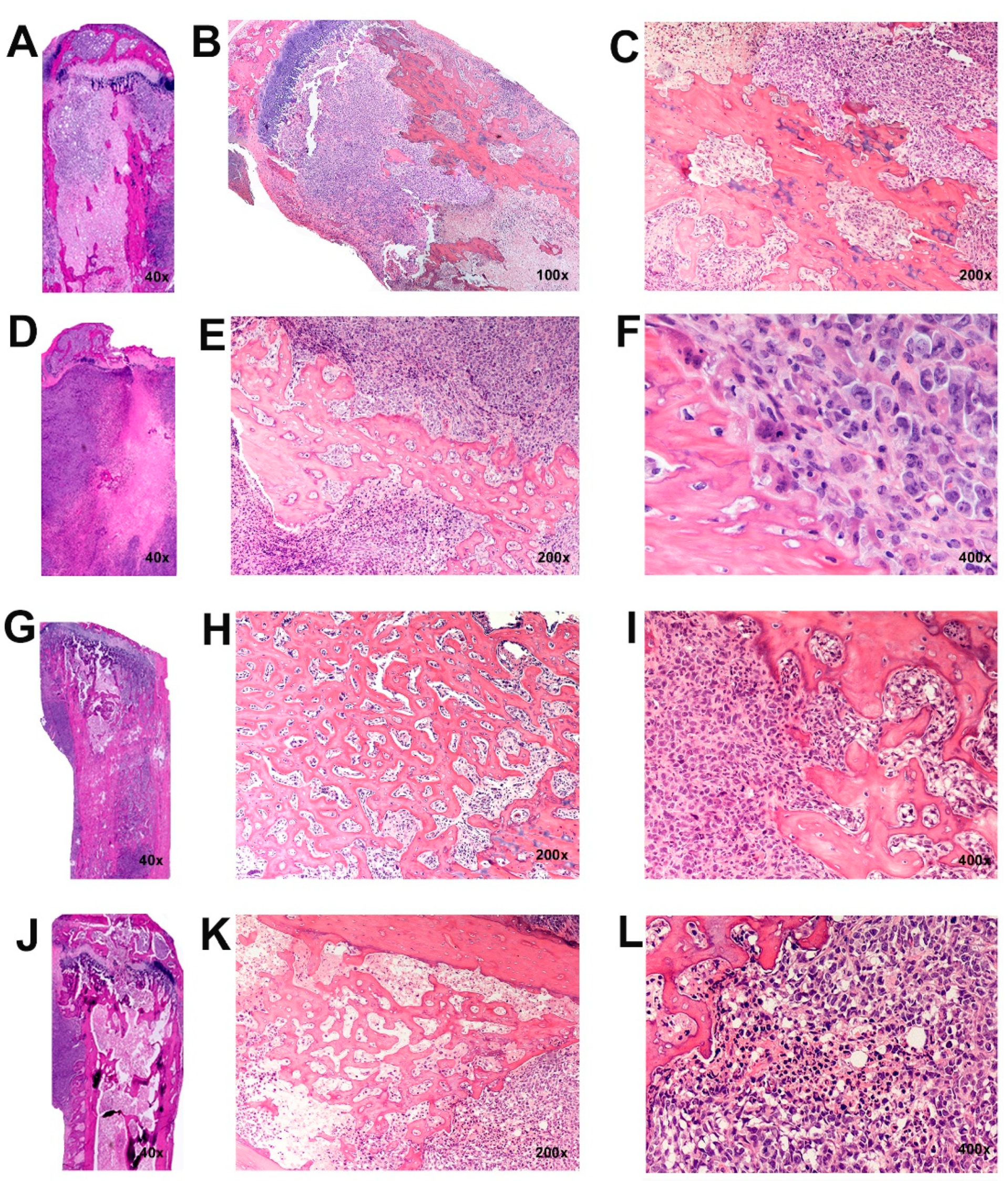
2.6. Histopathology
2.7. Radiographic Imaging
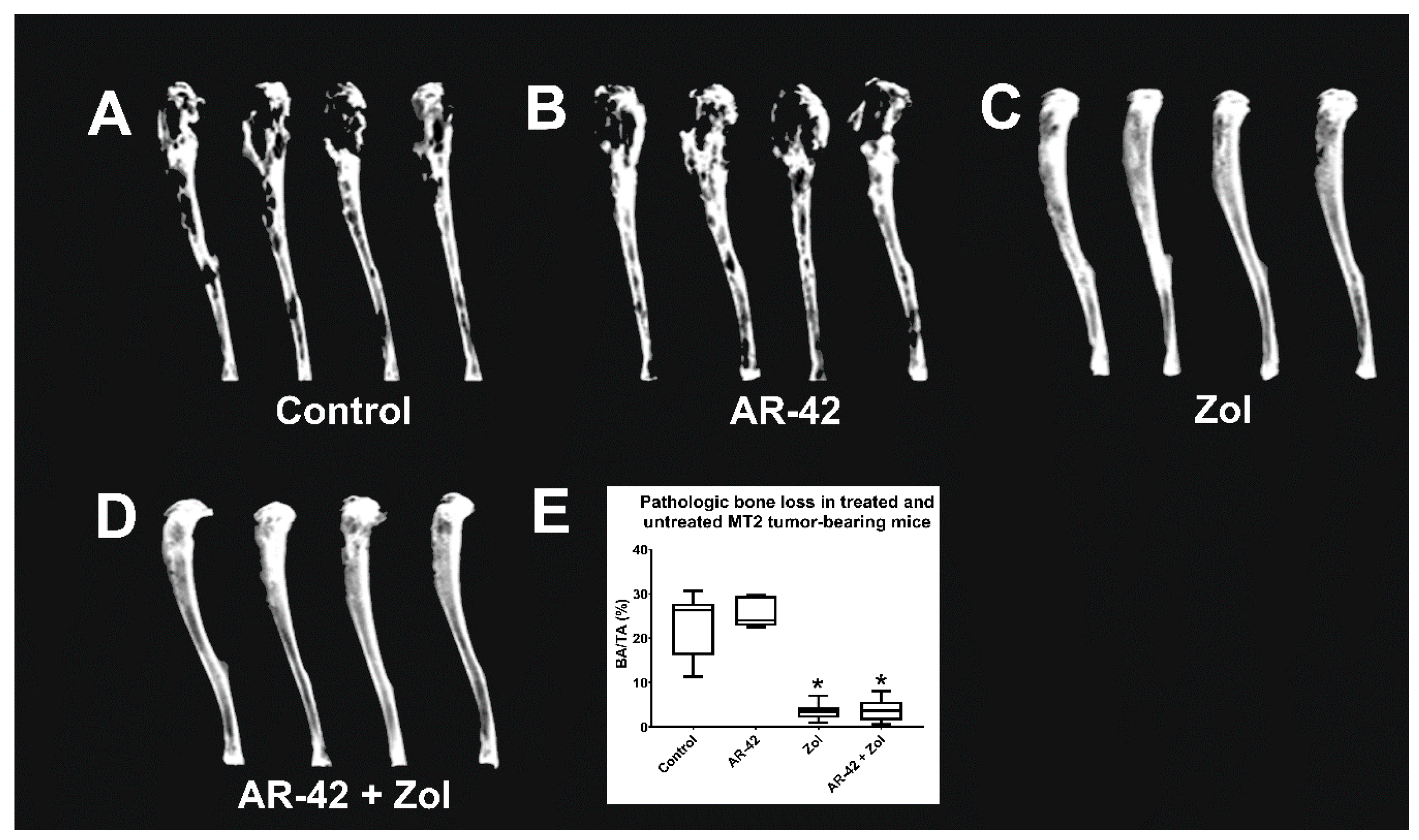
2.8. Microcomputed Tomography (μCT)
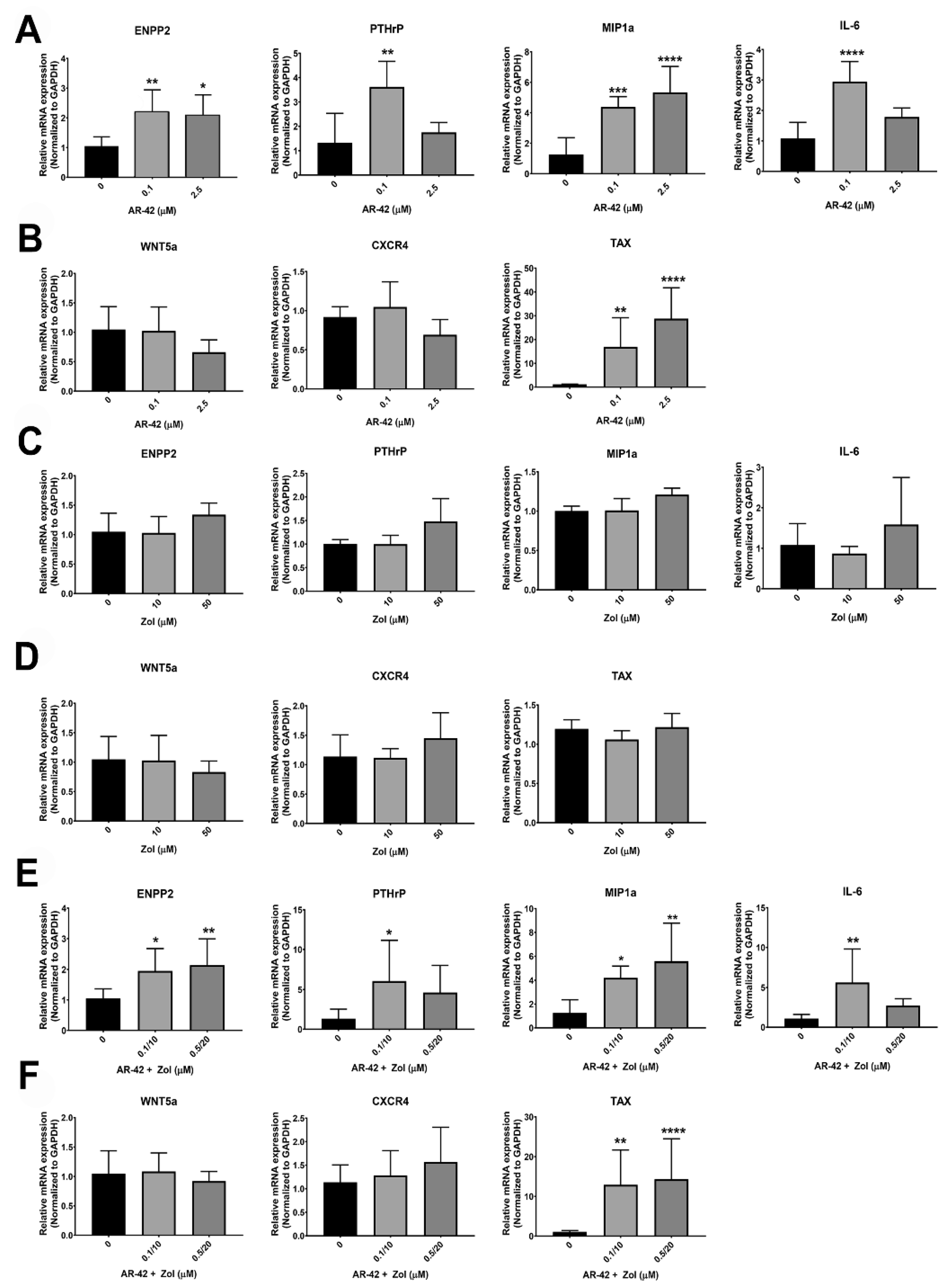
2.9. Real-Time Reverse Transcription-PCR (qRT-PCR)
2.10. Statistical Analysis
3. Results
3.1. MTT Viability Assays
3.2. Development of MT-2 Intratibial Mouse Model
3.3. Bioluminescence, Tumor Growth and Tibial Tumor Burden
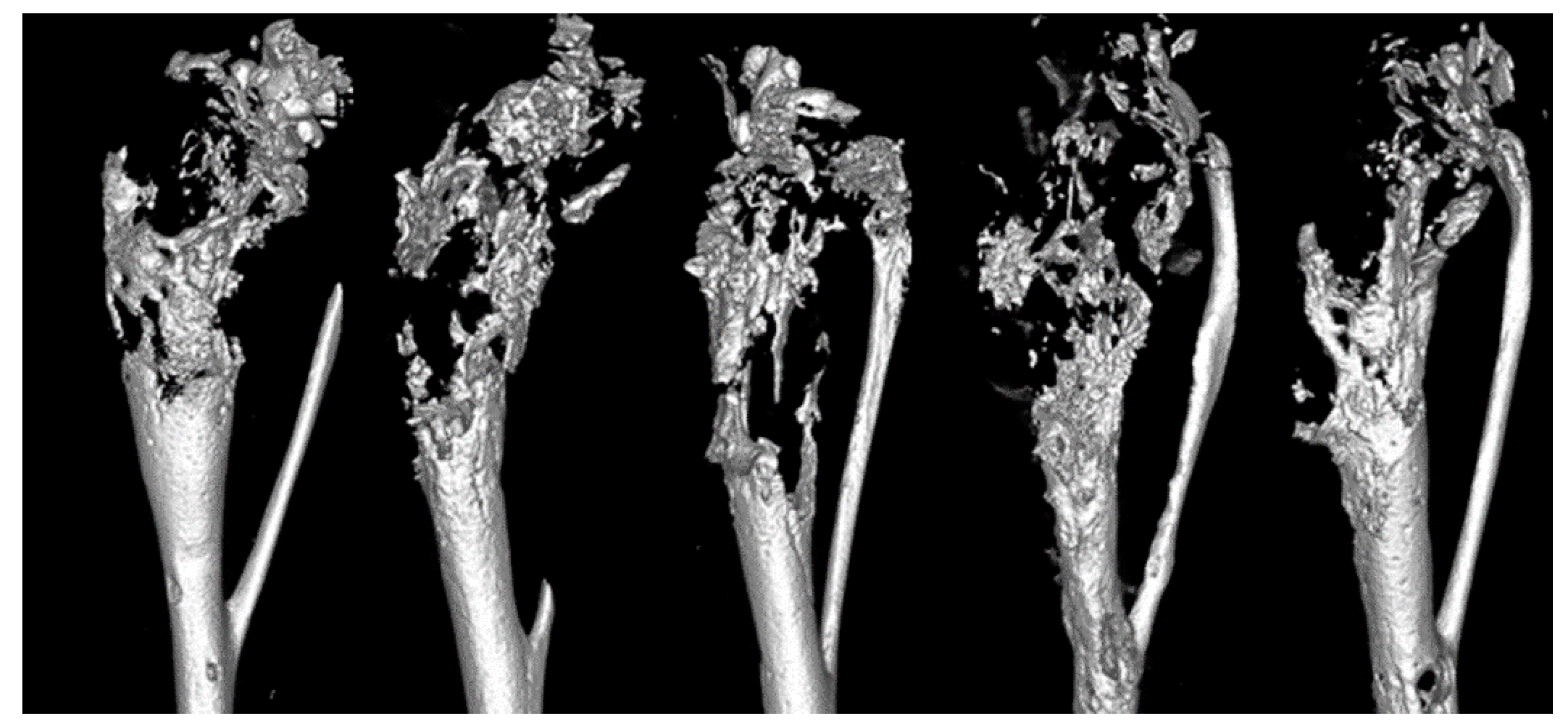
3.4. Faxitron Radiography and Analysis
3.5. Histopathology of Intratibial Tumors
3.6. qRT-PCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonçalves, D.U.; Proietti, F.A.; Ribas, J.G.; Araújo, M.G.; Pinheiro, S.R.; Guedes, A.C.; Carneiro-Proietti, A.B.F. Epidemiology, Treatment, and Prevention of Human T-Cell Leukemia Virus Type 1-Associated Diseases. Clin. Microbiol. Rev. 2010, 23, 577–589. [Google Scholar] [CrossRef]
- Mahieux, R.; Gessain, A. HTLV-1 and associated adult T-cell leukemia/lymphoma. Rev. Clin. Exp. Hematol. 2003, 7, 336–361. [Google Scholar] [PubMed]
- Shimoyama, M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. Br. J. Haematol. 1991, 79, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Prager, D.; Rosenblatt, J.D.; Ejima, E. Hypercalcemia, Parathyroid Hormone-Related Protein Expression and Human T-cell Leukemia Virus Infection. Leuk. Lymphoma 1994, 14, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.P.; Matsuoka, M. Natural history of adult T-cell leukemia/lymphoma and approaches to therapy. Oncogene 2005, 24, 6047–6057. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.T.; Martin, C.K.; Thudi, N.K.; Dirksen, W.P.; Rosol, T.J. Osteolytic bone resorption in adult T-cell leukemia/lymphoma. Leuk. Lymphoma 2010, 51, 702–714. [Google Scholar] [CrossRef]
- Azran, I.; Schavinsky-Khrapunsky, Y.; Aboud, M. Role of Tax protein in human T-cell leukemia virus type-I leukemogenicity. Retrovirology 2004, 1, 20. [Google Scholar] [CrossRef] [PubMed]
- Grossman, W.J.; Kimata, J.; Wong, F.H.; Zutter, M.; Ley, T.J.; Ratner, L. Development of leukemia in mice transgenic for the tax gene of human T-cell leukemia virus type I. Proc. Natl. Acad. Sci. USA 1995, 92, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Fujii, S.M.; Ikeda, Y.; Yamada, M.; Tomonaga, D.; Ballard, W.; Yamamoto, N. Constitutive Activation of Nf-Kappab in Primary Adult T-Cell Leukemia Cells. Blood 1999, 93, 2360–2368. [Google Scholar]
- Ma, G.; Yasunaga, J.-I.; Fan, J.; Yanagawa, S.; Matsuoka, M. HTLV-1 bZIP factor dysregulates the Wnt pathways to support proliferation and migration of adult T-cell leukemia cells. Oncogene 2012, 32, 4222–4230. [Google Scholar] [CrossRef]
- Mazák, J.; Vodicková, L.; Bláha, M.; Siroký, O. Practical possibilities and problems of thrombolytic therapy. Sbornik Vedeckych Praci Lekarske Fakulty Karlovy University v Hradci Kralove 1976, 19, 273–277. [Google Scholar] [PubMed]
- Bellon, M.; Ko, N.L.; Lee, M.-J.; Yao, Y.; Waldmann, T.A.; Trepel, J.B.; Nicot, C. Adult T-cell leukemia cells overexpress Wnt5a and promote osteoclast differentiation. Blood 2013, 121, 5045–5054. [Google Scholar] [CrossRef] [PubMed]
- Esser, A.K.; Rauch, D.A.; Xiang, J.; Harding, J.C.; Kohart, N.A.; Ross, M.H.; Su, X.; Wu, K.; Huey, D.; Xu, Y.; et al. HTLV-1 viral oncogene HBZ induces osteolytic bone disease in transgenic mice. Oncotarget 2017, 8, 69250–69263. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xiang, J.; Rauch, D.A.; Huey, D.D.; Panfil, A.R.; Cheng, X.; Esser, A.K.; Su, X.; Harding, J.C.; Xu, Y.; Fox, G.C.; et al. HTLV-1 viral oncogene HBZ drives bone destruction in adult T cell leukemia. JCI Insight 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Roodman, G.D. Mechanisms of Bone Metastasis. N. Engl. J. Med. 2004, 350, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Mitra-Kaushik, S.; Harding, J.C.; Hess, J.L.; Ratner, L. Effects of the proteasome inhibitor PS-341 on tumor growth in HTLV-1 Tax transgenic mice and Tax tumor transplants. Blood 2004, 104, 802–809. [Google Scholar] [CrossRef]
- Weilbaecher, K.N.; Guise, T.A.; McCauley, L.K. Cancer to bone: A fatal attraction. Nat. Rev. Cancer 2011, 11, 411–425. [Google Scholar] [CrossRef]
- Lucas, D.M.; Alinari, L.; West, D.A.; Davis, M.E.; Edwards, R.B.; Johnson, A.J.; Blum, K.A.; Hofmeister, C.C.; Freitas, M.A.; Parthun, M.R.; et al. The Novel Deacetylase Inhibitor AR-42 Demonstrates Pre-Clinical Activity in B-Cell Malignancies In Vitro and In Vivo. PLoS ONE 2010, 5, e10941. [Google Scholar] [CrossRef]
- Dickinson, M.; Johnstone, R.; Prince, H.M. Histone deacetylase inhibitors: Potential targets responsible for their anti-cancer effect. Investig. New Drugs 2010, 28, 3–20. [Google Scholar] [CrossRef]
- Shu, S.T.; Nadella, M.V.; Dirksen, W.P.; Fernandez, S.A.; Thudi, N.K.; Werbeck, J.L.; Lairmore, M.D.; Rosol, T.J. A Novel Bioluminescent Mouse Model and Effective Therapy for Adult T-Cell Leukemia/Lymphoma. Cancer Res. 2007, 67, 11859–11866. [Google Scholar] [CrossRef]
- Nosaka, K.; Miyamoto, T.; Sakai, T.; Mitsuya, H.; Suda, T.; Matsuoka, M. Mechanism of Hypercalcemia in Adult T-Cell Leukemia: Overexpression of Receptor Activator of Nuclear Factor Kappab Ligand on Adult T-Cell Leukemia Cells. Blood 2002, 99, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Satou, Y.; Nosaka, K.; Koya, Y.; Yasunaga, J.-I.; Toyokuni, S.; Matsuoka, M. Proteasome inhibitor, bortezomib, potently inhibits the growth of adult T-cell leukemia cells both in vivo and in vitro. Leukemia 2004, 18, 1357–1363. [Google Scholar] [CrossRef]
- Watanabe, T. Adult T-cell leukemia: Molecular basis for clonal expansion and transformation of HTLV-1–infected T cells. Blood 2017, 129, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.T.; Clarke, B.L.; Khosla, S. Bisphosphonates: Mechanism of Action and Role in Clinical Practice. Mayo Clin. Proc. 2008, 83, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Chuah, C.; Barnes, D.J.; Kwok, M.; Corbin, A.; Deininger, M.W.N.; Druker, B.; Melo, J.V. Zoledronate inhibits proliferation and induces apoptosis of imatinib-resistant chronic myeloid leukaemia cells. Leukemia 2005, 19, 1896–1904. [Google Scholar] [CrossRef][Green Version]
- Mundy, G.R.; Yoneda, T.; Hiraga, T. Preclinical Studies with Zoledronic Acid and Other Bisphosphonates: Impact on the Bone Microenvironment. Semin. Oncol. 2001, 28, 35–44. [Google Scholar] [CrossRef]
- Gao, L.; Deng, H.; Zhao, H.; Hirbe, A.; Harding, J.; Ratner, L.; Weilbaecher, K. HTLV-1 Tax transgenic mice develop spontaneous osteolytic bone metastases prevented by osteoclast inhibition. Blood 2005, 106, 4294–4302. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, B.; Niewiesk, S.; Lairmore, M.D. Mouse Models of Human T Lymphotropic Virus Type-1–Associated Adult T-Cell Leukemia/Lymphoma. Vet. Pathol. 2010, 47, 677–689. [Google Scholar] [CrossRef]
- Richard, V.; Lairmore, M.D.; Green, P.L.; Feuer, G.; Erbe, R.S.; Albrecht, B.; D’Souza, C.; Keller, E.T.; Dai, J.; Rosol, T.J. Humoral Hypercalcemia of Malignancy: Severe Combined Immunodeficient/Beige Mouse Model of Adult T-Cell Lymphoma Independent of Human T-Cell Lymphotropic Virus Type-1 Tax Expression. Am. J. Pathol. 2001, 158, 2219–2228. [Google Scholar] [CrossRef]
- Yoshida, M.; Miyoshi, I.; Hinuma, Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc. Natl. Acad. Sci. USA 1982, 79, 2031–2035. [Google Scholar] [CrossRef]
- Miyoshi, I.; Kubonishi, I.; Yoshimoto, S.; Shiraishi, Y. A T-cell line derived from normal human cord leukocytes by co-culturing with human leukemic T-cells. Gan 1981, 72, 978–981. [Google Scholar]
- Stewart, S.A.; Feuer, G.; Jewett, A.; Lee, F.V.; Bonavida, B.; Chen, I.S. HTLV-1 gene expression in adult T-cell leukemia cells elicits an NK cell response in vitro and correlates with cell rejection in SCID mice. Virology 1996, 226, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Panfil, A.R.; Al-Saleem, J.; Howard, C.; Mates, J.M.; Kwiek, J.J.; Baiocchi, R.A.; Green, P.L. PRMT5 Is Upregulated in HTLV-1-Mediated T-Cell Transformation and Selective Inhibition Alters Viral Gene Expression and Infected Cell Survival. Viruses 2015, 8, 7. [Google Scholar] [CrossRef]
- Takeda, S.; Maeda, S.M.; Morikawa, Y.; Taniguchi, J.; Yasunaga, K.; Nosaka, Y.T.; Matsuoka, M. Genetic and Epigenetic Inactivation of Tax Gene in Adult T-Cell Leukemia Cells. Int. J. Cancer 2004, 109, 559–567. [Google Scholar] [CrossRef]
- Simmons, J.K.; Dirksen, W.P.; Hildreth, B.E., 3rd; Dorr, C.C.; Williams, R.; Thomas, M.; Breen, R.E.T.; Rosol, T.J. Canine Prostate Cancer Cell Line (Probasco) Produces Osteoblastic Metastases in Vivo. Prostate 2014, 74, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Kohart, N.A.; Elshafae, S.M.; Supsahvad, W.; Alasonyalilar-Demirer, A.; Panfil, A.R.; Xiang, J.; Dirksen, W.P.; Veis, D.J.; Green, P.L.; Weilbaecher, K.N.; et al. Mouse model recapitulates the phenotypic heterogeneity of human adult T-cell leukemia/lymphoma in bone. J. Bone Oncol. 2019, 19, 100257. [Google Scholar] [CrossRef]
- Nadella, M.V.; Dirksen, W.P.; Nadella, K.S.; Shu, S.; Cheng, A.S.; Morgenstern, J.A.; Richard, V.; Fernandez, S.A.; Huang, T.H.; Guttridge, D.; et al. Transcriptional Regulation of Parathyroid Hormone-Related Protein Promoter P2 by Nf-Kappab in Adult T-Cell Leukemia/Lymphoma. Leukemia 2007, 21, 1752–1762. [Google Scholar] [CrossRef] [PubMed]
- Satou, Y.; Yasunaga, J.-I.; Zhao, T.; Yoshida, M.; Miyazato, P.; Takai, K.; Shimizu, K.; Ohshima, K.; Green, P.L.; Ohkura, N.; et al. HTLV-1 bZIP Factor Induces T-Cell Lymphoma and Systemic Inflammation In Vivo. PLOS Pathog. 2011, 7, e1001274. [Google Scholar] [CrossRef]
- Haratake, J.; Horie, A.; Oda, S.; Chiba, S.; Kobori, K.; Sato, H. A CLINICOPATHOLOGICAL REVIEW OF 12 AUTOPSIED CASES OF ADULT T-CELL LEUKEMIA. Pathol. Int. 1986, 36, 349–362. [Google Scholar] [CrossRef]
- Okada, Y.; Tsukada, J.; Nakano, K.; Tonai, S.; Mine, S.; Tanaka, Y. Macrophage Inflammatory Protein-1α Induces Hypercalcemia in Adult T-Cell Leukemia. J. Bone Miner. Res. 2004, 19, 1105–1111. [Google Scholar] [CrossRef]
- Matutes, E. Adult T-Cell Leukaemia/Lymphoma. J. Clin. Pathol. 2007, 60, 1373–1377. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, S.; Matsuoka, M.; Sakata, K.; Matsuzaki, H.; Akagi, K.; Yamaguchi, K.; Takatsuki, K. Primary adult T cell leukemia of bone: Two patients with primary bone lesion showing monoclonal integration of HTLV-I proviral DNA. Leukemia 1996, 10. [Google Scholar]
- Virk, M.S.; Lieberman, J.R. Tumor Metastasis to Bone. Arthritis Res. Ther. 2007, 9, S5. [Google Scholar] [CrossRef]
- Bazarbachi, A.; Suarez, F.; Fields, P.; Hermine, O. How I treat adult T-cell leukemia/lymphoma. Blood 2011, 118, 1736–1745. [Google Scholar] [CrossRef]
- Utsunomiya, A.; Choi, D.I.C.; Seto, M. Recent Advances in the Treatment of Adult T-Cell Leukemia-Lymphomas. Cancer Sci. 2015, 106, 344–351. [Google Scholar] [CrossRef]
- Panfil, A.R.; Al-Saleem, J.J.; Green, P.L. Animal Models Utilized in HTLV-1 Research. Virol. Res. Treat. 2013, 4, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Garea, A.V.; Esteller, M. Histone deacetylase inhibitors: Understanding a new wave of anticancer agents. Int. J. Cancer 2004, 112, 171–178. [Google Scholar] [CrossRef]
- Tabakin-Fix, Y.; Azran, I.; Schavinky-Khrapunsky, Y.; Levy, O.; Aboud, M. Functional inactivation of p53 by human T-cell leukemia virus type 1 Tax protein: Mechanisms and clinical implications. Carcinogenesis 2005, 27, 673–681. [Google Scholar] [CrossRef]
- Ratner, L. Pathogenesis and Treatment of Human T-Cell Leukemia Virus Infection. Immunol. Res. 2005, 32, 217–224. [Google Scholar] [CrossRef]
- Nishioka, C.; Ikezoe, T.; Yang, J.; Komatsu, N.; Bandobashi, K.; Taniguchi, A.; Kuwayama, Y.; Togitani, K.; Koeffler, H.P.; Taguchi, H. Histone Deacetylase Inhibitors Induce Growth Arrest and Apoptosis of Htlv-1-Infected T-Cells Via Blockade of Signaling by Nuclear Factor Kappab. Leuk. Res. 2008, 32, 287–296. [Google Scholar] [CrossRef]
- Zimmerman, B.; Sargeant, A.; Landes, K.; Fernandez, S.A.; Chen, C.-S.; Lairmore, M.D. Efficacy of novel histone deacetylase inhibitor, AR42, in a mouse model of, human T-lymphotropic virus type 1 adult T cell lymphoma. Leuk. Res. 2011, 35, 1491–1497. [Google Scholar] [CrossRef]
- Elshafae, S.M.; Kohart, N.A.; Altstadt, L.A.; Dirksen, W.P.; Rosol, T.J. The Effect of a Histone Deacetylase Inhibitor (AR-42) on Canine Prostate Cancer Growth and Metastasis. Prostate 2017, 77, 776–793. [Google Scholar] [CrossRef]
- Henderson, S.E.; Ding, L.-Y.; Mo, X.; Bekaii-Saab, T.; Kulp, S.K.; Chen, C.-S.; Huang, P.-H. Suppression of Tumor Growth and Muscle Wasting in a Transgenic Mouse Model of Pancreatic Cancer by the Novel Histone Deacetylase Inhibitor AR-42. Neoplasia 2016, 18, 765–774. [Google Scholar] [CrossRef]
- Pratap, J.; Akech, J.; Wixted, J.J.; Szabo, G.; Hussain, S.; McGee-Lawrence, M.; Li, X.; Bedard, K.; Dhillon, R.J.; Van Wijnen, A.J.; et al. The Histone Deacetylase Inhibitor, Vorinostat, Reduces Tumor Growth at the Metastatic Bone Site and Associated Osteolysis, but Promotes Normal Bone Loss. Mol. Cancer Ther. 2010, 9, 3210–3220. [Google Scholar] [CrossRef] [PubMed]
- Achachi, A.; Florins, A.; Gillet, N.; Debacq, C.; Urbain, P.; Foutsop, G.M.; Vandermeers, F.; Jasik, A.; Reichert, M.; Kerkhofs, P.; et al. Valproate Activates Bovine Leukemia Virus Gene Expression, Triggers Apoptosis, and Induces Leukemia/Lymphoma Regression in Vivo. Proc. Natl. Acad. Sci. USA 2005, 102, 10309–10314. [Google Scholar] [CrossRef] [PubMed]
- Belrose, G.; Gross, A.; Olindo, S.; Lézin, A.; Dueymes, M.; Komla-Soukha, I.; Smadja, D.; Tanaka, Y.; Willems, L.; Mesnard, J.-M.; et al. Effects of valproate on Tax and HBZ expression in HTLV-1 and HAM/TSP T lymphocytes. Blood 2011, 118, 2483–2491. [Google Scholar] [CrossRef]
- Matalon, S.; Rasmussen, T.A.; Dinarello, C.A. Histone Deacetylase Inhibitors for Purging HIV-1 from the Latent Reservoir. Mol. Med. 2011, 17, 466–472. [Google Scholar] [CrossRef]
- Schnell, A.P.; Kohrt, S.; Thoma-Kress, A.K. Latency Reversing Agents: Kick and Kill of Htlv-1? Int. J. Mol. Sci. 2021, 22, 5545. [Google Scholar] [CrossRef] [PubMed]
- Søgaard, O.; Graversen, M.E.; Leth, S.; Olesen, R.; Brinkmann, C.R.; Nissen, S.K.; Kjaer, A.; Schleimann, M.; Denton, P.; Hey-Cunningham, W.J.; et al. The Depsipeptide Romidepsin Reverses HIV-1 Latency In Vivo. PLOS Pathog. 2015, 11, e1005142. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Deng, K.; Shroff, N.S.; Durand, C.M.; Rabi, S.A.; Yang, H.-C.; Zhang, H.; Margolick, J.B.; Blankson, J.N.; Siliciano, R.F. Stimulation of HIV-1-Specific Cytolytic T Lymphocytes Facilitates Elimination of Latent Viral Reservoir after Virus Reactivation. Immunity 2012, 36, 491–501. [Google Scholar] [CrossRef]
- Ylisastigui, L.; Archin, N.M.; Lehrman, G.; Bosch, R.J.; Margolis, D.M. Coaxing Hiv-1 from Resting Cd4 T Cells: Histone Deacetylase Inhibition Allows Latent Viral Expression. AIDS 2004, 18, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Mates, J.M.; de Silva, S.M.; Lustberg, K.; Van Deusen, R.A.; Baiocchi, L.W.; Kwiek, J.J. A Novel Histone Deacetylase Inhibitor, Ar-42, Reactivates Hiv-1 from Chronically and Latently Infected Cd4+ T-Cells. Retrovirology 2015, 7, 1–5. [Google Scholar] [PubMed]
- Guise, T.A.; Mundy, G.R. Cancer and Bone. Endocr. Rev. 1998, 19, 18–54. [Google Scholar]
- Fisher, J.E.; Rogers, M.J.; Halasy, J.M.; Luckman, S.P.; Hughes, D.E.; Masarachia, P.J.; Wesolowski, G.; Russell, R.G.; Rodan, G.A.; Reszka, A.A. Alendronate Mechanism of Action: Geranylgeraniol, an Intermediate in the Mevalonate Pathway, Prevents Inhibition of Osteoclast Formation, Bone Resorption, and Kinase Activation in Vitro. Proc. Natl. Acad. Sci. USA 1999, 96, 133–138. [Google Scholar] [CrossRef]
- Luckman, S.P.; Hughes, D.E.; Coxon, F.P.; Russell, R.G.G.; Rogers, M. Nitrogen-Containing Bisphosphonates Inhibit the Mevalonate Pathway and Prevent Post-Translational Prenylation of GTP-Binding Proteins, Including Ras. J. Bone Miner. Res. 1998, 13, 581–589. [Google Scholar] [CrossRef]
- Roelofs, A.J.; Thompson, K.; Gordon, S.; Rogers, M. Molecular Mechanisms of Action of Bisphosphonates: Current Status. Clin. Cancer Res. 2006, 12, 6222s–6230s. [Google Scholar] [CrossRef]
- Mahtani, R.; Jahanzeb, M. Bisphosphonates as Anticancer Therapy for Early Breast Cancer. Clin. Breast Cancer 2010, 10, 359–366. [Google Scholar] [CrossRef]
- Hirbe, A.C.; Roelofs, A.J.; Floyd, D.H.; Deng, H.; Becker, S.N.; Lanigan, L.G.; Apicelli, A.J.; Xu, Z.; Prior, J.L.; Eagleton, M.C.; et al. The bisphosphonate zoledronic acid decreases tumor growth in bone in mice with defective osteoclasts. Bone 2009, 44, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Tsukasaki, K.; Hermine, O.; Bazarbachi, A.; Ratner, L.; Ramos, J.C.; Jr, W.H.; O’Mahony, D.; Janik, J.E.; Bittencourt, A.L.; Taylor, G.P.; et al. Definition, Prognostic Factors, Treatment, and Response Criteria of Adult T-Cell Leukemia-Lymphoma: A Proposal From an International Consensus Meeting. J. Clin. Oncol. 2009, 27, 453–459. [Google Scholar] [CrossRef]
- Sonnemann, J.; Bumbul, B.; Beck, J.F. Synergistic activity of the histone deacetylase inhibitor suberoylanilide hydroxamic acid and the bisphosphonate zoledronic acid against prostate cancer cells in vitro. Mol. Cancer Ther. 2007, 6, 2976–2984. [Google Scholar] [CrossRef] [PubMed]
- Bruzzese, F.; Pucci, B.; Milone, M.R.; Ciardiello, C.; Franco, R.; Chianese, M.I.; Rocco, M.; Di Gennaro, E.; Leone, A.; Luciano, A.; et al. Panobinostat synergizes with zoledronic acid in prostate cancer and multiple myeloma models by increasing ROS and modulating mevalonate and p38-MAPK pathways. Cell Death Dis. 2013, 4, e878. [Google Scholar] [CrossRef] [PubMed]


| Genes | Forward Primers | Reverse Primers |
|---|---|---|
| GAPDH | GCAAATTCCATGGCACCGTC | AGCATCGCCCCACTTGATTT |
| PTHrP | GTCTCAGCCGCCGCCTCAA | GGAAGAATCGTCGCCGTAAA |
| MIP-1α | CTGCATCACTTGCTGCTGACA | CACTGGCTGCTCGTCTCAAAG |
| ENPP2 (autotaxin) | GGAACGCAGATGGCATGTTG | TTATCAAATCCGTGGTCTCCCT |
| Wnt5a | CTACGAGAGTGCTCGCATCC | GCCAGGTTGTACACCGTCC |
| Interleukin-6 (IL-6) | GAGGAGACTTGCCTGGTGAAA | TGGCATTTGTGGTTGGGTCA |
| CXCR4 | CGGTTACCATGGAGGGGATCA | ATGACCAATCCATTGCCCACA |
| TAX | CCGCCGATCCCAAAGAAA | CCGAACATAGTCCCCCAGA |
| HBZ | AACTTACCTAGACGGCGGAC | CATGGCACAGGCAAGCATCG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elshafae, S.M.; Kohart, N.A.; Breitbach, J.T.; Hildreth, B.E., III; Rosol, T.J. The Effect of a Histone Deacetylase Inhibitor (AR-42) and Zoledronic Acid on Adult T-Cell Leukemia/Lymphoma Osteolytic Bone Tumors. Cancers 2021, 13, 5066. https://doi.org/10.3390/cancers13205066
Elshafae SM, Kohart NA, Breitbach JT, Hildreth BE III, Rosol TJ. The Effect of a Histone Deacetylase Inhibitor (AR-42) and Zoledronic Acid on Adult T-Cell Leukemia/Lymphoma Osteolytic Bone Tumors. Cancers. 2021; 13(20):5066. https://doi.org/10.3390/cancers13205066
Chicago/Turabian StyleElshafae, Said M., Nicole A. Kohart, Justin T. Breitbach, Blake E. Hildreth, III, and Thomas J. Rosol. 2021. "The Effect of a Histone Deacetylase Inhibitor (AR-42) and Zoledronic Acid on Adult T-Cell Leukemia/Lymphoma Osteolytic Bone Tumors" Cancers 13, no. 20: 5066. https://doi.org/10.3390/cancers13205066
APA StyleElshafae, S. M., Kohart, N. A., Breitbach, J. T., Hildreth, B. E., III, & Rosol, T. J. (2021). The Effect of a Histone Deacetylase Inhibitor (AR-42) and Zoledronic Acid on Adult T-Cell Leukemia/Lymphoma Osteolytic Bone Tumors. Cancers, 13(20), 5066. https://doi.org/10.3390/cancers13205066






