Lidocaine Suppresses Viability and Migration of Human Breast Cancer Cells: TRPM7 as a Target for Some Breast Cancer Cell Lines
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
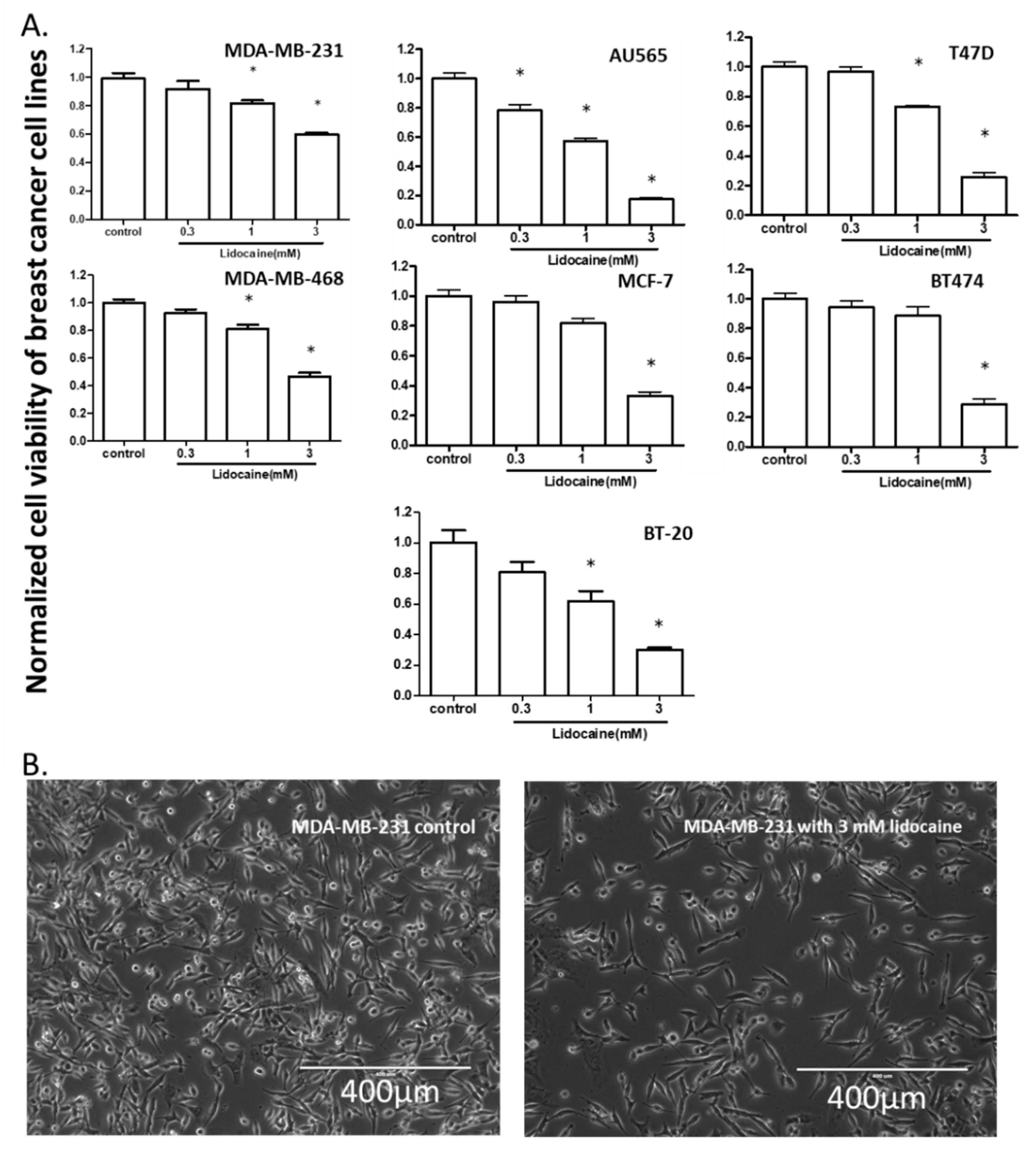
2.1. Lidocaine Reduced the Viability of Human Breast Cancer Cell Lines
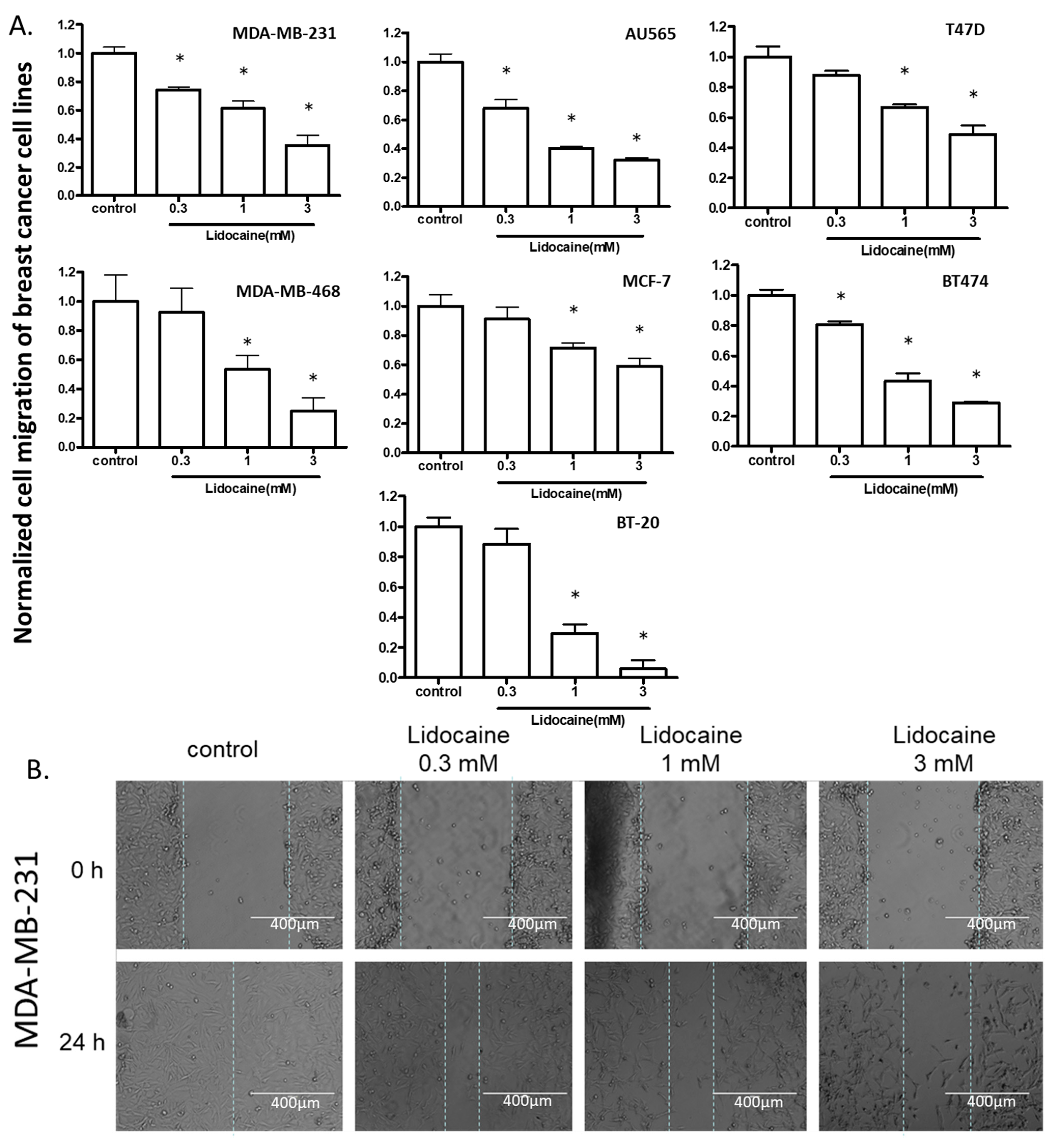
2.2. Lidocaine Suppressed the Migration of Breast Cancer Cell Lines
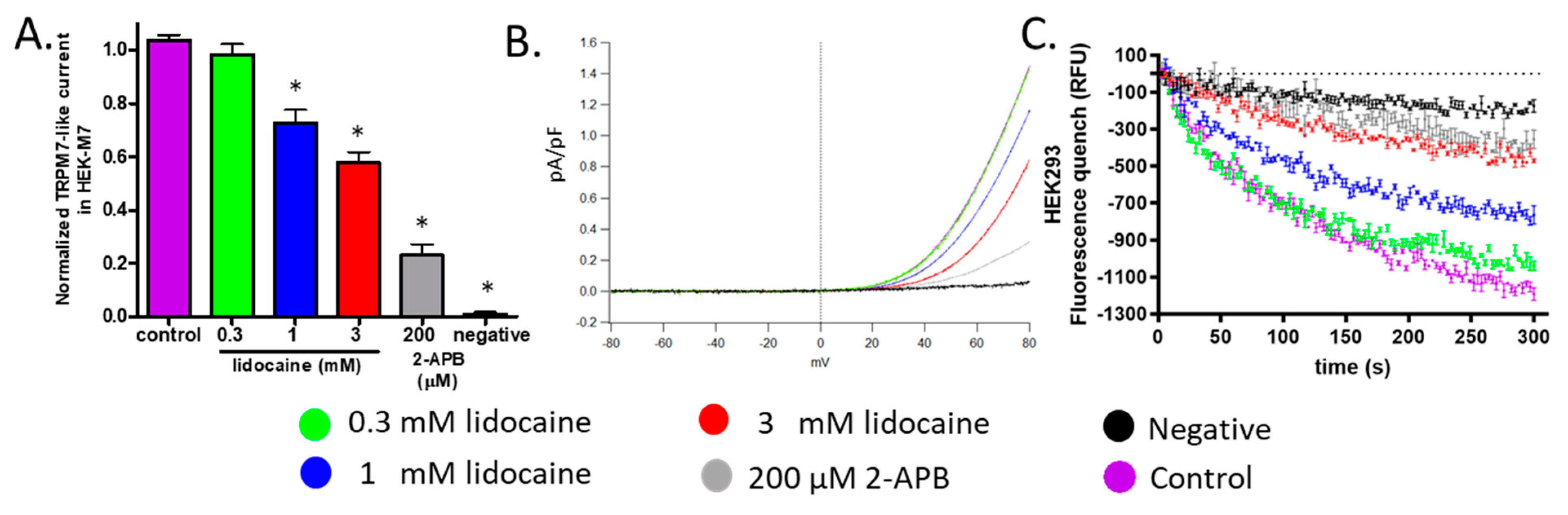
2.3. Lidocaine Suppressed the TRPM7 Channel Function in HEK293 Cells
2.4. Lidocaine Suppressed the TRPM7 Function in Human Breast Cancer Cell Lines
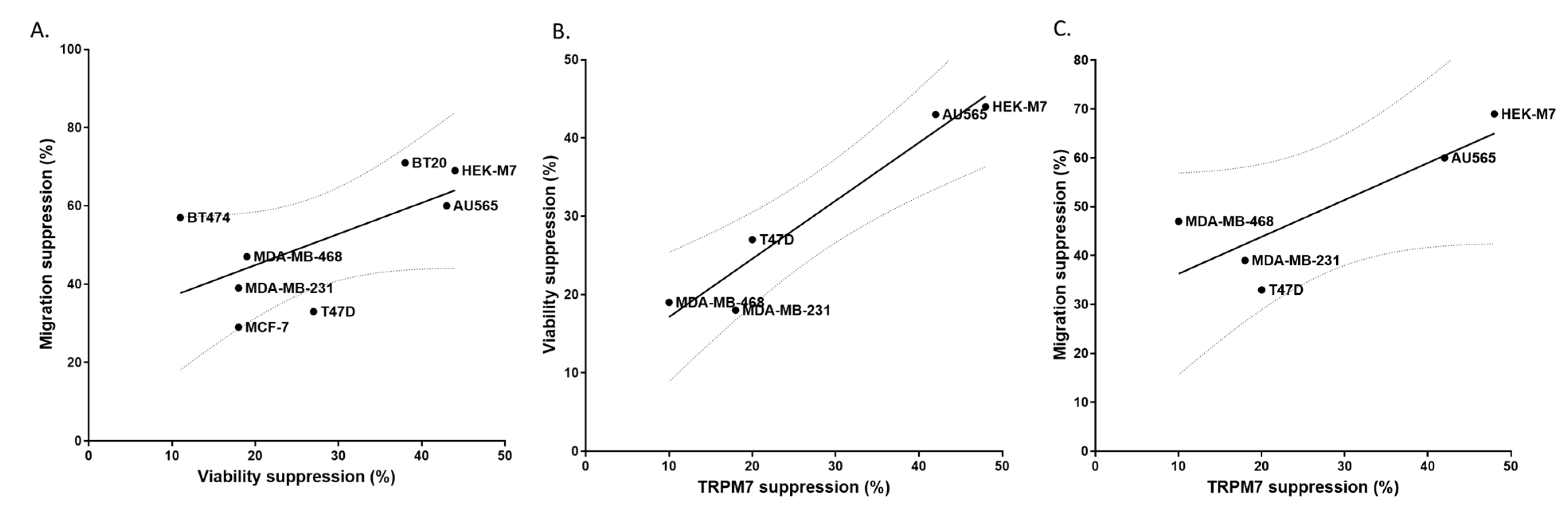
2.5. TRPM7 Was a Potential Common Target of Lidocaine for Breast Cancer Cells
2.6. TRPM7 Expression Increased the Sensitivity of HEK293 Cells to Lidocaine
2.7. Knockout of TRPM7 Decreased the Sensitivity of MDA-MB-231 Cells to Lidocaine
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Cell Culture
4.3. Drugs
4.4. Viability Assay
4.5. Migration Assay
4.6. Whole-Cell Patch-Clamping
4.7. Fura-2AM-Based Quench Assay
4.8. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Breast cancer statistics. CA Cancer J. Clin. 2019, 69, 438–451. [Google Scholar]
- Li, R.; Liu, H.; Dilger, J.P.; Lin, J. Effect of Propofol on breast Cancer cell, the immune system, and patient outcome. BMC Anesthesiol. 2018, 18, 1–8. [Google Scholar] [CrossRef]
- Li, R.; Huang, Y.; Liu, H.; Dilger, J.P.; Lin, J. Abstract 2162: Comparing volatile and intravenous anesthetics in a mouse model of breast cancer metastasis. Tumor Biol. 2018, 78, 2162. [Google Scholar] [CrossRef]
- Liu, H. A clinical mini-review: Clinical use of Local anesthetics in cancer surgeries. Gaz. Med. Sci. 2020, 1, 30–34. [Google Scholar] [CrossRef]
- Royds, J.; Khan, A.H.; Buggy, D.J. An Update on Existing Ongoing Prospective Trials Evaluating the Effect of Anesthetic and Analgesic Techniques During Primary Cancer Surgery on Cancer Recurrence or Metastasis. Int. Anesthesiol. Clin. 2016, 54, e76–e83. [Google Scholar] [CrossRef]
- Call, T.R.; Pace, N.L.; Thorup, D.B.; Maxfield, D.; Chortkoff, B.; Christensen, J.; Mulvihill, S.J. Factors associated with improved survival after resection of pancreatic AdenocarcinomaA multivariable model. J. Am. Soc. Anesthesiol. 2015, 122, 317–324. [Google Scholar]
- D’Agostino, G.; Saporito, A.; Cecchinato, V.; Silvestri, Y.; Borgeat, A.; Anselmi, L.; Uguccioni, M. Lidocaine inhibits cytoskeletal remodelling and human breast cancer cell migration. Br. J. Anaesth. 2018, 121, 962–968. [Google Scholar] [CrossRef]
- Li, R.; Xiao, C.; Liu, H.; Huang, Y.; Dilger, J.P.; Lin, J. Effects of local anesthetics on breast cancer cell viability and migration. BMC Cancer 2018, 18, 1–12. [Google Scholar] [CrossRef]
- Global Journal of Cancer Therapy. Glob. J. Cancer Ther. 2020, 6, 5. [CrossRef]
- Hengrui, L. A prospective for the role of two-pore channels in breast cancer cells. Glob. J. Cancer Ther. 2020, 6, 001–003. [Google Scholar] [CrossRef]
- Gautier, M.; Perrière, M.; Monet, M.; Vanlaeys, A.; Korichneva, I.; Dhennin-Duthille, I.; Ouadid-Ahidouch, H. Recent Advances in Oncogenic Roles of the TRPM7 Chanzyme. Curr. Med. Chem. 2016, 23, 4092–4107. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.-L.; Kong, C.-Z.; Zhang, Z.; Sheng-Lin, G.; Bi, J.-B.; Liu, X.-K. TRPM7 is overexpressed in bladder cancer and promotes proliferation, migration, invasion and tumor growth. Oncol. Rep. 2017, 38, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Yee, N.S. Role of TRPM7 in Cancer: Potential as Molecular Biomarker and Therapeutic Target. Pharmaceuticals 2017, 10, 39. [Google Scholar] [CrossRef]
- Jiang, J.; Li, M.-H.; Inoue, K.; Chu, X.-P.; Seeds, J.; Xiong, Z.-G. Transient Receptor Potential Melastatin 7–like Current in Human Head and Neck Carcinoma Cells: Role in Cell Proliferation. Cancer Res. 2007, 67, 10929–10938. [Google Scholar] [CrossRef]
- Rybarczyk, P.; Gautier, M.; Hague, F.; Dhennin-Duthille, I.; Chatelain, D.; Kerr-Conte, J.; Pattou, F.; Regimbeau, J.-M.; Sevestre, H.; Ouadid-Ahidouch, H. Transient receptor potential melastatin-related 7 channel is overexpressed in human pancreatic ductal adenocarcinomas and regulates human pancreatic cancer cell migration. Int. J. Cancer 2012, 131, E851–E861. [Google Scholar] [CrossRef]
- Freeman, J.; Crowley, P.D.; Foley, A.G.; Gallagher, H.C.; Arai, M.; Ma, D.; Buggy, D.J. Effect of Perioperative Lidocaine, Propofol and Steroids on Pulmonary Metastasis in a Murine Model of Breast Cancer Surgery. Cancers 2019, 11, 613. [Google Scholar] [CrossRef]
- Guilbert, A.; Gautier, M.; Dhennin-Duthille, I.; Haren, N.; Sevestre, H.; Ouadid-Ahidouch, H. Evidence that TRPM7 is required for breast cancer cell proliferation. Am. J. Physiol. Physiol. 2009, 297, C493–C502. [Google Scholar] [CrossRef]
- Dusmez, D.; Cengiz, B.; Yumrutas, O.; Demir, T.; Oztuzcu, S.; Demiryurek, S.; Tutar, E.; Bayraktar, R.; Bulut, A.; Şimşek, H.; et al. Effect of Verapamil and Lidocaine on TRPM and NaV1.9 Gene Expressions in Renal Ischemia-Reperfusion. Transplant. Proc. 2014, 46, 33–39. [Google Scholar] [CrossRef]
- Leng, T.-D.; Lin, J.; Sun, H.-W.; Zeng, Z.; O’Bryant, Z.; Inoue, K.; Xiong, Z. Local Anesthetic Lidocaine Inhibits TRPM7 Current and TRPM7-Mediated Zinc Toxicity. CNS Neurosci. Ther. 2014, 21, 32–39. [Google Scholar] [CrossRef]
- Leng, T.; Lin, S.; Xiong, Z.; Lin, J. Lidocaine suppresses glioma cell proliferation by inhibiting TRPM7 channels. Int. J. Physiol. Pathophysiol. Pharmacol. 2017, 9, 8–15. [Google Scholar] [PubMed]
- Liu, H.; Dilger, J.P.; Lin, J. The Role of Transient Receptor Potential Melastatin 7 (TRPM7) in Cell Viability: A Potential Target to Suppress Breast Cancer Cell Cycle. Cancers 2020, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Dilger, J.P.; Lin, J. Effects of local anesthetics on cancer cells. Pharmacol. Ther. 2020, 212, 107558. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Cai, C.; Wu, J.; Cai, S.; Ye, C.; Chen, H.; Yang, Z.; Zeng, H.; Shen, Q.; Zou, F. TRPM7 mediates breast cancer cell migration and invasion through the MAPK pathway. Cancer Lett. 2013, 333, 96–102. [Google Scholar] [CrossRef]
- Liu, L.; Wu, N.-Y.Y.; Wang, Y.; Zhang, X.; Xia, B.; Tang, J.; Cai, J.; Zhao, Z.; Liao, Q.; Wang, J. TRPM7 promotes the epithelial–mesenchymal transition in ovarian cancer through the calcium-related PI3K / AKT oncogenic signaling. J. Exp. Clin. Cancer Res. 2019, 38, 1–15. [Google Scholar] [CrossRef]
- Su, F.; Wang, B.-F.; Zhang, T.; Hou, X.-M.; Feng, M.-H. TRPM7 deficiency suppresses cell proliferation, migration, and invasion in human colorectal cancer via regulation of epithelial-mesenchymal transition. Cancer Biomark. 2019, 26, 451–460. [Google Scholar] [CrossRef]
- Wu, Y.; Sarkissyan, M.; Vadgama, J. Epithelial-Mesenchymal Transition and Breast Cancer. J. Clin. Med. 2016, 5, 13. [Google Scholar] [CrossRef]
- Song, C.; Bae, Y.; Jun, J.; Lee, H.; Kim, N.D.; Lee, K.-B.; Hur, W.; Park, J.-Y.; Sim, T. Identification of TG100-115 as a new and potent TRPM7 kinase inhibitor, which suppresses breast cancer cell migration and invasion. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 947–957. [Google Scholar] [CrossRef]
- Guilbert, A.; Gautier, M.; Dhennin-Duthille, I.; Rybarczyk, P.; Sahni, J.; Sevestre, H.; Scharenberg, A.; Ouadid-Ahidouch, H. Transient receptor potential melastatin 7 is involved in oestrogen receptor-negative metastatic breast cancer cells migration through its kinase domain. Eur. J. Cancer 2013, 49, 3694–3707. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Z.; Lis, A.; Penner, R.; Fleig, A. TRPM7 is regulated by halides through its kinase domain. Cell. Mol. Life Sci. 2013, 70, 2757–2771. [Google Scholar] [CrossRef]
- Su, L.-T.; Agapito, M.A.; Li, M.; Simonson, W.T.N.; Huttenlocher, A.; Habas, R.; Yue, L.; Runnels, L.W. TRPM7 Regulates Cell Adhesion by Controlling the Calcium-dependent Protease Calpain. J. Biol. Chem. 2006, 281, 11260–11270. [Google Scholar] [CrossRef] [PubMed]
- Zierler, S.; Yao, G.; Zhang, Z.; Kuo, W.C.; Poerzgen, P.; Penner, R.; Horgen, F.D.; Fleig, A. Waixenicin A Inhibits Cell Proliferation through Magnesium-dependent Block of Transient Receptor Potential Melastatin 7 (TRPM7) Channels. J. Biol. Chem. 2011, 286, 39328–39335. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Dilger, J.P.; Lin, J. Lidocaine Suppresses Viability and Migration of Human Breast Cancer Cells: TRPM7 as a Target for Some Breast Cancer Cell Lines. Cancers 2021, 13, 234. https://doi.org/10.3390/cancers13020234
Liu H, Dilger JP, Lin J. Lidocaine Suppresses Viability and Migration of Human Breast Cancer Cells: TRPM7 as a Target for Some Breast Cancer Cell Lines. Cancers. 2021; 13(2):234. https://doi.org/10.3390/cancers13020234
Chicago/Turabian StyleLiu, Hengrui, James P. Dilger, and Jun Lin. 2021. "Lidocaine Suppresses Viability and Migration of Human Breast Cancer Cells: TRPM7 as a Target for Some Breast Cancer Cell Lines" Cancers 13, no. 2: 234. https://doi.org/10.3390/cancers13020234
APA StyleLiu, H., Dilger, J. P., & Lin, J. (2021). Lidocaine Suppresses Viability and Migration of Human Breast Cancer Cells: TRPM7 as a Target for Some Breast Cancer Cell Lines. Cancers, 13(2), 234. https://doi.org/10.3390/cancers13020234






