Simple Summary
Although the role of multidisciplinary team meetings (MDT) in thoracic oncology is well established, its real impact on decisional process is not well known yet. The aim of this paper is to quantify the MDT impact on the decisional clinical pathway, assessing the modification rate of the initial out-patient evaluation. Our results show a mean modification rate of 10.6%; the clinical settings “solitary pulmonary nodule” and “proven or suspected recurrence” disclosed higher modification rates (14.6% and 13.3%, respectively). When histology is available at out-patient evaluation, “pulmonary carcinoid” is the group with the lowest modification rate when compared to other histologies. In the light of our results, we suggest multidisciplinary discussion even in departments where MDT is not always routinely performed. Moreover, when discussing clinical perspectives with patients belonging to groups with a higher modification rate, physicians should emphasize the possible decisional variability in order to prevent patients’ disorientation or controversies.
Abstract
Background: the aim of this paper is to quantify multidisciplinary team meeting (MDT) impact on the decisional clinical pathway of thoracic cancer patients, assessing the modification rate of the initial out-patient evaluation. Methods: the impact of MDT was classified as follows: confirmation: same conclusions as out-patient hypothesis; modification: change of out-patient hypothesis; implementation: definition of a clear clinical track/conclusion for patients that did not receive any clinical hypothesis; further exams required: the findings that emerged in the MDT meeting require further exams. Results: one thousand consecutive patients evaluated at MDT meetings were enrolled. Clinical settings of patients were: early stage lung cancer (17.4%); locally advanced lung cancer (27.4%); stage IV lung cancer (9.8%); mesothelioma (1%); metastases to the lung from other primary tumors (4%); histologically proven or suspected recurrence from previous lung cancer (15%); solitary pulmonary nodule (19.2%); mediastinal tumors (3.4%); other settings (2.8%). Conclusions: MDT meetings impact patient management in oncologic thoracic surgery by modifying the out-patient clinical hypothesis in 10.6% of cases; the clinical settings with the highest decisional modification rates are “solitary pulmonary nodule” and “proven or suspected recurrence” with modification rates of 14.6% and 13.3%, respectively.
1. Introduction
Oncologic diseases are complex clinical conditions requiring interaction between several specialists—with different skills and expertise—to offer the patients the best treatment strategies on the basis of the best available evidence [1]. A multidisciplinary team (MDT) consists of specialists with different backgrounds, skills and clinical experience, working together to recommend the best clinical pathway both in the case of planned treatments or to establish the most appropriate follow-up program [2]. MDT meetings in oncology can also be defined as tumor boards (TB); they offer several clinical benefits for global care: overall survival improvement [3,4], receipt of therapy [5,6], optimizing of treatment plan compared with pre-MDT hypotheses [7,8], staging accuracy [9] and global adherence to guidelines and international evidence-based recommendations [10,11,12,13].The MDT meeting can be considered as a common platform to coordinate the delivery of care by merging different clinical expertise in a single setting and can therefore be defined as a regularly scheduled discussion of clinical cases with the participation of physicians from different specialties such as surgeons, oncologists, radiotherapists, pulmonologists, pathologists, anesthesiologists, nurse specialists and other specialists when needed [14]. MDT meetings are suggested by many lung cancer treatment guidelines [15,16,17] but their organization and management is quite heterogeneous, varying across countries, hospitals and departments. Although the role of MDTs in lung cancer is well established today, their real impact on the decisional process is still not well known. The aim of this paper is to quantify the MDT impact on the decisional clinical pathway of thoracic cancer patients, assessing the modification rate of the initial out-patient evaluation, focusing on patients with different clinical settings referred to a high-volume oncologic thoracic surgical division.
2. Materials and Methods
The MDT meeting of the Division of Thoracic Surgery of the European Institute of Oncology is held weekly; attendees routinely include medical oncologists with a wide background in thoracic oncology, radiotherapists, interventional pulmonologists, thoracic surgeons, radiologists, a nurse case-manager and trainee specialists; other physicians are specifically invited on the basis of individual clinical cases, in particular pathologists, in case of unclear diagnosis. The meeting is coordinated by a senior physician; clinical cases are reviewed and presented by trainee specialists and all imaging exams are available on a maxi-screen. Each patient previously received an out-patient evaluation and then underwent a dedicated clinical track during which he/she was submitted to all exams, tests and procedures—both for oncologic and functional assessment—required by the referring physician at the time of out-patient access. After careful discussion, a final report is drawn up, the decision is recorded by a case manager and the patient is informed in person or by telephone about the results, depending on logistics and the patient’s preferences.
In the present study, the impact of the MDT on the previous out-patient program was classified as follows: (A) confirmation: same conclusions as the out-patient hypothesis (e.g., surgical indication confirmed); (B) modification: change of out-patient hypothesis (e.g., switch from surgical indication to radiotherapy or different treatment); (C) implementation: definition of a clear clinical track/conclusion for patients that did not receive any clinical hypothesis at out-patient access because of the lack of required exams or requiring further investigations before a definitive clinical conclusion (e.g., patient with no CT/PET/functional assessment or histology available at out-patient evaluation); (D) further exams required: the findings that emerged in the MDT meeting require further exams for a final decision.
With regard to clinical presentation at out-patient evaluation, patients were classified in the following “clinical settings”: (1) early stage lung cancer (stage I and II); (2) locally advanced lung cancer (stage IIIA and IIIB); (3) stage IV lung cancer; (4) mesothelioma; (5) metastases to the lung from other primary tumors; (6) histologically proven or suspected recurrence from previous lung cancer; (7) solitary pulmonary nodule (SPN); (8) mediastinal tumors; (9) other settings.
Written informed consent to undergo the procedures and for the use of clinical and imaging data for scientific or educational purposes, or both, were obtained from all patients; a blank copy of the written informed consent is provided.
Statistical Methods
Patients’ characteristics were summarized and tabulated either by counts and percent or mean, median, Standard Deviation (SD) and Interquartile Range (IQR) for categorical or continuous variables, respectively. The MDT percent changes for each level (confirmation, modification, implementation, further exams) were plotted according to the clinical setting alongside 95% Confidence Intervals (CIs) computed using the binomial exact method. For each clinical setting, the change of out-patient hypothesis (modification) entered a univariate logistic regression analysis as the event of interest against other MDT levels, using sex, availability of histology, age and the out-patient days to MDT evaluation as independent variables. Multivariable logistic regression was conducted using only those variables showing a significant association with the modification event at the univariate analysis. Results are presented as Odds Ratios with 95% CIs. Comparison of proportions for the categorical variables were tested using the chi-square test, continuous variables were tested using either the unpaired t-test or the two-sample Wilcoxon test. All tests were two-tailed and considered significant at the 5% level. All analyses were conducted using SAS 9.4 (Cary, NC, USA).
3. Results
One thousand consecutive patients evaluated in the MDT meetings of the Division of Thoracic Surgery in 2019 were enrolled. There were 590 (59%) male and 410 (41%) female patients; mean age was 67 years (standard deviation SD 11.1); mean time between out-patient evaluation and final MDT meeting decision was 33 days (interquartile range IQR 27.0–43.5).
Clinical settings of patients were: (1) early stage lung cancer (174 pts—17.4%); (2) locally advanced lung cancer (274 pts—27.4%); (3) stage IV lung cancer (98 pts—9.8%); (4) mesothelioma (10 pts—1%); (5) metastases to the lung from other primary tumors (40 pts—4%); (6) histologically proven or suspected recurrence from previous lung cancer (150 pts—15%); (7) solitary pulmonary nodule (SPN) (192 pts—19.2%); (8) mediastinal tumors (34 pts—3.4%); (9) other settings (28 pts—2.8%, including inflammatory/infective diseases, non-specific adenomegalies, isolated pleural lesions, neurogenic tumors, aspergillosis, chest wall tumors).
The overall impact of MDT discussion on the previous out-patient program was: confirmation (580 pts—58%); modification (106 patients—10.6%); implementation (234 pts—23.4%). Eighty patients (8%) required further exams at MDT: 18 patients (22.5%) required biopsy; 26 patients (32.5%) required biopsy plus further imaging exams; 10 patients (12.5%) required endoscopy (colonoscopy and/or esophago-gastroscopy); 18 patients (22.5%) required further imaging exams and 8 patients (10%) required specialist consultation (orthopedic, hepatologist, vascular/cardiac surgeon). After these additional exams, the out-patient hypothesis was confirmed in 38 patients (47.5%), modified in 24 patients (30%) and implemented in 18 patients (22.5%) (Table 1).

Table 1.
Patients’ demography, clinical setting and MDT impact summary statistics, N = 1000.
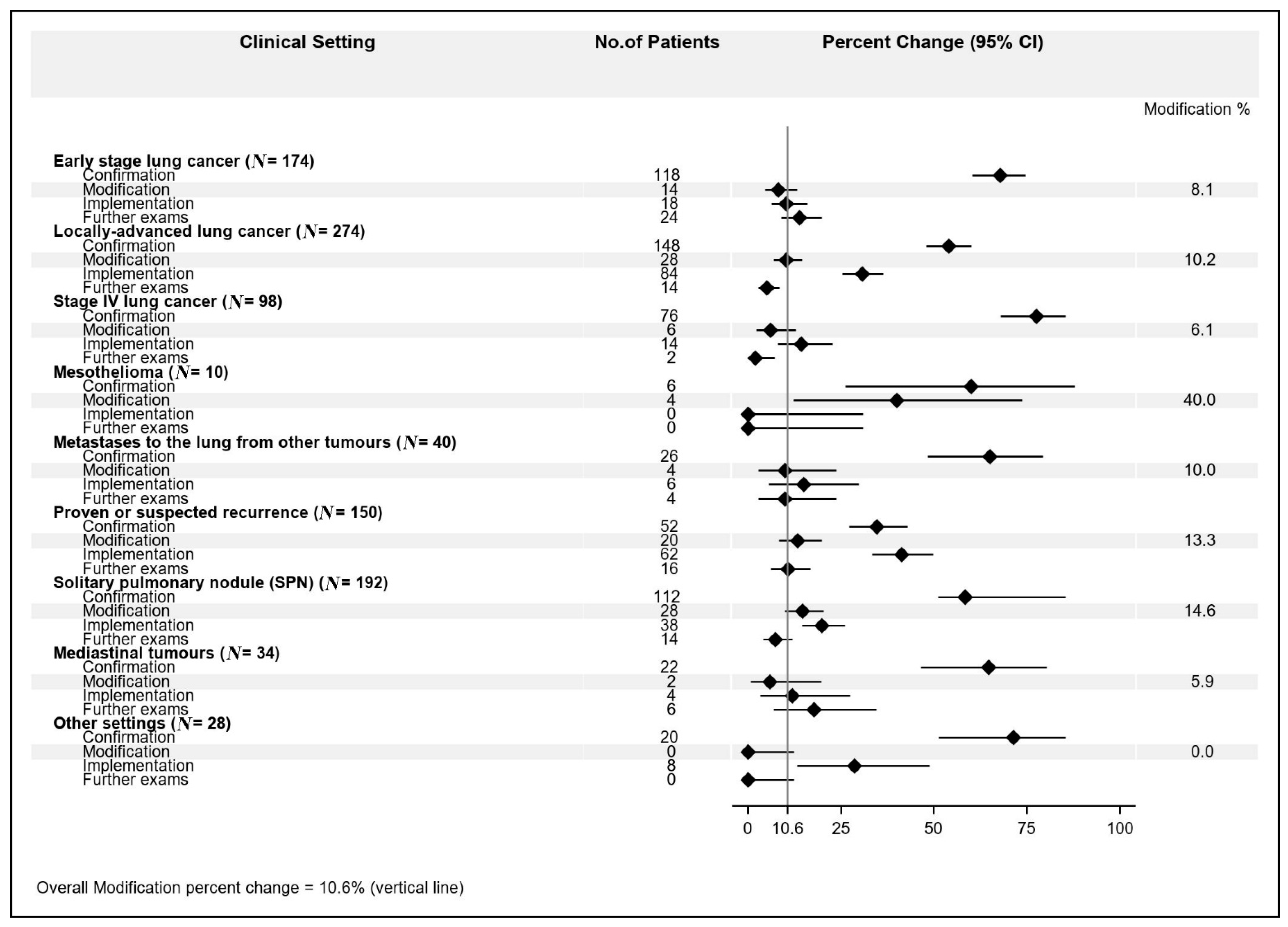
Among the different settings, we observed this MDT impact distribution: (1) early stage lung cancer: confirmation 67.8%; modification 8.1%; implementation 10.3%; further exams 13.8%; (2) locally advanced lung cancer: confirmation 54.1%; modification 10.2%; implementation 30.6%; further exams: 5.1%; (3) stage IV lung cancer: confirmation 77.6%; modification 6.1%; implementation 14.3%; further exams 2.0%; (4) mesothelioma: confirmation 60%; modification 40%; implementation 0%; further exams 0%; (5) metastases to the lung from other primary tumors: confirmation 65%; modification 10%; implementation 15%; further exams 4%; (6) histologically proven or suspected recurrence from previous lung cancer: confirmation 52%; modification 13.3%; implementation 41.3%; further exams 10.7%; (7) solitary pulmonary nodule (SPN): confirmation 58.3%; modification 14.6%; implementation 19.8%; further exams 7.3%; (8) mediastinal tumors: confirmation 64.7%; modification 5.9%; implementation 11.8%; further exams 17.6%; (9) other settings: confirmation 71.4%; modification 0%; implementation 28.6%; further exams 0% (Figure 1).
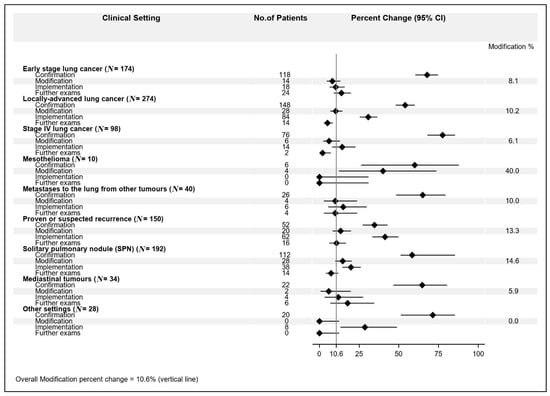
Figure 1.
Multidisciplinary team meeting (MDT) impact percent changes according to clinical setting.
The highest modification rate (40.0%) was observed for the “mesothelioma” setting, though in only 4 patients out of 10 (95% CI: 12.1–73.8%), followed by “solitary pulmonary nodule” (14.6%, 95% CI: 9.9–20.4%) and “histologically proven or suspected recurrence from previous lung cancer” (13.3%, 95% CI: 8.3–19.8%). Next, the modification rates ranged from 10.2% (95% CI: 6.9–14.4%) for the “locally advanced lung cancer” going down to 0% (one-sided 97.5% CI: 0–16.8%) for “other setting”.
It is the case that 776 patients (77.6%) did not have a histologically proven diagnosis when they received their out-patient evaluation. On the contrary, 224 patients (22.4%) already had a histologic characterization when they received their out-patient evaluation: 128 patients (57.1%) suffered from lung adenocarcinoma; 40 patients (18%) from squamous carcinoma; 12 patients (5.4%) from pulmonary carcinoid; 6 patients (2.7%) from small cell lung cancer; 38 patients (17.0%) presented different histologic types. Among patients with available histologically proven diagnoses at out-patient evaluation, those affected by pulmonary carcinoid had a significantly lower modification rate (0%) when compared with patients with lung adenocarcinoma (12.5%), squamous cell carcinoma (15.0%), small cell carcinoma (33.3%) and other histologies (5.3%) (p = 0.03) (Table 2).

Table 2.
Frequency distribution of histology type by MDT impact, N = 224.
The settings “early stage lung cancer” and “locally advanced lung cancer” showed a significant modification rate association with the availability of histology at the out-patient evaluation. Specifically, the initial out-patient hypothesis was modified for all 14 (100%) early stage lung cancer patients whose histology was not available, compared to 98 (61.3%) patients at other MDT levels (p = 0.002), while for the locally advanced lung cancer patients, the out-patient hypothesis was changed only for 8 (28.6%) patients whose histology was not available compared to 152 (61.8%) for other MDT levels (p = 0.001) (Table 3).

Table 3.
Frequency distribution of the availability of histology at out-patient evaluation according to the clinical setting.
Independent factors significantly associated with the modification event at the univariate analysis were the availability of histology vs. no availability (OR = 4.04, 95% CI: 1.71–4.05, p = 0.001) and the out-patient days to MDT evaluation (OR = 1.66, 95% CI: 1.15–2.41, p = 0.007), both for the locally advanced lung cancer setting and age (OR = 1.31, 95% CI: 1.03–1.68, p = 0.03) for the solitary pulmonary nodule setting (Table 4).

Table 4.
Univariate Analysis of factors associated with modification by clinical setting.
Multivariable analysis for the locally advanced lung cancer setting confirmed the significant association of both the availability of histology vs. no availability at out-patient evaluation (OR = 5.55, 95% CI: 2.23–13.7, p < 0.001) and days between out-patient evaluation and MDT discussion (OR = 1.04, 95% CI: 1.02–1.07, p < 0.001) (Table 5).

Table 5.
Multivariable analysis of factors associated with modification for the locally advanced lung cancer setting.
In this case, 222 patients (22.2%) did not receive any clinical hypothesis at out-patient access because of the lack of required exams.
4. Discussion
MDT meetings have been widely promoted to optimize the decision-making process for oncologic patients by improving coordination, communication and clinical discussion among physicians with different fields of expertise. However, the evidence that MDT meetings impact management was stronger than the evidence that they improve survival [3,18]. In a meta-analysis by Coory et coll., five studies on the impact of MDT on survival in lung cancer patients were found: among them, only two studies reported a modest 1-year survival increase in inoperable patients while the others did not disclose any advantage in terms of survival after the introduction of MDT meetings [19,20,21,22,23]. On the other hand, Pillay et coll.—in a systematic review on patient assessment, management and outcomes in oncology settings [6]—reported several studies among which the modification rate after MDT discussion ranged between 4% and 35% [24,25,26]. Moreover, very different scenarios have been observed in terms of MDT impact depending on each single specialty: for example, a modification rate of 27% was reported among gynecological patients after MDT discussion [27] and a modification rate of nearly 50% was reported among breast cancer patients after MDT meetings focusing on radiologic and pathologic data interpretation [28].
Our results show a global modification rate, after MDT discussion, of 10.6% which is consistent with the existing literature; moreover, the clinical settings “solitary pulmonary nodule” (SPN) and “proven or suspected recurrence” disclosed a higher modification rate of 14.6% and 13.3%, respectively. We may argue that the higher modification rate of these two settings may be due, on the one hand, to the intrinsic diagnostic challenge that both SPN and suspected recurrence represent by themselves; on the other hand, these are the clinical settings that most benefit from additional exams performed during diagnostic work-out, resulting in a more accurate diagnosis and clinical overview that may lead to modifying the out-patient initial decision.
The clinical setting “stage IV lung cancer” presents the lowest modification rate after MDT discussion (6.1%); this is probably due to the already clear clinical algorithm of a lung cancer metastatic patient at out-patient evaluation. In this setting, the surgical contribution is often limited to palliation of symptoms or diagnostic approach and can usually be well defined at out-patient approach; the clinical setting of “oligometastatic patients”—that may benefit from surgical treatment with curative intent [29,30]—was not considered in this paper.
Early stage lung cancer presents a very low modification rate after MDT of 8.1%; these patients are usually referred, at out-patient evaluation, for surgical therapy and a decisional switch at MDT is mainly due to cardio-pulmonary functional limitations, emerging during preoperative work-out, leading to radiotherapy as an alternative to surgical treatment [31,32]. Similarly, mediastinal tumors show a very low modification rate of 5.9%, due to clear surgical indications in the case of small and well-defined lesions as well as a clear multimodality treatment in the case of locally advanced tumors [33,34,35].
The vast majority of our patients (77.6%) had no histological diagnosis at out-patient evaluation, thus needing bronchoscopy or computed tomography(CT) -guided biopsy during clinical assessment before MDT discussion. Although—as expected—this further step conditioned a longer pre-MDT work-up compared with patients with available histology, the difference—albeit significant—was only 4 days (37 vs. 33, p < 0.0001).
In the group of patients with available histology at out-patient evaluation (22.4%), those with pulmonary carcinoid disclosed a significantly lower modification rate (0%) when compared to patients with lung adenocarcinoma (12.5%), squamous cell carcinoma (15.0%) small cell carcinoma (33.3%) and other histologies (5.3%) (p = 0.03). This was probably due to the fact that all patients in these groups (12 patients) belonged to early or locally advanced stages, thus the surgical indication formulated at out-patient evaluation was always confirmed during MDT discussion, as neither chemotherapy nor radiotherapy is indicated in this setting.
With regard to the “implementation” category, in this group we enrolled all patients (23.4%) for whom a clear clinical hypothesis was not possible, due to the lack of basic essential data provided at the time of out-patient evaluation. On the contrary, in the category “further exams required at MDT” (8.0%) we considered all patients that—despite receiving a clinical hypothesis and a complete diagnostic work-out—required additional new exams at the time of the MDT decision because of emerging unforeseen clinical conditions. In the vast majority of these latter cases, biopsies of new targets and further imaging exams were required, while in 10% of cases a specialist consultation was more rarely required to better study emerging vascular, cardiac, orthopedic and hepatic problems.
We have to point out some limitations of the study: as all of our patients are routinely presented and widely discussed at MDT meetings, we did not have any case-control group of patients not receiving MDT discussion, to search for an additional MDT value in terms of overall survival, disease-free survival or other clinical indicators, as reported in previous similar studies (Table 6) [36,37,38,39,40,41,42,43,44,45].

Table 6.
The role of MDT meeting in cancer settings in recent literature.
Moreover, because the population is quite heterogeneous with different tumors and stages, we could not compare disease outcomes of patients whose plan was changed with those without any change; we thus decided to focus on the decisional modification rate of MDT in order to identify the clinical settings that may most benefit from MDT discussion and for which we suggest clinical discussion even in departments where MDT is not always routinely performed. Moreover, when discussing clinical perspectives with patients belonging to clinical settings with higher modification rates, physicians should emphasize this aspect in order to prevent patient disorientation or controversies. Although this is a wide-population study, enrolling 1000 consecutive patients referred to a national high-volume referral cancer center, some clinical settings remain very infrequent, such as mesothelioma or other less frequent diseases, and so no clinical conclusion can be obtained for these groups of patients. Moreover, this paper is focused on the first-diagnosed cancer population and so some clinical scenarios of post treatment complications—such as post-resectional broncho-pleural fistula—are not evaluated [46].
5. Conclusions
MDT meetings impact on patient management in oncologic thoracic surgery by modifying the out-patient clinical hypothesis in 10.6% of cases, as similarly reported in other oncologic specialties. The clinical settings with the highest decisional modification rate are “solitary pulmonary nodule” and “proven or suspected recurrence” with modification rates of 14.6% and 13.3%, respectively. When histology is available at out-patient evaluation, “pulmonary carcinoid” is the group with the lowest modification rate when compared to other histologies. The modification rate in the settings “early stage lung cancer” and “locally advanced lung cancer” is significantly conditioned by the availability or not of histology at out-patient evaluation. When histology is not available at out-patient evaluation, the patients belonging to the “locally advanced lung cancer group” need more time (+4 days) to receive a definitive clinical decision.
In the light of our results, we suggest clinical discussion of these clinical settings even in departments where MDT is not always routinely performed; moreover, when discussing clinical perspectives with patients belonging to clinical settings with higher modification rates, physicians should emphasize this aspect in order to prevent patient disorientation or controversies.
Author Contributions
F.P. conceived the study; F.P., D.R., J.G., G.P., C.R., F.d.M. and L.S. contributed to the design and implementation of the research, to the analysis of the results and to the writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by the Italian Ministry of Health with “Ricerca Corrente”, “5 × 1000” funds and European Institute of Oncology Foundation.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki; Ethical review and approval were waived for this study, due to the retrospective nature of the study and the previous consents obtained.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Available on request.
Acknowledgments
The authors thank Susan Jane West for editing the English text. Medical doctors involved in clinical discussion of cases: Alessandro Borri, Luca Bertolaccini, Monica Casiraghi, Stefano Donghi, Niccolo’ Filippi, Domenico Galetta, Roberto Gasparri, Lara Girelli, Giorgio Lo Iacono, Antonio Mazzella, Chiara Matilde Catania, Ester Del Signore, Letizia Gianoncelli, Antonio Passaro, Gianluca Spitaleri, Valeria Stati, Chiara Maria Grana, Marzia Colandrea, Silvia Lidia Fracassi, Laura Giraldi, Paola Anna Rocca, Laura Lavinia Travaini.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Specchia, M.L.; Frisicale, E.M.; Carini, E. The impact of tumor board on cancer care: Evidence from an umbrella review. BMC Health Serv. Res. 2020, 20, 73. [Google Scholar] [CrossRef] [PubMed]
- Lesslie, M.; Parikh, J.R. Implementing a Multidisciplinary Tumor Board in the Community Practice Setting. Diagnostic 2017, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Stone, E.; Rankin, N.; Kerr, S.; Fong, K.; Currow, D.C.; Phillips, J.; Connon, T.; Zhang, L.; Shaw, T. Does presentation at multidisciplinary team meetings improve lung cancer survival? Findings from a consecutive cohort study. Lung Cancer 2018, 124, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Bilfinger, T.V.; Albano, D.; Perwaiz, M.; Keresztes, R.; Nemesure, B. Survival outcomes among lung cancer patients treated using a multidisciplinary team approach. Clin. Lung Cancer 2018, 19, 346–351. [Google Scholar] [CrossRef]
- Boxer, M.M.; Vinod, S.K.; Shafiq, J.; Duggan, K.J. Do multidisciplinary team meetings make a difference in the management of lung cancer? Cancer 2011, 117, 5112–5120. [Google Scholar] [CrossRef]
- Pillay, B.; Wootten, A.C.; Crowe, H.; Corcoran, N.; Tran, B.; Bowden, P.; Crowe, J.; Costello, A.J. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer Treat. Rev. 2016, 42, 56–72. [Google Scholar] [CrossRef]
- Ung, K.A.; Campbell, B.A.; Duplan, D.; Ball, D.; David, S. Impact of the lung oncology multidisciplinary team meetings on the management of patients with cancer. Asia Pac. J. Clin. Oncol. 2016, 12, e298–e304. [Google Scholar] [CrossRef]
- Conron, M.; Phuah, S.; Steinfort, D.; Dabscheck, E.; Wright, G.; Hart, D. Analysis of multidisciplinary lung cancer practice. Intern. Med. J. 2007, 37, 18–25. [Google Scholar] [CrossRef]
- Smeltzer, M.P.; Rugless, F.E.; Jackson, B.M.; Berryman, C.L.; Faris, N.R.; Ray, M.A.; Meadows, M.; Patel, A.A.; Roark, K.S.; Kedia, S.K.; et al. Pragmatic trial of a multidisciplinary lung cancer care model in a community healthcare setting: Study design, implementation evaluation, and baseline clinical results. Transl. Lung Cancer Res. 2018, 7, 88–102. [Google Scholar] [CrossRef]
- Lamprell, K.; Arnolda, G.; Delaney, G.P.; Liauw, W.; Braithwaite, J. The challenge of putting principles into practice: Resource tensions and real-world constraints in multidisciplinary oncology team meetings. Asia Pac. J. Clin. Oncol. 2019, 15, 199–207. [Google Scholar] [CrossRef]
- Campbell, B.A.; Ball, D.; Mornex, F. Multidisciplinary lung cancer meetings: Improving the practice of radiation oncology and facing future challenges. Respirology 2015, 20, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Stone, E.; Rankin, N.; Phillips, J.; Fong, K.; Currow, D.C.; Miller, A.; Largey, G.; Zielinski, R.; Flynn, P.; Shaw, T.; et al. Consensus minimum data set for lung cancer multidisciplinary teams: Results of a Delphi process. Respirology 2018, 23, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Fennell, M.L.; Das, I.P.; Clauser, S.; Petrelli, N.; Salner, A. The Organization of Multidisciplinary Care Teams: Modeling internal and external influences on Cancer care quality. J. Natl. Cancer Inst. Monogr. 2010, 40, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Kurpad, R.; Kim, W.; Rathmell, W.K.; Godley, P.; Whang, Y.; Fielding, J.; Smith, L.; Pettiford, A.; Schultz, H.; Nielsen, M.; et al. A multidisciplinary approach to the management of urologic malignancies: Does it influence diagnostic and treatment decisions? Urol. Oncol. Semin. Orig. Investig. 2011, 29, 378–382. [Google Scholar] [CrossRef] [PubMed]
- American College of Chest Physicians; Health and Science Policy Committee. Diagnosis and management of lung cancer: ACCP evidence-based guidelines. Chest 2003, 123 (Suppl. 1), 1S–19S. [Google Scholar] [CrossRef]
- Cancer Council Australia. Clinical Practice Guidelines for the Prevention, Diagnosis and Management of Lung Cancer. Cancer Council Australia. 2004. Available online: http://www.nhmrc.gov.au/publications/subjects/cancer.htm (accessed on 8 October 2020).
- The Lung Cancer Working Party of the British Thoracic Society Standards of Care Committee. BTS recommendations to respiratory physicians for organising the care of patients with lung cancer. The Lung Cancer Working Party of the British Thoracic Society Standards of Care Committee. Thorax 1998, 53 (Suppl. 1), S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Coory, M.; Gkolia, P.; Yang, I.A. Systematic review of multidisciplinary teams in the management of lung cancer. Lung Cancer 2008, 60, 4–21. [Google Scholar] [CrossRef]
- Forrest, L.M.; McMillan, D.C.; McArdle, C.S.; Dunlop, D.J. An evaluation of the impact of a multidisciplinary team, in a single centre, on treatment and survival in patients with inoperable non-smallcell lung cancer. Br. J. Cancer 2005, 93, 977–978. [Google Scholar] [CrossRef]
- Price, A.; Kerr, G.; Gregor, A.; Ironside, J.; Little, F. The impact of multidisciplinary teams and site specialisation on the use of radiotherapy in elderly people with non-small cell lung cancer (NSCLC). Radiother. Oncol. 2002, 64 (Suppl. 1). [Google Scholar]
- Murray, P.V.; O’Brien, M.E.R.; Sayer, R.; Cooke, N.; Knowles, A.C.; Miller, A.C.; Varney, V.; Rowell, N.P.; Padhani, A.R.; MacVicar, D.; et al. The pathway study: Results of a pilot feasibility study in patients suspected of having lung carcinoma investigated in a conventional chest clinic setting compared to a centralised two-stop pathway. Lung Cancer 2003, 42, 283–290. [Google Scholar] [CrossRef]
- Martin-Ucar, A.E.; Waller, D.A.; Atkins, J.L.; Swinson, D.; O’Byrne, K.J.; Peake, M.D. The beneficial effects of specialist thoracic surgery on the resection rate for non-small-cell lung cancer. Lung Cancer 2004, 46, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Dillman, R.O.; Chico, S.D. Cancer patient survival improvement is correlated with the opening of a community cancer centre: Comparisons with intramural and extramural benchmarks. J. Oncol. Pract. 2005, 1, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Wheless, S.A.; McKinney, K.A.; Zanation, A.M. A prospective study of the clinical impact of a multidisciplinary head and neck tumor board. Otolaryngol. Head Neck 2010, 143, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Gatcliffe, T.A.; Coleman, R.L. Tumor board: More than treatment planning: A 1-year prospective survey. J. Cancer Educ. 2008, 23, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Acher, P.L.; Young, A.J.; Etherington-Foy, R.; McCahy, P.J.; Deane, A.M. Improving outcomes in urological cancers: The impact of “multidisciplinary team meetings”. Int. J. Surg. 2005, 3, 121–123. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Greer, H.O.; Frederick, P.J.; Falls, N.M.; Tapley, E.B.; Samples, K.L.; Kimball, K.J.; Kendrick, J.E.; Conner, M.G.; Novak, L.; Michael, J.; et al. Impact of a weekly multidisciplinary tumor board conference on the management of women with gynecologic malignancies. Int. J. Gynecol. Cancer 2010, 20, 1321–1325. [Google Scholar]
- Newman, E.A.; Guest, A.B.; Helvie, M.A.; Roubidoux, M.A.; Chang, A.E.; Kleer, C.G.; Diehl, K.M.; Cimmino, V.M.; Pierce, L.; Hayes, D.; et al. Changes in surgical management resulting from case review at a breast cancer multidisciplinary tumor board. Cancer 2006, 107, 2346–2351. [Google Scholar] [CrossRef]
- Casiraghi, M.; Bertolaccini, L.; Sedda, G.; Petrella, F.; Galetta, D.; Guarize, J.; Maisonneuve, P.; De Marinis, F.; Spaggiari, L. Lung cancer surgery in oligometastatic patients: Outcome and survival. Eur. J. Cardiothorac. Surg. 2020, 57, 1173–1180. [Google Scholar] [CrossRef]
- Bini, A.; Grazia, M.; Petrella, F.; Chittolini, M. Multiple chondromatous hamartomas of the lung. Interact. Cardiovasc. Thorac. Surg. 2002, 1, 78–80. [Google Scholar] [CrossRef]
- Petrella, F.; Rizzo, S.; Radice, D.; Borri, A.; Galetta, D.; Gasparri, R.; Solli, P.; Veronesi, G.; Bellomi, M.; Spaggiari, L. Predicting prolonged air leak after standard pulmonary lobectomy: Computed tomography assessment and risk factors stratification. Surgeon 2011, 9, 72–77. [Google Scholar] [CrossRef]
- Petrella, F.; Chieco, P.; Solli, P.; Veronesi, G.; Borri, A.; Galetta, D.; Gasparri, R.; Spaggiari, L. Which factors affect pulmonary function after lung metastasectomy? Eur. J. Cardiothorac. Surg. 2009, 35, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, M.; Maisonneuve, P.; Piperno, G. Salvage Surgery after Definitive Chemoradiotherapy for Non–small Cell Lung Cancer. Semin. Thorac. Cardiovasc. Surg. 2017, 29, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Fanti, S.; Farsad, M.; Battista, G. Somatostatin Receptor Scintigraphy for Bronchial Carcinoid Follow-Up. Clin. Nucl. Med. 2003, 28, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, G.; Petrella, F.; Sandri, M.T.; Spaggiari, L.; Galetta, D.; Viale, G. A primary pure yolk sac tumor of the lung exhibiting CDX-2 immunoreactivity and increased serum levels of alkaline phosphatase intestinal isoenzyme. Int. J. Surg. Pathol. 2006, 14, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Klarenbeek, S.E.; Schuurbiers-Siebers, O.C.J.; van den Heuvel, M.M.; Prokop, M.; Tummers, M. Barriers and Facilitators for Implementation of a Computerized Clinical Decision Support System in Lung Cancer Multidisciplinary Team Meetings—A Qualitative Assessment. Biology 2020, 10, 9. [Google Scholar] [CrossRef]
- Shashi, K.K.; Madan, R.; Hammer, M.M.; van Hedent, S.; Byrne, S.C.; Schmidlin, E.J.; Mamon, H.; Hatabu, H.; Enzinger, P.C.; Gerbaudo, V.H. Contribution of FDG-PET/CT to the management of esophageal cancer patients at multidisciplinary tumor board conferences. Eur. J. Radiol. Open 2020, 7, 100291. [Google Scholar] [CrossRef]
- Crichi, B.; Sebuhyan, M.; Abdallah, N.A.; Montlahuc, C.; Bonnet, C.; Villiers, S.; Maignan, C.L.; Yannoutsos, A.; Farge, D. How to treat venous thromboembolism (TVE) in cancer patients: Ten years of multidisciplinary team meetings (MDTM) at Saint-Louis Hospital. J. Med. Vasc. 2020, 45, S24–S26. [Google Scholar]
- Graetz, D.E.; Chen, Y.; Devidas, M.; Antillon-Klussmann, F.; Fu, L.; Quintero, K.; Fuentes-Alabi, S.L.; Gassant, P.Y.; Kaye, E.C.; Baker, J.N. Interdisciplinary care of pediatric oncology patients in Central America and the Caribbean. Cancer 2020. [Google Scholar] [CrossRef]
- Karas, P.L.; Rankin, N.M.; Stone, E. Medicolegal Considerations in Multidisciplinary Cancer Care. JTO Clin. Res. Rep. 2020, 1, 100073. [Google Scholar] [CrossRef]
- Zhao, S.; Qi, W.; Chen, J. Role of a multidisciplinary team in administering radiotherapy for esophageal cancer. BMC Cancer 2020, 20, 974. [Google Scholar] [CrossRef]
- Dijkstra, S.; Kraal, K.C.J.M.; Ruijters, V.J.; Kremer, L.C.M.; Hoogerbrugge, P.M. Examining the Potential Relationship between Multidisciplinary Team Meetings and Patient Survival in Pediatric Oncology Settings: A Systematic Review. J. Pediatr. Hematol. Oncol. 2020, 102, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Warner, R.; Hoinville, L.; Pottle, E.; Taylor, C.; Green, J. Refocusing cancer multidisciplinary team meetings in the United Kingdom: Comparing urology with other specialties. Ann. R. Coll. Surg. Engl. 2021, 103, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Liam, C.K.; Liam, Y.S.; Poh, M.E.; Wong, C.K. Accuracy of lung cancer staging in the multidisciplinary team setting. Transl. Lung Cancer Res. 2020, 9, 1654–1666. [Google Scholar] [CrossRef] [PubMed]
- Pluyter, J.R.; Jacobs, I.; Langereis, S.; Cobben, D.; Williams, S.; Curfs, J.; van den Borne, B. Looking through the eyes of the multidisciplinary team: The design and clinical evaluation of a decision support system for lung cancer care. Transl. Lung Cancer Res. 2020, 9, 1422–1432. [Google Scholar] [CrossRef]
- Petrella, F.; Toffalorio, F.; Brizzola, S.; De Pas, T.M.; Rizzo, S.; Barberis, M.; Pelicci, P.; Spaggiari, L.; Acocella, F. Stem cell transplantation effectively occludes bronchopleural fistula in an animal model. Ann. Thorac. Surg. 2014, 97, 480–483. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).