Assessment of Cannabidiol and Δ9-Tetrahydrocannabiol in Mouse Models of Medulloblastoma and Ependymoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
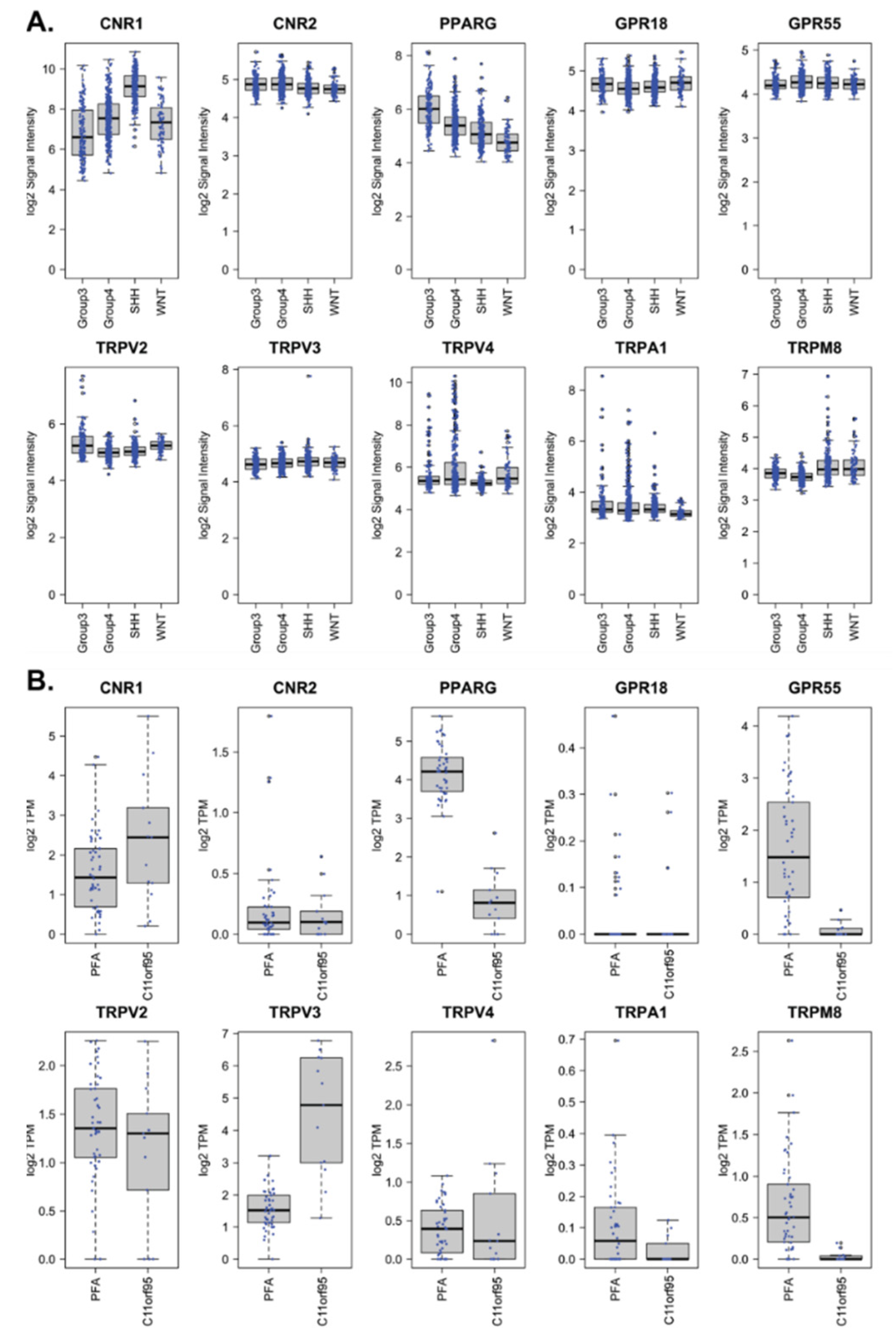
2.1. Cannabinoid Receptors Are Expressed in Pediatric Medulloblastoma and Ependymoma
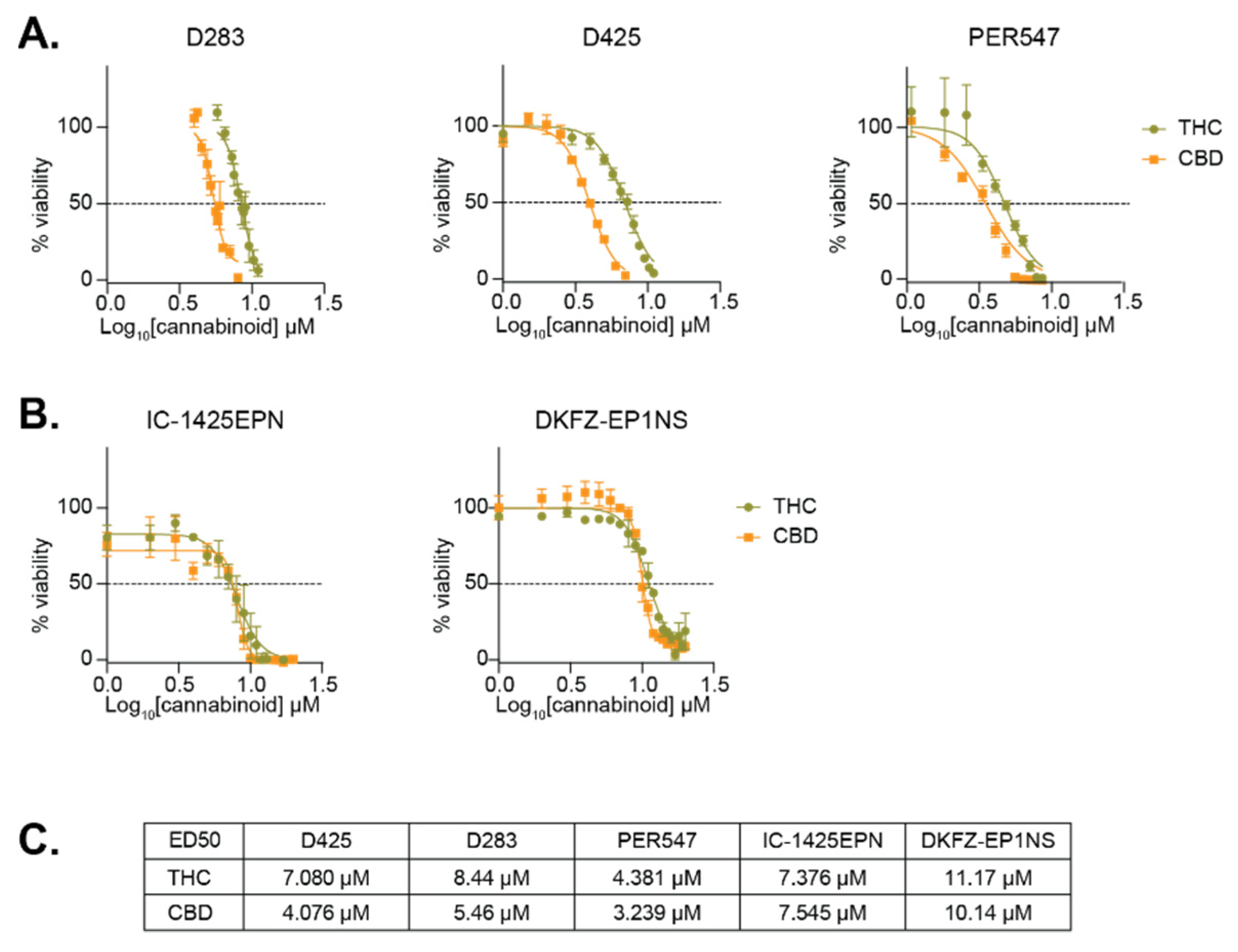
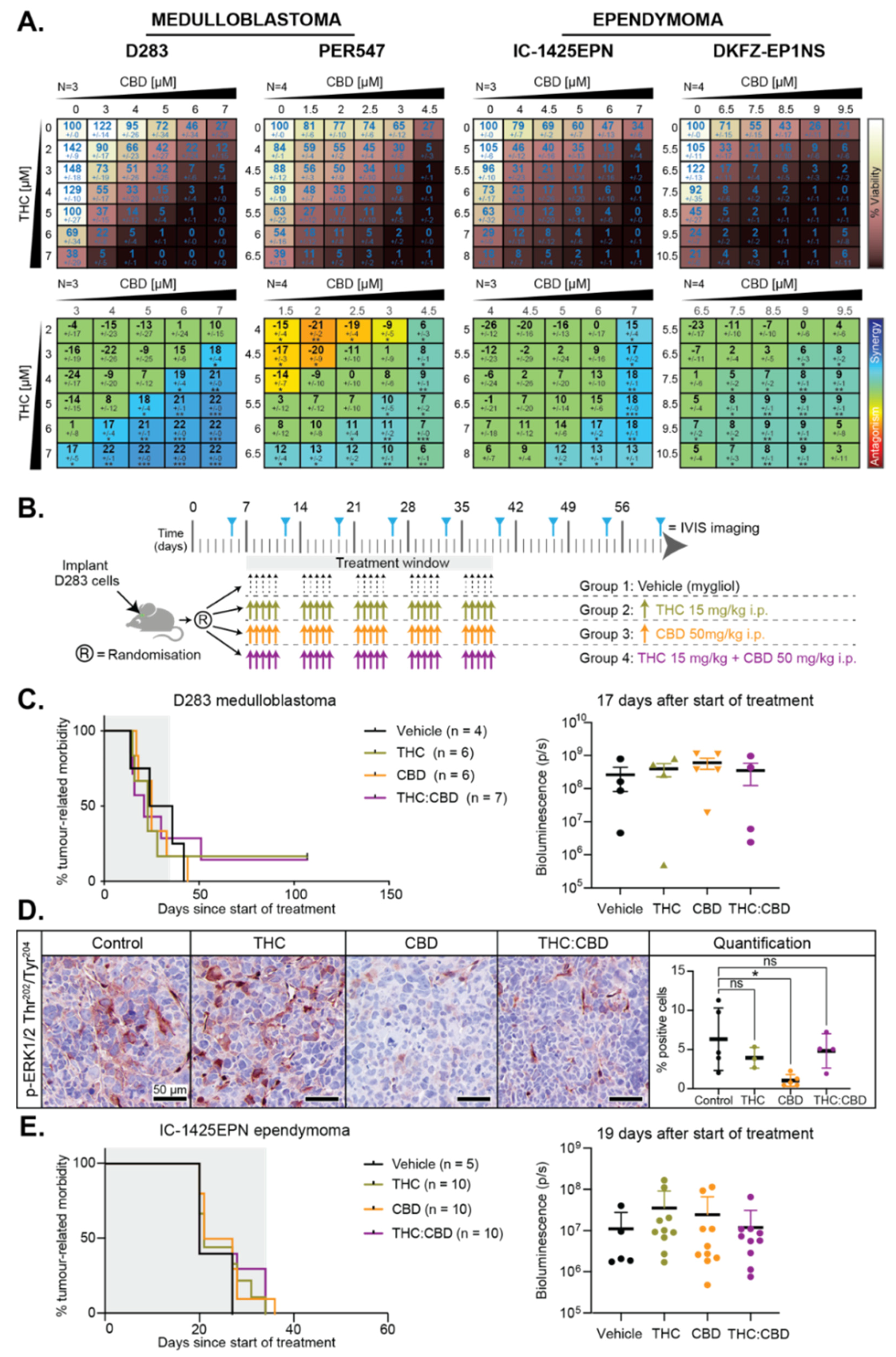
2.2. THC and CBD Have Cytotoxic Activity in Pediatric Brain Cancer Cell Lines
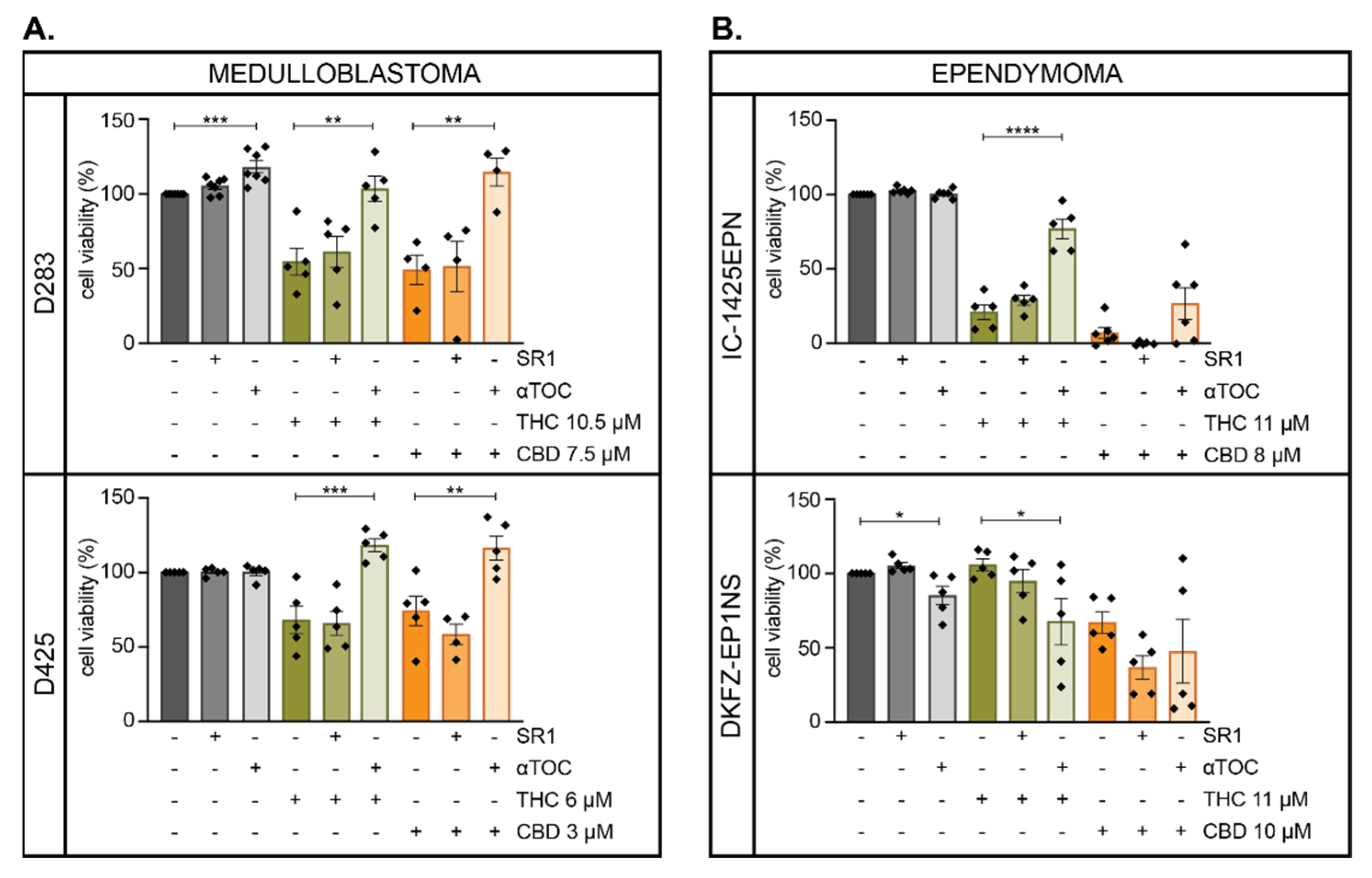
2.3. THC and CBD Mediated-Cytotoxicity May Be Mediated via the Production of Reactive Oxygen Species
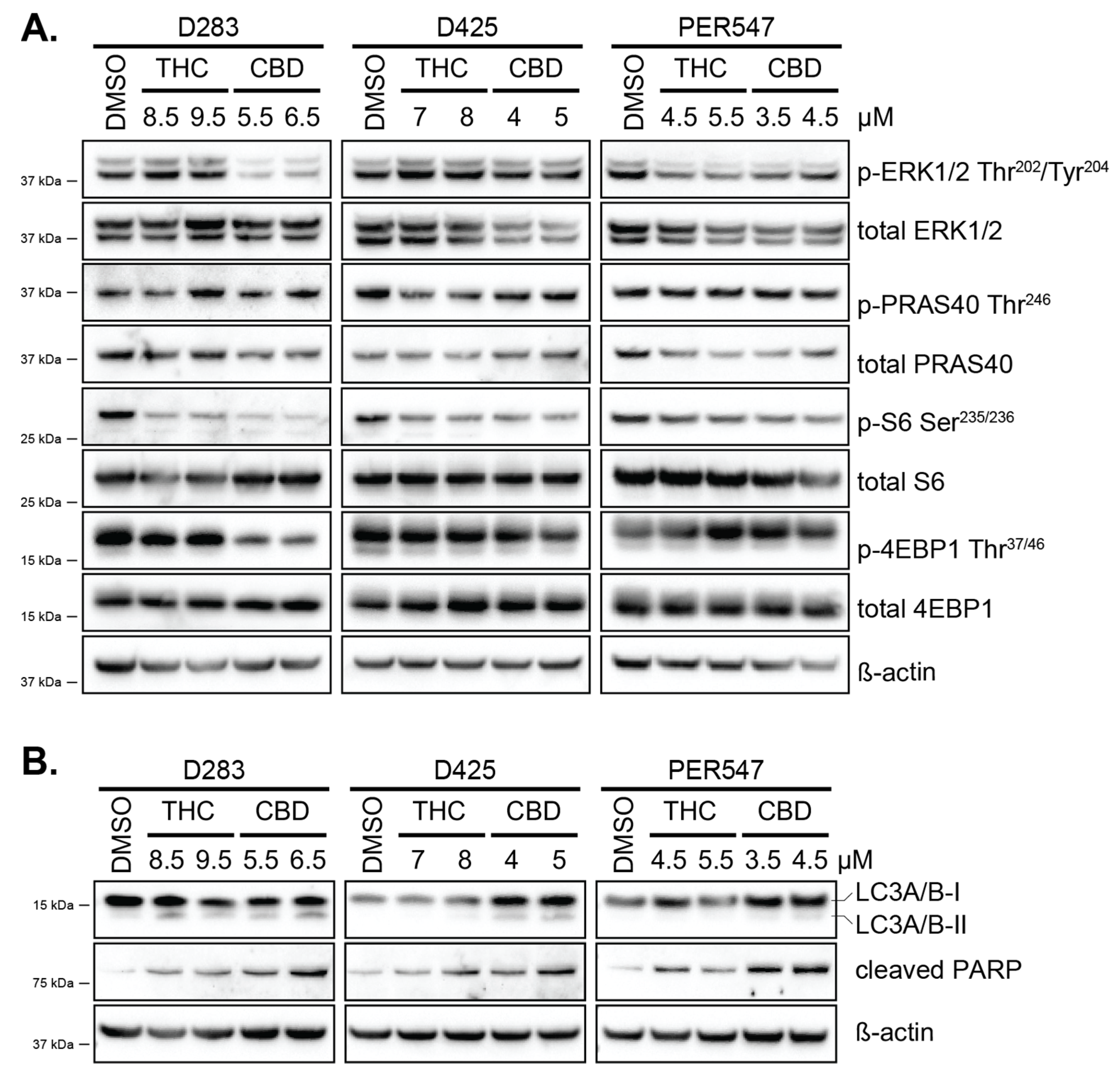
2.4. CBD Induces Autophagy and Apoptosis in Pediatric Brain Cancer Cells Potentially via MAPK or mTOR Signaling
2.5. THC, CBD, or the Combination of Both Does not Impact Survival of Mice with Pediatric Brain Cancer
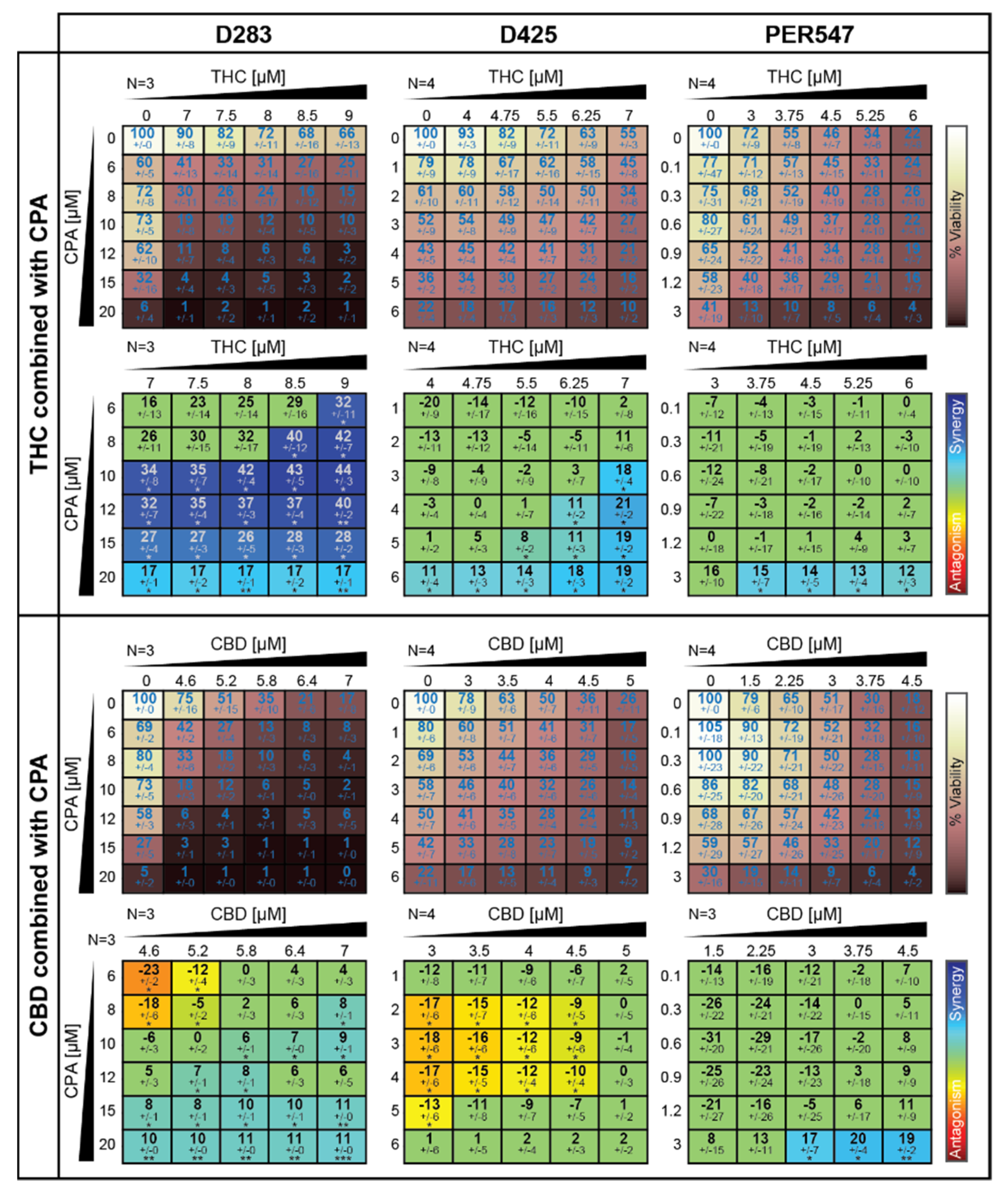
2.6. Cannabinoids Alter the Cytotoxic Effects of the Conventional Medulloblastoma Chemotherapeutic Cyclophosphamide In Vitro
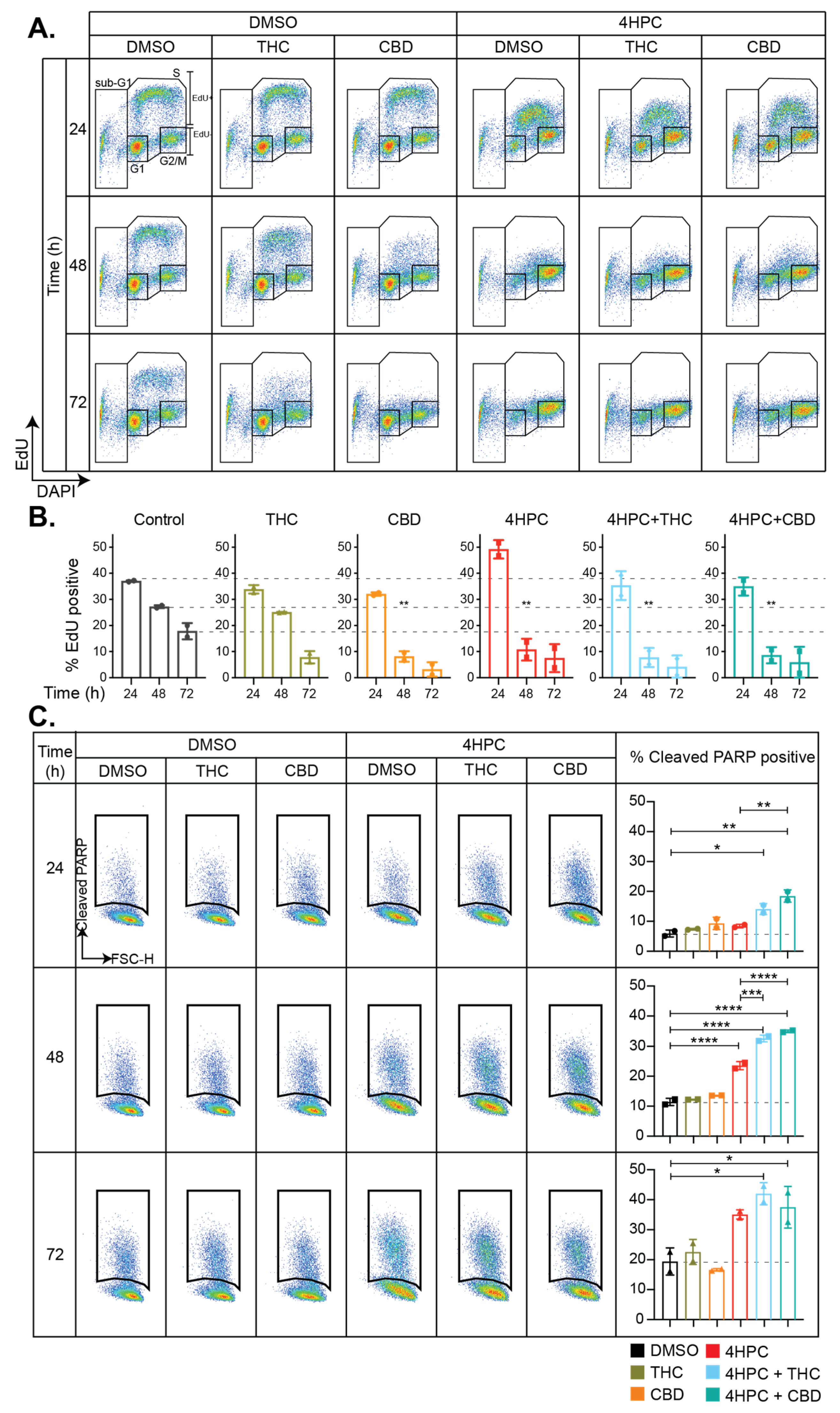
2.7. Cannabinoids Induce Cell Cycle Arrest and Enhance CPA-Induced Apoptosis
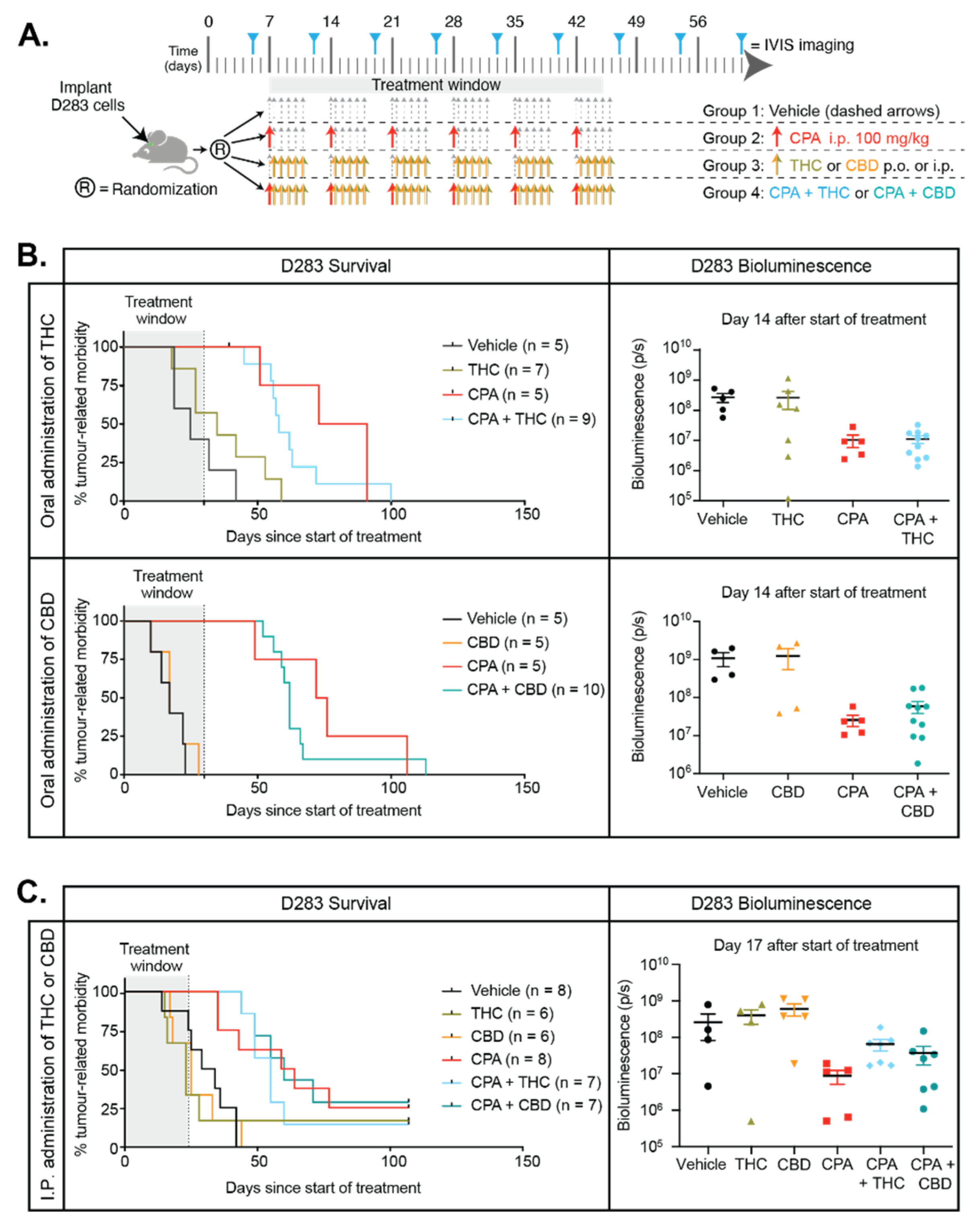
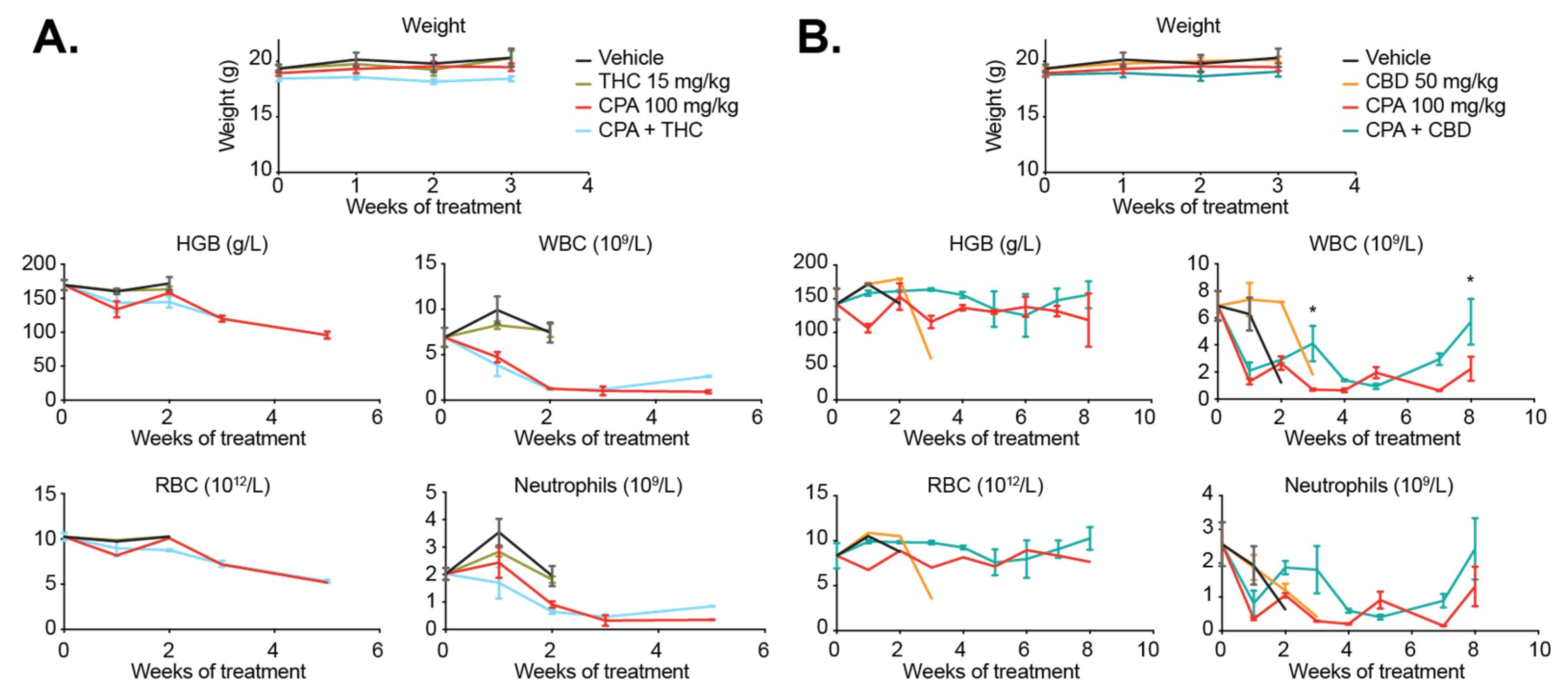
2.8. Cannabinoids Do Not Enhance the Efficacy of CPA In Vivo and Do Not Improve Animal Survival
3. Discussion
4. Materials and Methods
4.1. Analysis of Human Medulloblastoma and Ependymoma Expression Data
4.2. Medulloblastoma and Ependymoma Cell Lines and Culture Conditions
4.3. RNA Isolation and Transcriptome Profiling (RNA-Seq) from Cell Lines
4.4. Pre-Processing of RNA-Seq Data
4.5. Compounds
4.6. Drug Sensitivity and Drug Interaction Assays
4.7. Cannabinoid Receptor Antagonist Assays
4.8. Protein Analysis by Western Transfer and Immunoblotting
4.9. Flow Cytometry for Cell Cycle Distribution and Apoptosis
4.10. Orthotopic Implant Models of Medulloblastoma and Ependymoma
4.11. Immunohistochemistry
4.12. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Gittleman, H.; Fulop, J.; Liu, M.; Blanda, R.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2021. Neuro-Oncology 2015, 17, iv1–iv62. [Google Scholar] [CrossRef]
- Gottardo, N.G.; Gajjar, A. Chemotherapy for Malignant Brain Tumors of Childhood. J. Child Neurol. 2008, 23, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Hovestadt, V.; Ayrault, O.; Swartling, F.J.; Robinson, G.W.; Pfister, S.; Northcott, P.A. Medulloblastomics revisited: Biological and clinical insights from thousands of patients. Nat. Rev. Cancer 2019, 20, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Baxter, P.A.; Voicu, H.; Gurusiddappa, S.; Zhao, Y.; Adesina, A.; Man, T.-K.; Shu, Q.; Zhang, Y.-J.; Zhao, X.-M.; et al. A clinically relevant orthotopic xenograft model of ependymoma that maintains the genomic signature of the primary tumor and preserves cancer stem cells in vivo. Neuro-Oncology 2010, 12, 580–594. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.D.; Gajjar, A. Current Treatment Options for Pediatric and Adult Patients with Ependymoma. Curr. Treat. Options Oncol. 2012, 13, 465–477. [Google Scholar] [CrossRef]
- Ritzmann, T.A.; Rogers, H.A.; Paine, S.M.; Storer, L.C.; Jacques, T.S.; Chapman, R.J.; Ellison, D.; Donson, A.M.; Foreman, N.K.; Grundy, R.G. A retrospective analysis of recurrent pediatric ependymoma reveals extremely poor survival and ineffectiveness of current treatments across central nervous system locations and molecular subgroups. Pediatr. Blood Cancer 2020, 67. [Google Scholar] [CrossRef]
- NCT01096368. Maintenance Chemotherapy or Observation Following Induction Chemotherapy and Radiation Therapy in Treating Younger Patients with Newly Diagnosed Ependymoma. Bethesda (MD): National Library of Medicine (US). 2010. Available online: https://clinicaltrials.gov/ct2/show/NCT01096368 (accessed on 25 December 2020).
- NCT02265770. An International Clinical Program for the Diagnosis and Treatment of Children with Ependymoma (SIOP-EP-II). Bethesda (MD): National Library of Medicine (US). 2014. Available online: https://clinicaltrials.gov/ct2/show/NCT02265770 (accessed on 25 December 2020).
- Smith, A.; Onar-Thomas, A.; Ellison, D.; Owens-Pickle, E.; Wu, S.; Leary, S.E.S.; Fouladi, M.; Merchant, T.; Gajjar, A.; Foreman, N. EPEN-ACNS0831, PHASE III randomized trial of post-radiation chemotherapy in patients with newly diagnosed ependymoma ages 1 to 21 years. Neuro-Oncology 2020, 22, iii318–iii319. [Google Scholar] [CrossRef]
- Khatua, S.; Ramaswamy, V.; Bouffet, E. Current therapy and the evolving molecular landscape of pediatric ependymoma. Eur. J. Cancer 2017, 70, 34–41. [Google Scholar] [CrossRef]
- Pajtler, K.W.; Witt, H.; Sill, M.; Jones, D.T.; Hovestadt, V.; Kratochwil, F.; Wani, K.; Tatevossian, R.; Punchihewa, C.; Johann, P.; et al. Molecular Classification of Ependymal Tumors across All CNS Compartments, Histopathological Grades, and Age Groups. Cancer Cell 2015, 27, 728–743. [Google Scholar] [CrossRef]
- Ellison, D.W.; Aldape, K.D.; Capper, D.; Fouladi, M.; Gilbert, M.R.; Gilbertson, R.J.; Hawkins, C.; Merchant, T.E.; Pajtler, K.; Pratt, D.; et al. cIMPACT-NOW update 7: Advancing the molecular classification of ependymal tumors. Brain Pathol. 2020, 30, 863–866. [Google Scholar] [CrossRef]
- Whitehouse, J.P.; Howlett, M.; Hii, H.; Mayoh, C.; Wong, M.; Barahona, P.; Ajuyah, P.; White, C.L.; Buntine, M.K.; Dyke, J.M.; et al. A Novel Orthotopic Patient-Derived Xenograft Model of Radiation-Induced Glioma Following Medulloblastoma. Cancers 2020, 12, 2937. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.T.; Liu, Q.; Yasui, Y.; Huang, S.; Ness, K.K.; Leisenring, W.; Hudson, M.M.; Donaldson, S.S.; King, A.A.; Stovall, M.; et al. Long-Term Outcomes Among Adult Survivors of Childhood Central Nervous System Malignancies in the Childhood Cancer Survivor Study. J. Natl. Cancer Inst. 2009, 101, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Velasco, G.; Sánchez, C.; Guzmán, M. Towards the use of cannabinoids as antitumor agents. Nat. Rev. Cancer 2012, 12, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nat. Cell Biol. 1990, 346, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nat. Cell Biol. 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Howlett, A.C. International Union of Pharmacology. XXVII. Classification of Cannabinoid Receptors. Pharmacol. Rev. 2002, 54, 161–202. [Google Scholar] [CrossRef]
- Espejo-Porras, F.; Fernández-Ruiz, J.; Pertwee, R.G.; Mechoulam, R.; García, C. Motor effects of the non-psychotropic phytocannabinoid cannabidiol that are mediated by 5-HT1A receptors. Neuropharmacology 2013, 75, 155–163. [Google Scholar] [CrossRef]
- Bih, C.I.; Chen, T.; Nunn, A.V.W.; Bazelot, M.; Dallas, M.L.; Whalley, B.J. Molecular Targets of Cannabidiol in Neurological Disorders. Neurotherapeutics 2015, 12, 699–730. [Google Scholar] [CrossRef]
- Nabissi, M.; Morelli, M.B.; Santoni, M.; Santoni, G. Triggering of the TRPV2 channel by cannabidiol sensitizes glioblastoma cells to cytotoxic chemotherapeutic agents. Carcinogenesis 2013, 34, 48–57. [Google Scholar] [CrossRef]
- Hassan, S.; Eldeeb, K.; Millns, P.J.; Bennett, A.J.; Alexander, S.P.H.; Kendall, D. Cannabidiol enhances microglial phagocytosis via transient receptor potential (TRP) channel activation. Br. J. Pharmacol. 2014, 171, 2426–2439. [Google Scholar] [CrossRef]
- Velasco, G.; Sánchez, C.; Guzmán, M. Anticancer mechanisms of cannabinoids. Curr. Oncol. 2016, 23, S23–S32. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.; Lorente, M.; Rodríguez-Fornés, F.; Hernández-Tiedra, S.; Salazar, M.; García-Taboada, E.; Barcia, J.; Guzmán, M.; Velasco, G. A Combined Preclinical Therapy of Cannabinoids and Temozolomide against Glioma. Mol. Cancer Ther. 2011, 10, 90–103. [Google Scholar] [CrossRef] [PubMed]
- López-Valero, I.; Torres, S.; Salazar-Roa, M.; García-Taboada, E.; Hernández-Tiedra, S.; Guzmán, M.; Sepúlveda, J.M.; Velasco, G.; Lorente, M. Optimization of a preclinical therapy of cannabinoids in combination with temozolomide against glioma. Biochem. Pharmacol. 2018, 157, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.A.; Dalgleish, A.G.; Liu, W.M. The Combination of Cannabidiol and Δ9-Tetrahydrocannabinol Enhances the Anticancer Effects of Radiation in an Orthotopic Murine Glioma Model. Mol. Cancer Ther. 2014, 13, 2955–2967. [Google Scholar] [CrossRef] [PubMed]
- Ellert-Miklaszewska, A.; Ciechomska, I.A.; Kaminska, B. Cannabinoid Signaling in Glioma Cells. Adv. Exp. Med. Biol. 2020, 1202, 223–241. [Google Scholar] [PubMed]
- Dumitru, C.A.; Sandalcioglu, I.E.; Karsak, M. Cannabinoids in Glioblastoma Therapy: New Applications for Old Drugs. Front. Mol. Neurosci. 2018, 11, 159. [Google Scholar] [CrossRef]
- Salazar, M.; Carracedo, A.; Salanueva, Í.J.; Hernández-Tiedra, S.; Lorente, M.; Egia, A.; Vázquez, P.; Blázquez, C.; Torres, S.; García, S.; et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J. Clin. Investig. 2009, 119, 1359–1372. [Google Scholar] [CrossRef]
- Ryan, J.E.; Smeltzer, S.C.; Sharts-Hopko, N.C. Parents’ experiences using medical cannabis for their child. Nurs. Outlook 2020, 68, 337–344. [Google Scholar] [CrossRef]
- Adler, J.N.; Colbert, J.A. Medicinal Use of Marijuana—Polling Results. N. Engl. J. Med. 2013, 368, e30. [Google Scholar] [CrossRef]
- Skrypek, M.M.; Bostrom, B.; Bendel, A. Medical Cannabis Certification in a Large Pediatric Oncology Center. Children 2019, 6, 79. [Google Scholar] [CrossRef]
- Castillo, A.; Tolón, M.; Fernández-Ruiz, J.; Romero, J.; Martínez-Orgado, J. The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic–ischemic brain damage in mice is mediated by CB2 and adenosine receptors. Neurobiol. Dis. 2010, 37, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Kogan, N.M.; Mechoulam, R. Beyond THC and Endocannabinoids. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 637–659. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-C.; Mackie, K. Review of the Endocannabinoid System. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2020. [Google Scholar] [CrossRef]
- Cavalli, F.M.; Remke, M.; Rampasek, L.; Peacock, J.; Shih, D.J.; Luu, B.; Garzia, L.; Torchia, J.; Nor, C.; Morrissy, A.S.; et al. Intertumoral Heterogeneity within Medulloblastoma Subgroups. Cancer Cell 2017, 31, 737–754.e6. [Google Scholar] [CrossRef] [PubMed]
- Endersby, R.; Whitehouse, J.; Pribnow, A.; Kuchibhotla, M.; Hii, H.; Carline, B.; Gande, S.; Stripay, J.; Ancliffe, M.; Howlett, M.; et al. Small molecule screen reveals synergy of cell cycle checkpoint kinase inhibitors with DNA-damaging chemotherapies in medulloblastoma. Sci. Transl. Med. 2020, in press. [Google Scholar]
- Marcu, J.P.; Christian, R.T.; Lau, D.; Zielinski, A.J.; Horowitz, M.P.; Lee, J.; Pakdel, A.; Allison, J.; Limbad, C.; Moore, D.H.; et al. Cannabidiol Enhances the Inhibitory Effects of Δ9-Tetrahydrocannabinol on Human Glioblastoma Cell Proliferation and Survival. Mol. Cancer Ther. 2010, 9, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Massi, P.; Vaccani, A.; Bianchessi, S.; Costa, B.; Macchi, P.; Parolaro, D. The non-psychoactive cannabidiol triggers caspase activation and oxidative stress in human glioma cells. Cell. Mol. Life Sci. 2006, 63, 2057–2066. [Google Scholar] [CrossRef]
- Galve-Roperh, I.; Sánchez, C.; Cortés, M.L.; Del Pulgar, T.G.; Izquierdo, M.; Guzmán, M. Anti-tumoral action of cannabinoids: Involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat. Med. 2000, 6, 313–319. [Google Scholar] [CrossRef]
- Caffarel, M.M.; Andradas, C.; Mira, E.; Pérez-Gómez, E.; Cerutti, C.; Moreno-Bueno, G.; Flores, J.M.; García-Real, I.; Palacios, J.; Mañes, S.; et al. Cannabinoids reduce ErbB2-driven breast cancer progression through Akt inhibition. Mol. Cancer 2010, 9, 196. [Google Scholar] [CrossRef]
- Nascimento, E.B.; Snel, M.; Guigas, B.; Van Der Zon, G.C.; Kriek, J.; Maassen, J.A.; Jazet, I.M.; Diamant, M.; Ouwens, D.M. Phosphorylation of PRAS40 on Thr246 by PKB/AKT facilitates efficient phosphorylation of Ser183 by mTORC1. Cell Signal 2010, 22, 961–967. [Google Scholar] [CrossRef]
- Hernández-Tiedra, S.; Fabriàs, G.; Dávila, D.; Salanueva, Í.J.; Casas, J.; Montes, L.R.; Antón, Z.; García-Taboada, E.; Salazar-Roa, M.; Lorente, M.; et al. Dihydroceramide accumulation mediates cytotoxic autophagy of cancer cells via autolysosome destabilization. Autophagy 2016, 12, 2213–2229. [Google Scholar] [CrossRef]
- Loewe, S. The problem of synergism and antagonism of combined drugs. Arzneimittelforschung 1953, 3, 285–290. [Google Scholar]
- Di Veroli, G.Y.; Fornari, C.; Wang, D.; Mollard, S.; Bramhall, J.L.; Richards, F.M.; Jodrell, D.I. Combenefit: An interactive platform for the analysis and visualization of drug combinations. Bioinformatics 2016, 32, 2866–2868. [Google Scholar] [CrossRef] [PubMed]
- Von Bueren, A.O.; Kortmann, R.-D.; Von Hoff, K.; Friedrich, C.; Mynarek, M.; Müller, K.; Goschzik, T.; Mühlen, A.Z.; Gerber, N.; Warmuth-Metz, M.; et al. Treatment of Children and Adolescents with Metastatic Medulloblastoma and Prognostic Relevance of Clinical and Biologic Parameters. J. Clin. Oncol. 2016, 34, 4151–4160. [Google Scholar] [CrossRef] [PubMed]
- Gajjar, A.; Chintagumpala, M.; Ashley, D.; Kellie, S.; Kun, E.L.; Merchant, E.T.; Woo, S.; Wheeler, G.; Ahern, V.; Krasin, M.J.; et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): Long-term results from a prospective, multicentre trial. Lancet Oncol. 2006, 7, 813–820. [Google Scholar] [CrossRef]
- Branzei, D.; Foiani, M. Regulation of DNA repair throughout the cell cycle. Nat. Rev. Mol. Cell Biol. 2008, 9, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Deiana, S.; Watanabe, A.; Yamasaki, Y.; Amada, N.; Arthur, M.; Fleming, S.; Woodcock, H.; Dorward, P.; Pigliacampo, B.; Close, S.; et al. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ9-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive–compulsive behaviour. Psychopharmacology 2012, 219, 859–873. [Google Scholar] [CrossRef]
- Bergamaschi, M.M.; Queiroz, R.H.C.; Zuardi, A.W.; Crippa, J.A.S. Safety and Side Effects of Cannabidiol, a Cannabis sativa Constituent. Curr. Drug Saf. 2011, 6, 237–249. [Google Scholar] [CrossRef]
- Matsunaga, T.; Iwawaki, Y.; Watanabe, K.; Yamamoto, I.; Kageyama, T.; Yoshimura, H. Metabolism of Δ9-tetrahydrocannabinol by cytochrome P450 isozymes purified from hepatic microsomes of monkeys. Life Sci. 1995, 56, 2089–2095. [Google Scholar] [CrossRef]
- Friedman, H.S.; Mahaley, S.M.; Schold, C.S.; Vick, N.A.; Falletta, J.M.; Bullard, D.E.; D’souza, B.J.; Khandekar, J.D.; Lew, S.; Oakes, J.W.; et al. Efficacy of Vincristine and Cyclophosphamide in the Therapy of Recurrent Medulloblastoma. Neurosurgery 1986, 18, 335–340. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro-Oncology 2020, 22, iv1–iv96. [Google Scholar] [CrossRef] [PubMed]
- Gottardo, N.G.; Hansford, J.R.; McGlade, J.P.; Alvaro, F.; Ashley, D.M.; Bailey, S.; Baker, D.L.; Bourdeaut, F.; Cho, Y.-J.; Clay, M.; et al. Medulloblastoma Down Under 2013: A report from the third annual meeting of the International Medulloblastoma Working Group. Acta Neuropathol. 2014, 127, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Packer, R.J.; Gajjar, A.; Vezina, G.; Rorke-Adams, L.; Burger, P.C.; Robertson, P.L.; Bayer, L.; Lafond, D.; Donahue, B.R.; Marymont, M.H.; et al. Phase III Study of Craniospinal Radiation Therapy Followed by Adjuvant Chemotherapy for Newly Diagnosed Average-Risk Medulloblastoma. J. Clin. Oncol. 2006, 24, 4202–4208. [Google Scholar] [CrossRef] [PubMed]
- Ramer, R.; Hinz, B. Cannabinoids as Anticancer Drugs. Stud. Surf. Sci. Catal. 2017, 80, 397–436. [Google Scholar] [CrossRef]
- Nabbout, R.; Thiele, A.E. The role of cannabinoids in epilepsy treatment: A critical review of efficacy results from clinical trials. Epileptic Disord 2020, 22, 23–28. [Google Scholar]
- Friedman, H.S.; Burger, P.C.; Bigner, S.H.; Trojanowski, J.Q.; Wikstrand, C.J.; Halperin, E.C.; Bigner, D.D. Establishment and Characterization of the Human Medulloblastoma Cell Line and Transplantable Xenograft D283 Med. J. Neuropathol. Exp. Neurol. 1985, 44, 592–605. [Google Scholar] [CrossRef]
- Friedman, H.S.; Colvin, O.M.; Skapek, S.X.; Ludeman, S.M.; Elion, G.B.; Schold, S.C.; Jacobsen, P.F.; Muhlbaier, L.H.; Bigner, D.D. Experimental chemotherapy of human medulloblastoma cell lines and transplantable xenografts with bifunctional alkylating agents. Cancer Res. 1988, 48, 4189–4195. [Google Scholar]
- Holthouse, D.J.; Dallas, P.B.; Ford, J.; Fabian, V.; Murch, A.R.; Watson, M.; Wong, G.; Bertram, C.; Egli, S.; Baker, D.L.; et al. Classic and desmoplastic medulloblastoma: Complete case reports and characterizations of two new cell lines. Neuropathology 2009, 29, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Milde, T.; Kleber, S.; Korshunov, A.; Witt, H.; Hielscher, T.; Koch, P.; Kopp, H.-G.; Jugold, M.; Deubzer, H.E.; Oehme, I.; et al. A novel human high-risk ependymoma stem cell model reveals the differentiation-inducing potential of the histone deacetylase inhibitor Vorinostat. Acta Neuropathol. 2011, 122, 637–650. [Google Scholar] [CrossRef]
- Pertwee, R. Handbook of Cannabis; Oxford University Press (OUP): Oxford, UK, 2014; Volume 10. [Google Scholar]
- Blasco-Benito, S.; Seijo-Vila, M.; Caro-Villalobos, M.; Tundidor, I.; Andradas, C.; García-Taboada, E.; Wade, J.; Smith, S.; Guzmán, M.; Pérez-Gómez, E.; et al. Appraising the “entourage effect”: Antitumor action of a pure cannabinoid versus a botanical drug preparation in preclinical models of breast cancer. Biochem. Pharmacol. 2018, 157, 285–293. [Google Scholar] [CrossRef]
- López-Valero, I.; Saiz-Ladera, C.; Torres, S.; Hernández-Tiedra, S.; García-Taboada, E.; Rodríguez-Fornés, F.; Barba-Aliaga, M.; Dávila, D.; Salvador-Tormo, N.; Guzmán, M.; et al. Targeting Glioma Initiating Cells with A combined therapy of cannabinoids and temozolomide. Biochem. Pharmacol. 2018, 157, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Chang, T.; Du, Y.; Yu, C.; Tan, X.; Li, X. Pharmacokinetics of oral and intravenous cannabidiol and its antidepressant-like effects in chronic mild stress mouse model. Environ. Toxicol. Pharmacol. 2019, 70, 103202. [Google Scholar] [CrossRef] [PubMed]
- Endersby, R.; Whitehouse, J.; Hii, H.; Greenall, S.A.; Johns, T.G.; Gottardo, N.G. A Pre-Clinical Assessment of the Pan-ERBB Inhibitor Dacomitinib in Pediatric and Adult Brain Tumors. Neoplasia 2018, 20, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Singh, S.; Niyogi, R.G.; Lamont, G.J.; Wang, H.; Lamont, R.J.; Scott, A.D. Marijuana-Derived Cannabinoids Trigger a CB2/PI3K Axis of Suppression of the Innate Response to Oral Pathogens. Front. Immunol. 2019, 10, 2288. [Google Scholar] [CrossRef]
- Guindon, J.; Lai, Y.; Takacs, S.M.; Bradshaw, H.B.; Hohmann, A.G. Alterations in endocannabinoid tone following chemotherapy-induced peripheral neuropathy: Effects of endocannabinoid deactivation inhibitors targeting fatty-acid amide hydrolase and monoacylglycerol lipase in comparison to reference analgesics following cisplatin treatment. Pharmacol. Res. 2013, 67, 94–109. [Google Scholar] [CrossRef]
- Huang, Z.; Roy, P.; Waxman, D.J. Role of human liver microsomal CYP3A4 and CYP2B6 in catalyzing N-dechloroethylation of cyclophosphamide and ifosfamide. Biochem. Pharmacol. 2000, 59, 961–972. [Google Scholar] [CrossRef]
- Baumhäkel, M.; Kasel, D.; Rao-Schymanski, R.; Böcker, R.; Beckurts, K.; Zaigler, M.; Barthold, D.; Fuhr, U. Screening for inhibitory effects of antineoplastic agents on CYP3A4 in human liver microsomes. Int. J. Clin. Pharmacol. Ther. 2001, 39, 517–528. [Google Scholar] [CrossRef]
- Capper, D.; Jones, D.T.W.; Sill, M.; Hovestadt, V.; Schrimpf, D.; Sturm, D.; Koelsche, C.; Sahm, F.; Chavez, L.; Reuss, D.E.; et al. DNA methylation-based classification of central nervous system tumors. Nature 2018, 555, 469–474. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 25 December 2020).
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Anders, S.; McCarthy, D.J.; Chen, Y.; Okoniewski, M.; Smyth, G.K.; Huber, W.; Robinson, M.D. Count-based differential expression analysis of RNA sequencing data using R and Bioconductor. Nat. Protoc. 2013, 8, 1765–1786. [Google Scholar] [CrossRef]
- Lassmann, T.; Hayashizaki, Y.; Daub, C.O. SAMStat: Monitoring biases in next generation sequencing data. Bioinformatics 2010, 27, 130–131. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Kuzontkoski, P.M.; Groopman, J.E.; Prasad, A. Cannabidiol Induces Programmed Cell Death in Breast Cancer Cells by Coordinating the Cross-talk between Apoptosis and Autophagy. Mol. Cancer Ther. 2011, 10, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Endersby, R.; Zhu, X.; Hay, N.; Ellison, D.W.; Baker, S.J. Nonredundant Functions for Akt Isoforms in Astrocyte Growth and Gliomagenesis in an Orthotopic Transplantation Model. Cancer Res. 2011, 71, 4106–4116. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Fisher, T.; Golan, H.; Schiby, G.; Prichen, S.; Smoum, R.; Moshe, I.; Peshes-Yaloz, N.; Castiel, A.; Waldman, D.; Gallily, R.; et al. In vitro and in vivo efficacy of non-psychoactive cannabidiol in neuroblastoma. Curr. Oncol. 2016, 23, 15–22. [Google Scholar] [CrossRef]
- Oesch, S.; Walter, D.; Wachtel, M.; Pretre, K.; Salazar, M.; Guzmán, M.; Velasco, G.; Schäfer, B.W. Cannabinoid receptor 1 is a potential drug target for treatment of translocation-positive rhabdomyosarcoma. Mol. Cancer Ther. 2009, 8, 1838–1845. [Google Scholar] [CrossRef]
- Scott, K.A.; Dalgleish, A.G.; Liu, W.M. Anticancer effects of phytocannabinoids used with chemotherapy in leukemia cells can be improved by altering the sequence of their administration. Int. J. Oncol. 2017, 51, 369–377. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andradas, C.; Byrne, J.; Kuchibhotla, M.; Ancliffe, M.; Jones, A.C.; Carline, B.; Hii, H.; Truong, A.; Storer, L.C.D.; Ritzmann, T.A.; et al. Assessment of Cannabidiol and Δ9-Tetrahydrocannabiol in Mouse Models of Medulloblastoma and Ependymoma. Cancers 2021, 13, 330. https://doi.org/10.3390/cancers13020330
Andradas C, Byrne J, Kuchibhotla M, Ancliffe M, Jones AC, Carline B, Hii H, Truong A, Storer LCD, Ritzmann TA, et al. Assessment of Cannabidiol and Δ9-Tetrahydrocannabiol in Mouse Models of Medulloblastoma and Ependymoma. Cancers. 2021; 13(2):330. https://doi.org/10.3390/cancers13020330
Chicago/Turabian StyleAndradas, Clara, Jacob Byrne, Mani Kuchibhotla, Mathew Ancliffe, Anya C. Jones, Brooke Carline, Hilary Hii, Alexandra Truong, Lisa C. D. Storer, Timothy A. Ritzmann, and et al. 2021. "Assessment of Cannabidiol and Δ9-Tetrahydrocannabiol in Mouse Models of Medulloblastoma and Ependymoma" Cancers 13, no. 2: 330. https://doi.org/10.3390/cancers13020330
APA StyleAndradas, C., Byrne, J., Kuchibhotla, M., Ancliffe, M., Jones, A. C., Carline, B., Hii, H., Truong, A., Storer, L. C. D., Ritzmann, T. A., Grundy, R. G., Gottardo, N. G., & Endersby, R. (2021). Assessment of Cannabidiol and Δ9-Tetrahydrocannabiol in Mouse Models of Medulloblastoma and Ependymoma. Cancers, 13(2), 330. https://doi.org/10.3390/cancers13020330






