Simple Summary
Favorable survival outcomes for patients with advanced-stage ovarian cancer are associated with complete cytoreduction. Enlarged cardiophrenic lymph node (CPLN) is commonly observed in advanced-stage epithelial ovarian cancer (AEOC); however, the prognostic impact of CPLN adenopathy is inconclusive. In this study, we evaluate the clinical outcomes of CPLN adenopathy in AEOC patients who underwent cytoreductive surgery. This systematic review and meta-analysis demonstrated that enlarged CPLN in preoperative imaging is highly associated with metastatic involvement. Patients with CPLN adenopathy had a significantly increased risk of recurrence of disease and dying from the disease in comparison to those without adenopathy, a finding likely related to more advanced disease in this group. Currently, there are no data that definitively demonstrate a therapeutic benefit of CPLN resection. Further randomized controlled trials should be conducted to definitively demonstrate whether CPLN resection at the time of cytoreductive surgery is beneficial.
Abstract
Purpose: To evaluate the clinical outcomes of enlarged cardiophrenic lymph node (CPLN) in advanced-stage epithelial ovarian cancer (AEOC) patients who underwent cytoreductive surgery. Methods: The Embase, Medline, Web of Science, Cochrane Library, and Google Scholar databases were searched for articles from the database inception to June 2021. Meta-analysis was conducted to determine the prognostic impact of surgical outcome, postoperative complication, and survival using random-effects models. Results: A total of 15 studies involving 727 patients with CPLN adenopathy and 981 patients without CPLN adenopathy were included. The mean size of preoperative CPLN was 9.1± 3.75 mm. Overall, 82 percent of the resected CPLN were histologically confirmed pathologic nodes. Surgical outcomes and perioperative complications did not differ between both groups. The median OS time was 42.7 months (95% CI 10.8–74.6) vs. 47.3 months (95% CI 23.2–71.2), in patients with and without CPLN adenopathy, respectively. At 5 years, patients with CPLN adenopathy had a significantly increased risk of disease recurrence (HR 2.14, 95% CI 1.82–2.52, p < 0.001) and dying from the disease (HR 1.74, 95% CI 1.06–2.86, p = 0.029), compared with those without CPLN adenopathy. CPLN adenopathy was significantly associated with ascites (OR 3.30, 95% CI 1.90–5.72, p < 0.001), pleural metastasis (OR 2.58, 95% CI 1.37–4.82, p = 0.003), abdominal adenopathy (OR 2.30, 95% CI 1.53–3.46, p < 0.001) and extra-abdominal metastasis (OR 2.30, 95% CI 1.61–6.67, p = 0.001). Conclusions: Enlarged CPLN in preoperative imaging is highly associated with metastatic involvement. Patients with CPLN adenopathy had a lower survival rate, compared with patients without CPLN adenopathy. Further randomized controlled trials should be conducted to definitively demonstrate whether CPLN resection at the time of cytoreductive surgery is beneficial.
1. Introduction
Ovarian cancer is the second leading cause of death among gynecological malignancies [1]. According to the International Federation of Gynecology and Obstetrics (FIGO), the majority of women with epithelial ovarian cancer (EOC) patients are initially diagnosed with advanced stage III and IV disease [2,3]. The current standard management for patients with EOC includes surgical cytoreduction in combination with adjuvant platinum-based chemotherapy. An independent prognostic factor in EOC is the amount of residual disease following cytoreductive surgery [4,5]; the absence of residual disease is associated with the most favorable survival outcomes [5,6].
In 2014, FIGO published a new classification defining retroperitoneal lymph node metastasis between the inguinal ligament and the diaphragm as regional nodes, categorized as FIGO stage IIIA [7]. Over a decade, extensive pelvic and paraaortic systematic lymphadenectomy procedures have been performed with the intention of removing potentially metastatic retroperitoneal lymph nodes. However, the randomized lymphadenectomy in patients with advanced ovarian neoplasm (LION) trial showed no benefit of systematic lymph node dissection in patients with radiologically unsuspicious nodes [8].
Enlargement of cardiophrenic lymph nodes (CPLNs), also referred to paracardiac or supradiaphragmatic lymphadenopathy, is one of the most common manifestations of extra-abdominal disease in patients with FIGO stage IV [9]. CPLNs are located in fatty tissue at the basal portion of the mediastinum. Their afferent lymphatics arise from the pericardium, anterior thoracoabdominal wall, pleura, and diaphragm [9]. Current guidelines for ovarian cancer staging issued by the female imaging subcommittee of the European Society of Urogenital Radiology (ESUR) 2010 state that CPLN larger than 5 mm in short-axis diameter, seen on a computed tomography (CT) scan or magnetic resonance imaging (MRI) scan, should alert the clinician to metastatic involvement [10]. Although enlarged CPLNs are frequently observed during preoperative imaging, their prognostic impact is unclear. Holloway et al. demonstrated a significant association between peritoneal metastases and enlarged CPLN with a short-axis diameter >5 mm on CT scans. The presence of enlarged CPLN was found to be associated with poor survival, regardless of the presence of peritoneal metastases [9]. Currently, the surgical feasibility of CPLN resection has been reported [11,12]. However, there is still no consensus on the survival benefit of these issues. We, therefore, performed a systematic review and meta-analysis to summarize available evidence. The primary objective of the study was to evaluate the clinical outcomes of advanced-stage EOC patients with CPLN adenopathy vs. to those without adenopathy who underwent cytoreductive surgery. The secondary objective was to evaluate the potential role of CPLN resection in EOC patients who had also undergone cytoreduction.
2. Material and Methods
2.1. Data Sources and Searches
The methods in this review are described based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Checklist [13], the PRISMA-S extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews [14], and meta-analysis of observational studies (MOOSE) checklist [15]. The study was registered in PROSPERO, Registration Number 276488. The search was developed in Embase.com and then translated to other databases. The search was carried out on 1 June 2021 in the databases Embase.com, Medline ALL via Ovid, Web of Science Core Collection, and the Cochrane Central Register of Controlled Trials via Wiley. Additionally, a search was performed in Google Scholar from which the 200 most relevant references were downloaded using the software Publish or Perish [16]. See Table S1 for more information.
The search contained terms for (1) cardiophrenic lymph node and (2) ovarian cancer (Table S1). The searches in Embase, Medline, and Web of Science were limited to exclude conference papers and animal-only articles. No study registries were searched, but Cochrane Central retrieves the contents of ClinicalTrials.gov and the World Health Organization’s International Clinical Trials Registry Platform. No authors or subject experts were contacted, and we did not browse unindexed journals in the field.
2.2. Study Selection
Studies published in the English language with adequate information according to our including criteria and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement [17], were included in the review. The titles and abstracts retrieved from the search strategy were independently screened by two authors (M.K. and G.N.). Then, they retrieved and reviewed the full texts of the seemingly relevant articles. Any disagreements between M.K. and G.N. were resolved through discussion and arbitration by a third author (H.B.). The reference lists of retrieved articles were searched for possibly missed relevant studies. We included retrospective and prospective cohort studies as well as clinical trials that evaluated the survival of advanced-stage epithelial ovarian cancer patients, by preoperative CPLN adenopathy status, who underwent cytoreductive surgery for primary disease. Only studies that reported the associated factors and survival outcomes were included. Studies presenting results for patients treated only by surgery were excluded unless details regarding surgical outcomes—, e.g., residual disease or perioperative complications—were provided. Studies published as conference abstracts, narrative review, editorials, letters, and short communications were excluded
2.3. Data Extraction and Quality Assessment
Preoperative CPLN adenopathy was defined as CPLN ≥ 5 mm on the short axis, diagnosed by preoperative CT or MRI scan, or fluor-18-deoxyglucose (FDG) uptake in positron emission tomography/computer tomography (PET/CT) [10]. Study characteristics extracted were the following: name of the first author, year of publication, country, study center, sample size, and study design. Patient-related characteristics extracted were mean age, mean cancer antigen (CA)125, mean size of CPLN, percentage of histologically proven metastatic lymph nodes, FIGO stage, histology, American Society of Anesthesiologists (ASA) classification, surgical outcome, presence of extra-abdominal metastasis, and postoperative complication rate. Primary outcomes were median progression-free survival (PFS) time, median overall survival (OS) time, 5-year PFS, and OS probabilities. PFS was defined as the time elapsed between the date of diagnosis to the date of recurrence. Overall survival was defined as the time elapsed from diagnosis to the date of death or last follow-up. All survival outcomes and patient characteristics were extracted from patients with a primary diagnosis of advanced-stage ovarian cancer. Two authors (M.K. and G.N.) independently evaluated the quality and the risk of bias in the observational studies included in the meta-analysis based on The Newcastle-Ottawa Quality Assessment Scale [18], and discrepancies were resolved in the manner described for article review.
2.4. Data Synthesis and Analysis
Results were pooled using random-effects meta-analyses to compute weighted mean differences (WMD) of continuous variables and pooled odds ratios (ORs) of binary variables. The pooled estimations are displayed with 95% confidence intervals (CIs). If the mean and standard deviation (SD) were not provided in the original articles, the method described by Wan et al. was used for calculation [19]. Pooled median survival was analyzed by the method of Simes et al. [20]. To calculate the individual study hazard ratios (HR), we used the methods described by Tierney et al. [21]. Heterogeneity in study effect sizes was assessed using the I2 index and Cochran’s Q-test. I2 indices of 25%, 50%, and 75% indicate mild, medium, and high heterogeneity, respectively. Categorical variables are presented as number and percentage and continuous variables were presented as mean ± SD. Each variable was considered statistical significance when a p-value < 0.05. Publication bias was formally assessed using the Egger test. The analyses were performed using Stata Statistical Software Release 16.1 (StataCorp LLC, College Station, TX, USA).
3. Results
3.1. General Characteristic
The initial search retrieved 1468 articles. After the removal of duplicates, the titles and abstracts of 957 articles were screened, and 45 full-text articles were retrieved for a comprehensive review. In total, 15 articles were included in the review based on inclusion and exclusion criteria (Figure S1). All studies had adequate quality for analysis according to The Newcastle-Ottawa Quality Assessment Scale (Table S2).
Thirteen articles described retrospective cohort studies [9,11,22,23,24,25,26,27,28,29,30,31,32,33], one was a case report [34], and one a case series [35]. The studies spanned the period from 1997 to 2020. CPLN adenopathy was defined as CPLN ≥ 5 mm in six studies, ≥7 mm in four studies, and >8 mm on preoperative scans and/or bulky lymph nodes during surgery in three studies, and >10 mm in a single study. One study did not mention a threshold cutoff to define CPLN adenopathy. From these studies, 727 patients with CPLN adenopathy and 981 patients without CPLN adenopathy were included in our analyses (Table S3).
The pooled baseline characteristics of patients by the CPLN adenopathy group are presented in Table 1. The pooled mean age of patients with and those without CPLN adenopathy was 59.17 ± 11.87 years and 60 ± 12.64 years, respectively. There was no difference in pooled mean CA125 level between the two groups when results were available. Overall, 43 percent of each group had ASA classification ≥ 3. In each group, the majority of patients had high-grade serous carcinoma. In addition, 73% of patients with CPLN adenopathy had been diagnosed with FIGO stage IV, vs. 23% of patients without CPLN adenopathy. Note that stage IV disease was defined as metastases other than CLPN involvement, for example, liver or spleen parenchymal involvement, extra-abdominal metastases, etc. The proportion of ascites, pleural metastases, pleural effusion, upper abdominal metastases, pelvic and abdominal adenopathy, carcinomatosis peritonitis, and other extra-abdominal metastases were highest in the CPLN adenopathy group. The proportion of patients achieving complete abdominal cytoreduction did not differ between the groups. Surgical complexity—reflected by the description of procedures including diaphragmatic stripping, splenectomy, and hepatectomy—was higher in the CPLN adenopathy group. Despite this finding, only a slightly higher proportion of perioperative complications was found for the CPLN adenopathy group (26% vs. 23%). Eight studies provided survival outcomes [9,11,24,25,26,29,32,33]. The pooled median PFS time was 14.6 months (95% CI 4.9–24.4) vs. 27.8 months (95% CI 3.2–52.5) in patients with and without CPLN adenopathy, respectively. The pooled median OS time was 42.7 months (95% CI 10.8–74.6) vs. 47.3 months (95% CI 23.2–71.2), respectively.

Table 1.
Pooled baseline characteristics of patients included in the systematic review.
3.2. Intervention
Regarding the impact of CPLN resection during cytoreductive surgery, nine studies reported the mean size of preoperative suspicious nodes [11,23,24,25,26,28,29,32,34]. The baseline characteristics are presented in Table 2. The mean size of preoperative CPLN was 9.1 ± 3.75 mm. Four studies evaluated the CPLN location; right, left, bilateral and midline, and unknown [25,28,31,34]. Nearly 50% of the enlarged nodes were located in the right mediastinum. In total, 194 patients with enlarged CPLN underwent CPLN resection. Among them, 82% of patients had histologically proven CPLN metastases (Table S4). The most commonly used surgical technique was the transdiaphragmatic approach, followed by VATS and the substernal approach, respectively. Four studies reported postoperative complications [24,27,28,35]. Overall, 30 percent of patients were diagnosed with Clavien–Dindo grade IIIa or above [36]. No studies reported 30-day mortality. Nine studies provided adequate data regarding survival outcomes [9,11,24,25,26,29,31,32,33]. The pooled median PFS time was 17.7 months (95% CI 7.9–27.4), and the median OS time was 54.7 months (95% CI 15.2–94.3) in patients who underwent CPLN resection, compared with 15.3 months (95% CI 3.5–27.1) and 35.6 months (95% CI 19.0–52.2), respectively, in patients with CPLN adenopathy but without CPLN resection.

Table 2.
Pooled baseline characteristic of patients who underwent CPLN resection.
3.3. Meta-Analysis
In the meta-analysis, patients with CPLN adenopathy vs. patients without CPLN adenopathy had a significantly increased risk of ascites (OR 3.30, 95% CI 1.90–5.72), pleural metastases (OR 2.58, 95% CI 1.37–4.82), pleural effusion (OR 1.78, 95% CI 1.17–2.69), abdominal adenopathy (OR 2.30, 95% CI 1.53–3.46), and extra-abdominal metastases (OR 3.27, 95% CI 1.61–6.67). Neither the risk of upper abdominal metastases (OR 4.38, 95% CI 0.36–54.01) nor the prevalence of complete abdominal cytoreduction (OR 0.69, 95% CI 0.17–2.82) differed between groups (Table 3). Six studies provided 5-year overall survival rates [9,22,25,31,32,33] (Figure 1). Patients with CPLN adenopathy had a significantly increased risk of dying from the disease, compared with those without adenopathy (HR of death at 5 years; 1.74, 95% CI 1.06–2.86, p = 0.029). Two studies provided 5-year mortality in patients undergoing optimal abdominal cytoreductive surgery [22,27] (Figure 2). Those with CPLN adenopathy had a 1.42 times higher risk of dying from the disease than those without adenopathy (95% CI 1.08–1.85, p = 0.011). The forest plot displaying hazard ratios at 5-year disease-free survival in patients with CPLN adenopathy relative to those with no adenopathy is displayed in Figure 3. Funnel plots of these results are presented in Supplementary Data (Figures S2–S4). Unfortunately, data to inform survival times and probabilities in patients undergoing abdominally complete cytoreductive surgery in relation to CPLN resection were not provided in the individual studies.

Table 3.
Meta-analysis of clinical characteristics of patients with CPLN adenopathy, compared with patients without CPLN adenopathy.
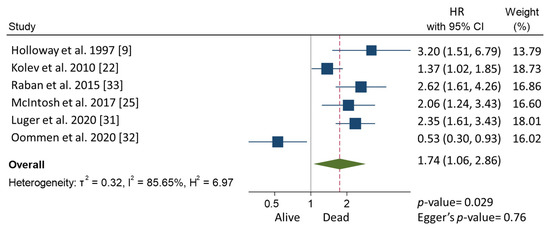
Figure 1.
Forest plot displaying hazard ratios for mortality at 5 years in patients with CPLN adenopathy relative to those with no adenopathy.
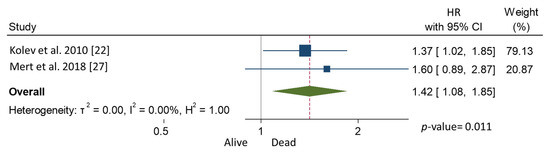
Figure 2.
Forest plot displaying hazard ratios for mortality at 5 years after optimal abdominal cytoreduction surgery in patients with CPLN adenopathy relative to those with no adenopathy.
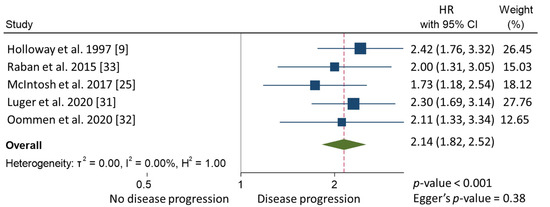
Figure 3.
Forest plot displaying hazard ratios at 5-year disease-free survival in patients with CPLN adenopathy relative to those with no adenopathy.
4. Discussion
Among advanced-stage EOC, 12–33% of patients will present with extra-abdominal and or visceral metastases [37]. Evidence generated over decades of research shows that complete cytoreduction at primary surgery is the cornerstone of favorable survival outcomes for advanced-stage ovarian carcinoma [6,38,39]. Of 208 patients with stage IV EOC from seven referral centers in France, patients on whom no operation was performed had significantly worse overall survival than those who had undergone primary debulking surgery or interval debulking surgery after neoadjuvant chemotherapy (NACT). The authors concluded that the presence of distant metastases should not deter surgeons from performing a radical procedure if it could be tolerated by the patient. In this study, patients who received chemotherapy only were in poor general condition and judged not suitable for surgery, or developed progressive disease during chemotherapy [40].
In the studies included in this review, the prevalence of radiologically suspected CPLN adenopathy varied from 11 to 50% in advanced-stage EOC. Three studies described suspected CPLN when the size was ≥5 mm in short-axis diameter according to ESUR2010 [22,27,31]. Luger et al. demonstrated that CPLN ≥ 5 mm in short axis correctly predicted carcinomatosis of the upper abdomen in 94% of cases [31]. In our meta-analysis, we additionally found that patients with CPLN adenopathy had a significantly increased likelihood of having ascites, abdominal adenopathy, and extra-abdominal metastases, compared with patients without CPLN adenopathy. We found that 82.4% of patients who underwent CPLN resection had pathologically confirmed metastatic lymph nodes. It must be noted that cutoff sizes for defining enlarged CPLN, and different diagnostic imaging modalities were included in our analysis. The percentage of pathologic lymph nodes found on imaging varied from 57.1% to 94.4%. Kim et al. purposed that the threshold of 7 mm in a short axis on CT scans was the optimal cutoff for predicting CPLN metastasis, with 63% sensitivity and 83% specificity [23]. Another study also reported the probability of detecting CPLN metastases of approximately 85% if the short axis of the node exceeded 7 mm [33]. In contrast, Pinelli et al. found metastases in only 57.14% of CPLN after interval debulking surgery (IDS) when using the same cutoff value. However, all patients in the study had received 3–6 cycles of NACT before IDS. The authors suggested that the lower detection rate was a consequence of chemotherapy-induced fibrosis after tumor regression [31]. Surprisingly, Cowan et al. reported the highest detection rate when the cutoff size was >5 mm in the short axis [24]. However, the mean size of CPLN in this study was as large as 15 ± 0.5 mm, while in other studies, the mean size ranged from 7 to 10 mm [23,26,29]. Lopes et al. reported that 85.7% of EOC patients were upstaged from stage IIIC to IV, based on histologically confirmed CPLN metastases when CPLN were ≥8 mm on preoperative imaging and/or exhibited 18-fluorine fluorodeoxyglucose uptake on PET/CT. Interestingly, all patients with PET/CT uptake in CPLN in this study— including those who had received NACT—had histologically positive lymph nodes [11]. Currently, many studies have demonstrated that PET/CT imaging increases the likelihood of finding CPLN metastases, in comparison with CT imaging, especially when they are <10 mm in size [26,27,41]. However, another study suggested that CPLN size ≥ 7 mm in a short axis on CT imaging has 85.7% positive predictive value and 58.8% negative predictive value [23], which is useful in settings where PET/CT is not always available. It also implies that PET/CT may be best reserved for patients with an unclear diagnosis, or for patients who have already received chemotherapy.
In some centers, gynecologic oncologists employ CPLN resection—most commonly with a transdiaphragmatic approach—with the aim to maximize the degree of cytoreduction. Many advantages of transdiaphragmatic approaches have been described. First, the procedure is feasible for both gynecologic oncologists and surgeons. Second, the patient’s position during surgery need not be changed. Third, it is not always necessary to insert a chest tube after the procedure [42,43]. In 2017, Cowan et al. reported on the largest cohort of EOC patients who underwent CPLN resection at primary cytoreductive surgery [24]. This cohort included patients who underwent CPLN resection via VATS and a transdiaphragmatic approach by a gynecologic oncologist or another consulting surgeon. The postoperative complication rate was 35%. Only 7% of the major postoperative complications were of a pulmonary nature—namely, pulmonary embolism, chylothorax, pleural effusion, and acute respiratory disease syndrome [24]. In our analysis, about 30% of CPLN-resected patients had ≥Clavien–Dindo grade IIIa. The authors of the various publications concluded, however, that the procedure was feasible, the morbidity rate acceptable, and no procedure-related deaths had occurred [23,24,28,34,44].
Although CPLN adenopathy is associated with extensive intra- and extra-abdominal tumor burden, it does not affect the surgical outcome. In our pooled analysis, abdominally complete cytoreductive surgery was accomplished in nearly 60% of patients with or without CPLN adenopathy. Notably, all studies had been conducted in tertiary centers, and surgery was highly complex in 65–85% of the patients. Our findings confirm that CPLN adenopathy is associated with a lower PFS and worse survival prognosis. On the other hand, the pooled median PFS and the OS of patients who underwent CPLN resection was superior to those of patients with CPLN adenopathy but without CPLN resection, suggesting a possible benefit of CPLN resection. Unfortunately, only two studies had directly compared survival outcomes in patients by whether or not they had CPLN resection. The authors reported no significant benefit of CPLN resection with regard to PFS and OS [26,29].
Strength and Limitations
To our knowledge, this is the first systematic review and meta-analysis describing the clinical impact of CPLN adenopathy in patients with ovarian cancer. Still, some limitations of the study need to be addressed. First, most of the included studies were retrospective cohort studies, which carry the risk of missing data, and the possibility that significant biases may have occurred in the selection of controls. Second, although we presented the pooled median survival times in our study, these values could not be derived from every patient in all studies in the meta-analysis, because of inadequate information reported in the original studies. Third, adequate data regarding the overall survival of patients who underwent abdominally complete cytoreductive surgery and CPLN resection were not yet available for meta-analysis.
5. Conclusions
This systematic review and meta-analysis demonstrated that patients with CPLN adenopathy had a significantly higher risk of having ascites and intra- and extra-abdominal metastases than patients without CPLN adenopathy. Furthermore, PET/CT increases the detection rate of pathological CPLN metastases. CPLN exceeding 7 mm in the short axis on CT or MRI scans, with a positive predictive value for detecting metastatic lesions of approximately 85%. Based on these lines of evidence, it would seem appropriate that patients with CPLN of this size should therefore be considered candidates in future studies of the prognostic importance of CPLN dissection. PET/CT imaging should be reserved for patients with an inconclusive diagnosis, or a specific group of patients, for example, those who have received chemotherapy before imaging or patients in a healthcare setting where resources are limited. Patients with CPLN adenopathy had a significantly increased risk of recurrence of disease and dying from the disease in comparison to those without adenopathy. Currently, there are no data that definitively demonstrate a therapeutic benefit of CPLN resection, although this review and meta-analysis indicated a possible benefit of CPLN resection. For now, we recommend that EOC patients with suspected CPLN involvement be referred to a gynecologic oncologist to receive maximal cytoreduction. A randomized controlled trial should be conducted to demonstrate the potential benefit of CPLN debulking in cytoreductive surgery.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers13195017/s1, Table S1: The search strings, Table S2: Quality assessment of the included studies using the Newcastle-Ottawa Quality Assessment Scale, Table S3: Review of literatures, Table S4: The association between radiologically suspicious CPLN and histologically confirmed CPLN, Figure S1: Flow diagram of study selection, Figure S2: Funnel plot, using data from 6 studies of 5-year mortality in advanced stage EOC with CPLN adenopathy, Figure S3: Funnel plot, using data from 2 studies of 5-year mortality in advanced stage EOC with CPLN adenopathy after optimal abdominal cytoreductive surgery, Figure S4: Funnel plot, using data from 5 studies of 5-year disease free survival in advanced stage EOC with CPLN adenopathy.
Author Contributions
Study design, M.K., G.M.N.-d.B., A.H.J.B. and H.J.v.B.; systematic searching, C.D.N. and M.K.; literature review and data collection, M.K. and G.M.N.-d.B.; data analysis, M.K., G.M.N.-d.B., S.U., S.J.K. and H.J.v.B.; writing—original daft preparation, M.K. and G.M.N.-d.B. All authors had reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
There is no financial support in this study.
Acknowledgments
The authors would like to thank the collaborators of the Medical Library of the Erasmus Medical Center, Rotterdam, for their help with the systematic literature search.
Conflicts of Interest
The authors have no conflict of interest to declare.
Abbreviations
| ASA | American Society of Anesthesiologists |
| AEOC | Advanced-stage epithelial ovarian cancer |
| CPLN | Cardiophrenic lymph nodes |
| CT | Computed tomography scan |
| EOC | Epithelial ovarian cancer |
| ESGO | European Society of Gynecological Oncology |
| ESUR | European Society of Urogenital Radiology |
| FIGO | International Federation of Gynecology and Obstetrics |
| IDS | Interval debulking surgery |
| NACT | Neoadjuvant chemotherapy |
| MRI | Magnetic resonance imaging scan |
| OS | Overall survival |
| PFS | Progression-free survival |
| VATS | Video-assisted thoracic surgery |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Steinberga, I.; Jansson, K.; Sorbe, B. Quality Indicators and Survival Outcome in Stage IIIB-IVB Epithelial Ovarian Cancer Treated at a Single Institution. In Vivo 2019, 33, 1521–1530. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar]
- Bristow, R.E.; Tomacruz, R.S.; Armstrong, D.K.; Trimble, E.L.; Montz, F.J. Survival effect of maximal cytoreductive surgery for ad-vanced ovarian carcinoma during the platinum era: A meta-analysis. J. Clin. Oncol. 2002, 20, 1248–1259. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-J.; Hodeib, M.; Chang, J.; Bristow, R.E. Survival impact of complete cytoreduction to no gross residual disease for advanced-stage ovarian cancer: A meta-analysis. Gynecol. Oncol. 2013, 130, 493–498. [Google Scholar] [CrossRef]
- Colombo, P.-E.; Mourregot, A.; Fabbro, M.; Gutowski, M.; Saint-Aubert, B.; Quenet, F.; Gourgou, S.; Rouanet, P. Aggressive surgical strategies in advanced ovarian cancer: A monocentric study of 203 stage IIIC and IV patients. Eur. J. Surg. Oncol. 2009, 35, 135–143. [Google Scholar] [CrossRef]
- Berek, J.S.; Kehoe, S.T.; Kumar, L.; Friedlander, M. Cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynecol. Obstet. 2018, 143, 59–78. [Google Scholar] [CrossRef]
- Harter, P.; Sehouli, J.; Lorusso, D.; Reuss, A.; Vergote, I.; Marth, C.; Kim, J.-W.; Raspagliesi, F.; Lampe, B.; Aletti, G.; et al. A Randomized Trial of Lymphadenectomy in Patients with Advanced Ovarian Neoplasms. N. Engl. J. Med. 2019, 380, 822–832. [Google Scholar] [CrossRef]
- Holloway, B.; Gore, M.; A’Hern, R.; Parsons, C. The significance of paracardiac lymph node enlargement in ovarian cancer. Clin. Radiol. 1997, 52, 692–697. [Google Scholar] [CrossRef]
- Forstner, R.; Sala, E.; Kinkel, K.; Spencer, J.A. ESUR guidelines: Ovarian cancer staging and follow-up. Eur. Radiol. 2010, 20, 2773–2780. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.; Costa, R.L.R.; Di Paula, R.; Anton, C.; Calheiros, Y.; Sartorelli, V.; Kanashiro, Y.M.; De Lima, J.A.; Yamada, A.; Pinto, G.L.D.S.; et al. Cardiophrenic lymph node resection in cytoreduction for primary advanced or recurrent epithelial ovarian carcinoma: A cohort study. Int. J. Gynecol. Cancer 2019, 29, 188–194. [Google Scholar] [CrossRef]
- LaFargue, C.J.; Bristow, R.E. Transdiaphragmatic cardiophrenic lymph node resection for Stage IV ovarian cancer. Gynecol. Oncol. 2015, 138, 762–763. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Rethlefsen, M.L.; PRISMA-S Group; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B. PRISMA-S: An extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B.; et al. Meta-analysis of Observational Studies in Epidemiology. A Proposal for Reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Harzing, A.W. Publish or Perish. 2007. Available online: https://harzing.com/resources/publish-or-perish (accessed on 1 June 2021).
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [Green Version]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [Green Version]
- Simes, R.J. Confronting publication bias: A cohort design for meta-analysis. Stat. Med. 1987, 6, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolev, V.; Mironov, S.; Mironov, O.; Ishill, N.; Moskowitz, C.S.; Gardner, G.J.; Levine, D.A.; Hricak, H.; Barakat, R.R.; Chi, D.S. Prognostic Significance of Supradiaphragmatic Lymphadenopathy Identified on Preoperative Computed Tomography Scan in Patients Undergoing Primary Cytoreduction for Advanced Epithelial Ovarian Cancer. Int. J. Gynecol. Cancer 2010, 20, 979–984. [Google Scholar] [CrossRef]
- Kim, T.-H.; Lim, M.C.; Kim, S.I.; Seo, S.-S.; Kim, S.H.; Park, S.-Y. Preoperative Prediction of Cardiophrenic Lymph Node Metastasis in Advanced Ovarian Cancer Using Computed Tomography. Ann. Surg. Oncol. 2015, 23, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Cowan, R.A.; Tseng, J.; Murthy, V.; Srivastava, R.; Roche, K.C.L.; Zivanovic, O.; Gardner, G.J.; Chi, D.S.; Park, B.J.; Sonoda, Y. Feasibility, safety and clinical outcomes of cardiophrenic lymph node resection in advanced ovarian cancer. Gynecol. Oncol. 2017, 147, 262–266. [Google Scholar] [CrossRef]
- McIntosh, L.J.; O’Neill, A.C.; Bhanusupriya, S.; Matalon, S.A.; Abbeele, A.D.V.D.; Ramaiya, N.H.; Shinagare, A.B. Prognostic significance of supradiaphragmatic lymph nodes at initial presentation in patients with stage III high-grade serous ovarian cancer. Abdom. Radiol. 2017, 64, 9–2520. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.O.; Lee, J.Y.; Kim, H.J.; Nam, E.J.; Kim, S.; Kim, S.W. Prognostic Significance of Supradiaphragmatic Lymph Node Metastasis Detected by 18F-FDG PET/CT in Advanced Epithelial Ovarian Cancer; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Mert, I.; Kumar, A.; Sheedy, S.P.; Weaver, A.L.; McGree, M.E.; Kim, B.; Cliby, W.A. Clinical significance of enlarged cardiophrenic lymph nodes in advanced ovarian cancer: Implications for survival. Gynecol. Oncol. 2018, 148, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Salehi, S.; Mohammar, R.; Suzuki, C.; Joneborg, U.; Hjerpe, E.; Torbrand, C.; Falconer, H. Cardiophrenic lymph node resection in advanced ovarian cancer: Surgical outcomes, pre- and postoperative imaging. Acta Oncol. 2018, 57, 820–824. [Google Scholar] [CrossRef] [Green Version]
- Prader, S.; Vollmar, N.; du Bois, A.; Heitz, F.; Schneider, S.; Ataseven, B.; Bommert, M.; Waltering, K.-U.; Heikaus, S.; Koch, J.A.; et al. Pattern and impact of metastatic cardiophrenic lymph nodes in advanced epithelial ovarian cancer. Gynecol. Oncol. 2019, 152, 76–81. [Google Scholar] [CrossRef]
- Komatsu, H.; Iida, Y.; Osaku, D.; Shimogai, R.; Chikumi, J.; Sato, S.; Oishi, T.; Harada, T. Effects of pretreatment radiological and pathological lymph node statuses on prognosis in patients with ovarian cancer who underwent interval debulking surgery with lymphadenectomy following neoadjuvant chemotherapy. J. Obstet. Gynaecol. Res. 2021, 47, 152–158. [Google Scholar] [CrossRef]
- Luger, A.K.; Steinkohl, F.; Aigner, F.; Jaschke, W.; Marth, C.; Zeimet, A.G.; Reimer, D. Enlarged cardiophrenic lymph nodes predict disease involvement of the upper abdomen and the outcome of primary surgical debulking in advanced ovarian cancer. Acta Obstet. Gynecol. Scand. 2020, 99, 1092–1099. [Google Scholar] [CrossRef] [Green Version]
- Oommen, I.; Chandramohan, A.; Raji, P.S.; Thomas, A.; Joel, A.; Ram, T.S.; Peedicayil, A. Clinical significance of CT detected enlarged cardiophrenic nodes in ovarian cancer patients. Abdom. Radiol. 2021, 46, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Raban, O.; Peled, Y.; Krissi, H.; Goldberg, N.; Aviram, A.; Sabah, G.; Levavi, H.; Eitan, R. The significance of paracardiac lymph-node enlargement in patients with newly diagnosed stage IIIC ovarian cancer. Gynecol. Oncol. 2015, 138, 259–262. [Google Scholar] [CrossRef]
- Nuruzzaman, H.S.M.; Tan, G.H.C.; Nadarajah, R.; Teo, M. Relevance of enlarged cardiophrenic lymph nodes in determining prognosis of patients with advanced ovarian cancer. BMJ Case Rep. 2018, 2018. [Google Scholar] [CrossRef]
- Pinelli, C.; Morotti, M.; Casarin, J.; Tozzi, R.; Alazzam, M.; Mavroeidis, V.K.; Majd, H.S. The Feasibility of Cardiophrenic Lymphnode Assessment and Removal in Patients Requiring Diaphragmatic Resection during Interval Debulking Surgery for Ovarian Cancer. J. Investig. Surg. 2021, 34, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Ataseven, B.; Chiva, L.M.; Harter, P.; Gonzalez-Martin, A.; du Bois, A. FIGO stage IV epithelial ovarian, fallopian tube and peritoneal cancer revisited. Gynecol. Oncol. 2016, 142, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Ataseven, B.; Grimm, C.; Harter, P.; Heitz, F.; Traut, A.; Prader, S.; du Bois, A. Prognostic impact of debulking surgery and residual tumor in patients with epithelial ovarian cancer FIGO stage IV. Gynecol. Oncol. 2016, 140, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Delga, B.; Classe, J.-M.; Houvenaeghel, G.; Blache, G.; Sabiani, L.; El Hajj, H.; Andrieux, N.; Lambaudie, E. 30 Years of Experience in the Management of Stage III and IV Epithelial Ovarian Cancer: Impact of Surgical Strategies on Survival. Cancers 2020, 12, 768. [Google Scholar] [CrossRef] [Green Version]
- Dabi, Y.; Huchon, C.; Ouldamer, L.; Bendifallah, S.; Collinet, P.; Bricou, A.; Daraï, E.; Ballester, M.; Lavoue, V.; Haddad, B.; et al. Patients with stage IV epithelial ovarian cancer: Understanding the determinants of survival. J. Transl. Med. 2020, 18, 134. [Google Scholar] [CrossRef] [Green Version]
- Farmakis, S.; Vejdani, K.; Muzaffar, R.; Parkar, N.; Osman, M.M. Detection of Metastatic Disease in Cardiophrenic Lymph Nodes: FDG PET/CT versus Contrast-Enhanced CT and Implications for Staging and Treatment of Disease. Front. Oncol. 2013, 3, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, H.J.; Lim, M.C.; Song, Y.J.; Jung, Y.-S.; Kim, S.H.; Yoo, C.W.; Park, S.-Y. Transabdominal cardiophrenic lymph node dissection (CPLND) via incised diaphragm replace conventional video-assisted thoracic surgery for cytoreductive surgery in advanced ovarian cancer. Gynecol. Oncol. 2013, 129, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.; Park, B.; Stine, J.; Flores, R.; Sonoda, Y.; Abu-Rustum, N.; Barakat, R.; Chi, D. The feasibility of mediastinal lymphadenectomy in the management of advanced and recurrent ovarian carcinoma. Gynecol. Oncol. 2011, 120, S80. [Google Scholar] [CrossRef]
- LaFargue, C.; Sawyer, B.; Bristow, R. Short-term morbidity in transdiaphragmatic cardiophrenic lymph node resection for advanced stage gynecologic cancer. Gynecol. Oncol. Rep. 2016, 17, 33–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).