First Evaluation of Temporal and Spatial Fractionation in Proton Minibeam Radiation Therapy of Glioma-Bearing Rats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tumor Inoculation
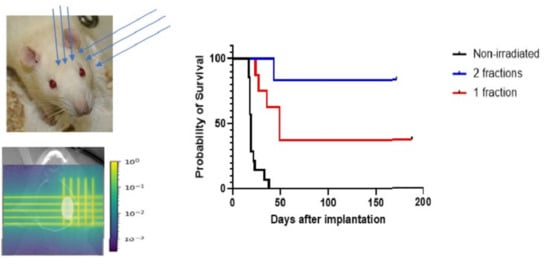
2.2. Irradiations and Dosimetry
2.3. Animal Follow-Up
3. Results
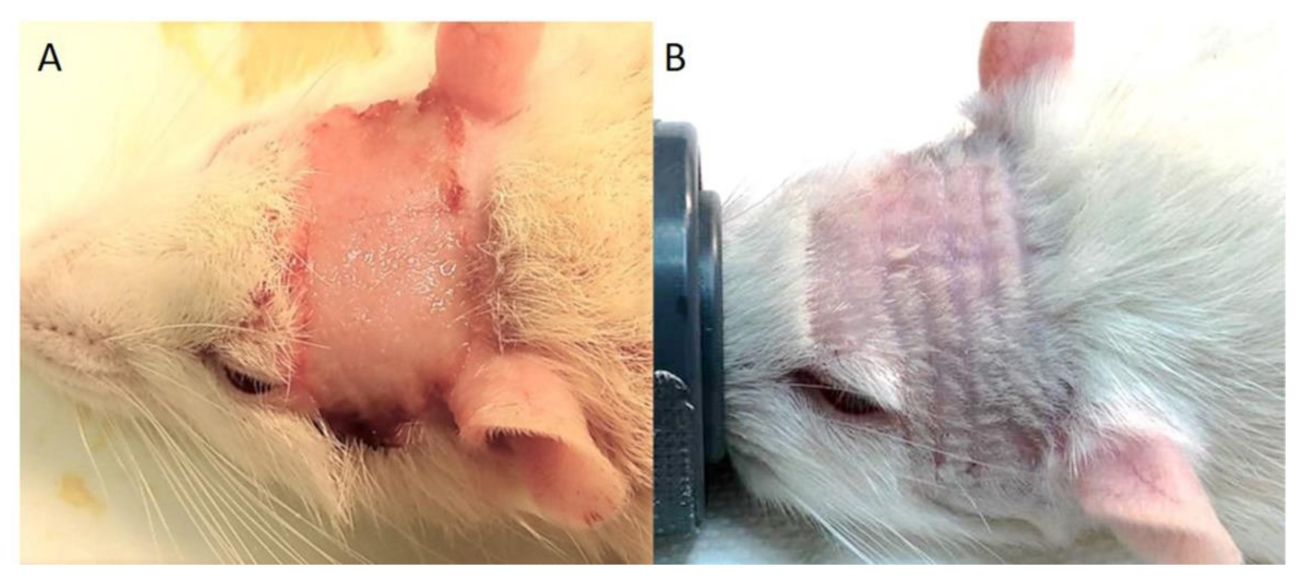
3.1. Clinical Symptoms
3.2. Survival Curves
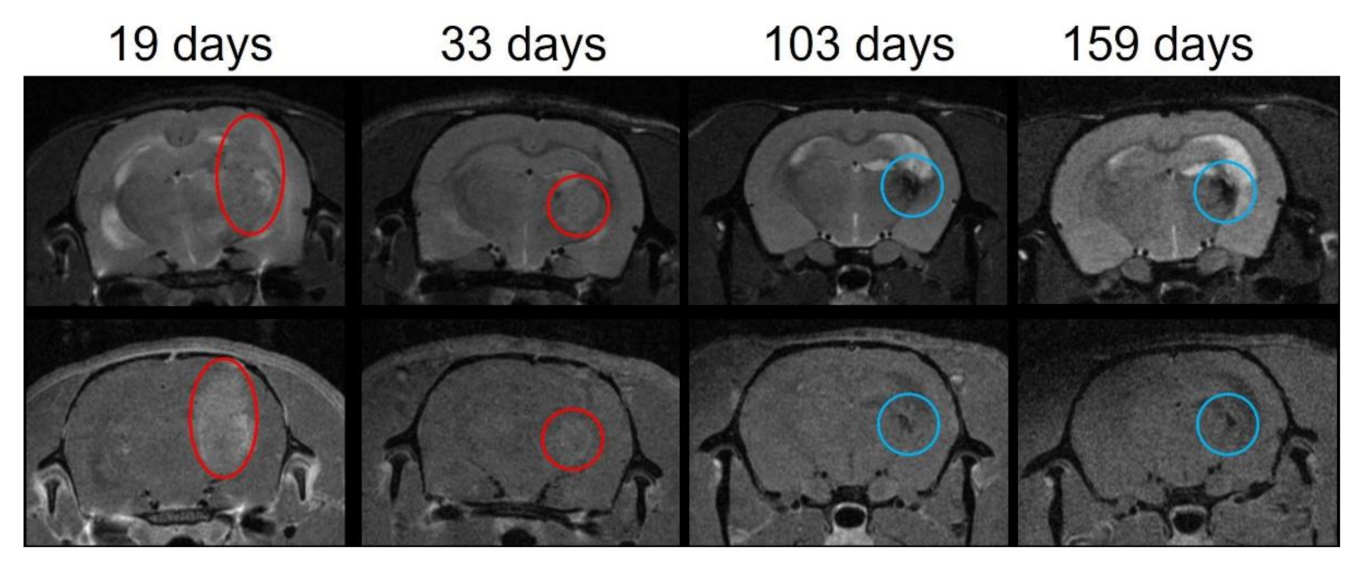
3.3. MRI Follow-Up
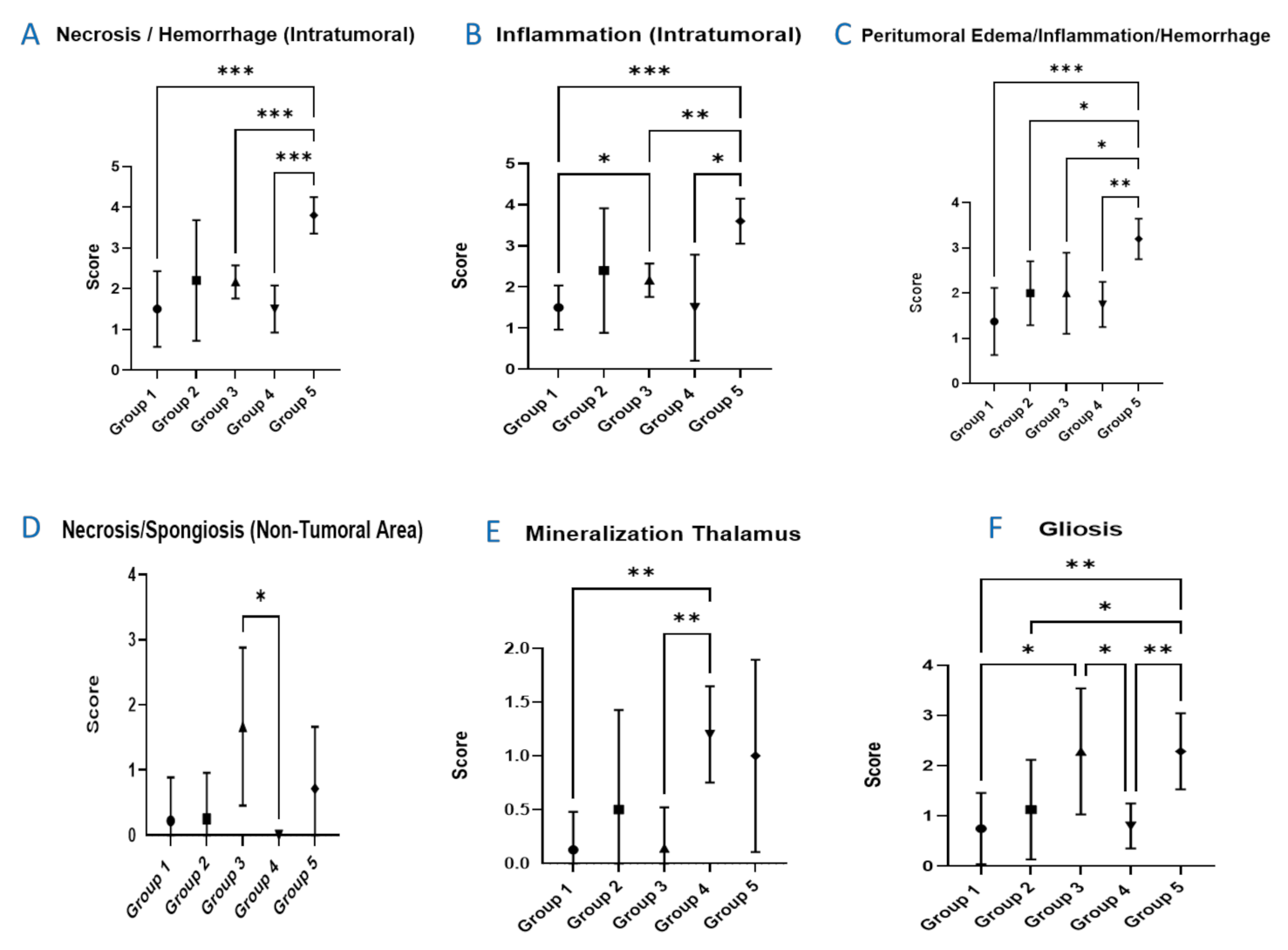
3.4. Histopathological Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Grade 1, Minimal | This corresponds to a histopathologic change ranging from inconspicuous to barely noticeable but so minor, small, or infrequent as to warrant no more than the least assignable grade. For multifocal or diffusely distributed lesions, this grade was used for processes where less than approximately 10% of the tissue in an average high-power field was involved. |
| Grade 2, Slight | This corresponds to a histopathologic change that is a noticeable but not a prominent feature of the tissue. For multifocal or diffusely distributed lesions, this grade was used for processes where between approximately 10% and 25% of the tissue in an average high-power field was involved. |
| Grade 3, Moderate | This corresponds to a histopathologic change that is a prominent but not a dominant feature of the tissue. For multifocal or diffusely distributed lesions, this grade was used for processes where between approximately 25% and 50% of the tissue in an average high-power field was involved. |
| Grade 4, Marked | This corresponds to a histopathologic change that is a dominant but not an overwhelming feature of the tissue. For multifocal or diffusely distributed lesions, this grade was used for processes where between approximately 50% and 95% of the tissue in an average high-power field was involved. |
| Grade 5, Severe | This corresponds to a histopathologic change that is an overwhelming feature of the tissue. For multifocal or diffusely distributed lesions, this grade was used for processes where greater than approximately 95% of the tissue in an average high-power field was involved. |
References
- Prezado, Y.; Fois, G.R. Proton-minibeam radiation therapy: A proof of concept. Med. Phys. 2013, 40, 031712. [Google Scholar] [CrossRef]
- Peucelle, C.; Nauraye, C.; Patriarca, A.; Hierso, E.; Fournier-Bidoz, N.; Martinez-Rovira, I.; Prezado, Y. Proton minibeam radiation therapy: Experimental dosimetry evaluation. Med. Phys. 2015, 42, 7108–7113. [Google Scholar] [CrossRef]
- Guardiola, C.; De Marzi, L.; Prezado, Y. Verification of a Monte Carlo dose calculation engine in proton minibeam radiotherapy in a passive scattering beamline for preclinical trials. Br. J. Radiol. 2020, 93, 20190578. [Google Scholar] [CrossRef]
- Meyer, J.; Eley, J.; Schmid, T.E.; Combs, S.E.; Dendale, R.; Prezado, Y. Spatially fractionated proton minibeams. Br. J. Radiol. 2019, 92, 20180466. [Google Scholar] [CrossRef]
- Girst, S.; Greubel, C.; Reindl, J.; Siebenwirth, C.; Zlobinskaya, O.; Walsh, D.W.M.; Ilicic, K.; Aichler, M.; Walch, A.; Wilkens, J.J.; et al. Proton minibeam radiation therapy reduces side effects in an in vivo mouse ear model. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 234–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zlobinskaya, O.; Girst, S.; Greubel, C.; Hable, V.; Siebenwirth, C.; Walsh, D.W.M.; Multhoff, G.; Wilkens, J.J.; Schmid, T.E.; Dollinger, G.; et al. Reduced side effects by proton microchannel radiotherapy: Study in a human skin model. Radiat. Environ. Biophys. 2013, 52, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Lamirault, C.; Doyere, V.; Juchaux, M.; Pouzoulet, F.; Labiod, D.; Dendale, R.; Patriarca, A.; Nauraye, C.; Le Dudal, M.; Jouvion, G.; et al. Short and long-term evaluation of the impact of proton minibeam radiation therapy on motor, emotional and cognitive functions. Sci. Rep. 2020, 10, 13511. [Google Scholar] [CrossRef] [PubMed]
- Prezado, Y.; Jouvion, G.; Hardy, D.; Patriarca, A.; Nauraye, C.; Bergs, J.; Gonzalez, W.; Guardiola, C.; Juchaux, M.; Labiod, D.; et al. Proton minibeam radiation therapy spares normal rat brain: Long-term clinical, radiological and histopathological, analysis. Sci. Rep. 2017, 7, 14403. [Google Scholar]
- Prezado, Y.; Jouvion, G.; Patriarca, A.; Nauraye, C.; Guardiola, C.; Juchaux, M.; Lamirault, C.; Labiod, D.; Jourdain, L.; Sebrie, C.; et al. Proton minibeam radiation therapy widens the therapeutic index for high-grade gliomas. Sci. Rep. 2018, 8, 16479. [Google Scholar] [CrossRef]
- Prezado, Y.; Jouvion, G.; Guardiola, C.; Gonzalez, W.; Juchaux, M.; Bergs, J.; Nauraye, C.; Labiod, D.; De Marzi, L.; Pouzoulet, F.; et al. Tumor control in RG2 glioma-bearing rats: A comparison between proton minibeam therapy and standard proton therapy. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 266–271. [Google Scholar] [CrossRef]
- Lamirault, C.; Brisebard, E.; Patriarca, A.; Juchaux, M.; Crepin, D.; Labiod, D.; Pouzoulet, F.; Sebrie, C.; Jourdain, L.; Le Dudal, M.; et al. Spatially modulated proton minibeams results in the same increase of lifespan as a uniform target dose coverage in F98-glioma-bearing rats. Radiat. Res. 2020, 194, 715–723. [Google Scholar] [CrossRef]
- Steel, G.G.; McMillan, T.J.; Peacock, J.H. The 5Rs of radiobiology. Int. J. Radiat. Biol. 1989, 56, 1045–1048. [Google Scholar] [CrossRef] [Green Version]
- Serduc, R.; Brauer-Krisch, E.; Bouchet, A.; Renaud, L.; Brochard, T.; Bravin, A.; Laissue, J.A.; Le Duc, G. First trial of spatial and temporal fractionations of the delivered dose using synchrotron microbeam radiation therapy. J. Synchrotron. Radiat. 2009, 16 Pt 4, 587–590. [Google Scholar] [CrossRef]
- De Marzi, L.; Da Fonseca, A.; Moignier, C.; Patriarca, A.; Goudjil, F.; Mazal, A.; Buvat, I.; Hérault, J. Experimental characterisation of a proton kernel model for pencil beam scanning techniques. Phys. Med. 2019, 64, 195–203. [Google Scholar] [CrossRef] [PubMed]
- De Marzi, L.; Patriarca, A.; Nauraye, C.; Hierso, E.; Dendale, R.; Guardiola, C.; Prezado, Y. Implementation of planar proton minibeam radiation therapy using a pencil beam scanning system: A proof of concept study. Med. Phys. 2018, 45, 5305–5316. [Google Scholar] [CrossRef] [PubMed]
- Brauer-Krisch, E.; Requardt, H.; Regnard, P.; Corde, S.; Siegbahn, E.; LeDuc, G.; Brochard, T.; Blattmann, H.; Laissue, J.; Bravin, A.; et al. New irradiation geometry for microbeam radiation therapy. Phys. Med. Biol. 2005, 50, 3103–3111. [Google Scholar] [CrossRef] [PubMed]
- Perl, J.; Shin, J.; Schumann, J.; Faddegon, B.; Paganetti, H. TOPAS: An innovative proton Monte Carlo platform for research and clinical applications. Med. Phys. 2012, 39, 6818–6837. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.S.; Schultz, C.J.; Li, X.A. An estimation of radiobiologic parameters from clinical outcomes for radiation treatment planning of brain tumor. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 1570–1580. [Google Scholar] [CrossRef]
- Prezado, Y.; Dos Santos, M.; González, W.; Jouvion, G.; Guardiola, C.; Heinrich, S.; Labiod, D.; Juchaux, M.; Jourdain, L.; Sébrié, C.; et al. Transfer of minibeam radiation therapy into a cost-effective equipment for radiobiological studies: A proof of concept. Sci. Rep. 2017, 7, 17295. [Google Scholar]
- Bolon, B.; Garman, R.H.; Pardo, I.D.; Jensen, K.; Sills, R.C.; Roulois, A.; Radovsky, A.; Bradley, A.; Andrews-Jones, L.; Butt, M.; et al. STP position paper: Recommended practices for sampling and processing the nervous system (brain, spinal cord, nerve, and eye) during nonclinical general toxicity studies. Toxicol. Pathol. 2013, 41, 1028–1048. [Google Scholar] [CrossRef] [Green Version]
- Bernier, J.; Hall, E.J.; Giaccia, A. Radiation oncology: A century of achievements. Nat. Rev. Cancer 2004, 4, 737–747. [Google Scholar] [CrossRef]
- Garibaldi, C.; Jereczek-Fossa, B.A.; Marvaso, G.; Dicuonzo, S.; Rojas, D.P.; Cattani, F.; Starzyńska, A.; Ciardo, D.; Surgo, A.; Leonardi, M.C.; et al. Recent advances in radiation oncology. Ecancer Med. Sci. 2017, 11, 785. [Google Scholar] [CrossRef] [Green Version]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Fitzek, M.M.; Thornton, A.F.; Rabinov, J.D.; Lev, M.H.; Pardo, F.S.; Munzenrider, J.E.; Okunieff, P.; Bussière, M.; Braun, I.; Hochberg, F.H.; et al. Accelerated fractionated proton/photon irradiation to 90 cobalt gray equivalent for glioblastoma multiforme: Results of a phase II prospective trial. J. Neurosurg. 1999, 91, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Shibamoto, Y.; Iwata, H. The quest for optimal fractionation schedules in stereotactic radiotherapy. Cureus 2020, 12, e6777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Palomo, C.; Fazzari, J.; Trappetti, V.; Smyth, L.; Janka, H.; Laissue, J.; Djonov, V. Animal models in microbeam radiation therapy: A scoping review. Cancers 2020, 12, 527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Palomo, C.; Trappetti, V.; Potez, M.; Pellicioli, P.; Krisch, M.; Laissue, J.; Djonov, V. Complete remission of mouse melanoma after temporally fractionated microbeam radiotherapy. Cancers 2020, 12, 2656. [Google Scholar] [CrossRef]
- Abayomi, O.; Chun, M.S.; Kelly, K. Cerebral calcification and learning disabilities following cranial irradiation for medulloblastoma. J. Natl. Med. Assoc. 1990, 82, 833–836. [Google Scholar]
- Carl, U.M.; Hartmann, K.A. Heterotopic calcification as a late radiation effect: Report of 15 cases. Br. J. Radiol. 2002, 75, 460–463. [Google Scholar] [CrossRef]
- Sammer, M.; Dombrowsky, A.C.; Schauer, J.; Oleksenko, K.; Bicher, S.; Schwarz, B.; Rudigkeit, S.; Matejka, N.; Reindl, J.; Bartzsch, S.; et al. Normal tissue response of combined temporal and spatial fractionation in proton minibeam radiation therapy. Int. J. Radiat. Oncol Biol. Phys. 2021, 109, 76–83. [Google Scholar] [CrossRef]



| Type of Irradiation | Group | Fractions | Dose Per Array (Gy) | Average Dose in the Target (Gy) | BED Target (Gy) | Dpeak-peak (Gy) | Dpeak-valley (Gy) | Dvalley_valley (Gy) |
|---|---|---|---|---|---|---|---|---|
| Ctrl | 1 | 0 | 0 | 0 | 0 | - | - | - |
| pMBRT crossfire (2 arrays) | 2 | 1 | 15 ± 1 | 30 ± 1 | 120 ± 6 | 59 ± 2 | 36 ± 1 | 10.6 ± 0.5 |
| 3 | 2 | 15 ± 1 | 30 ± 1 | 75 ± 4 | 59 ± 2 | 36 ± 1 | 10.6 ± 0.5 | |
| 4 | 2 | 20 ± 1 | 40 ± 2 | 120 ± 6 | 79 ± 2 | 46 ± 1 | 14.2 ± 0.5 | |
| Broad beam (1 array) | 5 | 1 | 30 ± 2 | 30 ± 2 | 120 ± 6 | − | − | − |
| Type of Irradiation | Dose (Gy) Per Array | Group | Transitory Weight Loss | Radiation Dermatitis | Radiation-Induced Alopecia |
|---|---|---|---|---|---|
| Ctrl | − | 1 | − | − | − |
| pMBRT crossfire | 15 Gy | 2 (1 fraction) | 4/8 3 to 6 days | 0/7 | 7/7 |
| 15 Gy | 3 (2 fractions) | 7/7 3 to 5 days | 0/8 | 8/8 | |
| 20 Gy | 4 (2 fractions) | 6/6 1 to 3 days | 0/8 | 6/6 | |
| Broad beam (1 array) | 30 Gy | 5 (1 fraction) | 4/8 2 to 4 days | 8/8 11 to 12 days | − |
| Group | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | *** | * | *** | *** | |
| 2 | *** | * | ns | ns | |
| 3 | * | * | *** | *** | |
| 4 | *** | *** | *** | *** | |
| 5 | *** | ns | * | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertho, A.; Ortiz, R.; Juchaux, M.; Gilbert, C.; Lamirault, C.; Pouzoulet, F.; Polledo, L.; Liens, A.; Warfving, N.; Sebrie, C.; et al. First Evaluation of Temporal and Spatial Fractionation in Proton Minibeam Radiation Therapy of Glioma-Bearing Rats. Cancers 2021, 13, 4865. https://doi.org/10.3390/cancers13194865
Bertho A, Ortiz R, Juchaux M, Gilbert C, Lamirault C, Pouzoulet F, Polledo L, Liens A, Warfving N, Sebrie C, et al. First Evaluation of Temporal and Spatial Fractionation in Proton Minibeam Radiation Therapy of Glioma-Bearing Rats. Cancers. 2021; 13(19):4865. https://doi.org/10.3390/cancers13194865
Chicago/Turabian StyleBertho, Annaïg, Ramon Ortiz, Marjorie Juchaux, Cristèle Gilbert, Charlotte Lamirault, Frederic Pouzoulet, Laura Polledo, Alethea Liens, Nils Warfving, Catherine Sebrie, and et al. 2021. "First Evaluation of Temporal and Spatial Fractionation in Proton Minibeam Radiation Therapy of Glioma-Bearing Rats" Cancers 13, no. 19: 4865. https://doi.org/10.3390/cancers13194865
APA StyleBertho, A., Ortiz, R., Juchaux, M., Gilbert, C., Lamirault, C., Pouzoulet, F., Polledo, L., Liens, A., Warfving, N., Sebrie, C., Jourdain, L., Patriarca, A., de Marzi, L., & Prezado, Y. (2021). First Evaluation of Temporal and Spatial Fractionation in Proton Minibeam Radiation Therapy of Glioma-Bearing Rats. Cancers, 13(19), 4865. https://doi.org/10.3390/cancers13194865