A Modified Intraperitoneal Chemotherapy Regimen for Ovarian Cancer: Technique and Treatment Outcomes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics
3.2. Safety Outcomes and Completion Rates
3.3. Survival Outcomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, S.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Lim, M.C.; Won, Y.J.; Ko, M.J.; Kim, M.; Shim, S.H.; Suh, D.H.; Kim, J.W. Incidence of cervical, endometrial, and ovarian cancer in Korea during 1999-2015. J. Gynecol. Oncol. 2019, 30, e38. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.K.; Bundy, B.; Wenzel, L.; Huang, H.Q.; Baergen, R.; Lele, S.; Copeland, L.J.; Walker, J.L.; Burger, R.A. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N. Engl. J. Med. 2006, 354, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Alberts, D.S.; Liu, P.Y.; Hannigan, E.V.; O’Toole, R.; Williams, S.D.; Young, J.A.; Franklin, E.W.; Clarke-Pearson, D.L.; Malviya, V.K.; DuBeshter, B. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N. Engl. J. Med. 1996, 335, 1950–1955. [Google Scholar] [CrossRef] [PubMed]
- Markman, M.; Bundy, B.N.; Alberts, D.S.; Fowler, J.M.; Clark-Pearson, D.L.; Carson, L.F.; Wadler, S.; Sickel, J. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: An intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2001, 19, 1001–1007. [Google Scholar] [CrossRef]
- Walker, J.L.; Armstrong, D.K.; Huang, H.Q.; Fowler, J.; Webster, K.; Burger, R.A.; Clarke-Pearson, D. Intraperitoneal catheter outcomes in a phase III trial of intravenous versus intraperitoneal chemotherapy in optimal stage III ovarian and primary peritoneal cancer: A Gynecologic Oncology Group Study. Gynecol. Oncol. 2006, 100, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Teefey, P.; Zgheib, N.B.; Apte, S.M.; Gonzalez-Bosquet, J.; Judson, P.L.; Roberts, W.S.; Lancaster, J.M.; Wenham, R.M. Factors associated with improved toxicity and tolerability of intraperitoneal chemotherapy in advanced-stage epithelial ovarian cancers. Am. J. Obstet. Gynecol. 2013, 208, 501.e1–501.e7. [Google Scholar] [CrossRef] [PubMed]
- Barlin, J.N.; Dao, F.; Bou Zgheib, N.; Ferguson, S.E.; Sabbatini, P.J.; Hensley, M.L.; Bell-McGuinn, K.M.; Konner, J.; Tew, W.P.; Aghajanian, C.; et al. Progression-free and overall survival of a modified outpatient regimen of primary intravenous/intraperitoneal paclitaxel and intraperitoneal cisplatin in ovarian, fallopian tube, and primary peritoneal cancer. Gynecol. Oncol. 2012, 125, 621–624. [Google Scholar] [CrossRef]
- Skaznik-Wikiel, M.E.; Lesnock, J.L.; McBee, W.C.; Beriwal, S.; Zorn, K.K.; Richard, S.D.; Krivak, T.C.; Edwards, R.P. Intraperitoneal chemotherapy for recurrent epithelial ovarian cancer is feasible with high completion rates, low complications, and acceptable patient outcomes. Int. J. Gynecol. Cancer 2012, 22, 232–237. [Google Scholar] [CrossRef]
- May, T.; Altman, A.; McGee, J.; Lu, L.; Xu, W.; Lane, K.; Ghatage, P.; Rosen, B. Examining Survival Outcomes of 852 Women With Advanced Ovarian Cancer: A Multi-institutional Cohort Study. Int. J. Gynecol. Cancer 2018, 28, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Markman, M.; Brady, M.F.; Spirtos, N.M.; Hanjani, P.; Rubin, S.C. Phase II trial of intraperitoneal paclitaxel in carcinoma of the ovary, tube, and peritoneum: A Gynecologic Oncology Group Study. J. Clin. Oncol. 1998, 16, 2620–2624. [Google Scholar] [CrossRef]
- Lim, M.C.; Lee, H.S.; Jung, D.C.; Choi, J.Y.; Seo, S.S.; Park, S.Y. Pathological diagnosis and cytoreduction of cardiophrenic lymph node and pleural metastasis in ovarian cancer patients using video-assisted thoracic surgery. Ann. Surg. Oncol. 2009, 16, 1990–1996. [Google Scholar] [CrossRef] [PubMed]
- Chambers, L.M.; Son, J.; Radeva, M.; DeBernardo, R. Evaluation of non-completion of intraperitoneal chemotherapy in patients with advanced epithelial ovarian cancer. J. Gynecol. Oncol. 2019, 30, e93. [Google Scholar] [CrossRef] [PubMed]
- Gockley, A.A.; Fiascone, S.; Courant, K.H.; Pepin, K.; Carmen, M.D.; Clark, R.M.; Goldberg, J.; Horowitz, N.; Berkowitz, R.; Worley, W., Jr. Clinical characteristics and outcomes after bowel surgery and ostomy formation at the time of debulking surgery for advanced-stage epithelial ovarian carcinoma. Int. J. Gynecol. Cancer 2019, 29, 585–592. [Google Scholar] [CrossRef]
- López-López, V.; Lynn, P.B.; Gil, J.; García-Salom, M.; Gil, E.; González, A.; Muñoz, I.P.; Cascales-Campos, P.A. Effect of Paclitaxel-based Hyperthermic Intraperitoneal Chemotherapy (HIPEC) on colonic anastomosis in a rat model. Clin. Transl. Oncol. 2019, 21, 505–511. [Google Scholar] [CrossRef]
- Makrin, V.; Lev-Chelouche, D.; Even Sapir, E.; Paran, H.; Rabau, M.; Gutman, M. Intraperitoneal heated chemotherapy affects healing of experimental colonic anastomosis: An animal study. J. Surg. Oncol. 2005, 89, 18–22. [Google Scholar] [CrossRef]
- Arikan, A.Y.; Günal, O.; Pehlivan, M.; Alper, M. The effect of intraperitoneal paclitaxel administration on colonic anastomosis. Hepatogastroenterology 2000, 47, 1273–1276. [Google Scholar]
- Kanellos, D.; Pramateftakis, M.G.; Demetriades, H.; Zacharakis, E.; Angelopoulos, S.; Mantzoros, I.; Kanellos, I.; Despoudi, K.; Zaraboukas, T.; Koliakos, G.; et al. Healing of colonic anastomoses after immediate postoperative intraperitoneal administration of oxaliplatin. Int. J. Colorectal. Dis. 2008, 23, 1185–1191. [Google Scholar] [CrossRef]
- Kay, A.H.; Urban, R.R.; Gray, H.J. Intraperitoneal ports placed at the time of bowel resection for treatment of ovarian cancer: Complications and surgical outcomes. Gynecol. Oncol. 2019, 155, 220–223. [Google Scholar] [CrossRef]
- Nica, A.; Covens, A.; Parra-Herran, C.; May, T. Does timing of intraperitoneal chemotherapy initiation following primary cytoreductive surgery with bowel resection impact outcomes in patients with advanced ovarian cancer? Gynecol. Oncol. 2020, 158, 622–630. [Google Scholar] [CrossRef]
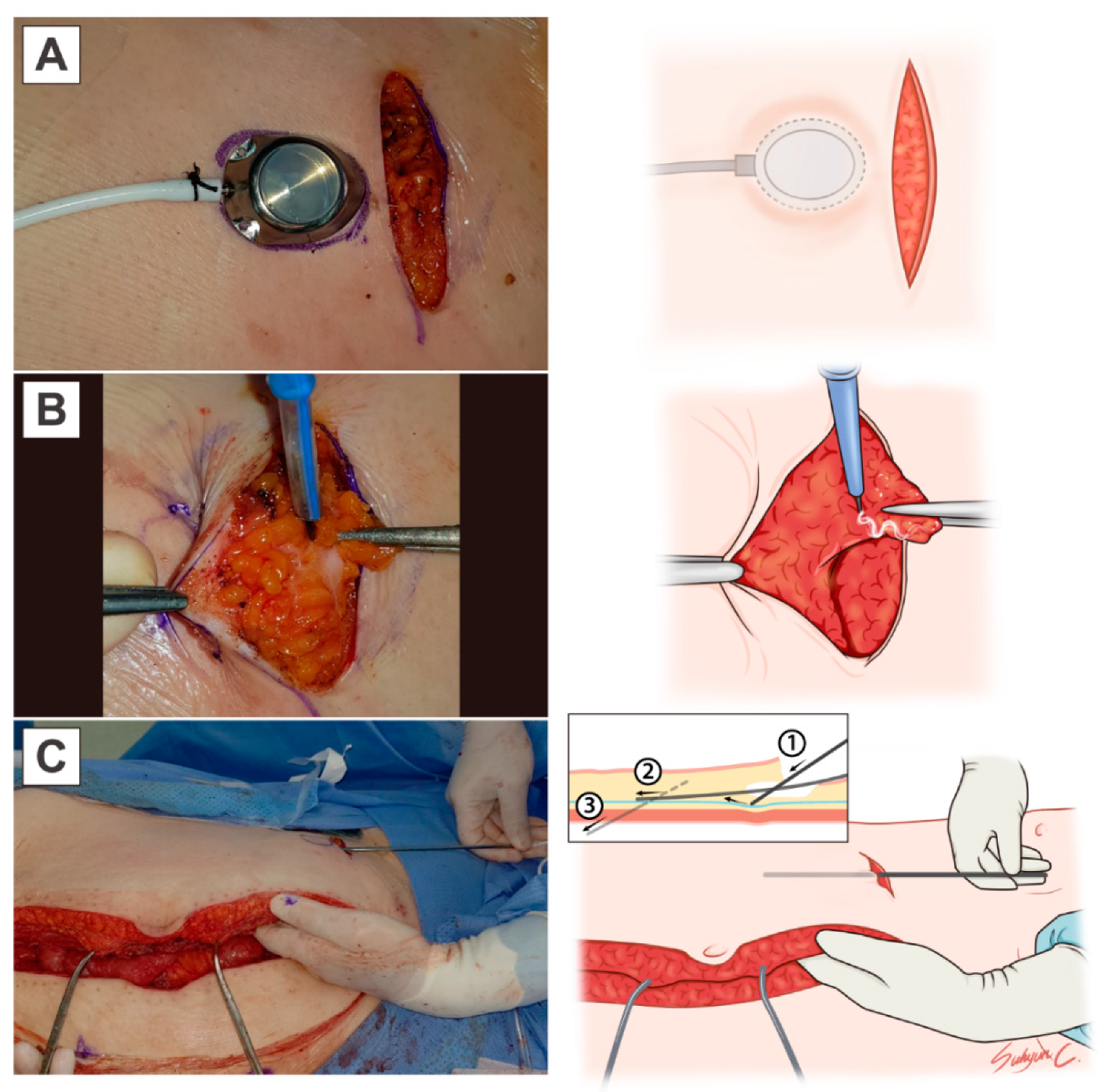

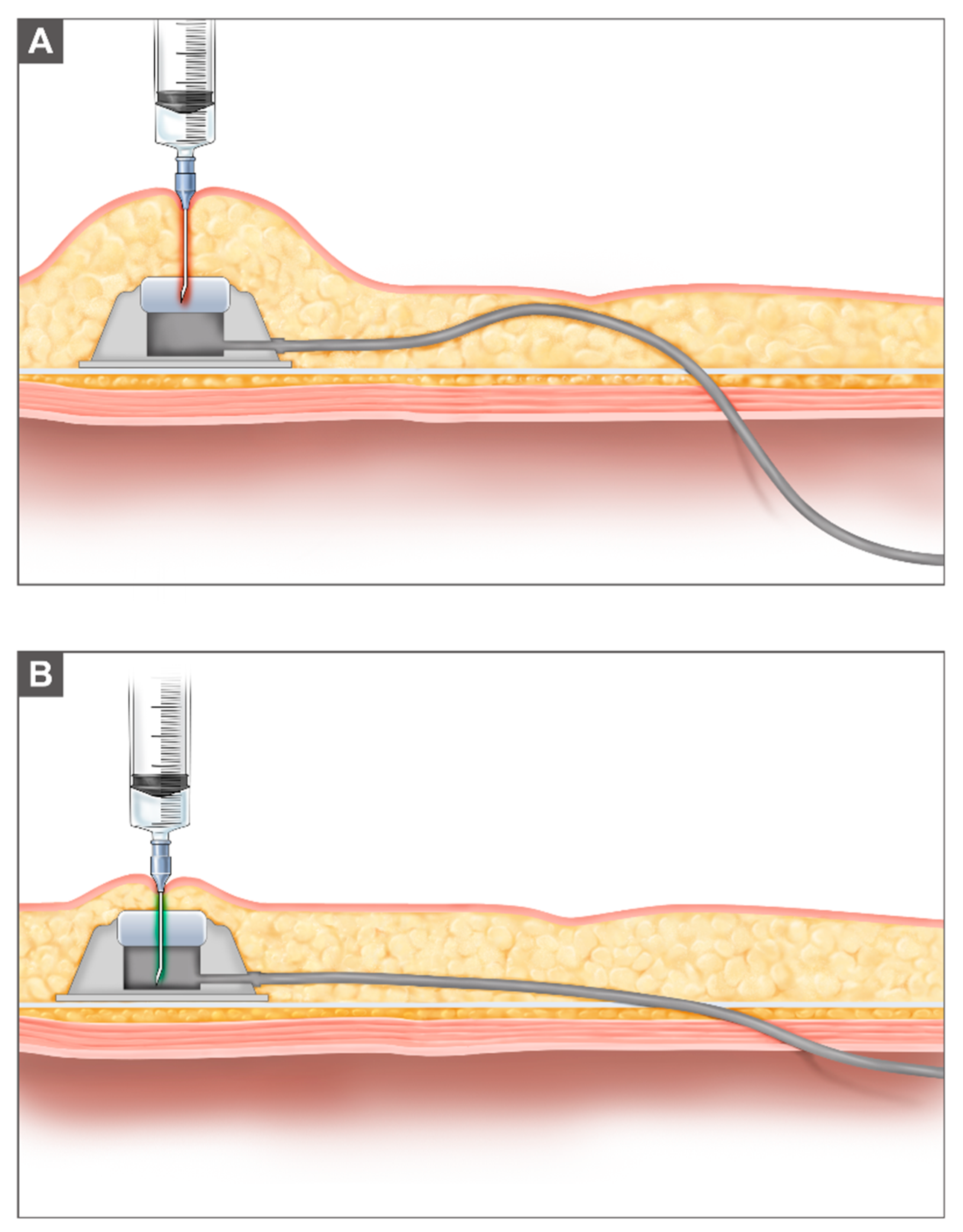
| Characteristics | Number of Patients (%) |
|---|---|
| N = 27 | |
| Age (years) | |
| Mean ± SD (years) | 60.9 ± 12.0 |
| Median(min–max) (years) | 59.0 (34.0–80.0) |
| FIGO surgical stage (FIGO stage 2014) | |
| IIIA | 2 (7.4%) |
| IIIB | 7 (25.9%) |
| IIIC | 15 (55.6%) |
| IVB | 3 (11.1%) |
| Histology (No.) | |
| High grade serous | 25 (92.6%) |
| Endometrioid | 0 (0.0%) |
| Clear cell carcinoma | 0 (0.0%) |
| Mucinous | 0 (0.0%) |
| Low grade serous | 0 (0.0%) |
| Others | 2 (7.4%) |
| Histologic grade | |
| Grade 1 | 1 (3.7%) |
| Grade 2 | 6 (22.2%) |
| Grade 3 | 20 (74.1%) |
| Primary disease site (No.) | |
| Ovary | 27 (100%) |
| Peritoneum | 0 (0.0%) |
| Fallopian tube | 0 (0.0%) |
| Previous surgery | |
| Primary | |
| Primary cytoreductive surgery | 13 (48.2%) |
| Interval debulking surgery | 4 (14.8%) |
| Secondary | 10 (37.0%) |
| Residual tumor | |
| R0 (no residual tumor) | 23 (85.2%) |
| R1 (microscopic) | 4 (14.8%) |
| R2 (macroscopic) | 0 (0.0%) |
| Left colonic surgery | |
| No | 12 (44.4%) |
| Yes | 15 (55.6%) |
| FIGO, International Federation of Gynecology and Obstetrics |
| Variables | N = 27 |
|---|---|
| Institutional Modification | |
| Modified GOG 172 regimen | 27 (100%) |
| Warmed dextrose IP infusion | 26 (96.3%) |
| IV cisplatin Day 2 when left colonic surgery | 23 (85.2%) |
| Cycle Completion–All | |
| Cycle ≤ 2 (number, %) | 8 (29.6%) |
| 2 < cycle < 6 (number, %) | 2 (7.4%) |
| Cycle 6 (number, %) | 17 (63.0%) |
| Cycle Completion–Primary | |
| Cycle ≤ 2 (number, %) | 3 (17.7%) |
| 2 < cycle < 6 (number, %) | 1 (5.9%) |
| Cycle 6 (number, %) | 13 (76.5%) |
| Reason for Discontinuation of IP chemotherapy–All | |
| Grade 3–4 Gastrointestinal disorder | 3 (11.1%) |
| Loss to follow-up | 3 (11.1%) |
| IP port obstruction | 2 (7.4%) |
| IP port infection | 1 (3.7%) |
| Disease progression | 1 (3.7%) |
| Left Colonic Surgery | p-Value (1) | ||
|---|---|---|---|
| No. of Cycles | No. (N = 12) | Yes (N = 15) | |
| 1 | 2 (16.7%) | 2 (13.3%) | 0.8691 |
| 2 | 1 (8.3%) | 3 (20.0%) | |
| 3 | 0 (0.0%) | 1 (6.7%) | |
| 4 | 0 (0.0%) | 0 (0.0%) | |
| 5 | 0 (0.0%) | 1 (6.7%) | |
| 6 | 9 (75.0%) | 8 (53.3%) | |
| Adverse Event | N = 27 |
|---|---|
| Anemia | 0 (0.0%) |
| Neutropenia | 8 (29.6%) |
| Thrombocytopenia | 0 (0.0%) |
| aFever | 6 (22.2%) |
| Gastrointestinal | 9 (33.3%) |
| Infection | 8 (29.6%) |
| Renal | 1 (3.7%) |
| Metabolic | 0 (0.0%) |
| Neurologic | 0 (0.0%) |
| IP port related | |
| IP port obstruction | 2 (7.4%) |
| IP port infection | 1 (3.7%) |
| IP port leakage | 1 (3.7%) |
| CTCAE 5.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Ha, H.I.; Kim, M.H.; Han, M.R.; Park, S.-Y.; Lim, M.C. A Modified Intraperitoneal Chemotherapy Regimen for Ovarian Cancer: Technique and Treatment Outcomes. Cancers 2021, 13, 4886. https://doi.org/10.3390/cancers13194886
Kim JH, Ha HI, Kim MH, Han MR, Park S-Y, Lim MC. A Modified Intraperitoneal Chemotherapy Regimen for Ovarian Cancer: Technique and Treatment Outcomes. Cancers. 2021; 13(19):4886. https://doi.org/10.3390/cancers13194886
Chicago/Turabian StyleKim, Ji Hyun, Hyeong In Ha, Min Hae Kim, Mi Ra Han, Sang-Yoon Park, and Myong Cheol Lim. 2021. "A Modified Intraperitoneal Chemotherapy Regimen for Ovarian Cancer: Technique and Treatment Outcomes" Cancers 13, no. 19: 4886. https://doi.org/10.3390/cancers13194886
APA StyleKim, J. H., Ha, H. I., Kim, M. H., Han, M. R., Park, S.-Y., & Lim, M. C. (2021). A Modified Intraperitoneal Chemotherapy Regimen for Ovarian Cancer: Technique and Treatment Outcomes. Cancers, 13(19), 4886. https://doi.org/10.3390/cancers13194886






