Level of Combined Estrogen and Progesterone Receptor Expression Determines the Eligibility for Adjuvant Endocrine Therapy in Breast Cancer Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. IHC Staining
2.3. Statistical Analyses
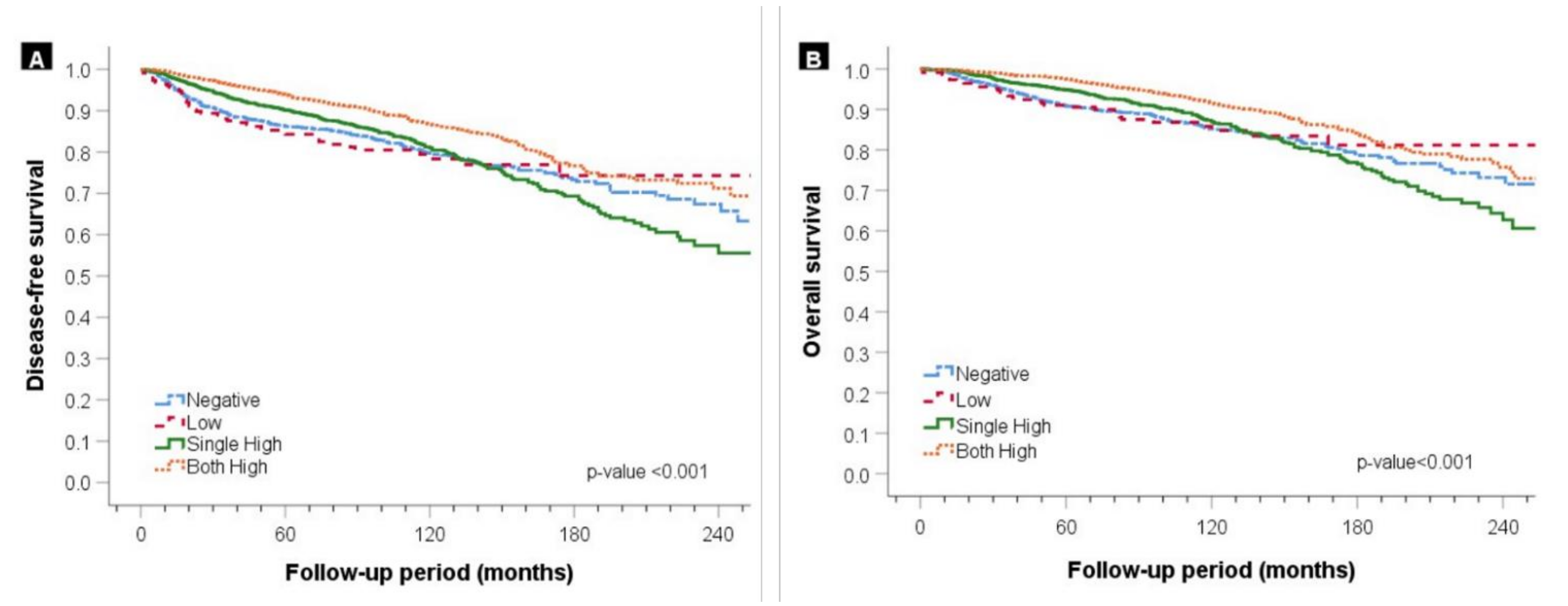
3. Results
3.1. Patient Characteristics
3.2. Survival Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Breast cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Acheampong, T.; Kehm, R.D.; Terry, M.B.; Argov, E.L.; Tehranifar, P. Incidence Trends of Breast Cancer Molecular Subtypes by Age and Race/Ethnicity in the US From 2010 to 2016. JAMA Netw Open 2020, 3, e2013226. [Google Scholar] [CrossRef]
- Harvey, J.M.; Clark, G.M.; Osborne, C.K.; Allred, D.C. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J. Clin. Oncol. 1999, 17, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.M.; Brookes, C.L.; Robson, T.; van de Velde, C.J.; Billingham, L.J.; Campbell, F.M.; Grant, M.; Hasenburg, A.; Hille, E.T.; Kay, C.; et al. Estrogen receptor and progesterone receptor as predictive biomarkers of response to endocrine therapy: A prospectively powered pathology study in the Tamoxifen and Exemestane Adjuvant Multinational trial. J. Clin. Oncol. 2011, 29, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J.; Lacchetti, C.; Anderson, H.; Buchholz, T.A.; Davidson, N.E.; Gelmon, K.A.; Giordano, S.H.; Hudis, C.A.; Solky, A.J.; Stearns, V.; et al. Adjuvant Endocrine Therapy for Women With Hormone Receptor-Positive Breast Cancer: ASCO Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2019, 37, 423–438. [Google Scholar] [CrossRef]
- Hammond, M.E.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 2010, 28, 2784–2795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, D.S.; Troester, M.A.; Usary, J.; Hu, Z.; He, X.; Fan, C.; Wu, J.; Carey, L.A.; Perou, C.M. Estrogen-regulated genes predict survival in hormone receptor-positive breast cancers. J. Clin. Oncol. 2006, 24, 1656–1664. [Google Scholar] [CrossRef] [Green Version]
- Prat, A.; Perou, C.M. Deconstructing the molecular portraits of breast cancer. Mol. Oncol. 2011, 5, 5–23. [Google Scholar] [CrossRef]
- Park, S.; Koo, J.S.; Kim, M.S.; Park, H.S.; Lee, J.S.; Lee, J.S.; Kim, S.I.; Park, B.W. Characteristics and outcomes according to molecular subtypes of breast cancer as classified by a panel of four biomarkers using immunohistochemistry. Breast 2012, 21, 50–57. [Google Scholar] [CrossRef]
- Deroo, B.J.; Korach, K.S. Estrogen receptors and human disease. J. Clin. Invest 2006, 116, 561–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, C.; Godwin, J.; Gray, R.; Clarke, M.; Cutter, D.; Darby, S.; McGale, P.; Pan, H.C.; Taylor, C.; Wang, Y.C.; et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet 2011, 378, 771–784. [Google Scholar] [PubMed] [Green Version]
- Iwamoto, T.; Booser, D.; Valero, V.; Murray, J.L.; Koenig, K.; Esteva, F.J.; Ueno, N.T.; Zhang, J.; Shi, W.; Qi, Y.; et al. Estrogen receptor (ER) mRNA and ER-related gene expression in breast cancers that are 1% to 10% ER-positive by immunohistochemistry. J. Clin. Oncol. 2012, 30, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.J. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Glick, J.H.; Gelber, R.D.; Coates, A.S.; Thürlimann, B.; Senn, H.J. Meeting highlights: International expert consensus on the primary therapy of early breast cancer 2005. Ann. Oncol. 2005, 16, 1569–1583. [Google Scholar] [CrossRef]
- Raghav, K.P.; Hernandez-Aya, L.F.; Lei, X.; Chavez-Macgregor, M.; Meric-Bernstam, F.; Buchholz, T.A.; Sahin, A.; Do, K.A.; Hortobagyi, G.N.; Gonzalez-Angulo, A.M. Impact of low estrogen/progesterone receptor expression on survival outcomes in breast cancers previously classified as triple negative breast cancers. Cancer 2012, 118, 1498–1506. [Google Scholar] [CrossRef] [Green Version]
- Balduzzi, A.; Bagnardi, V.; Rotmensz, N.; Dellapasqua, S.; Montagna, E.; Cardillo, A.; Viale, G.; Veronesi, P.; Intra, M.; Luini, A.; et al. Survival outcomes in breast cancer patients with low estrogen/progesterone receptor expression. Clin. Breast Cancer 2014, 14, 258–264. [Google Scholar] [CrossRef]
- Park, S.; Park, B.W.; Kim, T.H.; Jeon, C.W.; Kang, H.S.; Choi, J.E.; Hwang, K.T.; Kim, I.C. Lack of either estrogen or progesterone receptor expression is associated with poor survival outcome among luminal A breast cancer subtype. Ann. Surg. Oncol. 2013, 20, 1505–1513. [Google Scholar] [CrossRef]
- Dauphine, C.; Moazzez, A.; Neal, J.C.; Chlebowski, R.T.; Ozao-Choy, J. Single Hormone Receptor-Positive Breast Cancers Have Distinct Characteristics and Survival. Ann. Surg. Oncol. 2020, 27, 4687–4694. [Google Scholar] [CrossRef]
- Li, Y.; Yang, D.; Yin, X.; Zhang, X.; Huang, J.; Wu, Y.; Wang, M.; Yi, Z.; Li, H.; Li, H. Clinicopathological characteristics and breast cancer-specific survival of patients with single hormone receptor-positive breast cancer. JAMA Netw. Open 2020, 3, e1918160. [Google Scholar] [CrossRef] [Green Version]
- Edge, S.B.; Byrd, D.R.; Carducci, M.A.; Compton, C.C.; Fritz, A.; Greene, F. AJCC Cancer Staging Manual; Springer: New York, NY, USA, 2010; Volume 7, Available online: https://scholar.google.com/scholar_lookup?title=AJCC%20cancer%20staging%20manual&publication_year=2010 (accessed on 4 October 2021).
- Wolff, A.C.; Hammond, M.E.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. 2013, 31, 3997–4013. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Wood, W.C.; Coates, A.S.; Gelber, R.D.; Thürlimann, B.; Senn, H.J. Strategies for subtypes—Dealing with the diversity of breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann. Oncol. 2011, 22, 1736–1747. [Google Scholar] [CrossRef]
- Anderson, W.F.; Chen, B.E.; Jatoi, I.; Rosenberg, P.S. Effects of estrogen receptor expression and histopathology on annual hazard rates of death from breast cancer. Breast Cancer Res. Treat. 2006, 100, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Blows, F.M.; Driver, K.E.; Schmidt, M.K.; Broeks, A.; Van Leeuwen, F.E.; Wesseling, J.; Cheang, M.C.; Gelmon, K.; Nielsen, T.O.; Blomqvist, C. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: A collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010, 7, e1000279. [Google Scholar] [CrossRef]
- Colleoni, M.; Sun, Z.; Price, K.N.; Karlsson, P.; Forbes, J.F.; Thürlimann, B.; Gianni, L.; Castiglione, M.; Gelber, R.D.; Coates, A.S.; et al. Annual Hazard Rates of Recurrence for Breast Cancer During 24 Years of Follow-Up: Results From the International Breast Cancer Study Group Trials I to V. J. Clin. Oncol. 2016, 34, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, K.B.; Koseki, Y.; McGuire, W.L. Estrogen control of progesterone receptor in human breast cancer: Role of estradiol and antiestrogen. Endocrinology 1978, 103, 1742–1751. [Google Scholar] [CrossRef]
- Hilton, H.N.; Clarke, C.L.; Graham, J.D. Estrogen and progesterone signalling in the normal breast and its implications for cancer development. Mol. Cell Endocrinol. 2018, 466, 2–14. [Google Scholar] [CrossRef]
- Kunc, M.; Biernat, W.; Senkus-Konefka, E. Estrogen receptor-negative progesterone receptor-positive breast cancer—“Nobody’s land” or just an artifact? Cancer Treat. Rev. 2018, 67, 78–87. [Google Scholar] [CrossRef]
- De Maeyer, L.; Van Limbergen, E.; De Nys, K.; Moerman, P.; Pochet, N.; Hendrickx, W.; Wildiers, H.; Paridaens, R.; Smeets, A.; Christiaens, M.-R. Does estrogen receptor negative/progesterone receptor positive breast carcinoma exist? J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 335–338. [Google Scholar] [CrossRef]
- Hefti, M.M.; Hu, R.; Knoblauch, N.W.; Collins, L.C.; Haibe-Kains, B.; Tamimi, R.M.; Beck, A.H. Estrogen receptor negative/progesterone receptor positive breast cancer is not a reproducible subtype. Breast Cancer Res. 2013, 15, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Guideline Update. Arch. Pathol. Lab. Med. 2020, 144, 545–563. [Google Scholar] [CrossRef] [Green Version]
- Bae, S.Y.; Kim, S.; Lee, J.H.; Lee, H.C.; Lee, S.K.; Kil, W.H.; Kim, S.W.; Lee, J.E.; Nam, S.J. Poor prognosis of single hormone receptor- positive breast cancer: Similar outcome as triple-negative breast cancer. BMC Cancer 2015, 15, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kok, M.; Koornstra, R.H.; Mook, S.; Hauptmann, M.; Fles, R.; Jansen, M.P.; Berns, E.M.; Linn, S.C.; Van’t Veer, L.J. Additional value of the 70-gene signature and levels of ER and PR for the prediction of outcome in tamoxifen-treated ER-positive breast cancer. Breast 2012, 21, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Truin, W.; Roumen, R.M.H.; Siesling, S.; van de Vijver, K.K.; Tjan-Heijnen, V.C.G.; Voogd, A.C. Estrogen and progesterone receptor expression levels do not differ between lobular and ductal carcinoma in patients with hormone receptor-positive tumors. Breast Cancer Res. Treat. 2017, 164, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Borras, M.; Lacroix, M.; Legros, N.; Leclercq, G. Estrogen receptor-negative/progesterone receptor-positive Evsa-T mammary tumor cells: A model for assessing the biological property of this peculiar phenotype of breast cancers. Cancer Lett. 1997, 120, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Kiani, J.; Khan, A.; Khawar, H.; Shuaib, F.; Pervez, S. Estrogen receptor alpha-negative and progesterone receptor-positive breast cancer: Lab error or real entity? Pathol. Oncol. Res. 2006, 12, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.C.; Botero, M.L.; Schnitt, S.J. Bimodal frequency distribution of estrogen receptor immunohistochemical staining results in breast cancer: An analysis of 825 cases. Am. J. Clin. Pathol. 2005, 123, 16–20. [Google Scholar] [CrossRef]

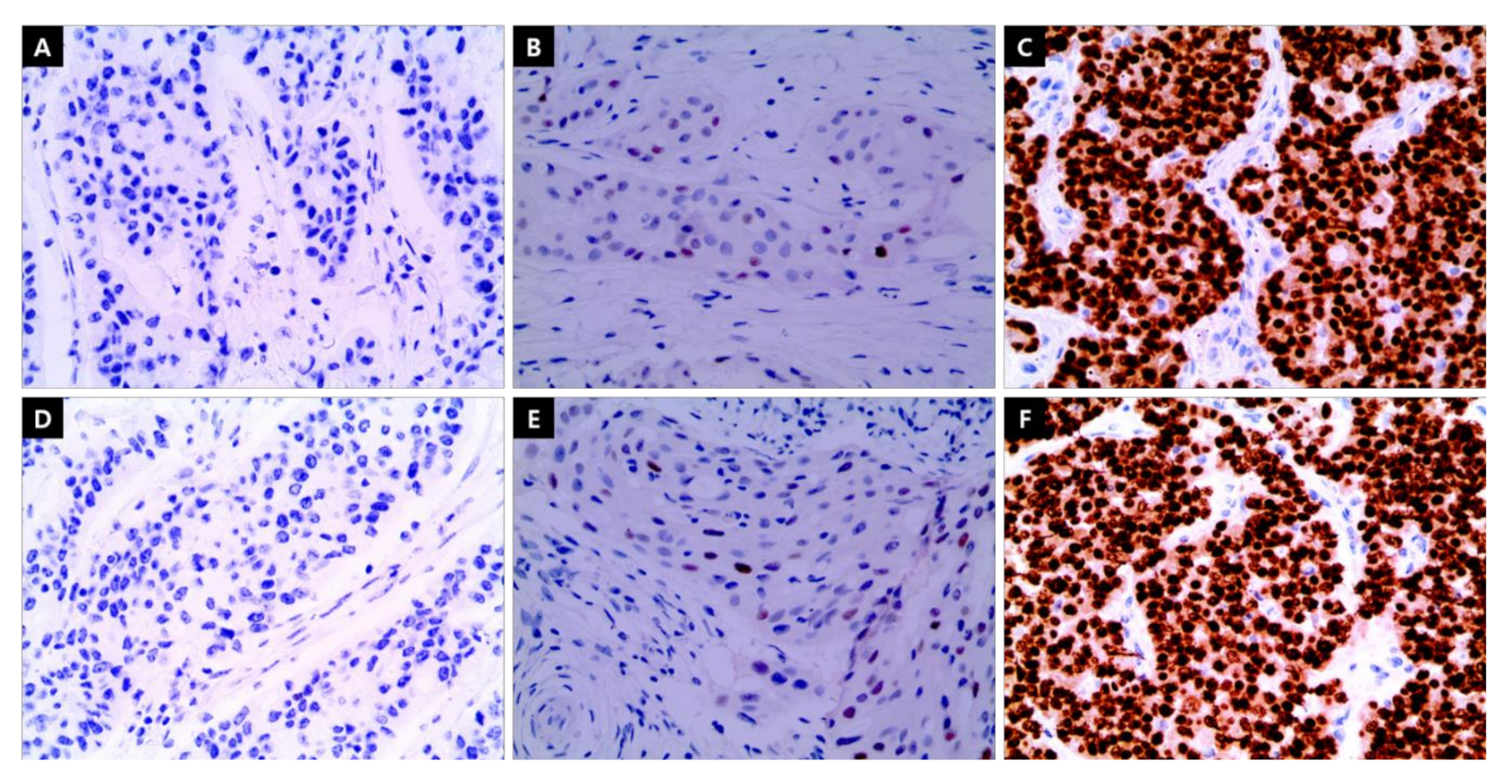
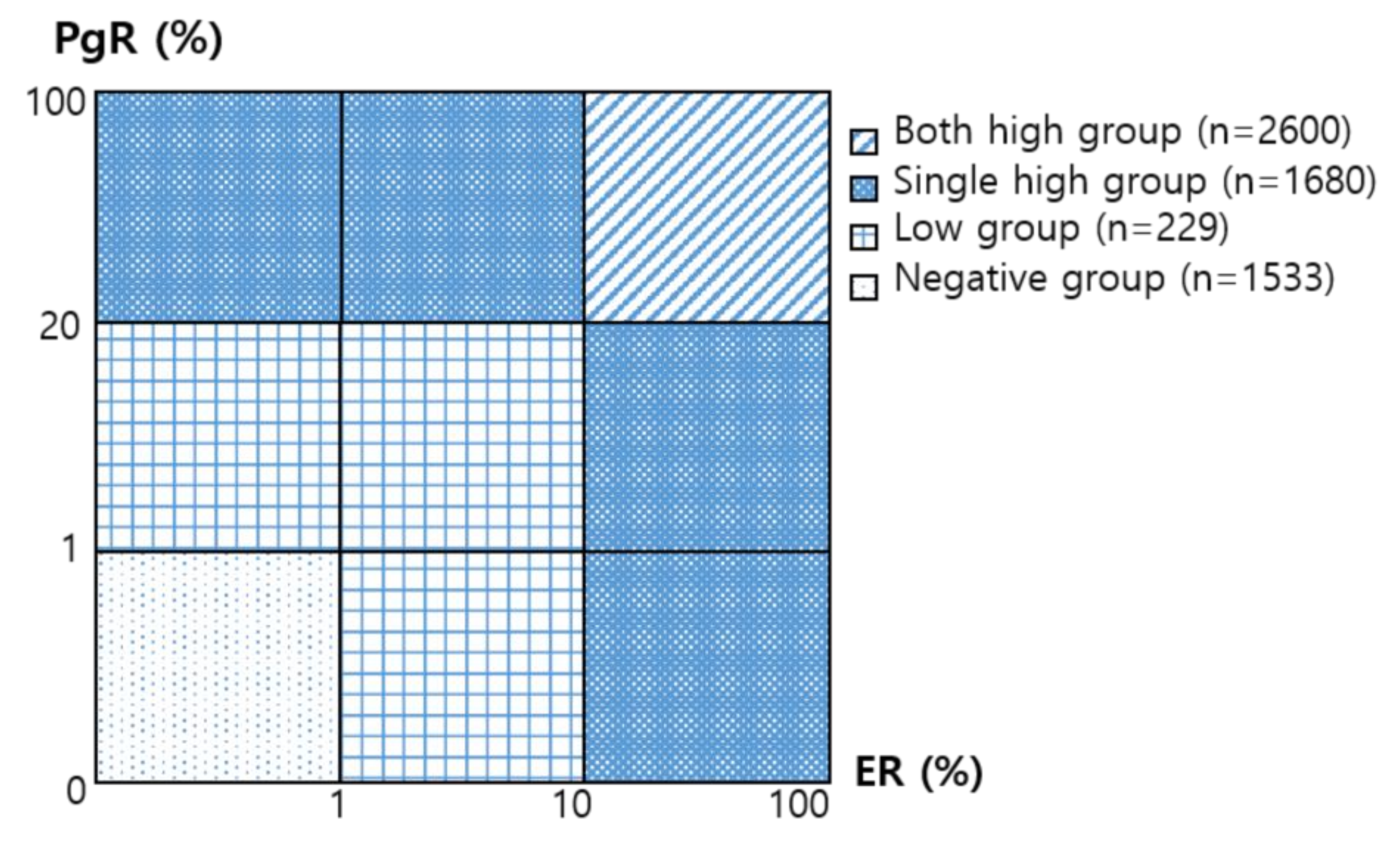
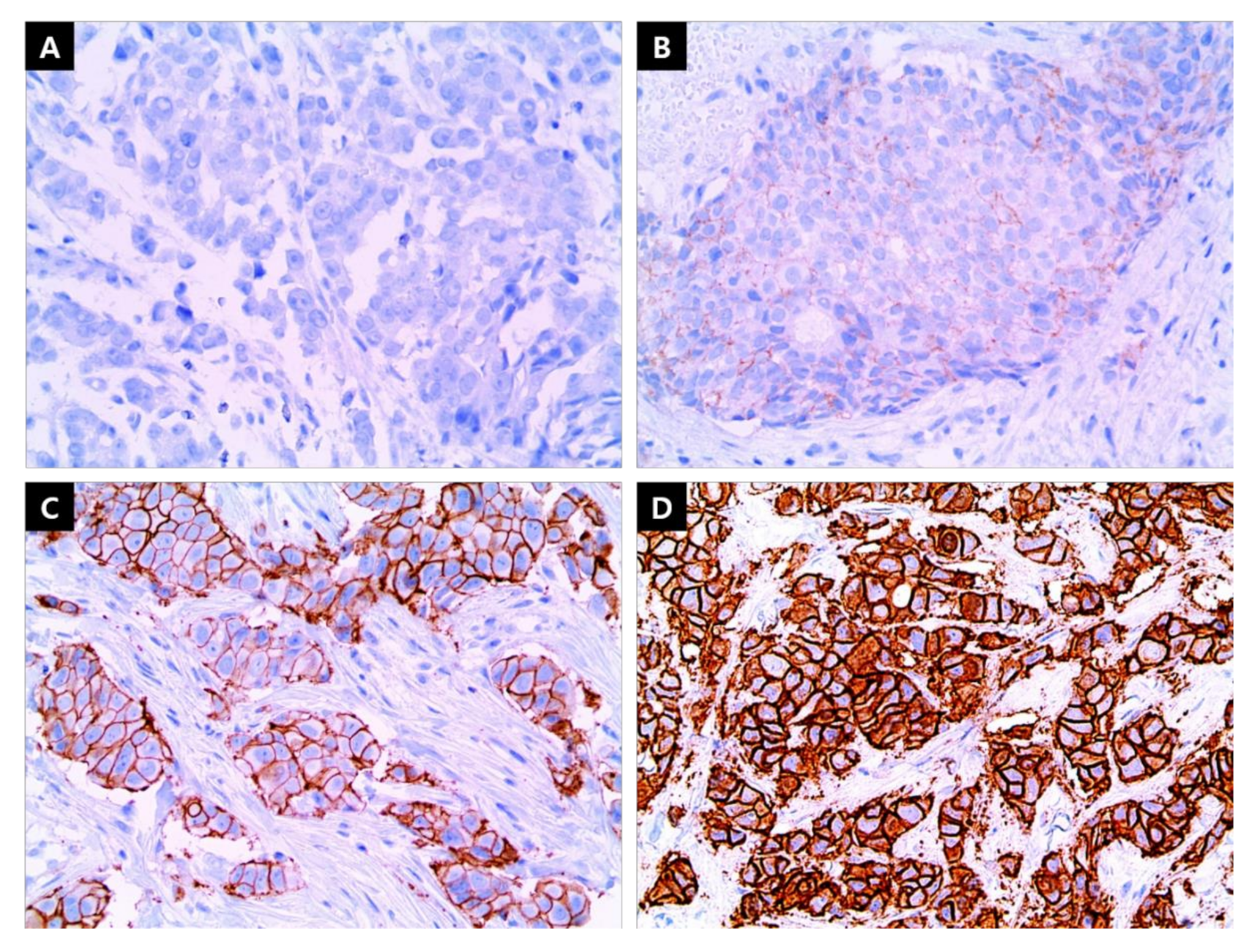
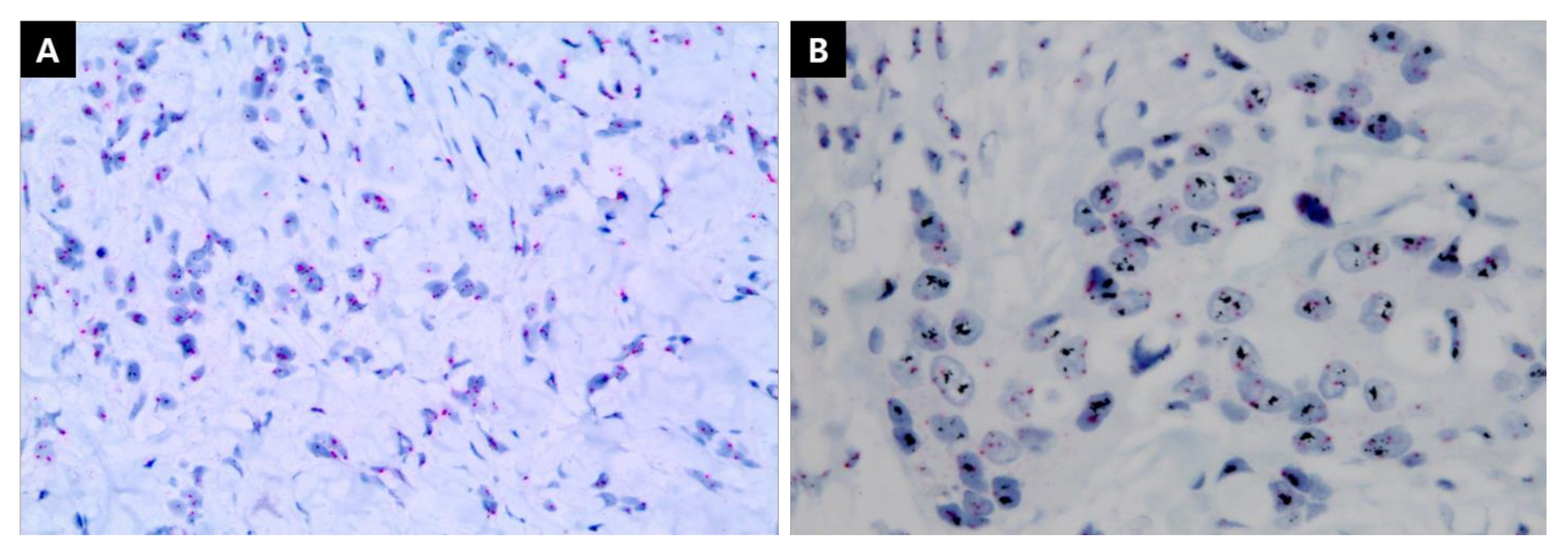
| Group of ER/PgR Level Combination | |||||
|---|---|---|---|---|---|
| Negative n = 1533 (%) | Low n = 229 (%) | Single High n = 1680 (%) | Both High n = 2600 (%) | p-Value | |
| Patient Age (Years) | <0.001 | ||||
| <50 | 709 (46.2) | 110 (48.0) | 691 (41.1) | 1595 (61.3) | |
| ≥50 | 824 (53.8) | 119 (52.0) | 989 (58.9) | 1005 (38.7) | |
| BMI (kg/m2) | <0.001 | ||||
| <23 | 746 (48.7) | 104 (45.4) | 815 (48.5) | 1362 (54.4) | |
| ≥23 | 739 (48.2) | 120 (52.4) | 836 (49.8) | 1210 (46.5) | |
| Unknown | 48 (3.1) | 5 (2.2) | 29 (1.7) | 28 (1.1) | |
| Tumor Histology | <0.001 | ||||
| Ductal | 1366 (89.1) | 208 (90.8) | 1463 (87.1) | 2261 (87.0) | |
| Lobular | 15 (1.0) | 5 (2.2) | 60 (3.6) | 132 (5.0) | |
| Other | 152 (9.9) | 16 (7.0) | 157 (9.3) | 207 (8.0) | |
| Tumor Stage | <0.001 | ||||
| T1 | 857 (55.9) | 132 (57.6) | 1129 (67.2) | 1810 (69.6) | |
| T2 | 638 (41.6) | 90 (39.3) | 522 (31.1) | 764 (29.4) | |
| T3, T4 | 38 (2.5) | 7 (3.1) | 29 (1.7) | 26 (1.0) | |
| Node Stage | <0.001 | ||||
| N0 | 1150 (75.0) | 163 (71.2) | 1141 (67.9) | 1782 (68.5) | |
| N1 | 272 (17.7) | 39 (17.0) | 383 (22.8) | 602 (23.2) | |
| N2 | 70 (4.6) | 17 (7.4) | 92 (5.5) | 153 (5.9) | |
| N3 | 41 (2.7) | 10 (4.4) | 64 (3.8) | 63 (2.4) | |
| Histologic Grade | <0.001 | ||||
| I | 84 (5.4) | 17 (7.5) | 447 (26.6) | 919 (35.3) | |
| II | 530 (34.6) | 110 (48.0) | 876 (52.1) | 1297 (49.9) | |
| III | 909 (59.3) | 93 (40.6) | 300 (17.9) | 290 (11.2) | |
| Unknown | 10 (0.7) | 9 (3.9) | 57 (3.4) | 94 (3.6) | |
| HER2 Overexpression | <0.001 | ||||
| Negative | 1013 (66.1) | 139 (60.7) | 1307 (77.8) | 2191 (84.3) | |
| Positive | 414 (27.0) | 72 (31.4) | 252 (15.0) | 225 (8.7) | |
| Undetermined | 106 (6.9) | 18 (7.9) | 121 (7.2) | 184 (7.1) | |
| Breast Surgery (Mastectomy) | 0.133 | ||||
| Partial | 689 (44.9) | 93 (40.6) | 801 (47.7) | 1215 (46.7) | |
| Total | 844 (55.1) | 136 (59.4) | 879 (52.3) | 1385 (53.3) | |
| Radiation Therapy | 0.093 | ||||
| None | 710 (46.3) | 110 (48.0) | 713 (42.4) | 1139 (43.8) | |
| Performed | 823 (53.7) | 119 (52.0) | 967 (57.6) | 1461 (56.2) | |
| Adjuvant Chemotherapy | <0.001 | ||||
| None | 314 (20.5) | 41 (17.9) | 624 (37.1) | 1042 (40.1) | |
| Performed | 1219 (79.5) | 188 (82.1) | 1056 (62.9) | 1558 (59.9) | |
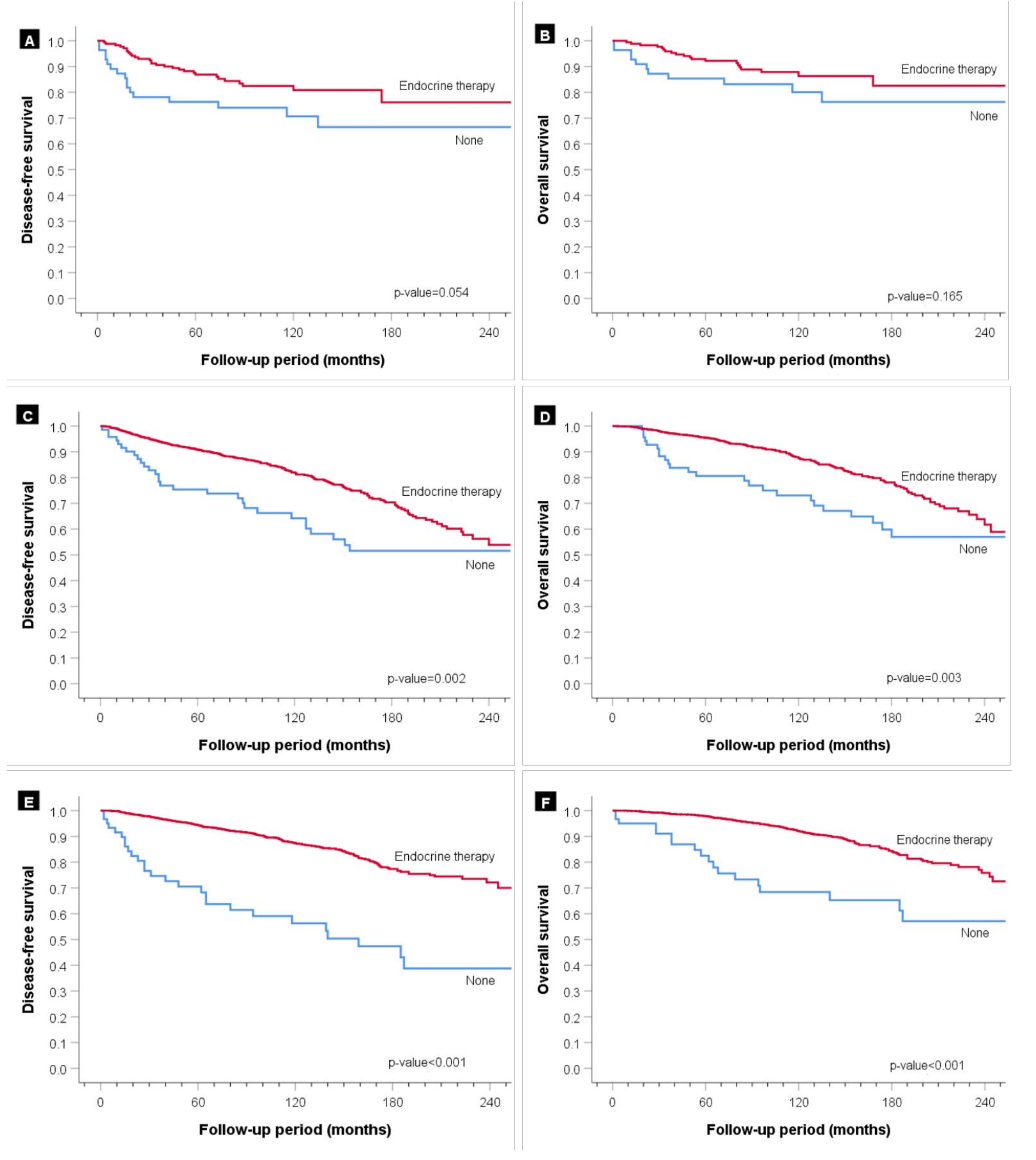
| Endocrine Therapy | <0.001 | ||||
| None | 1480 (96.5) | 56 (24.5) | 72 (4.3) | 66 (2.5) | |
| Performed | 53 (3.5) | 173 (75.5) | 1608 (95.7) | 2534 (97.5) | |
| Disease-Free Survival | Overall Survival | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| Patient Age (Years) | 0.486 | <0.001 | ||
| <50 | 1 | 1 | ||
| ≥50 | 1.05 (0.92–1.20) | 1.48 (1.25–1.74) | ||
| BMI (kg/m2) | <0.001 | <0.001 | ||
| <23 | 1 | 1 | ||
| ≥23, Unknown | 1.41 (1.24–1.61) | 1.43 (1.22–1.68) | ||
| Tumor Size | <0.001 | <0.001 | ||
| ≤2 cm | 1 | 1 | ||
| >2 cm | 1.73 (1.50–1.99) | 1.65 (1.40–1.95) | ||
| Node Metastasis | <0.001 | <0.001 | ||
| Negative | 1 | 1 | ||
| Positive | 1.97 (1.69–2.29) | 1.98 (1.65–2.37) | ||
| Histologic Grade | 0.193 | 0.682 | ||
| I, II | 1 | 1 | ||
| III, Unknown | 1.10 (0.95–1.28) | 1.04 (0.87–1.23) | ||
| Group of ER/PR Level | ||||
| Negative | 0.71 (0.51–0.99) | 0.046 | 1.04 (0.73–1.49) | 0.810 |
| Low | 1.25 (0.89–1.75) | 0.204 | 1.27 (0.85–1.90) | 0.238 |
| Single high | 1.33 (1.14–1.56) | <0.001 | 1.42 (1.18–1.72) | <0.001 |
| Both high | 1 | 1 | ||
| HER2 Overexpression | ||||
| Negative | 1 | 1 | ||
| Positive | 0.81 (0.67–0.97) | 0.021 | 0.92 (0.74–1.14) | 0.463 |
| Undetermined | 1.98 (1.62–2.42) | <0.001 | 1.48 (1.18–1.86) | 0.001 |
| Breast Surgery (Mastectomy) | <0.001 | <0.001 | ||
| Partial | 1 | 1 | ||
| Total | 1.89 (1.53–2.33) | 1.97 (1.53–2.54) | ||
| Radiation Therapy | 0.640 | 0.239 | ||
| None | 1 | 1 | ||
| Performed | 0.96 (0.79–1.15) | 1.14 (0.92–1.41) | ||
| Adjuvant Chemotherapy | 0.002 | <0.001 | ||
| None | 1 | 1 | ||
| Performed | 0.77 (0.65–0.91) | 0.64 (0.53–0.79) | ||
| Endocrine Therapy | <0.001 | 0.001 | ||
| None | 1 | 1 | ||
| Performed | 0.41 (0.30–0.55) | 0.60 (0.44–0.82) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, J.H.; Choi, S.B.; Park, J.M.; Kim, J.Y.; Park, H.S.; Kim, S.I.; Park, B.-W.; Park, S. Level of Combined Estrogen and Progesterone Receptor Expression Determines the Eligibility for Adjuvant Endocrine Therapy in Breast Cancer Patients. Cancers 2021, 13, 5007. https://doi.org/10.3390/cancers13195007
Ahn JH, Choi SB, Park JM, Kim JY, Park HS, Kim SI, Park B-W, Park S. Level of Combined Estrogen and Progesterone Receptor Expression Determines the Eligibility for Adjuvant Endocrine Therapy in Breast Cancer Patients. Cancers. 2021; 13(19):5007. https://doi.org/10.3390/cancers13195007
Chicago/Turabian StyleAhn, Jee Hyun, Soon Bo Choi, Jung Min Park, Jee Ye Kim, Hyung Seok Park, Seung Il Kim, Byeong-Woo Park, and Seho Park. 2021. "Level of Combined Estrogen and Progesterone Receptor Expression Determines the Eligibility for Adjuvant Endocrine Therapy in Breast Cancer Patients" Cancers 13, no. 19: 5007. https://doi.org/10.3390/cancers13195007
APA StyleAhn, J. H., Choi, S. B., Park, J. M., Kim, J. Y., Park, H. S., Kim, S. I., Park, B.-W., & Park, S. (2021). Level of Combined Estrogen and Progesterone Receptor Expression Determines the Eligibility for Adjuvant Endocrine Therapy in Breast Cancer Patients. Cancers, 13(19), 5007. https://doi.org/10.3390/cancers13195007






