Image-Guided Proton Therapy for Elderly Patients with Hepatocellular Carcinoma: High Local Control and Quality of Life Preservation
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patient and Treatment Characteristics
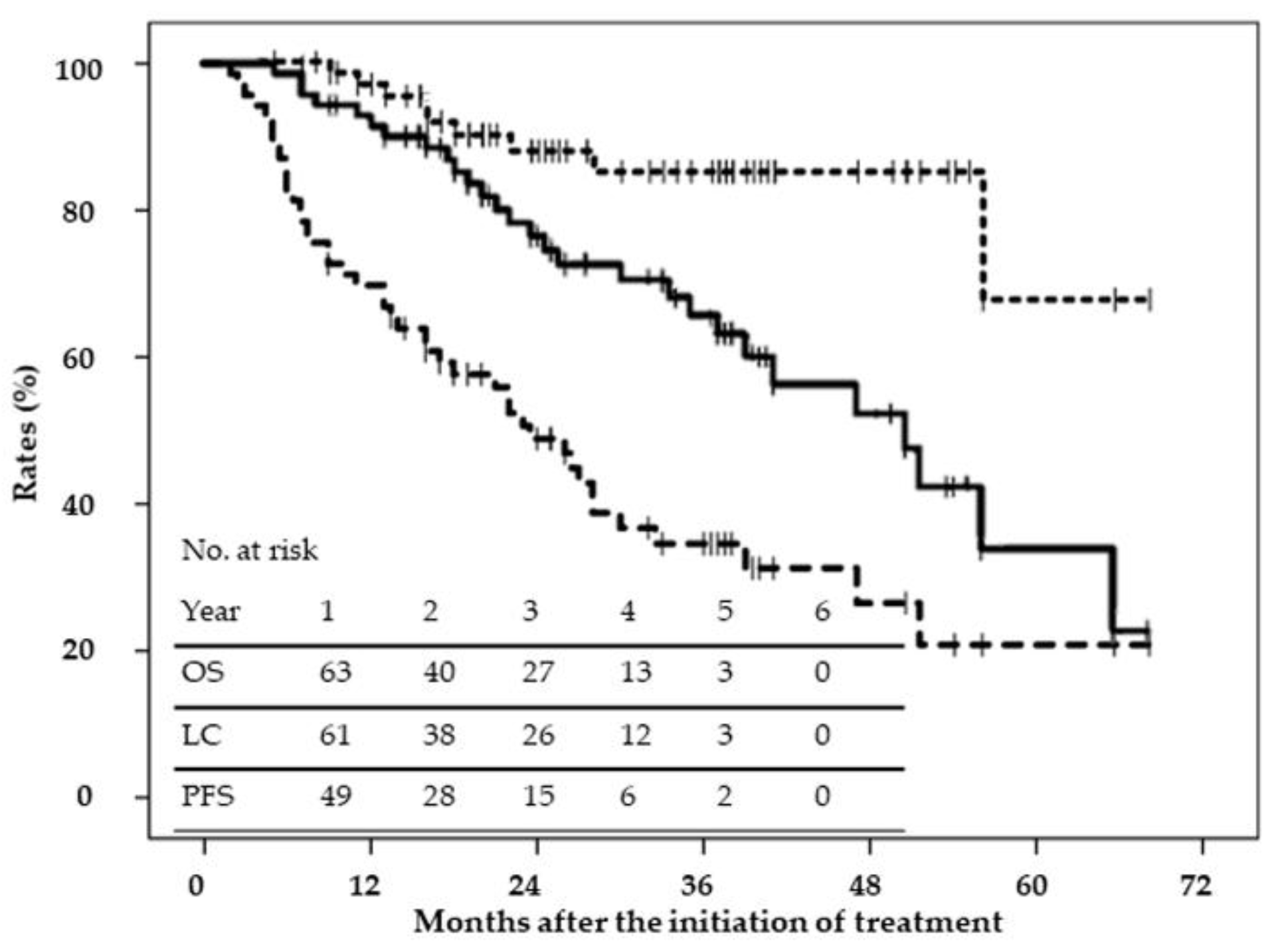
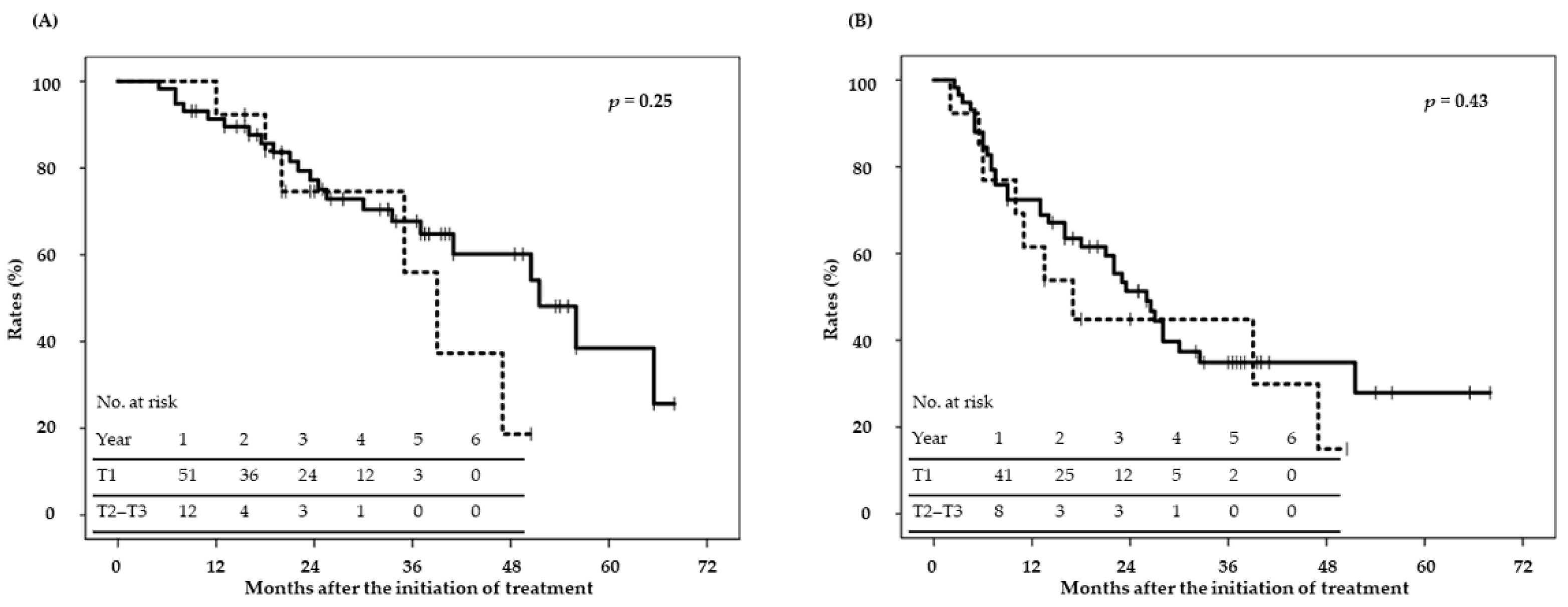
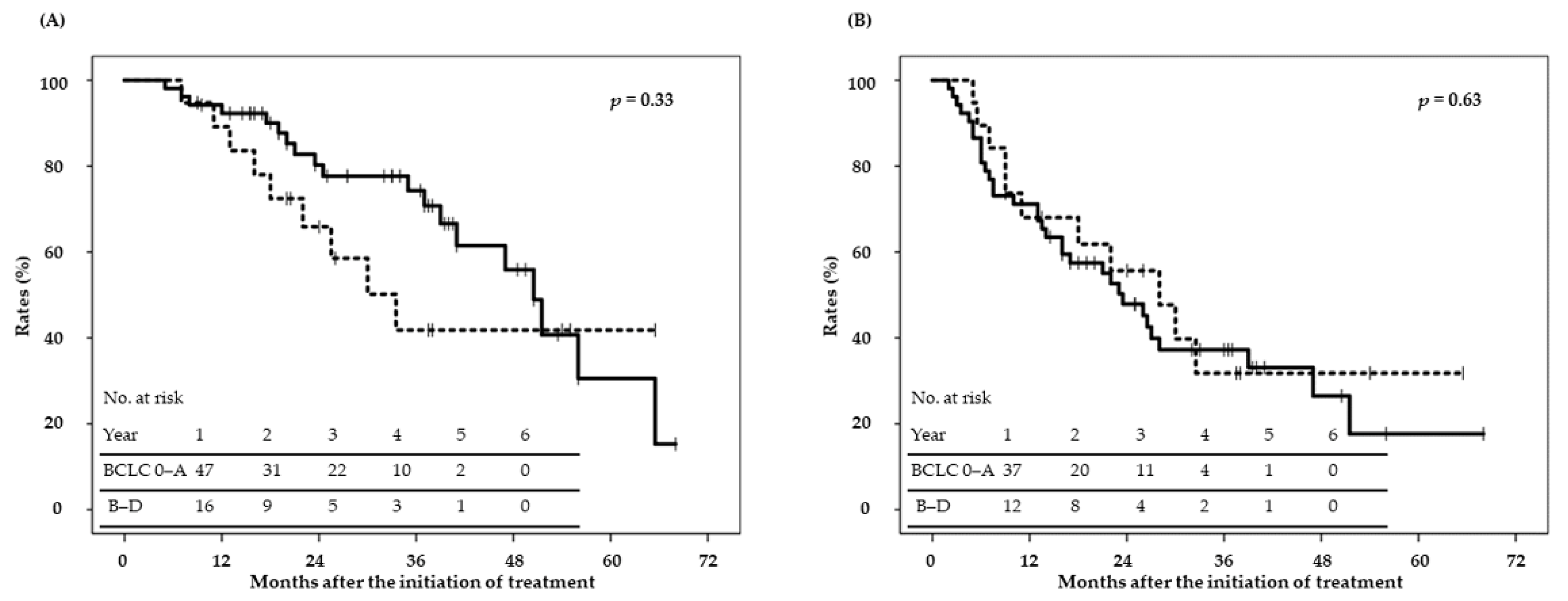
2.2. Disease Control and Survival
2.3. Complications and QOL Scores
3. Discussion
4. Materials and Methods
4.1. Study Design and Patient Eligibility
4.2. Treatment Protocols and Systems
4.3. Treatment Planning
4.4. Follow-Up Evaluation and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quaglia, A.; Tavilla, A.; Shack, L.; Brenner, H.; Janssen-Heijnenm, M.; Allemani, C.; Colonna, M.; Grande, E.; Grosclaude, P.; Vercelli, M.; et al. The cancer survival gap between elderly and middle-aged patients in Europe is widening. Eur. J. Cancer 2009, 45, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Dohmen, K.; Shirahama, M.; Shigematsu, H.; Irie, K.; Ishibashi, H. Optimal treatment strategy for elderly patients with hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2004, 19, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Campion, E.W. The oldest old. N. Engl. J. Med. 1994, 330, 1819–1820. [Google Scholar] [CrossRef] [PubMed]
- Weir, H.K.; Thompson, T.D.; Soman, A.; Møller, B.; Leadbetter, S. The past, present, and future of cancer incidence in the United States: 1975 through 2020. Cancer 2015, 121, 1827–1837. [Google Scholar] [CrossRef]
- Kaibori, M.; Yoshii, K.; Hasegawa, K.; Ogawa, A.; Kubo, S.; Tateishi, R.; Izumi, N.; Kadoya, M.; Kudo, M.; Kumada, T.; et al. Cancer Study Group of Japan. Treatment optimization for hepatocellular carcinoma in elderly patients in a Japanese nationwide cohort. Ann. Surg. 2019, 270, 121–130. [Google Scholar] [CrossRef]
- Shin, I.S.; Kim, D.G.; Cha, S.W.; Kang, S.H.; Kim, S.H.; Kim, M.Y.; Baik, S.K. Hepatocellular carcinoma in old age: Are there any benefits of liver resection in old age? Ann. Surg. Treat. Res. 2020, 99, 65–71. [Google Scholar] [CrossRef]
- Iida, H.; Kaibori, M.; Matsui, K.; Ishizaki, M.; Kon, M. Assessing the feasibility of clinicopathological features of hepatic resection for hepatocellular carcinoma in patients over 80 years of age. Mol. Clin. Oncol. 2017, 6, 29–38. [Google Scholar] [CrossRef]
- Khuri, S.F.; Henderson, W.G.; DePalma, R.G.; Mosca, C.; Healey, N.A.; Kumbhani, D.J. Participants in the VA National Surgical Quality Improvement Program. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann. Surg. 2005, 242, 326–343. [Google Scholar]
- Turrentine, F.E.; Wang, H.; Simpson, V.B.; Jones, R.S. Surgical risk factors, morbidity, and mortality in elderly patients. J. Am. Coll. Surg. 2006, 203, 865–877. [Google Scholar] [CrossRef]
- Sato, S.; Tanaka, K.; Nojiri, K.; Kumamoto, T.; Mori, R.; Taniguchi, K.; Matsuyama, R.; Takeda, K.; Ueda, M.; Akiyama, H.; et al. Hepatic resection for hepatocellular carcinoma in the elderly: Selecting hepatectomy procedures based on patient age. Anticancer Res. 2015, 35, 6855–6860. [Google Scholar]
- Makovski, T.T.; Le Coroller, G.; Putrik, P.; Choi, Y.H.; Zeegers, M.P.; Stranges, S.; Ruiz Castell, M.; Huiart, L.; van den Akker, M. Role of clinical, functional and social factors in the association between multimorbidity and quality of life: Findings from the Survey of Health, Ageing and Retirement in Europe (SHARE). PLoS ONE 2020, 15, e0240024. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, M.; Ikai, I.; Matsuyama, Y.; Yamaoka, Y.; Makuuchi, M. Staging of hepatocellular carcinoma: Assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann. Surg. 2007, 245, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Mo, F.; Hui, E.P.; Chan, S.L.; Koh, J.; Tang, N.L.S.; Yu, S.C.H.; Yeo, W. The association of liver function and quality of life of patients with liver cancer. BMC Gastroenterol. 2019, 19, 66. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.A.; Kim, H.I.; Chang, S.; An, J.; Lee, D.; Lee, H.C.; Han, S.; Shim, J.H. A prospective evaluation of the reliability and utility of quality of life measures in patients with hepatocellular carcinoma. Am. J. Clin. Oncol. 2019, 42, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Takeda, A.; Sanuki, N.; Ariyoshi, K.; Yamaguchi, T.; Imagumbai, T.; Katoh, N.; Eriguchi, T.; Oku, Y.; Ozawa, S.; et al. Multicenter prospective study of stereotactic body radiotherapy for previously untreated solitary primary hepatocellular carcinoma: The STRSPH study. Hepatol. Res. 2020, article in press. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.M.; Kim, S.Y.; Lim, Y.S.; Kim, K.M.; Shim, J.H.; Lee, D.; An, J.; Jung, J.; Kim, J.H.; Lee, H.C. Stereotactic body radiation therapy for small (≤5 cm) hepatocellular carcinoma not amenable to curative treatment: Results of a single-arm, phase II clinical trial. Clin. Mol. Hepatol. 2020, 26, 506–515. [Google Scholar] [CrossRef]
- Igaki, H.; Mizumoto, M.; Okumura, T.; Hasegawa, K.; Kokudo, N.; Sakurai, H. A systematic review of publications on charged particle therapy for hepatocellular carcinoma. Int. J. Clin. Oncol. 2018, 23, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Iwata, H.; Ogino, H.; Hattori, Y.; Hashimoto, S.; Nakajima, K.; Hayashi, K.; Toshito, T.; Sasaki, S.; Baba, F.; Kuwabara, Y.; et al. Phase II study of image-guided proton therapy for solitary primary hepatocellular carcinoma with indication for standard treatment. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, S132–S133. [Google Scholar] [CrossRef]
- Gambert, S.R.; Tsitouras, P.D.; Duthie, E.H., Jr. Interpretation of laboratory results in the elderly. 2. A clinician’s guide to endocrine tests. Postgrad. Med. 1982, 72, 251–256. [Google Scholar] [CrossRef]
- Schmucker, D.L. Aging and the liver: An update. J. Gerontol. A Biol. Sci. Med. Sci. 1998, 53, B315–B320. [Google Scholar] [CrossRef]
- Zoli, M.; Magalotti, D.; Bianchi, G.; Gueli, C.; Orlandini, C.; Grimaldi, M.; Marchesini, G. Total and functional hepatic blood flow decrease in parallel with ageing. Age Ageing 1999, 28, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.J.; Liang, X.; Ding, X.; Zhu, T.C.; Ben-Josef, E.; Plastaras, J.P.; Metz, J.M.; Both, S.; Apisarnthanarax, S. Clinical decision tool for optimal delivery of liver stereotactic body radiation therapy: Photons versus protons. Pr. Radiat. Oncol. 2015, 5, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.E.; Venkatesulu, B.P.; Lee, C.H.; Hung, S.P.; Wong, P.F.; Aithala, S.P.; Kim, B.K.; Rao, A.; Tung-Chieh Chang, J.; Tsang, N.M.; et al. Predictors of radiation-induced liver disease in eastern and western patients with hepatocellular carcinoma undergoing proton beam therapy. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 73–86. [Google Scholar] [CrossRef]
- Portolani, N.; Baiocchi, G.L.; Coniglio, A.; Tiberio, G.A.; Prestini, K.; Gheza, F.; Benetti, A.; Giulini, S.M. Limited liver resection: A good indication for the treatment of hepatocellular carcinoma in elderly patients. Jpn. J. Clin. Oncol. 2011, 41, 1358–1365. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Chen, Q.L.; Sun, L.L.; He, S.P.; Luo, X.F.; Huang, L.S.; Huang, J.H.; Xiong, C.M.; Zhong, C. Laparoscopic versus open major liver resection for hepatocellular carcinoma: Systematic review and meta-analysis of comparative cohort studies. BMC Cancer 2019, 19, 1047. [Google Scholar] [CrossRef]
- Nishikawa, H.; Osaki, Y.; Iguchi, E.; Takeda, H.; Ohara, Y.; Sakamoto, A.; Hatamaru, K.; Henmi, S.; Saito, S.; Nasu, A.; et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma: Clinical outcome and safety in elderly patients. J. GastroIntestin. Liver Dis. 2012, 21, 397–405. [Google Scholar]
- Imamura, H.; Takami, Y.; Ryu, T.; Wada, Y.; Sasaki, S.; Ureshino, H.; Saitsu, H. Feasibility and safety of surgical microwave ablation for hepatocellular carcinoma in elderly patients: A single center analysis in Japan. Sci. Rep. 2020, 10, 14215. [Google Scholar] [CrossRef]
- Teraoka, Y.; Kimura, T.; Aikata, H.; Daijo, K.; Osawa, M.; Honda, F.; Nakamura, Y.; Morio, K.; Morio, R.; Hatooka, M.; et al. Clinical outcomes of stereotactic body radiotherapy for elderly patients with hepatocellular carcinoma. Hepatol. Res. 2018, 48, 193–204. [Google Scholar] [CrossRef]
- Okamura, Y.; Takeda, S.; Fujii, T.; Sugimoto, H.; Nomoto, S.; Nakao, A. Prognostic significance of postoperative complications after hepatectomy for hepatocellular carcinoma. J. Surg. Oncol. 2011, 104, 814–821. [Google Scholar] [CrossRef]
- Chen, Y.L.; Lin, H.C.; Lin, K.H.; Lin, L.S.; Hsieh, C.E.; Ko, C.J.; Hung, Y.J.; Lin, P.Y. Low hemoglobin level is associated with the development of delirium after hepatectomy for hepatocellular carcinoma patients. PLoS ONE 2015, 10, e0119199. [Google Scholar] [CrossRef]
- Janssen, T.L.; Steyerberg, E.W.; van Hoof-de Lepper, C.C.H.A.; Seerden, T.C.J.; de Lange, D.C.; Wijsman, J.H.; Ho, G.H.; Gobardhan, P.D.; van der Laan, L. Long-term outcomes of major abdominal surgery and postoperative delirium after multimodal prehabilitation of older patients. Surg. Today 2020, 50, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.W.C.; Chan, A.L.F. Cost-utility of stereotactic radiation therapy versus proton beam therapy for inoperable advanced hepatocellular carcinoma. Oncotarget 2017, 8, 75568–75576. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, S.; Suzukamo, Y. Manual of SF-36v2 Japanese Version, 4th ed.; iHope International Inc.: Kyoto, Japan, 2019. [Google Scholar]
- Chiu, C.C.; Lee, K.T.; Wang, J.J.; Sun, D.P.; Lee, H.H.; Shi, H.Y. Health-related quality of life before and after surgical resection of hepatocellular carcinoma: A prospective study. Asian Pac. J. Cancer Prev. 2018, 19, 65–72. [Google Scholar]
- Hiraoka, A.; Otsuka, Y.; Kawasaki, H.; Izumoto, H.; Ueki, H.; Kitahata, S.; Aibiki, T.; Okudaira, T.; Yamago, H.; Miyamoto, Y.; et al. Impact of muscle volume and muscle function decline in patients undergoing surgical resection for hepatocellular carcinoma. J. Gastroenterol. Hepatol 2018, 33, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, M.; Gow, P.J.; Grossmann, M.; Angus, P.W. Review article: Sarcopenia in cirrhosis--aetiology, implications and potential therapeutic interventions. Aliment. Pharm. Ther. 2016, 43, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.; Dawson, L.A.; Jiang, H.; Kim, J.; Dinniwell, R.; Brierley, J.; Wong, R.; Lockwood, G.; Ringash, J. Prospective longitudinal assessment of quality of life for liver cancer patients treated with stereotactic body radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.J.; Chen, C.H.; Yao, G.; Chung, C.W.; Sheu, J.C.; Lee, P.H.; Tsai, Y.J.; Wang, J.D. Quality of life in patients with hepatocellular carcinoma received surgical resection. J. Surg. Oncol. 2007, 95, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Chie, W.C.; Yu, F.; Li, M.; Baccaglini, L.; Blazeby, J.M.; Hsiao, C.F.; Chiu, H.C.; Poon, R.T.; Mikoshiba, N.; Al-Kadhimi, G.; et al. Quality of life changes in patients undergoing treatment for hepatocellular carcinoma. Qual. Life Res. 2015, 24, 2499–2506. [Google Scholar] [CrossRef]
- Iwata, H.; Ogino, H.; Hashimoto, S.; Yamada, M.; Shibata, H.; Yasui, K.; Toshito, T.; Omachi, C.; Tatekawa, K.; Manabe, Y.; et al. Spot scanning and passive scattering proton therapy: Relative biological effectiveness and oxygen enhancement ratio in cultured cells. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 95–102. [Google Scholar] [CrossRef]
- Toshito, T.; Omachi, C.; Kibe, Y.; Sugai, H.; Hayashi, K.; Shibata, H.; Yasui, K.; Tanaka, K.; Yamamoto, T.; Yoshida, A.; et al. A proton therapy system in Nagoya Proton Therapy Center. Australas Phys. Eng. Sci. Med. 2016, 39, 645–654. [Google Scholar] [CrossRef]
- Nakajima, K.; Iwata, H.; Ogino, H.; Hattori, Y.; Hashimoto, S.; Toshito, T.; Hayashi, K.; Akita, K.; Baba, F.; Nakamae, K.; et al. Clinical outcomes of image-guided proton therapy for histologically confirmed stage I non-small cell lung cancer. Radiat. Oncol. 2018, 13, 199. [Google Scholar] [CrossRef] [PubMed]
- Ohta, K.; Shimohira, M.; Murai, T.; Nishimura, J.; Iwata, H.; Ogino, H.; Hashizume, T.; Shibamoto, Y. Percutaneous fiducial marker placement prior to stereotactic body radiotherapy for malignant liver tumors: An initial experience. J. Radiat. Res. 2016, 57, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Moyers, M.F.; Miller, D.W.; Bush, D.A.; Slater, J.D. Methodologies and tools for proton beam design for lung tumors. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 1429–1438. [Google Scholar] [CrossRef]
- Marks, L.B.; Yorke, E.D.; Jackson, A.; Ten Haken, R.K.; Constine, L.S.; Eisbruch, A.; Bentzen, S.M.; Nam, J.; Deasy, J.O. Use of normal tissue complication probability models in the clinic. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S10–S19. [Google Scholar] [CrossRef]
- Urata, K.; Kawasaki, S.; Matsunami, H.; Hashikura, Y.; Ikegami, T.; Ishizone, S.; Momose, Y.; Komiyama, A.; Makuuchi, M. Calculation of child and adult standard liver volume for liver transplantation. Hepatology 1995, 21, 1317–1321. [Google Scholar] [CrossRef]
- Shibamoto, Y.; Miyakawa, A.; Otsuka, S.; Iwata, H. Radiobiology of hypofractionated stereotactic radiotherapy: What are the optimal fractionation schedules? J. Radiat. Res. 2016, S57, i76–i82. [Google Scholar] [CrossRef]
| Variable | Level | Total [%] |
|---|---|---|
| Number | N | 71 |
| Age | Median (range) | 82 (80–96) |
| Sex | Male/Female | 43 (61)/28 (39) |
| Performance status | 0/1/2/3 | 44 (62)/20 (28)/4 (6)/3 (4) |
| Major underlying liver disease | HBV/HCV/Alcoholic/NAFLD | 7 (10)/28 (39)/5 (7)/31 (44) |
| Treatment options | Operable/RFA indicated/TACE indicated | 27 (38)/12 (17)/46 (65) |
| Treatment history | −(primary tumor)/+(recurrence or IM) | 46 (65)/25 (35) |
| Longest tumor diameter (mm) | Median (range) | 32 (8–111) |
| Tumor volume (cc) | Median (range) | 25.6 (1.2–602.5) |
| Child–Pugh classification | A5/A6/B7-9 | 49 (69)/15 (21)/7 (10) |
| TNM (UICC 8th) | T1N0M0/T2N0M0/T3bN0M0 | 58 (82)/11 (15)/2 (3) |
| BCLC staging system | 0/A/B/C/D | 7 (10)/45 (63)/1 (2)/15 (21)/3 (4) |
| Okuda staging system | I/II | 62 (87)/9 (13) |
| Liver damage 1 | A/B/C | 47 (66)/22 (31)/2 (3) |
| 99mTc-GSA hepatic scintigraphy | HH15/LHL15 Median (range) | 0.60 (0.48––0.85)/0.88 (0.69–0.94) |
| ICGR15 (%) | Median (range) | 18.3 (5.0–47.4) |
| K-ICG (%) | Median (range) | 0.12 (0.01–0.20) |
| ALBI grade | 1/2–3 | 35 (49)/36 (51) |
| FIB-4 index | Median (range) | 4.3 (1.6–15.2) |
| Serum hyaluronate level (ng/mL) | Median (range) | 101 (5–1920) |
| Serum type IV collagen (ng/mL) | Median (range) | 5.5 (2.9–495.0) |
| Alpha fetoprotein (ng/mL) | Median (range) | 6.8 (1.6–55149.6) |
| Alpha fetoprotein-L3 (%) | Median (range) | 1.4 (<0.5–96.3) |
| PIVKA-II (mAU/mL) | Median (range) | 57 (8–91900) |
| Variable | Level | Total [%] |
|---|---|---|
| Dose fractionation (peripheral/central) | 66 GyRBE/10 Fr/72.6 GyRBE/22 Fr | 47 (66)/24 (34) |
| Number of beam portals | 2/3/4 | 8 (11)/61 (86)/2 (3) |
| Vicinity to the gastrointestinal tract | ≤1 cm | 25 (31) |
| PTV D98 (%) | Median (interquartile range) | 90.5 (65.0–97.4) |
| PTV D95 (%) | Median (interquartile range) | 95.9 (74.1–98.5) |
| Homogeneity index | Median (interquartile range) | 0.11 (0.05–0.36) |
| Conformity index | Median (interquartile range) | 1.26 (0.97–1.42) |
| Mean liver dose (Liver-GTV) (GyRBE) | Median (range) | 12.3 (2.0–21.6) |
| Liver-GTV V25/V30 (peripheral) 1 (%) | Median | 15.6/14.0 |
| Liver-GTV V32/V38 (central) 2 (%) | Median | 21.6/19.4 |
| V < 1 GyRBE remnant liver volume | Standard liver volume ≥ 35% | 71 (100)/0 (0) |
| Skin V80%/V50% (cc) | Median (range) | 0 (0–22.8)/2.9 (0–53.7) |
| Colon D1cc/D10cc (GyRBE) | Median (range) | 1.0 (0–53.5)/0 (0–47.9) |
| Stomach D1cc/D10cc (GyRBE) | Median (range) | 0 (0–50.8)/0 (0–40.9) |
| Duodenum D1cc/D10cc (GyRBE) | Median (range) | 0 (0–52.6)/0 (0–27.3) |
| Factor | N of Data | N of event (%) | Level | Univariate HR (95% CI) | p | Multivariate HR (95% CI) | p |
|---|---|---|---|---|---|---|---|
| Age | 71 | 28 (39) | ≥ vs. <82 years old 1 | 0.75 (0.29, 1.97) | 0.57 | ||
| Sex | 71 | 28 (39) | Male vs. Female | 2.86 (1.01, 8.13) | 0.048 | 4.38 (1.02, 18.9) | 0.047 |
| Performance status | 71 | 28 (39) | ≥1 vs. 0 | 5.10 (1.81, 14.4) | 0.002 | 7.66 (1.57, 37.3) | 0.012 |
| Major underlying liver disease | 71 | 28 (39) | NAFLD vs. Others | 0.74 (0.28, 1.96) | 0.55 | ||
| Treatment history | 71 | 28 (39) | +(recurrence or IM) vs.−(primary tumor) | 2.91 (1.06, 7.98) | 0.038 | 5.44 (0.95, 31.1) | 0.057 |
| Longest tumor diameter | 71 | 28 (39) | ≥ vs. <32 mm 1 | 0.87 (0.34, 2.23) | 0.77 | ||
| Tumor volume | 71 | 28 (39) | ≥ vs. <25.6 cc 1 | 1.05 (0.40, 2.71) | 0.92 | ||
| Child–Pugh score | 71 | 28 (39) | A6, B7 vs. A5 | 3.27 (1.15, 9.31) | 0.026 | 2.02 (0.48, 8.42) | 0.34 |
| TNM (UICC8th) | 71 | 28 (39) | ≥T2 vs. T1 | 1.40 (0.42, 4.72) | 0.58 | ||
| Okuda staging | 71 | 28 (39) | II vs. I | 2.12 (0.52, 8.70) | 0.30 | ||
| BCLC staging | 71 | 28 (39) | B–D vs. 0, A | 1.56 (0.54, 4.53) | 0.41 | ||
| Liver damage | 71 | 28 (39) | B vs. A | 2.52 (0.92, 6.93) | 0.073 | ||
| HH15 | 71 | 28 (39) | >0.55 vs. ≤0.55 2 | 1.26 (0.41, 3.91) | 0.69 | ||
| LHL15 | 71 | 28 (39) | <0.92 vs. ≥0.92 2 | 1.62 (0.38, 6.88) | 0.51 | ||
| ICGR15 | 71 | 28 (39) | ≥10 vs. <10 % 2 | 1.71 (0.31, 9.50) | 0.54 | ||
| K-ICG | 71 | 28 (39) | <0.15 vs. ≥0.15, ≤0.22 % | 1.62 (0.38, 6.88) | 0.58 | ||
| ALBI grade | 71 | 28 (39) | 2, 3 vs. 1 | 1.53 (0.59, 4.00) | 0.38 | ||
| FIB-4 index | 71 | 28 (39) | ≥2.67 vs. <2.67 | 0.86 (0.18, 4.15) | 0.85 | ||
| Treatment options | 71 | 28 (39) | Inoperable vs. operable | 6.90 (2.05, 23.3) | 0.002 | 2.03 (0.43, 9.59) | 0.37 |
| 71 | 28 (39) | RFA untreatable vs. treatable | 0.89 (0.25, 3.16) | 0.86 | |||
| 71 | 28 (39) | TACE untreatable vs. treatable | 2.24 (0.83, 6.07) | 0.11 | |||
| Dose fractionation | 71 | 28 (39) | 72.6 GyRBE/22 Fr vs. 66 GyRBE/10 Fr | 1.49 (0.55, 4.06) | 0.43 | ||
| Vicinity to the gastrointestinal tract | 71 | 28 (39) | ≤ vs. >1 cm | 1.28 (0.47, 3.52) | 0.63 | ||
| Alpha fetoprotein | 71 | 28 (39) | >20 vs. ≤20 ng/mL 2 | 3.36 (1.22, 9.22) | 0.019 | 2.05 (0.43, 9.73) | 0.37 |
| PIVKA-II | 71 | 28 (39) | >40 vs. ≤40 mAU/mL 2 | 2.88 (1.04, 7.94) | 0.042 | 3.55 (0.81, 15.5) | 0.092 |
| Local control | 71 | 28 (39) | Failure vs. control | 3.64 (0.83. 16.0) | 0.09 |
| Factor | N of Data | N of Event (%) | Level | Univariate HR (95% CI) | P | Multivariate HR (95% CI) | P |
|---|---|---|---|---|---|---|---|
| Age | 71 | 9 (13) | ≥ vs. <82 years old 1 | 2.88 (0.55, 15.0) | 0.21 | ||
| Sex | 71 | 9 (13) | Male vs. Female | 0.74 (0.17, 3.24) | 0.69 | ||
| Performance status | 71 | 9 (13) | ≥1 vs. 0 | 0.26 (0.06, 1.13) | 0.07 | ||
| Major underlying liver disease | 71 | 9 (13) | NAFLD vs. Others | 0.96 (0.24, 3.94) | 0.96 | ||
| Treatment history | 71 | 9 (13) | +(recurrence or IM) vs.-(primary tumor) | 1.04 (0.25, 4.24) | 0.96 | ||
| Longest tumor diameter | 71 | 9 (13) | ≥ vs. <32 mm1 | 9.10 (1.07, 77.2) | 0.043 | 1.10 (0.02, 73.5) | 0.96 |
| Tumor volume | 71 | 9 (13) | ≥ vs. <25.6 cc 1 | 10.4 (1.22, 88.0) | 0.032 | 0.11 (0.002, 6.94) | 0.30 |
| Child–Pugh score | 71 | 9 (13) | A6, B7 vs. A5 | 0.24 (0.03, 2.08) | 0.20 | ||
| TNM (UICC8th) | 71 | 9 (13) | ≥T2 vs. T1 | 0.52 (0.06, 4.57) | 0.57 | ||
| Okuda staging | 71 | 9 (13) | II vs. I | 0.84 (0.09, 7.67) | 0.88 | ||
| BCLC staging | 71 | 9 (13) | B–D vs. 0, A | 2.51 (0.60, 10.6) | 0.21 | ||
| Liver damage | 71 | 9 (13) | B vs. A | 0.21 (0.03, 1.81) | 0.16 | ||
| HH15 | 71 | 9 (13) | >0.55 vs. ≤0.55 2 | 0.33 (0.08, 1.41) | 0.14 | ||
| LHL15 | 71 | 9 (13) | <0.92 vs. ≥0.92 2 | 1.36 (0.15, 12.2) | 0.78 | ||
| ICGR15 | 71 | 9 (13) | ≥10 vs. <10 %2 | 0.86 (0.09, 8.08) | 0.89 | ||
| K-ICG | 71 | 9 (13) | <0.15 vs. ≥0.15, ≤0.22% | 1.36 (0.15, 12.2) | 0.78 | ||
| ALBI grade | 71 | 9 (13) | 2, 3 vs. 1 | 0.44 (0.10, 1.92) | 0.27 | ||
| FIB-4 index | 71 | 9 (13) | ≥2.67 vs. <2.67 | 0.86 (0.09, 8.08) | 0.89 | ||
| Treatment options | 71 | 9 (13) | Inoperable vs. operable | 2.37 (0.45, 12.3) | 0.31 | ||
| 71 | 9 (13) | RFA untreatable vs. treatable | - 3 | - 3 | |||
| 71 | 9 (13) | TACE untreatable vs. treatable | 1.56 (0.38, 6.44) | 0.54 | |||
| Dose fractionation | 71 | 9 (13) | 72.6 GyRBE/22 Fr vs. 66 GyRBE/10 Fr | 2.83 (0.68, 11.72) | 0.15 | ||
| Vicinity to the gastrointestinal tract | 71 | 9 (13) | ≤ vs. >1 cm | 5.29 (1.19, 23.6) | 0.029 | 0.25 (0.05, 1.22) | 0.08 |
| Alpha fetoprotein | 71 | 9 (13) | > 20 vs. ≤20 ng/mL 2 | 2.34 (0.59, 10.1) | 0.22 | ||
| PIVKA-II | 71 | 9 (13) | > 40 vs. ≤40 mAU/mL 2 | 3.08 (0.59, 16.0) | 0.18 |
| Adverse Event | BCLC 0–A 52 Patients (Grade 2/3) | BCLC B–D 19 Patients (Grade 2/3) | Total Number (Grade 2/3) |
|---|---|---|---|
| Acute toxicity | |||
| Hepatobiliary enzyme | 0/0 | 1/0 | 1/0 |
| Cytopenia | 0/0 | 0/0 | 0/0 |
| Dermatitis | 2/0 | 2/0 | 4/0 |
| Decrease in Child–Pugh score ≥ 2 | 0 | 0 | 0 |
| Radiation pneumonitis1 | 0/0 | 0/0 | 0/0 |
| Late toxicity | |||
| Hepatobiliary enzyme | 0/0 | 0/0 | 0/0 |
| Cytopenia | 0/0 | 0/0 | 0/0 |
| Dermatitis | 0/0 | 0/1 | 0/1 |
| Decrease in Child–Pugh score ≥ 2 | 1 2 | 3 2 | 4 2 |
| Radiation pneumonitis 1 | 0/0 | 0/0 | 0/0 |
| Soft-tissue inflammation | 1/0 | 0/0 | 1/0 |
| Rib fracture | 1/0 | 0/0 | 1/0 |
| Variables | Pre-IGPT (N = 71) | 6 Months (N = 58) | p1 | 12 Months (N = 46) | p1 | p2 |
|---|---|---|---|---|---|---|
| EORTC QLQ-C30 | ||||||
| Global health status/QOL | 51.4 (20.5) | 52.0 (22.0) | 0.91, 0.71 | 55.4 (22.2) | 0.41, 0.12 | 0.69, 0.19 |
| Functional scales | ||||||
| Physical functioning | 65.4 (24.6) | 66.1 (26.7) | 0.75, 0.78 | 62.6 (28.7) | 0.81, 0.37 | 0.86, 0.66 |
| Role functioning | 72.3 (31.9) | 66.5 (32.9) | 0.18, 0.49 | 62.8 (34.5) | 0.15, 0.78 | 0.25, 0.53 |
| Emotional functioning | 70.4 (23.5) | 75.0 (21.8) | 0.21, 0.53 | 75.2 (20.1) | 0.33, 0.26 | 0.40, 0.37 |
| Cognitive functioning | 68.8 (23.0) | 72.8 (20.3) | 0.44, 0.22 | 65.5 (25.8) | 0.59, 0.35 | 0.48, 0.54 |
| Social functioning | 76.5 (25.9) | 77.5 (25.0) | 0.89, 0.99 | 76.7 (28.7) | 0.69, 0.08 | 0.93, 0.21 |
| Symptom scales/items | ||||||
| Fatigue | 44.4 (25.1) | 45.0 (24.3) | 0.77, 0.53 | 43.7 (25.0) | 0.93, 0.22 | 0.96, 0.28 |
| Nausea and vomiting | 3.3 (9.2) | 4.1 (9.6) | 0.46, 0.78 | 5.8 (10.2) | 0.08, 0.95 | 0.21, 0.96 |
| Pain | 26.1 (27.7) | 34.5 (33.5) | 0.20, 0.83 | 30.2 (29.4) | 0.45, 0.45 | 0.42, 0.75 |
| Dyspnea | 29.1 (26.4) | 33.3 (25.2) | 0.26, 0.23 | 33.3 (26.2) | 0.31, 0.99 | 0.44, 0.38 |
| Insomnia | 27.2 (27.2) | 28.1 (30.7) | 0.93, 0.90 | 30.2 (33.2) | 0.89, 0.99 | 0.98, 0.95 |
| Appetite loss | 23.0 (23.6) | 25.1 (26.9) | 0.78, 0.33 | 24.0 (29.4) | 0.79, 0.43 | 0.90, 0.64 |
| Constipation | 29.6 (34.1) | 28.7 (29.8) | 0.86, 0.95 | 26.4 (27.8) | 0.90, 0.76 | 0.96, 0.97 |
| Diarrhea | 13.1 (22.2) | 12.3 (24.1) | 0.55, 0.88 | 8.5 (14.7) | 0.38, 0.26 | 0.65, 0.30 |
| Financial difficulties | 19.2 (26.2) | 13.5 (21.7) | 0.20, 0.24 | 17.1 (25.6) | 0.62, 0.20 | 0.44, 0.19 |
| EORTC QLQ-HCC18 | ||||||
| Symptom scale/items | ||||||
| Fatigue | 34.1 (23.7) | 40.7 (28.3) | 0.24, 0.59 | 41.3 (27.4) | 0.21, 0.52 | 0.35, 0.85 |
| Body image | 31.2 (23.2) | 34.5 (26.5) | 0.55, 0.99 | 32.6 (20.2) | 0.64, 0.76 | 0.81, 0.78 |
| Jaundice | 15.0 (17.6) | 18.4 (18.0) | 0.25, 0.38 | 15.9 (17.4) | 0.83, 0.95 | 0.51, 0.80 |
| Nutrition | 17.7 (16.1) | 18.8 (14.3) | 0.48, 0.95 | 19.4 (13.7) | 0.36, 0.68 | 0.61, 0.67 |
| Pain | 14.6 (17.6) | 20.2 (22.2) | 0.14, 0.53 | 19.4 (19.9) | 0.14, 0.85 | 0.22, 0.51 |
| Fever | 5.4 (11.5) | 10.8 (18.0) | 0.04, 0.27 | 10.5 (19.9) | 0.16, 0.97 | 0.10, 0.65 |
| Single items | ||||||
| Abdominal swelling | 23.9 (28.8) | 25.7 (32.1) | 0.94, 0.57 | 24.0 (24.5) | 0.66, 0.43 | 0.91, 0.55 |
| Sex life | 84.0 (33.7) | 87.1 (30.0) | 0.74, 0.73 | 84.5 (35.1) | 0.81, 0.61 | 0.94, 0.74 |
| SF-36 | ||||||
| Physical functioning | 60.0 (26.3) | 59.6 (27.7) | 0.98, 0.88 | 55.9 (29.5) | 0.55, 0.63 | 0.80, 0.75 |
| Role physical | 60.7 (31.4) | 60.3 (33.2) | 0.94, 0.85 | 53.5 (35.8) | 0.33, 0.30 | 0.59, 0.57 |
| Bodily pain | 64.4 (25.9) | 65.1 (29.8) | 0.70, 0.75 | 64.4 (32.0) | 0.91, 0.58 | 0.94, 0.85 |
| General health | 46.4 (19.2) | 51.4 (20.4) | 0.22, 0.98 | 53.9 (21.2) | 0.07, 0.63 | 0.17, 0.87 |
| Vitality | 50.3 (24.6) | 53.3 (22.8) | 0.52, 0.80 | 50.9 (26.3) | 0.82, 0.51 | 0.82, 0.55 |
| Social functioning | 69.5 (29.1) | 70.8 (28.9) | 0.77, 0.78 | 68.3 (30.4) | 0.84, 0.76 | 0.91, 0.88 |
| Role emotional | 62.3 (34.4) | 66.1 (33.1) | 0.55, 0.90 | 57.4 (37.3) | 0.57, 0.38 | 0.53, 0.64 |
| Mental health | 60.1 (24.1) | 64.0 (21.0) | 0.46, 0.80 | 63.8 (20.9) | 0.52, 0.71 | 0.71, 0.96 |
| 3-Physical CS 3 | 37.8 (12.6) | 37.5 (12.9) | 0.81, 0.93 | 36.8 (13.4) | 0.82, 0.71 | 0.96, 0.89 |
| 3-Mental CS 3 | 51.7 (9.6) | 54.0 (9.4) | 0.20, 0.93 | 55.7 (8.6) | 0.03, 0.96 | 0.10, 0.94 |
| 3-Role-social CS 3 | 42.0 (13.6) | 42.1 (14.7) | 0.98, 0.88 | 37.9 (15.1) | 0.14, 0.27 | 0.28, 0.49 |
| 2-Physical CS 3 | 34.9 (15.1) | 34.6 (16.1) | 0.93, 0.95 | 31.0 (16.5) | 0.24, 0.34 | 0.46, 0.56 |
| 2-Mental CS 3 | 50.2 (10.0) | 52.4 (9.5) | 0.21, 0.75 | 53.3 (8.8) | 0.13, 0.84 | 0.24, 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwata, H.; Ogino, H.; Hattori, Y.; Nakajima, K.; Nomura, K.; Hayashi, K.; Toshito, T.; Sasaki, S.; Hashimoto, S.; Mizoe, J.-e.; et al. Image-Guided Proton Therapy for Elderly Patients with Hepatocellular Carcinoma: High Local Control and Quality of Life Preservation. Cancers 2021, 13, 219. https://doi.org/10.3390/cancers13020219
Iwata H, Ogino H, Hattori Y, Nakajima K, Nomura K, Hayashi K, Toshito T, Sasaki S, Hashimoto S, Mizoe J-e, et al. Image-Guided Proton Therapy for Elderly Patients with Hepatocellular Carcinoma: High Local Control and Quality of Life Preservation. Cancers. 2021; 13(2):219. https://doi.org/10.3390/cancers13020219
Chicago/Turabian StyleIwata, Hiromitsu, Hiroyuki Ogino, Yukiko Hattori, Koichiro Nakajima, Kento Nomura, Kensuke Hayashi, Toshiyuki Toshito, Shigeru Sasaki, Shingo Hashimoto, Jun-etsu Mizoe, and et al. 2021. "Image-Guided Proton Therapy for Elderly Patients with Hepatocellular Carcinoma: High Local Control and Quality of Life Preservation" Cancers 13, no. 2: 219. https://doi.org/10.3390/cancers13020219
APA StyleIwata, H., Ogino, H., Hattori, Y., Nakajima, K., Nomura, K., Hayashi, K., Toshito, T., Sasaki, S., Hashimoto, S., Mizoe, J.-e., & Shibamoto, Y. (2021). Image-Guided Proton Therapy for Elderly Patients with Hepatocellular Carcinoma: High Local Control and Quality of Life Preservation. Cancers, 13(2), 219. https://doi.org/10.3390/cancers13020219





