Effectiveness of Cell-Free and Concentrated Ascites Reinfusion Therapy in the Treatment of Malignancy-Related Ascites: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Overview
2.2. Study Search
2.3. Inclusion and Exclusion Criteria
2.4. Risk of Bias
2.5. Outcomes
2.6. Data Extraction
2.7. Statistics
3. Results
3.1. Study Search and Study Characteristics
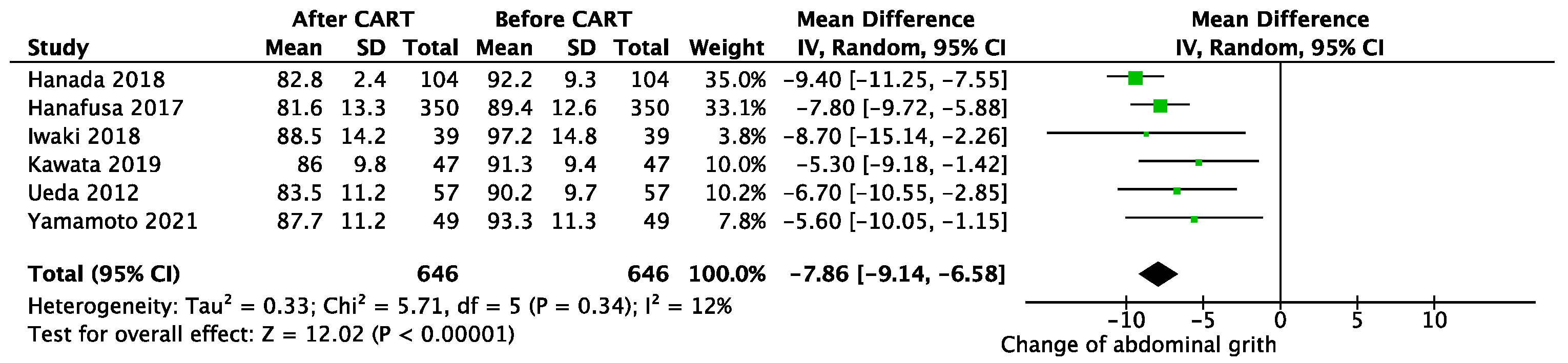
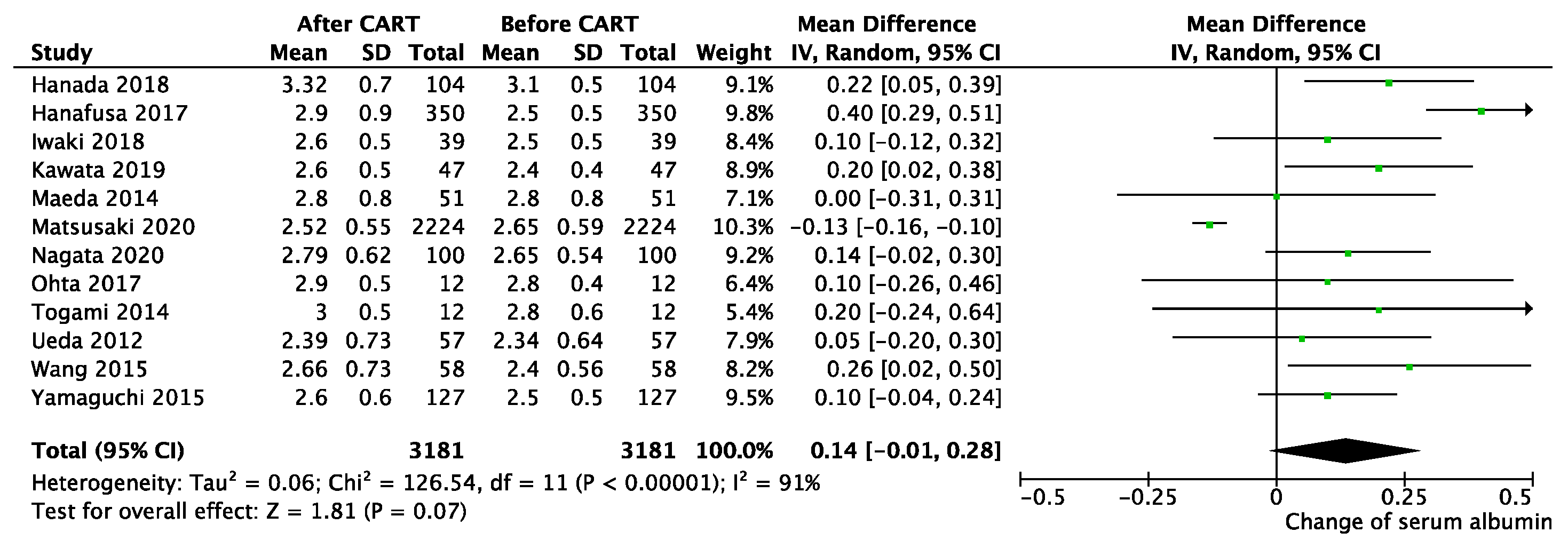
3.2. Efficiency of CART
3.3. Adverse Events in CART
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Becker, G. Acites. In Encyclopedia of Cancer; Schwab, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 16–18. [Google Scholar]
- Stukan, M. Drainage of malignant ascites: Patient selection and perspectives. Cancer Manag. Res. 2017, 9, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Sangisetty, S.L.; Miner, T.J. Malignant ascites: A review of prognostic factors, pathophysiology and therapeutic measures. World J. Gastrointest Surg. 2012, 4, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Saif, M.W.; Siddiqui, I.A.; Sohail, M.A. Management of ascites due to gastrointestinal malignancy. Ann. Saudi Med. 2009, 29, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Becker, G.; Galandi, D.; Blum, H.E. Malignant ascites: Systematic review and guideline for treatment. Eur. J. Cancer 2006, 42, 589–597. [Google Scholar] [CrossRef]
- Kietpeerakool, C.; Rattanakanokchai, S.; Jampathong, N.; Srisomboon, J.; Lumbiganon, P. Management of drainage for malignant ascites in gynaecological cancer. Cochrane Database Syst. Rev. 2019, 12, CD007794. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Yokomichi, N.; Ishiki, H.; Kawaguchi, T.; Masuda, K.; Tsukuura, H.; Funaki, H.; Suzuki, K.; Oya, K.; Nakagawa, J.; et al. Optimal Paracentesis Volume for Terminally Ill Cancer Patients With Ascites. J. Pain Symptom Manag. 2021, 6, 202. [Google Scholar] [CrossRef]
- Hodge, C.; Badgwell, B.D. Palliation of malignant ascites. J. Surg. Oncol. 2019, 120, 67–73. [Google Scholar] [CrossRef]
- Desiderio, J.; Chao, J.; Melstrom, L.; Warner, S.; Tozzi, F.; Fong, Y.; Parisi, A.; Woo, Y. The 30-year experience—A meta-analysis of randomised and high-quality non-randomised studies of hyperthermic intraperitoneal chemotherapy in the treatment of gastric cancer. Eur. J. Cancer 2017, 79, 1–14. [Google Scholar] [CrossRef]
- Cianci, S.; Riemma, G.; Ronsini, C.; De Franciscis, P.; Torella, M.; Schiattarella, A.; La Verde, M.; Colacurci, N. Hyperthermic intraperitoneal chemotherapy (HIPEC) for ovarian cancer recurrence: Systematic review and meta-analysis. Gland. Surg. 2020, 9, 1140–1148. [Google Scholar] [CrossRef]
- Ito, T.; Hanafusa, N. CART: Cell-free and Concentrated Ascites Reinfusion Therapy against malignancy-related ascites. Transfus. Apher. Sci. 2017, 56, 703–707. [Google Scholar] [CrossRef]
- Kloft, C.; Wallin, J.; Henningsson, A.; Chatelut, E.; Karlsson, M.O. Population Pharmacokinetic-Pharmacodynamic Model for Neutropenia with Patient Subgroup Identification: Comparison across Anticancer Drugs. Clin. Cancer Res. 2006, 12, 5481–5490. [Google Scholar] [CrossRef] [PubMed]
- Urrútia, G.; Bonfill, X. PRISMA declaration: A proposal to improve the publication of systematic reviews and meta-analyses. Med. Clin. 2010, 135, 507–511. [Google Scholar] [CrossRef]
- University Hospital Medical Information Network. Available online: https://uploaduminacjp/cgi-open-bin/ctr_e/ctr_viewcgi?recptno=R000050886 (accessed on 30 June 2021).
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Hanada, R.; Yokomichi, N.; Kato, C.; Miki, K.; Oyama, S.; Morita, T.; Kawahara, R. Efficacy and safety of reinfusion of concentrated ascitic fluid for malignant ascites: A concept-proof study. Support. Care Cancer 2017, 26, 1489–1497. [Google Scholar] [CrossRef]
- Hanafusa, N.; Isoai, A.; Ishihara, T.; Inoue, T.; Ishitani, K.; Utsugisawa, T.; Yamaka, T.; Ito, T.; Sugiyama, H.; Arakawa, A.; et al. Safety and efficacy of cell-free and concentrated ascites reinfusion therapy (CART) in refractory ascites: Post-marketing surveillance results. PLoS ONE 2017, 12, e0177303. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Hanafusa, N.; Iwase, S.; Noiri, E.; Nangaku, M.; Nakagawa, K.; Miyagawa, K. Effects of cell-free and concentrated ascites reinfusion therapy (CART) on symptom relief of malignancy-related ascites. Int. J. Clin. Oncol. 2014, 20, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Hanafusa, N.; Iwase, S.; Noiri, E.; Nangaku, M.; Nakagawa, K.; Miyagawa, K. Ascitic IL-10 Concentration Predicts Prognosis of Patients Undergoing Cell-Free and Concentrated Ascites Reinfusion Therapy. Ther. Apher. Dial. 2019, 24, 90–95. [Google Scholar] [CrossRef]
- Iwaki, R.; Shindo, K.; Kawahara, H.; Nanba, N.; Kuramoto, Y.; Beppu, T.; Uesugi, T.; Tanaka, Y.; Uesugi, Y. Cell-Free Ascites Reinfusion Therapy(CART)Modified by Keisuke Matsusaki(KM-CART)at Our Hospital. Gan Kagaku Ryoho. Cancer Chemother. 2018, 45, 2165–2167. [Google Scholar]
- Kawata, Y.; Nagasaka, K.; Matsumoto, Y.; Oda, K.; Tanikawa, M.; Sone, K.; Mori-Uchino, M.; Tsuruga, T.; Arimoto, T.; Osuga, Y.; et al. Usefulness of cell-free and concentrated ascites reinfusion therapy in the therapeutic management of advanced ovarian cancer patients with massive ascites. Int. J. Clin. Oncol. 2018, 24, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Maeda, O.; Ando, T.; Ishiguro, K.; Watanabe, O.; Miyahara, R.; Nakamura, M.; Funasaka, K.; Kazuhiro, F.; Ando, Y.; Goto, H. Safety of repeated cell-free and concentrated ascites reinfusion therapy for malignant ascites from gastrointestinal cancer. Mol. Clin. Oncol. 2014, 2, 1103–1106. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matsusaki, K.; Orihashi, K. Feasibility, efficacy, and safety of cell-free and concentrated ascites reinfusion therapy (KM-CART) for malignant ascites. Artif. Organs 2020, 44, 1090–1097. [Google Scholar] [CrossRef]
- Nagata, Y.; Kato, K.; Miyamoto, T.; Hirano, H.; Shoji, H.; Iwasa, S.; Honma, Y.; Takashima, A.; Hamaguchi, T.; Matsushita, H.; et al. Safety and efficacy of cell-free and concentrated ascites reinfusion therapy (CART) in gastrointestinal cancer patients with massive ascites treated with systemic chemotherapy. Support. Care Cancer 2020, 28, 5861–5869. [Google Scholar] [CrossRef] [PubMed]
- Ohta, K.; Ikenaga, M.; Ueda, M.; Iwaki, R.; Kinoshita, T.; Mishima, K.; Shindo, K.; Chinen, Y.; Itakura, H.; Takayama, T.; et al. Cell-Free and Concentrated Ascites Reinfusion Therapy for Malignant Intractable Ascites from Colorectal Cancer. Gan Kagaku Ryoho. Cancer Chemother. 2017, 44, 1556–1558. [Google Scholar]
- Togami, S.; Hori, S.; Kamio, M.; Matsuo, T.; Yoshinaga, M.; Douchi, T. Clinical usefulness of concentrated ascites reinfusion therapy (CART) for gynecological cancer patients with refractory massive ascites due to cancerous peritonitis. Eur. J. Gynaecol. Oncol. 2014, 35, 301–303. [Google Scholar]
- Ueda, T.; Maehara, M.; Takahashi, Y.; Nakayama, N.; Kondo, H.; Shirota, K.; Yoshizato, T.; Miyamoto, S. Clinical significance of cell-free and concentrated ascites re-infusion therapy for advanced and recurrent gynecological cancer. Anticancer. Res. 2012, 32, 2353–2357. [Google Scholar]
- Wang, L.; Okubo, T.; Shinsaka, M.; Kobayashi, A.; Ogasawara, M.; Sakaguchi, R.; Nagai, T.; Seki, H. Efficacy and safety of cell-free and concentrated ascites reinfusion therapy (CART) in gynecologic cancer patients with a large volume of ascites. J. Obstet. Gynaecol. Res. 2015, 41, 1614–1620. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Kitayama, J.; Emoto, S.; Ishigami, H.; Ito, T.; Hanafusa, N.; Watanabe, T. Cell-free and concentrated ascites reinfusion therapy (CART) for management of massive malignant ascites in gastric cancer patients with peritoneal metastasis treated with intravenous and intraperitoneal paclitaxel with oral S. Eur. J. Surg. Oncol. 2015, 41, 875–880. [Google Scholar] [CrossRef]
- Yamamoto, K.; Nagao, S.; Tsu, T.; Matsushima, T.; Ishido, Y.; Narita, M.; Suzuki, K.; Nakazawa, H.; Shibutani, T.; Jimi, T.; et al. Quality of life assessment of cell-free and concentrated ascites reinfusion therapy during initial treatment for advanced ovarian cancer: A prospective cohort study. J. Obstet. Gynaecol. Res. 2021, 47, 1536–1543. [Google Scholar] [CrossRef]
- Ross, G.; Kessler, H.; Gatenby, R.; Hartz, W.; Ross, L. Sonographically guided paracentesis for palliation of symptomatic malignant ascites. Am. J. Roentgenol. 1989, 153, 1309–1311. [Google Scholar] [CrossRef]
- Heiss, M.M.; Murawa, P.; Koralewski, P.; Kutarska, E.; Kolesnik, O.O.; Ivanchenko, V.V.; Dudnichenko, A.S.; Aleknaviciene, B.; Razbadauskas, A.; Gore, M.; et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: Results of a prospective randomized phase II/III trial. Int. J. Cancer 2010, 127, 2209–2221. [Google Scholar] [CrossRef]
- Jatoi, A.; Nieva, J.J.; Qin, R.; Loprinzi, C.L.; Wos, E.J.; Novotny, P.J.; Moore, J.D.F.; Mowat, R.B.; Bechar, N.; Pajon, J.E.R.; et al. A Pilot Study of Long-Acting Octreotide for Symptomatic Malignant Ascites. Oncology 2012, 82, 315–320. [Google Scholar] [CrossRef]
- Ishitani, K.; Isoai, A.; Ito, T.; Sugiyama, H.; Arakawa, A.; Yamada, Y.; Onodera, H.; Kobayashi, R.; Torii, N.; Soneda, N.; et al. Clinical usefulness of cell-free and concentrated ascites reinfusion therapy (CART) in combination with chemotherapy for malignant ascites: A post-marketing surveillance study. Int. J. Clin. Oncol. 2021, 26, 1130–1138. [Google Scholar] [CrossRef]

| First Author | Year | Tumors | Types | Patients | Chemotherapy | Age (y) | Procedures | Collection (mL) (SD) | Reinfusion (mL) (SD) | Protein (g) (SD) |
|---|---|---|---|---|---|---|---|---|---|---|
| Hanada | 2018 | mixed | CART | 51 | 14 | 64 | 104 | 5855 (1790) | 764 (320) | ND |
| Hanafusa | 2017 | mixed | mixed | 142 | ND | 65.7 | 350 | 3709 (1730) | 491 (320) | 66.8 (32.4) |
| Ito | 2015 | mixed | CART | 37 | ND | 59.7 | 100 | 3197 (1424) | 302 (150) | 93.1 (51.62) |
| Ito | 2020 | mixed | CART | 43 | ND | 58.7 | 123 | 3207 (1427) | 299 (152) | 91.3 (53) |
| Iwaki | 2018 | mixed | KM-CART | 19 | ND | 62.8 | 39 | 7000 (2600) | ND | ND |
| Kawata | 2019 | Gyn | CART | 29 | 2 | 56.6 | 47 | 2937 (820) | 272 (84) | 85 (33.2) |
| Maeda | 2014 | Gas | CART | 5 | ND | 63.6 | 51 | 4007 (1304) | 561 (205) | 75 (29.8) |
| Matsusaki | 2020 | mixed | KM-CART | 2109 | ND | 60.7 | 2224 † | 6200 (2600) | 610 (300) | 67.3 (44.5) |
| Nagata | 2020 | Gas | CART | 30 | 30 | 59.5 | 100 | 4000 (200) | ND | ND |
| Ohta | 2017 | Gas | CART | 6 | ND | 73.8 | 12 | 3850 | 485 | ND |
| Togami | 2014 | Gyn | NA | 4 | ND | ND | 15 | 3190 (1086) | 538 (249) | ND |
| Ueda | 2012 | Gyn | CART | 22 | 14 | ND | 57 | 3290 (1200) | NA | ND |
| Wang | 2015 | Gyn | CART | 9 | 6 | 67.7 | 58 | 7730 (3390) | 920 (470) | 161.2 (89.1) |
| Yamaguchi | 2015 | Gas | CART | 30 | 30 | 58 | 127 | 3056 (1250) | 334 (162) | 85.5 (46.9) |
| Yamamoto | 2021 | Gyn | CART | 31 | 11 | 66.4 | 49 | 3009 (1253) | 392 (190) | ND |
| Symptoms | Before (95% CI) | After (95% CI) | Mean Difference (95% CI) | p Value |
|---|---|---|---|---|
| Abdominal distension | 8.10 (7.78, 8.42) | 2.12 (1.80, 2.44) | 6.00 (5.49–6.51) | <0.01 |
| Dyspnea | 4.40 (3.03, 5.77) | 1.67 (0.89, 2.45) | 2.66 (2.05–3.28) | <0.01 |
| Fatigue | 6.17 (4.11, 8.23) | 3.54 (2.27, 4.82) | 2.64 (1.86–3.42) | <0.01 |
| Lack of appetite | 6.15 (4.56, 7.73) | 3.62 (3.04, 4.20) | 2.58 (1.53–3.63) | <0.01 |
| Abdominal pain | 3.90 (2.53, 5.27) | 2.15 (1.26, 3.03) | 1.74 (1.14–2.35) | <0.01 |
| Nausea and vomiting | 3.21 (1.05, 5.36) | 1.79 (0.01, 4.14) | 1.40 (0.86–1.95) | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Ishihara, M.; Horita, N.; Tanzawa, S.; Kazahari, H.; Ochiai, R.; Sakamoto, T.; Honda, T.; Ichikawa, Y.; Watanabe, K.; et al. Effectiveness of Cell-Free and Concentrated Ascites Reinfusion Therapy in the Treatment of Malignancy-Related Ascites: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 4873. https://doi.org/10.3390/cancers13194873
Chen H, Ishihara M, Horita N, Tanzawa S, Kazahari H, Ochiai R, Sakamoto T, Honda T, Ichikawa Y, Watanabe K, et al. Effectiveness of Cell-Free and Concentrated Ascites Reinfusion Therapy in the Treatment of Malignancy-Related Ascites: A Systematic Review and Meta-Analysis. Cancers. 2021; 13(19):4873. https://doi.org/10.3390/cancers13194873
Chicago/Turabian StyleChen, Hao, Masashi Ishihara, Nobuyuki Horita, Shigeru Tanzawa, Hiroki Kazahari, Ryusuke Ochiai, Takahiko Sakamoto, Takeshi Honda, Yasuko Ichikawa, Kiyotaka Watanabe, and et al. 2021. "Effectiveness of Cell-Free and Concentrated Ascites Reinfusion Therapy in the Treatment of Malignancy-Related Ascites: A Systematic Review and Meta-Analysis" Cancers 13, no. 19: 4873. https://doi.org/10.3390/cancers13194873
APA StyleChen, H., Ishihara, M., Horita, N., Tanzawa, S., Kazahari, H., Ochiai, R., Sakamoto, T., Honda, T., Ichikawa, Y., Watanabe, K., & Seki, N. (2021). Effectiveness of Cell-Free and Concentrated Ascites Reinfusion Therapy in the Treatment of Malignancy-Related Ascites: A Systematic Review and Meta-Analysis. Cancers, 13(19), 4873. https://doi.org/10.3390/cancers13194873









