Reversal of Bortezomib-Induced Neurotoxicity by Suvecaltamide, a Selective T-Type Ca-Channel Modulator, in Preclinical Models
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. In Vivo BTZ-Induced CIPN Reversal Study
2.1.1. BTZ-Induced CIPN Rat Model
2.1.2. NCV Measurement
2.1.3. MT Measurement
2.1.4. Tissue Sample Collection
2.1.5. β-Tubulin Polymerization Assay
2.1.6. IENF Density
2.1.7. Proteasome Inhibition Assay
2.2. In Vitro BTZ Cytotoxicity Study
2.2.1. Chemicals and Drugs
2.2.2. MCLs
2.2.3. Cell Survival Assay
2.2.4. In Vivo BTZ Anti-Tumor Activity Study
2.3. Ethical Standards
2.4. Statistical Analysis
3. Results
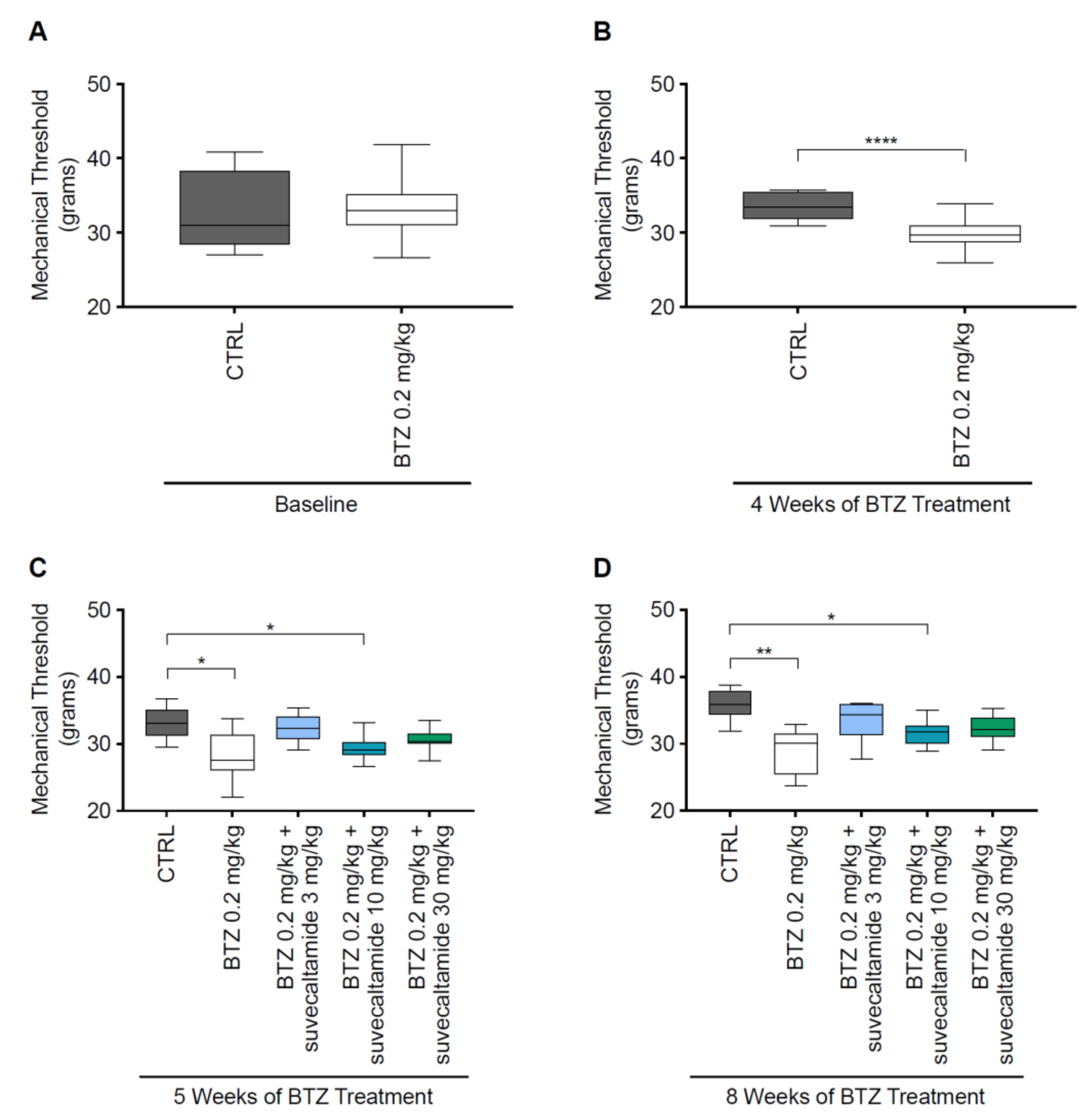
3.1. Effect of Suvecaltamide on BTZ-Induced CIPN
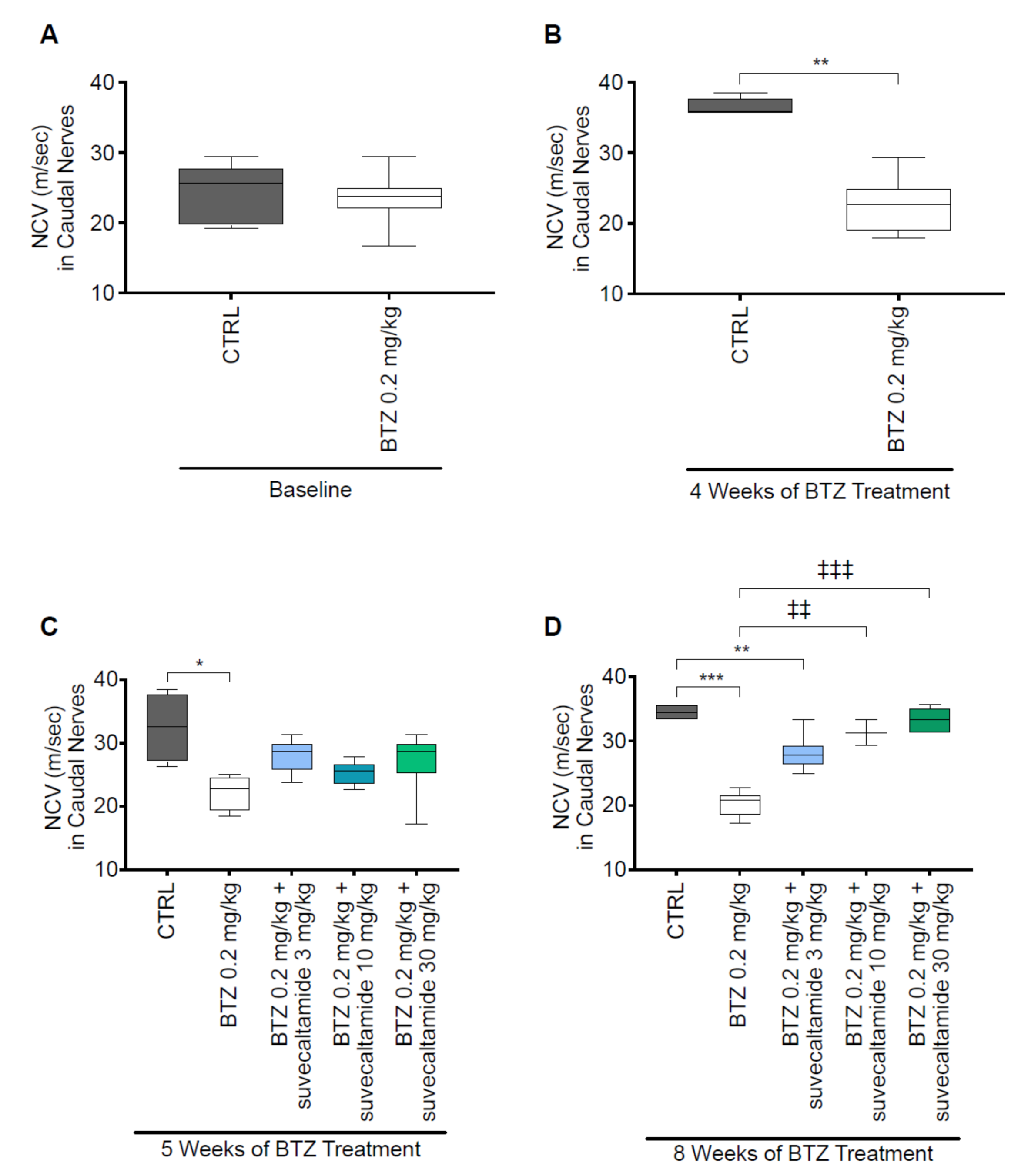
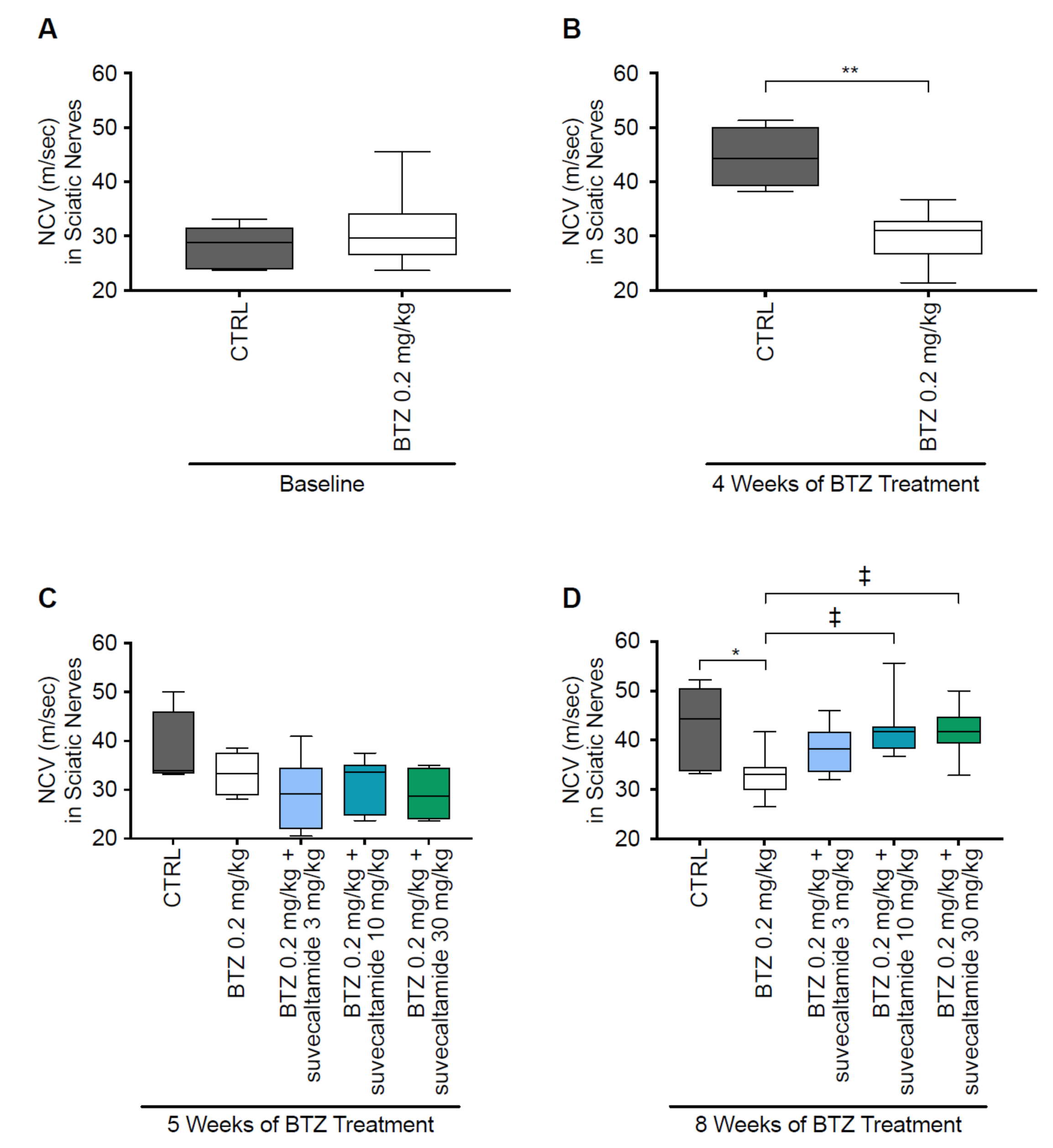
3.1.1. NCV and MT
3.1.2. Tissue Assessments
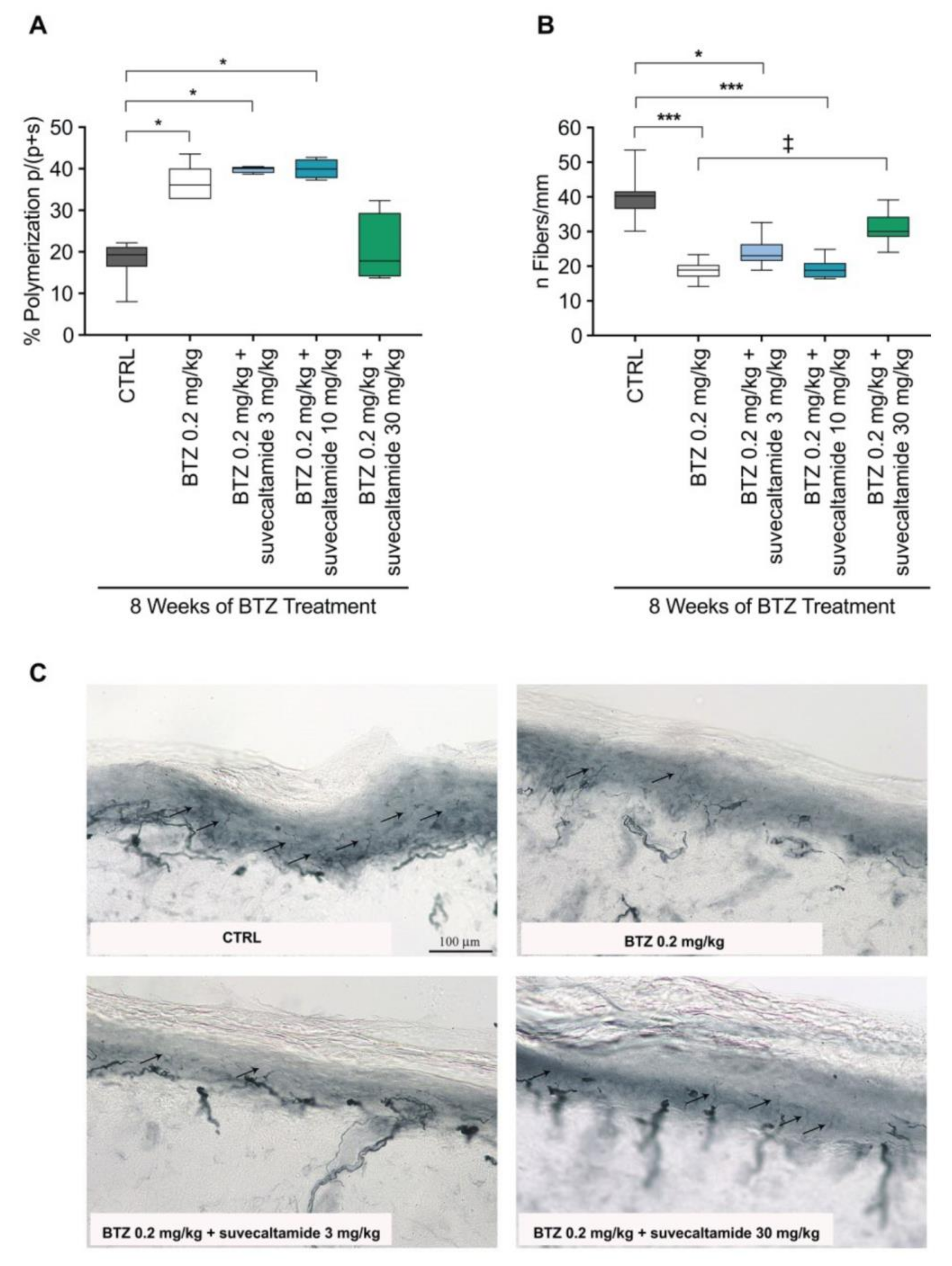
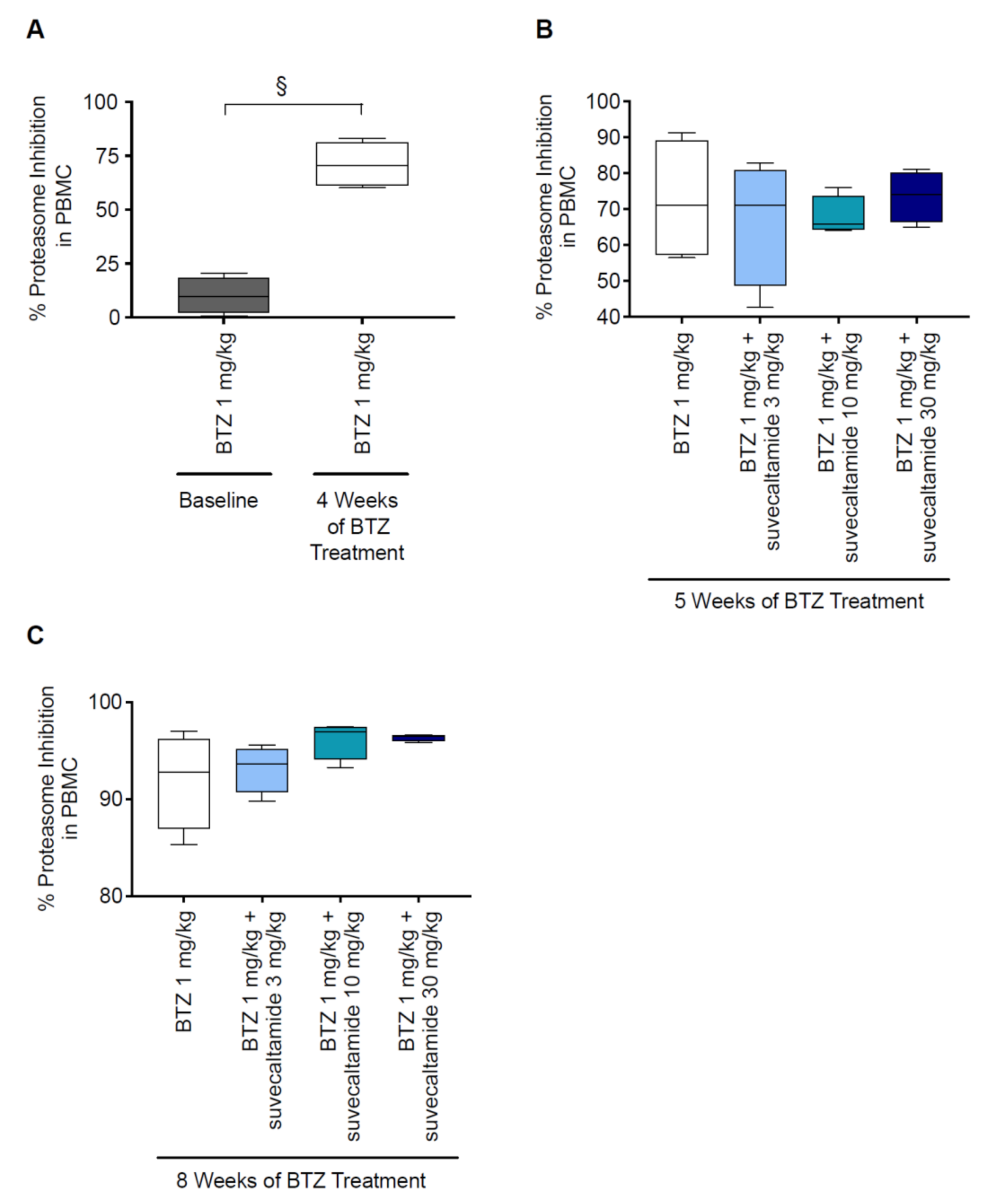
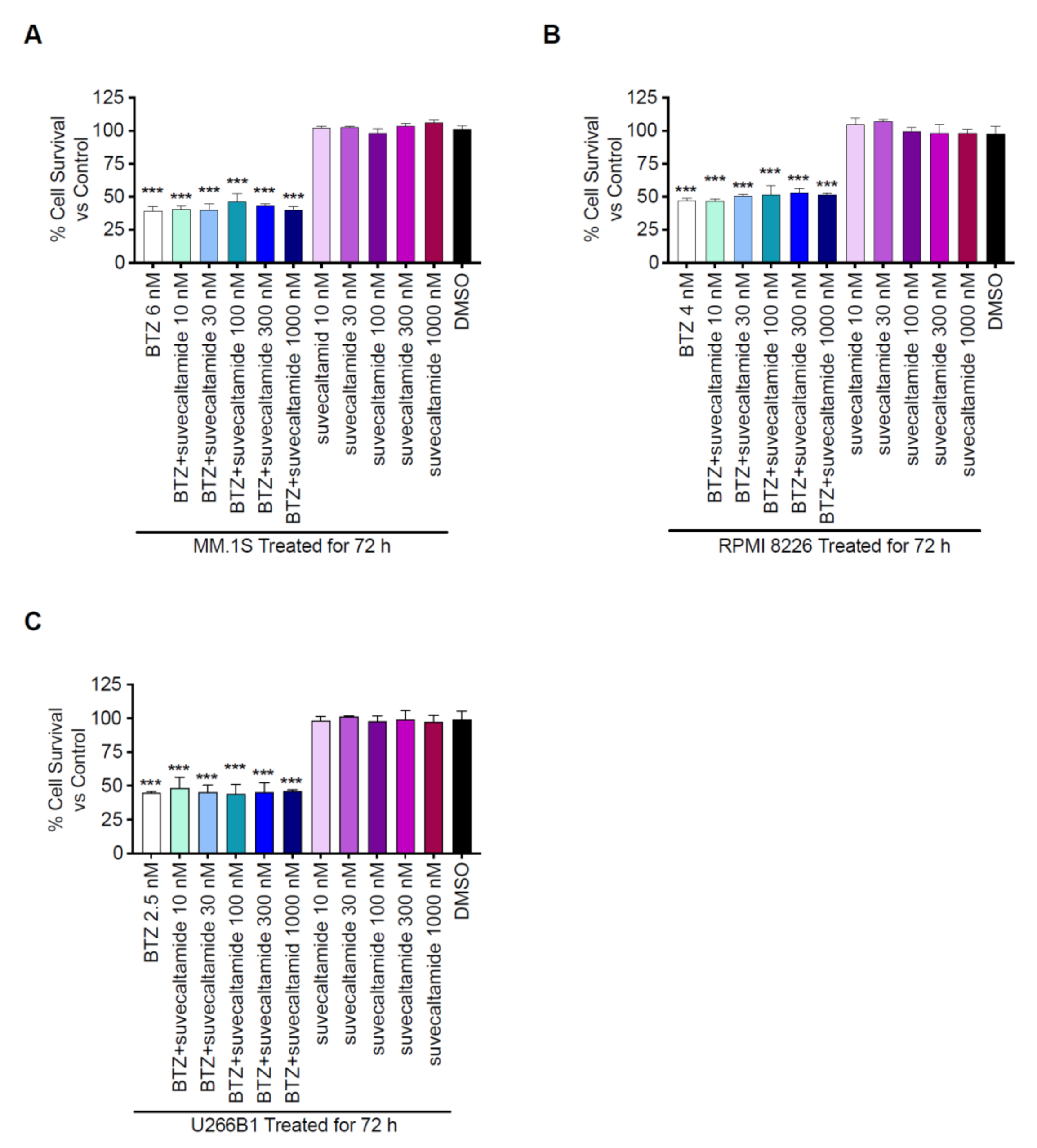
3.2. Effect of Suvecaltamide on BTZ Anti-Cancer Activity
3.2.1. Proteasome Inhibition
3.2.2. Cytotoxicity
3.2.3. Anti-Tumor Activity
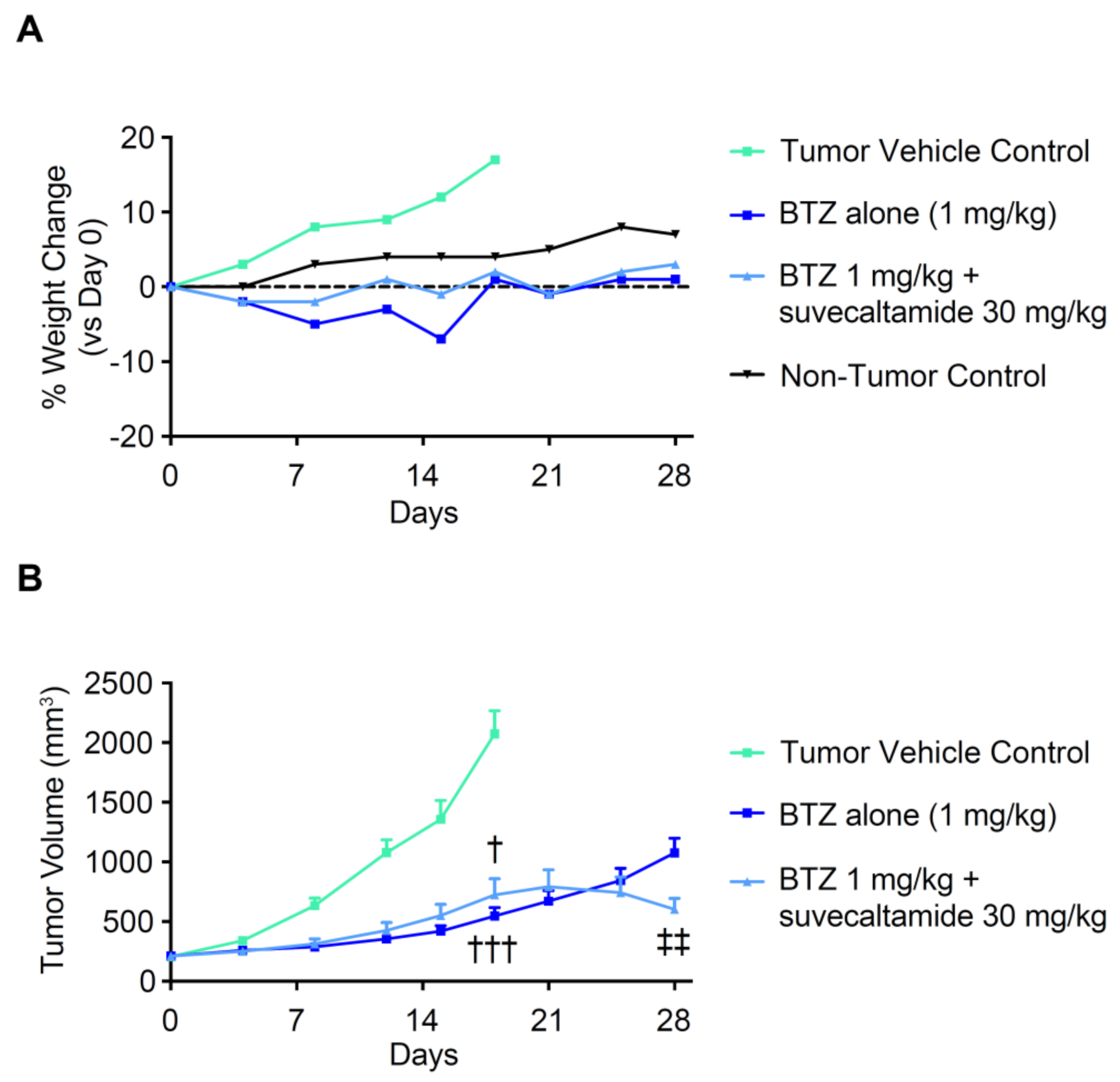
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaplan, G.S.; Torcun, C.C.; Grune, T.; Ozer, N.K.; Karademir, B. Proteasome inhibitors in cancer therapy: Treatment regimen and peripheral neuropathy as a side effect. Free Radic. Biol. Med. 2017, 103, 1–13. [Google Scholar] [CrossRef]
- Chen, D.; Frezza, M.; Schmitt, S.; Kanwar, J.; Dou, Q.P. Bortezomib as the first proteasome inhibitor anticancer drug: Current status and future perspectives. Curr. Cancer Drug Targets 2011, 11, 239–253. [Google Scholar] [CrossRef] [Green Version]
- Knopf, K.B.; Duh, M.S.; Lafeuille, M.H.; Gravel, J.; Lefebvre, P.; Niculescu, L.; Ba-Mancini, A.; Ma, E.; Shi, H.; Comenzo, R.L. Meta-analysis of the efficacy and safety of bortezomib re-treatment in patients with multiple myeloma. Clin. Lymphoma Myeloma Leuk. 2014, 14, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, P.M.; de Mendonça Lima, T.; Colleoni, G.W.B.; Storpirtis, S. Efficacy and safety of bortezomib, thalidomide, and lenalidomide in multiple myeloma: An overview of systematic reviews with meta-analyses. Crit. Rev. Oncol. Hematol. 2017, 113, 195–212. [Google Scholar] [CrossRef]
- Sun, C.Y.; Li, J.Y.; Chu, Z.B.; Zhang, L.; Chen, L.; Hu, Y. Efficacy and safety of bortezomib maintenance in patients with newly diagnosed multiple myeloma: A meta-analysis. Biosci. Rep. 2017, 37, BSR20170304. [Google Scholar] [CrossRef] [Green Version]
- Argyriou, A.A.; Bruna, J.; Marmiroli, P.; Cavaletti, G. Chemotherapy-induced peripheral neurotoxicity (CIPN): An update. Crit. Rev. Oncol. Hematol. 2012, 82, 51–77. [Google Scholar] [CrossRef]
- Cavaletti, G.; Marmiroli, P. Chemotherapy-induced peripheral neurotoxicity. Nat. Rev. Neurol. 2010, 6, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Grisold, W.; Cavaletti, G.; Windebank, A.J. Peripheral neuropathies from chemotherapeutics and targeted agents: Diagnosis, treatment, and prevention. Neuro. Oncol. 2012, 14 (Suppl. 4), iv45–iv54. [Google Scholar] [CrossRef] [Green Version]
- Flatters, S.J.L.; Dougherty, P.M.; Colvin, L.A. Clinical and preclinical perspectives on chemotherapy-induced peripheral neuropathy (CIPN): A narrative review. Br. J. Anaesth. 2017, 119, 737–749. [Google Scholar] [CrossRef] [Green Version]
- Millennium Pharmaceuticals. Velcade [Package Insert]; Millennium Pharmaceuticals: Cambridge, MA, USA, 2019. [Google Scholar]
- Carozzi, V.A.; Renn, C.L.; Bardini, M.; Fazio, G.; Chiorazzi, A.; Meregalli, C.; Oggioni, N.; Shanks, K.; Quartu, M.; Serra, M.P.; et al. Bortezomib-induced painful peripheral neuropathy: An electrophysiological, behavioral, morphological and mechanistic study in the mouse. PLoS ONE 2013, 8, e72995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavaletti, G.; Gilardini, A.; Canta, A.R.; Rigamonti, L.; Rodriguez-Menendez, V.; Ceresa, C.; Marmiroli, P.; Bossi, M.; Oggioni, N.; D’Incalci, M.; et al. Bortezomib-induced peripheral neurotoxicity: A neurophysiological and pathological study in the rat. Exp. Neurol. 2007, 204, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Gilardini, A.; Avila, R.L.; Oggioni, N.; Rodriguez-Menendez, V.; Bossi, M.; Canta, A.; Cavaletti, G.; Kirschner, D.A. Myelin structure is unaltered in chemotherapy-induced peripheral neuropathy. Neurotoxicology 2012, 33, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Quartu, M.; Carozzi, V.A.; Dorsey, S.G.; Serra, M.P.; Poddighe, L.; Picci, C.; Boi, M.; Melis, T.; Del Fiacco, M.; Meregalli, C.; et al. Bortezomib treatment produces nocifensive behavior and changes in the expression of TRPV1, CGRP, and substance P in the rat DRG, spinal cord, and sciatic nerve. BioMed Res. Int. 2014, 2014, 180428. [Google Scholar] [CrossRef]
- Starobova, H.; Vetter, I. Pathophysiology of chemotherapy-induced peripheral neuropathy. Front. Mol. Neurosci. 2017, 10, 174. [Google Scholar] [CrossRef]
- Talley, E.M.; Cribbs, L.L.; Lee, J.H.; Daud, A.; Perez-Reyes, E.; Bayliss, D.A. Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J. Neurosci. 1999, 19, 1895–1911. [Google Scholar] [CrossRef]
- Iftinca, M.C. Neuronal T-type calcium channels: What’s new? Iftinca: T-type channel regulation. J. Med. Life 2011, 4, 126–138. [Google Scholar]
- Rose, K.; Lunardi, N.; Boscolo, A.; Dong, X.; Erisir, A.; Jevtovic-Todorovic, V.; Todorovic, S. Immunohistological demonstration of CaV3.2 T-type voltage-gated calcium channel expression in soma of dorsal root ganglion neurons and peripheral axons of rat and mouse. Neuroscience 2013, 250, 263–274. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Peng, S.; Xiao, L.; Cheng, X.; Kuang, H.; Zhu, M.; Zhang, D.; Jiang, C.; Liu, T. Cell-type specific distribution of T-type calcium currents in lamina II neurons of the rat spinal cord. Front. Cell Neurosci. 2018, 12, 370. [Google Scholar] [CrossRef]
- Sekiguchi, F.; Kawabata, A. T-type calcium channels: Functional regulation and implication in pain signaling. J. Pharmacol. Sci. 2013, 122, 244–250. [Google Scholar] [CrossRef] [Green Version]
- Todorovic, S.M.; Jevtovic-Todorovic, V. The role of T-type calcium channels in peripheral and central pain processing. CNS Neurol. Disord. Drug Targets 2006, 5, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Zamponi, G.W.; Striessnig, J.; Koschak, A.; Dolphin, A.C. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol. Rev. 2015, 67, 821–870. [Google Scholar] [CrossRef] [Green Version]
- Todorovic, S.M.; Jevtovic-Todorovic, V.; Meyenburg, A.; Mennerick, S.; Perez-Reyes, E.; Romano, C.; Olney, J.W.; Zorumski, C.F. Redox modulation of T-type calcium channels in rat peripheral nociceptors. Neuron 2001, 31, 75–85. [Google Scholar] [CrossRef] [Green Version]
- Dogrul, A.; Gardell, L.R.; Ossipov, M.H.; Tulunay, F.C.; Lai, J.; Porreca, F. Reversal of experimental neuropathic pain by T-type calcium channel blockers. Pain 2003, 105, 159–168. [Google Scholar] [CrossRef]
- Bourinet, E.; Alloui, A.; Monteil, A.; Barrere, C.; Couette, B.; Poirot, O.; Pages, A.; McRory, J.; Snutch, T.P.; Eschalier, A.; et al. Silencing of the Cav3.2 T-type calcium channel gene in sensory neurons demonstrates its major role in nociception. EMBO J. 2005, 24, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Tomita, S.; Sekiguchi, F.; Deguchi, T.; Miyazaki, T.; Ikeda, Y.; Tsubota, M.; Yoshida, S.; Nguyen, H.D.; Okada, T.; Toyooka, N.; et al. Critical role of Ca(v)3.2 T-type calcium channels in the peripheral neuropathy induced by bortezomib, a proteasome-inhibiting chemotherapeutic agent, in mice. Toxicology 2019, 413, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Shipe, W.D.; Barrow, J.C.; Yang, Z.-Q.; Lindsley, C.W.; Yang, F.V.; Schlegel, K.-A.S.; Shu, Y.; Rittle, K.E.; Bock, M.G.; Hartman, G.D.; et al. Design, synthesis, and evaluation of a novel 4-aminomethyl-4-fluoropiperidine as a T-type Ca2+ channel antagonist. J. Med. Chem. 2008, 51, 3692–3695. [Google Scholar] [CrossRef]
- Egan, M.F.; Zhao, X.; Smith, A.; Troyer, M.D.; Uebele, V.N.; Pidkorytov, V.; Cox, K.; Murphy, M.; Snavely, D.; Lines, C.; et al. Randomized controlled study of the T-type calcium channel antagonist MK-8998 for the treatment of acute psychosis in patients with schizophrenia. Hum. Psychopharmacol. 2013, 28, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Papapetropoulos, S.; Lee, M.S.; Boyer, S.; Krouse, A.; Pahwa, R.; Lyons, K.; Ondo, W.; Jinnah, H.A.; Elble, R.J.; T-CALM Investigators. Proof-of-concept, double-blind, placebo-controlled study for CX-8998 a state-dependent T-type calcium (Cav3) channel antagonist in essential tremor patients (T-CALM): Efficacy and safety results. In Proceedings of the (Abstract presented at) Annual International Meeting of Parkinson’s Disease and Movement Disorders, Hong Kong, China, 5–9 October 2018. [Google Scholar]
- Meregalli, C.; Canta, A.; Carozzi, V.A.; Chiorazzi, A.; Oggioni, N.; Gilardini, A.; Ceresa, C.; Avezza, F.; Crippa, L.; Marmiroli, P.; et al. Bortezomib-induced painful neuropathy in rats: A behavioral, neurophysiological and pathological study in rats. Eur. J. Pain 2010, 14, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Meregalli, C.; Monza, L.; Chiorazzi, A.; Scali, C.; Guarnieri, C.; Fumagalli, G.; Alberti, P.; Pozzi, E.; Canta, A.; Ballarini, E.; et al. Human intravenous immunoglobulin alleviates neuropathic symptoms in a rat model of paclitaxel-induced peripheral neurotoxicity. Int. J. Mol. Sci. 2021, 22, 1058. [Google Scholar] [CrossRef] [PubMed]
- Canta, A.; Chiorazzi, A.; Carozzi, V.A.; Meregalli, C.; Oggioni, N.; Bossi, M.; Rodriguez-Menendez, V.; Avezza, F.; Crippa, L.; Lombardi, R.; et al. Age-related changes in the function and structure of the peripheral sensory pathway in mice. Neurobiol. Aging 2016, 45, 136–148. [Google Scholar] [CrossRef]
- Meregalli, C.; Chiorazzi, A.; Carozzi, V.A.; Canta, A.; Sala, B.; Colombo, M.; Oggioni, N.; Ceresa, C.; Foudah, D.; La Russa, F.; et al. Evaluation of tubulin polymerization and chronic inhibition of proteasome as citotoxicity mechanisms in bortezomib-induced peripheral neuropathy. Cell Cycle 2014, 13, 612–621. [Google Scholar] [CrossRef] [Green Version]
- Staff, N.P.; Podratz, J.L.; Grassner, L.; Bader, M.; Paz, J.; Knight, A.M.; Loprinzi, C.L.; Trushina, E.; Windebank, A.J. Bortezomib alters microtubule polymerization and axonal transport in rat dorsal root ganglion neurons. Neurotoxicology 2013, 39, 124–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meister, S.; Schubert, U.; Neubert, K.; Herrmann, K.; Burger, R.; Gramatzki, M.; Hahn, S.; Schreiber, S.; Wilhelm, S.; Herrmann, M.; et al. Extensive immunoglobulin production sensitizes myeloma cells for proteasome inhibition. Cancer Res. 2007, 67, 1783–1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loprinzi, C.L.; Lacchetti, C.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Hertz, D.L.; Kelley, M.R.; Lavino, A.; Lustberg, M.B.; Paice, J.A.; et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. J. Clin. Oncol. 2020, 38, 3325–3348. [Google Scholar] [CrossRef]
- Watanabe, M.; Ueda, T.; Shibata, Y.; Kumamoto, N.; Shimada, S.; Ugawa, S. Expression and regulation of Cav3.2 T-type calcium channels during inflammatory hyperalgesia in mouse dorsal root ganglion neurons. PLoS ONE 2015, 10, e0127572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obradovic, A.; Hwang, S.M.; Scarpa, J.; Hong, S.J.; Todorovic, S.M.; Jevtovic-Todorovic, V. CaV3.2 T-type calcium channels in peripheral sensory neurons are important for mibefradil-induced reversal of hyperalgesia and allodynia in rats with painful diabetic neuropathy. PLoS ONE 2014, 9, e91467. [Google Scholar] [CrossRef]
- Todorovic, S.M.; Jevtovic-Todorovic, V. Targeting of CaV3.2 T-type calcium channels in peripheral sensory neurons for the treatment of painful diabetic neuropathy. Pflug. Arch. 2014, 466, 701–706. [Google Scholar] [CrossRef]
- Francois, A.; Kerckhove, N.; Meleine, M.; Alloui, A.; Barrere, C.; Gelot, A.; Uebele, V.N.; Renger, J.J.; Eschalier, A.; Ardid, D.; et al. State-dependent properties of a new T-type calcium channel blocker enhance Ca(V)3.2 selectivity and support analgesic effects. Pain 2013, 154, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Tuohy, P.; Ma, C.; Kitamura, N.; Gomez, K.; Zhou, Y.; Ran, D.; Bellampalli, S.S.; Yu, J.; Luo, S.; et al. A modulator of the low-voltage activated T-type calcium channel that reverses HIV glycoprotein 120-, paclitaxel-, and spinal nerve ligation-induced peripheral neuropathies. Pain 2020, 161, 2551–2570. [Google Scholar] [CrossRef]
- Nagi, S.S.; Dunn, J.S.; Birznieks, I.; Vickery, R.M.; Mahns, D.A. The effects of preferential A- and C-fibre blocks and T-type calcium channel antagonist on detection of low-force monofilaments in healthy human participants. BMC Neurosci. 2015, 16, 52. [Google Scholar] [CrossRef] [Green Version]
- Kardosh, A.; Golden, E.B.; Pyrko, P.; Uddin, J.; Hofman, F.M.; Chen, T.C.; Louie, S.G.; Petasis, N.A.; Schonthal, A.H. Aggravated endoplasmic reticulum stress as a basis for enhanced glioblastoma cell killing by bortezomib in combination with celecoxib or its non-coxib analogue, 2,5-dimethyl-celecoxib. Cancer Res. 2008, 68, 843–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nawrocki, S.T.; Carew, J.S.; Pino, M.S.; Highshaw, R.A.; Dunner, K.; Huang, P.; Abbruzzese, J.L.; McConkey, D.J. Bortezomib sensitizes pancreatic cancer cells to endoplasmic reticulum stress-mediated apoptosis. Cancer Res. 2005, 65, 11658–11666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obeng, E.A.; Carlson, L.M.; Gutman, D.M.; Harrington, W.J., Jr.; Lee, K.P.; Boise, L.H. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood 2006, 107, 4907–4916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, G.J.; Doyle, T.; Salvemini, D. Mitotoxicity in distal symmetrical sensory peripheral neuropathies. Nat. Rev. Neurol. 2014, 10, 326–336. [Google Scholar] [CrossRef] [Green Version]
- Jacus, M.O.; Uebele, V.N.; Renger, J.J.; Todorovic, S.M. Presynaptic Cav3.2 channels regulate excitatory neurotransmission in nociceptive dorsal horn neurons. J. Neurosci. 2012, 32, 9374–9382. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meregalli, C.; Maricich, Y.; Cavaletti, G.; Canta, A.; Carozzi, V.A.; Chiorazzi, A.; Newbold, E.; Marmiroli, P.; Ceresa, C.; Diani, A.; et al. Reversal of Bortezomib-Induced Neurotoxicity by Suvecaltamide, a Selective T-Type Ca-Channel Modulator, in Preclinical Models. Cancers 2021, 13, 5013. https://doi.org/10.3390/cancers13195013
Meregalli C, Maricich Y, Cavaletti G, Canta A, Carozzi VA, Chiorazzi A, Newbold E, Marmiroli P, Ceresa C, Diani A, et al. Reversal of Bortezomib-Induced Neurotoxicity by Suvecaltamide, a Selective T-Type Ca-Channel Modulator, in Preclinical Models. Cancers. 2021; 13(19):5013. https://doi.org/10.3390/cancers13195013
Chicago/Turabian StyleMeregalli, Cristina, Yuri Maricich, Guido Cavaletti, Annalisa Canta, Valentina A. Carozzi, Alessia Chiorazzi, Evan Newbold, Paola Marmiroli, Cecilia Ceresa, Arthur Diani, and et al. 2021. "Reversal of Bortezomib-Induced Neurotoxicity by Suvecaltamide, a Selective T-Type Ca-Channel Modulator, in Preclinical Models" Cancers 13, no. 19: 5013. https://doi.org/10.3390/cancers13195013
APA StyleMeregalli, C., Maricich, Y., Cavaletti, G., Canta, A., Carozzi, V. A., Chiorazzi, A., Newbold, E., Marmiroli, P., Ceresa, C., Diani, A., Papapetropoulos, S., & Lee, M. S. (2021). Reversal of Bortezomib-Induced Neurotoxicity by Suvecaltamide, a Selective T-Type Ca-Channel Modulator, in Preclinical Models. Cancers, 13(19), 5013. https://doi.org/10.3390/cancers13195013







