Artificial Light at Night Reduces Anxiety-like Behavior in Female Mice with Exacerbated Mammary Tumor Growth
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Animals and Experimental Outline
2.2. Cell lines and Orthotopic Injections
2.3. Behavioral Testing
2.4. Statistics
3. Results
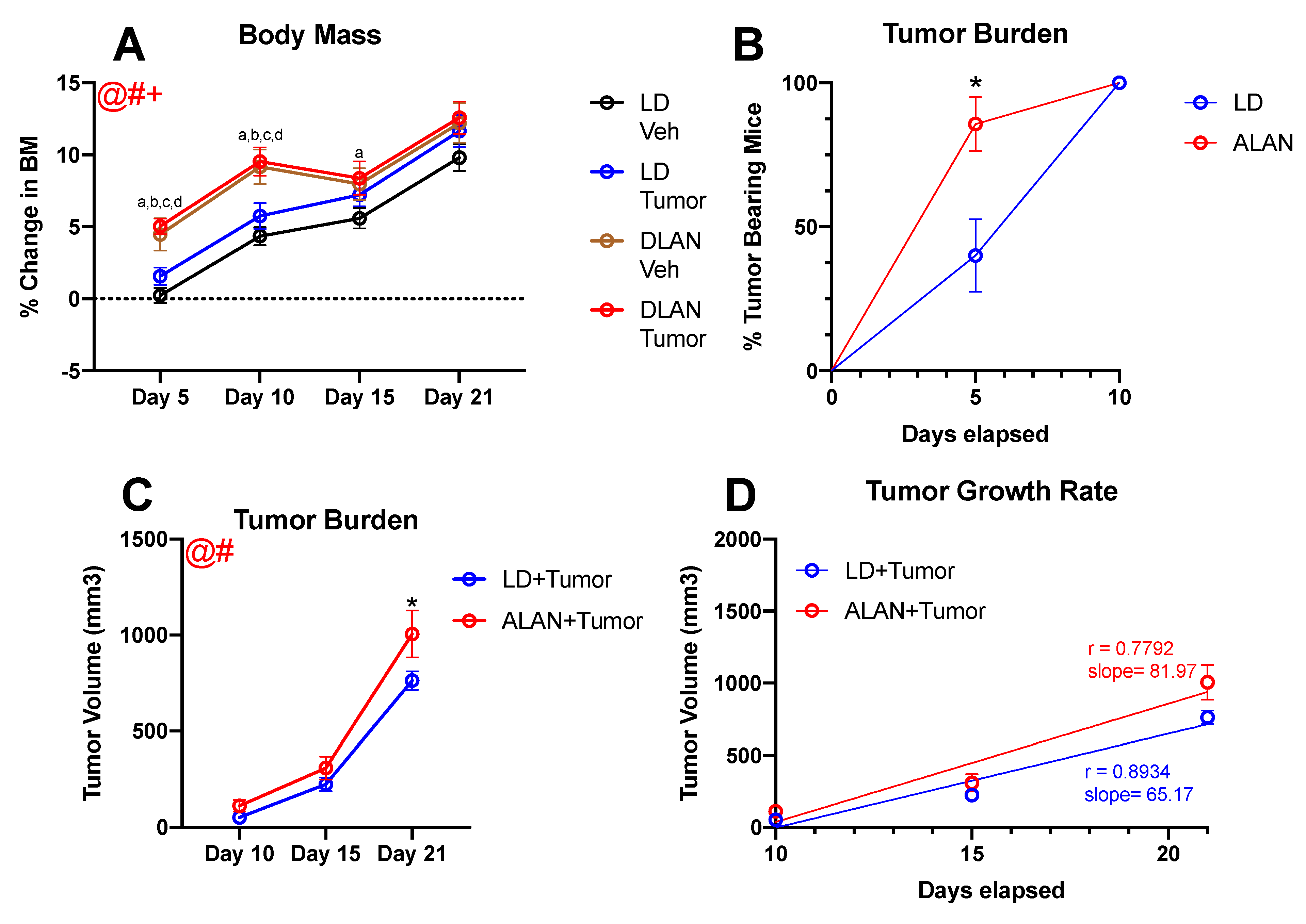
3.1. ALAN Accelerates Body Mass Gain
3.2. ALAN Stimulates Tumor Growth
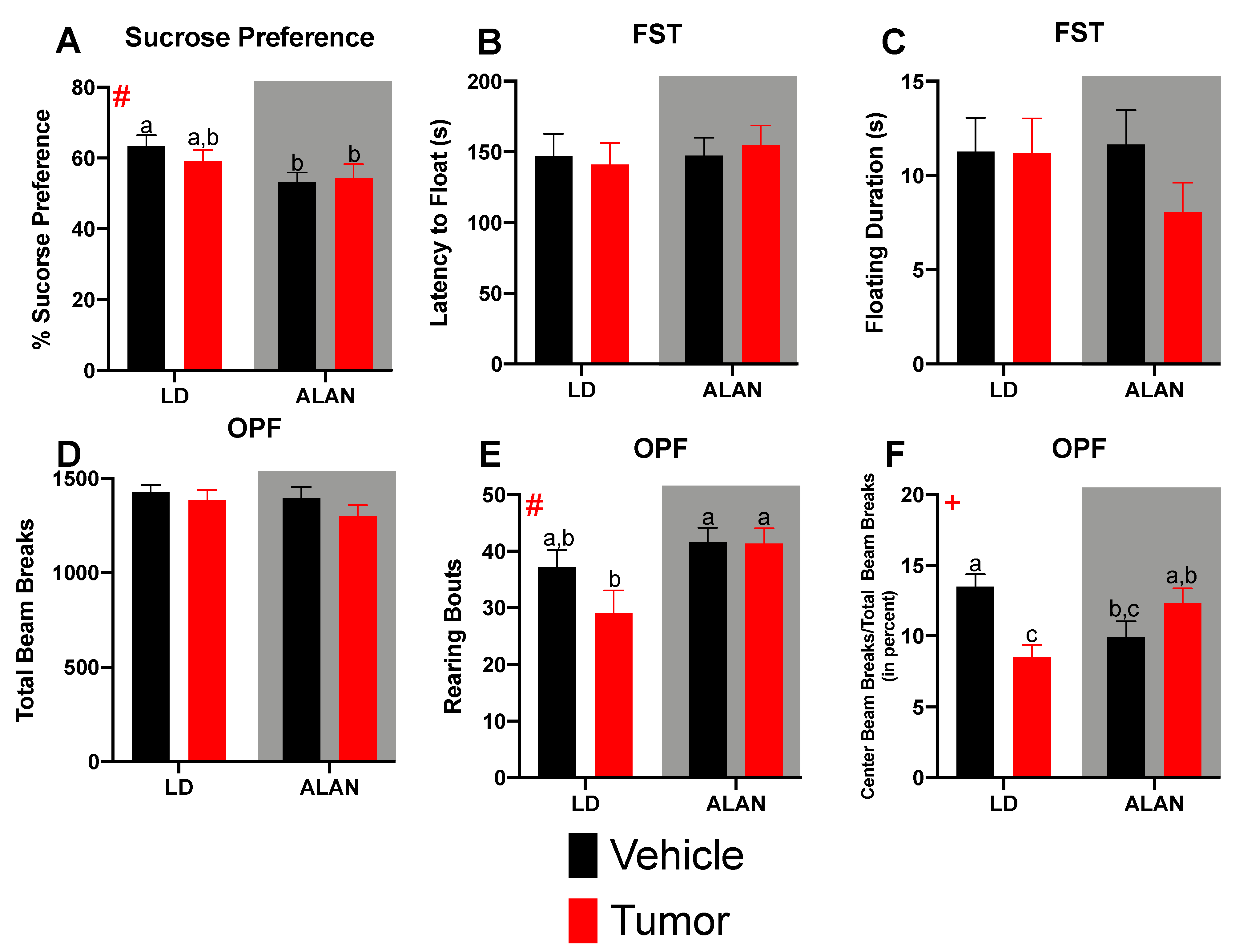
3.3. Mammary Tumors and ALAN Alter Behavior
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bailey, M.; Silver, R. Sex differences in circadian timing systems: Implications for disease. Front. Neuroendocrinol. 2013, 35, 111–139. [Google Scholar] [CrossRef] [PubMed]
- Mistlberger, R.E.; Skene, D.J. Social influences on mammalian circadian rhythms: Animal and human studies. Biol. Rev. 2004, 79, 533–556. [Google Scholar] [CrossRef]
- Castillo, M.R.; Hochstetler, K.J.; Tavernier, R.J.; Greene, D.M.; Bult-Ito, A. Entrainment of the master circadian clock by scheduled feeding. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Buhr, E.D.; Yoo, S.H.; Takahashi, J.S. Temperature as a universal resetting cue for mammalian circadian oscillators. Science 2010, 330, 379–385. [Google Scholar] [CrossRef]
- Wright, K.P.; McHill, A.W.; Birks, B.R.; Griffin, B.R.; Rusterholz, T.; Chinoy, E.D. Entrainment of the human circadian clock to the natural light-dark cycle. Curr. Biol. 2013, 23, 1554–1558. [Google Scholar] [CrossRef] [PubMed]
- Ginty, D.D.; Kornhauser, J.M.; Thompson, M.A.; Bading, H.; Mayo, K.E.; Takahashi, J.S.; Greenberg, M.E. Regulation of CREB phosphorylation in the suprachiasmatic nucleus by light and a circadian clock. Science 1993, 260, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Kornhauser, J.M.; Ginty, D.D.; Greenberg, M.E.; Mayo, K.E.; Takahashi, J.S. Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 1996; Volume 111, pp. 133–146. [Google Scholar]
- Golombek, D.A.; Rosenstein, R.E. Physiology of circadian entrainment. Physiol. Rev. 2020, 90, 1063–1102. [Google Scholar] [CrossRef] [PubMed]
- Falchi, F.; Cinzano, P.; Duriscoe, D.; Kyba, C.C.M.; Elvidge, C.D.; Baugh, K.; Portnov, B.A.; Rybnikova, N.A.; Furgoni, R. The new world atlas of artificial night sky brightness. Sci. Adv. 2016, 2, e1600377. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.G. Light-at-night, circadian disruption and breast cancer: Assessment of existing evidence. Int. J. Epidemiol. 2009, 38, 963–970. [Google Scholar] [CrossRef]
- Walker, W.H.; Bumgarner, J.R.; Walton, J.C.; Liu, J.A.; Meléndez-Fernández, O.H.; Nelson, R.J.; Devries, A.C. Light pollution and cancer. Int. J. Mol. Sci. 2020, 21, 9360. [Google Scholar] [CrossRef]
- Walker, W.H.; Walton, J.C.; DeVries, A.C.; Nelson, R.J. Circadian rhythm disruption and mental health. Transl. Psychiatry. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Kohyama, J. A newly proposed disease condition produced by light exposure during night: Asynchronization. Brain Dev. 2009, 31, 255–273. [Google Scholar] [CrossRef]
- Driesen, K.; Jansen, N.W.H.; Kant, I.; Mohren, D.C.L.; van Amelsvoort, L.G.P.M. Depressed mood in the working population: Associations with work schedules and working hours. Chronobiol. Int. 2010, 27, 1062–1079. [Google Scholar] [CrossRef]
- Cos, S.; Mediavilla, D.; Martínez-Campa, C.; González, A.; Alonso-González, C.; Sánchez-Barceló, E.J. Exposure to light-at-night increases the growth of DMBA-induced mammary adenocarcinomas in rats. Cancer Lett. 2006, 235, 266–271. [Google Scholar] [CrossRef]
- Wu, J.; Dauchy, R.T.; Tirrell, P.C.; Wu, S.S.; Lynch, D.T.; Jitawatanarat, P.; Burrington, C.M.; Dauchy, E.M.; Blask, D.E.; Greene, M.W. Light at night activates IGF-1R/PDK1 signaling and accelerates tumor growth in human breast cancer xenografts. Cancer Res. 2011, 71, 2622–2631. [Google Scholar] [CrossRef]
- Blask, D.E.; Brainard, G.C.; Dauchy, R.T.; Hanifin, J.P.; Davidson, L.K.; Krause, J.A.; Sauer, L.A.; Rivera-Bermudez, M.A.; Dubocovich, M.L.; Jasser, S.A.; et al. Melatonin-depleted blood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Res. 2005, 65, 11174–11184. [Google Scholar] [CrossRef] [PubMed]
- Zubidat, E.; Fares, B.; Fares, F.; Haim, A. Artificial light at night of different spectral compositions differentially affects tumor growth in mice: Interaction with melatonin and epigenetic pathways. Cancer Control 2018, 25. [Google Scholar] [CrossRef] [PubMed]
- Agbaria, S.; Haim, A.; Fares, F.; Zubidat, A.E. Epigenetic modification in 4T1 mouse breast cancer model by artificial light at night and melatonin–the role of DNA-methyltransferase. Chronobiol. Int. 2019, 36, 629–643. [Google Scholar] [CrossRef] [PubMed]
- Blask, D.E.; Dauchy, R.T.; Dauchy, E.M.; Mao, L.; Hill, S.M.; Greene, M.W.; Belancio, V.P.; Sauer, L.A.; Davidson, L. Light exposure at night disrupts host/cancer circadian regulatory dynamics: Impact on the Warburg effect, lipid signaling and tumor growth prevention. PLoS ONE 2014, 9, e102776. [Google Scholar] [CrossRef]
- Bedrosian, T.A.; Weil, Z.M.; Nelson, R.J. Chronic dim light at night provokes reversible depression-like phenotype: Possible role for TNF. Mol. Psychiatry 2013, 18, 930–936. [Google Scholar] [CrossRef]
- Fonken, L.K.; Workman, J.L.; Walton, J.C.; Weil, Z.M.; Morris, J.S.; Haim, A.; Nelson, R.J. Light at night increases body mass by shifting the time of food intake. Proc. Natl. Acad. Sci. USA 2010, 107, 18664–18669. [Google Scholar] [CrossRef]
- Fonken, L.K.; Kitsmiller, E.; Smale, L.; Nelson, R.J. Dim nighttime light impairs cognition and provokes depressive-like responses in a diurnal rodent. J. Biol. Rhythms. 2012, 27, 319–327. [Google Scholar] [CrossRef]
- Walker, W.H.; Borniger, J.C.; Gaudier-Diaz, M.M.; Meléndez-Fernández, O.H.; Pascoe, J.L.; DeVries, A.C.; Nelson, R.J. Acute exposure to low-level light at night is sufficient to induce neurological changes and depressive-like behavior. Mol. Psychiatry 2020, 25, 1080–1093. [Google Scholar] [CrossRef] [PubMed]
- George, M.A.; Lustberg, M.B.; Orchard, T.S. Psychoneurological symptom cluster in breast cancer: The role of inflammation and diet. Breast Cancer Res. Treat. 2020, 184, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.A.; Pace, T.W.; Liu, T.; Felger, J.C.; Mister, D.; Doho, G.H.; Kohn, J.N.; Barsevick, A.M.; Long, Q.; Miller, A.H. Predictors of depression in breast cancer patients treated with radiation: Role of prior chemotherapy and nuclear factor kappa B. Cancer 2013, 119, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Jehn, C.F.; Flath, B.; Strux, A.; Krebs, M.; Possinger, K.; Pezzutto, A.; Lüftner, D. Influence of age, performance status, cancer activity, and IL-6 on anxiety and depression in patients with metastatic breast cancer. Breast Cancer Res. Treat. 2012, 136, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Pyter, L.M.; Cochrane, S.F.; Ouwenga, R.L.; Patel, P.N.; Pineros, V.; Prendergast, B.J. Mammary tumors induce select cognitive impairments. Brain. Behav. Immun. 2010, 24, 903–907. [Google Scholar] [CrossRef]
- Pyter, L.M.; Pineros, V.; Galang, J.A.; McClintock, M.K.; Prendergast, B.J. Peripheral tumors induce depressive-like behaviors and cytokine production and alter hypothalamic-pituitary-adrenal axis regulation. Proc. Natl. Acad. Sci. USA 2009, 106, 9069–9074. [Google Scholar] [CrossRef] [PubMed]
- Norden, D.M.; Bicer, S.; Clark, Y.; Jing, R.; Henry, C.J.; Wold, L.E.; Reiser, P.J.; Godbout, J.P.; McCarthy, D.O. Tumor growth increases neuroinflammation, fatigue and depressive-like behavior prior to alterations in muscle function. Brain. Behav. Immun. 2015, 43, 76–85. [Google Scholar] [CrossRef]
- Walker, W.H., II; Borniger, J.C.; Surbhi; Zalenski, A.A.; Muscarella, S.L.; Fitzgerald, J.A.; Zhang, N.; Gaudier-Diaz, M.M.; DeVries, A.C. Mammary tumors induce central pro-inflammatory cytokine expression, but not behavioral deficits in balb/c mice. Sci. Rep. 2017, 7, 8152. [Google Scholar] [CrossRef]
- Vichaya, E.G.; Vermeer, D.W.; Christian, D.L.; Molkentine, J.M.; Mason, K.A.; Lee, J.H.; Dantzer, R. Neuroimmune mechanisms of behavioral alterations in a syngeneic murine model of human papilloma virus-related head and neck cancer. Psychoneuroendocrinology 2017, 79, 59–66. [Google Scholar] [CrossRef]
- Pinquart, M.; Duberstein, P.R. Depression and cancer mortality: A meta-analysis. Psychol. Med. 2010, 40, 1797–1810. [Google Scholar] [CrossRef]
- Reich, M.; Lesur, A.; Perdrizet-Chevallier, C. Depression, quality of life and breast cancer: A review of the literature. Breast Cancer Res. Treat. 2008, 110, 9–17. [Google Scholar] [CrossRef]
- Satin, J.R.; Linden, W.; Phillips, M.J. Depression as a predictor of disease progression and mortality in cancer patients: A meta-analysis. Cancer 2009, 115, 5349–5361. [Google Scholar] [CrossRef]
- Bower, J.E. Behavioral symptoms in patients with breast cancer and survivors. J. Clin. Oncol. 2008, 26, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Fonken, L.K.; Weil, Z.M.; Nelson, R.J. Mice exposed to dim light at night exaggerate inflammatory responses to lipopolysaccharide. Brain. Behav. Immun. 2013, 34, 159–163. [Google Scholar] [CrossRef]
- Tomayko, M.M.; Reynolds, C.P. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother. Pharmacol. 1989, 24, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Borniger, J.C.; Walker, W.H., II; Emmer, K.M.; Zhang, N.; Zalenski, A.A.; Muscarella, S.L.; Fitzgerald, J.A.; Smith, A.N.; Braam, C.J.; TinKai, T.; et al. A role for hypocretin/orexin in metabolic and sleep abnormalities in a mouse model of non-metastatic breast cancer. Cell Metab. 2018, 28, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Blask, D.E.; Sauer, L.A.; Dauchy, R.T.; Holowachuk, E.W.; Ruhoff, M.S.; Kopff, H.S. Melatonin inhibition of cancer growth in vivo involves suppression of tumor fatty acid metabolism via melatonin receptor-mediated signal transduction events 1. CANCER Res. 1999, 59, 4693–4701. [Google Scholar]
- Nakano, N. Establishment of cell lines in vitro from a mammary ascites tumor of mouse and biological properties of the established lines in a serum containing medium. Tohoku J. Exp. Med. 1966, 88, 69–84. [Google Scholar] [CrossRef]
- Sukhbaatar, A.; Sakamoto, M.; Mori, S.; Kodama, T. Analysis of tumor vascularization in a mouse model of metastatic lung cancer. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Mikada, M.; Sukhbaatar, A.; Miura, Y.; Horie, S.; Sakamoto, M.; Mori, S.; Kodama, T. Evaluation of the enhanced permeability and retention effect in the early stages of lymph node metastasis. Cancer Sci. 2017, 108, 846–852. [Google Scholar] [CrossRef]
- Akram, M.; Iqbal, M.; Daniyal, M.; Khan, A.U. Awareness and current knowledge of breast cancer. Biol. Res. 2017, 50, 1–23. [Google Scholar] [CrossRef]
- Walker, W.H., II; Meléndez-Fernández, O.H.; Pascoe, J.L.; Zhang, N.; Devries, A.C. Social enrichment attenuates chemotherapy induced pro-inflammatory cytokine production and affective behavior via oxytocin signaling. Brain. Behav. Immun. 2020, 89, 451–464. [Google Scholar] [CrossRef]
- Sandadi, S.; Frasure, H.E.; Broderick, M.J.; Waggoner, S.E.; Miller, J.A.; von Gruenigen, V.E. The effect of sleep disturbance on quality of life in women with ovarian cancer. Gynecol. Oncol. 2011, 123, 351–355. [Google Scholar] [CrossRef]
- Chen, M.L.; Yu, C.T.; Yang, C.H. Sleep disturbances and quality of life in lung cancer patients undergoing chemotherapy. Lung Cancer. 2008, 62, 391–400. [Google Scholar] [CrossRef]
- Li, J.; Bentzen, S.M.; Li, J.; Renschler, M.; Mehta, M.P. Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Panagiotou, M.; Deboer, T. Effects of chronic dim-light-at-night exposure on sleep in young and aged mice. Neuroscience 2020, 426, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.H.; Yoon, H.K.; Kang, S.G.; Kim, L.; Lee, E.I.; Lee, H.J. Impact of exposure to dim light at night on sleep in female and comparison with male subjects. Psychiatry Investig. 2018, 15, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Aubrecht, T.G.; Jenkins, R.; Nelson, R.J. Dim light at night increases body mass of female mice. Chronobiol. Int. 2015, 32, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Borniger, J.C.; Maurya, S.K.; Periasamy, M.; Nelson, R.J. Acute dim light at night increases body mass, alters metabolism, and shifts core body temperature circadian rhythms. Chronobiol. Int. 2014, 31, 917–925. [Google Scholar] [CrossRef]
- Fonken, L.K.; Lieberman, R.A.; Weil, Z.M.; Nelson, R.J. Dim light at night exaggerates weight gain and inflammation associated with a high-fat diet in male mice. Endocrinology 2013, 154, 3817–3825. [Google Scholar] [CrossRef]
- Fonken, L.K.; Weil, Z.M.; Nelson, R.J. Dark nights reverse metabolic disruption caused by dim light at night. Obesity 2013, 21, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Dauchy, R.T.; Dauchy, E.M.; Tirrell, R.P.; Hill, C.R.; Davidson, L.K.; Greene, M.W.; Tirrell, P.C.; Wu, J.; Sauer, L.A.; Blask, D.E. Dark-phase light contamination disrupts circadian rhythms in plasma measures of endocrine physiology and metabolism in rats. Comp. Med. 2010, 60, 348–356. [Google Scholar] [PubMed]
- Kloog, I.; Haim, A.; Portnov, B.A. Using kernel density function as an urban analysis tool: Investigating the association between nightlight exposure and the incidence of breast cancer in Haifa, Israel. Comput. Environ. Urban. Syst. 2009, 33, 55–63. [Google Scholar] [CrossRef]
- Kloog, I.; Haim, A.; Stevens, R.G.; Barchana, M.; Portnov, B.A. Light at night co-distributes with incident breast but not lung cancer in the female population of Israel. Chronobiol. Int. 2008, 25, 65–81. [Google Scholar] [CrossRef] [PubMed]
- James, P.; Bertrand, K.A.; Hart, J.E.; Schernhammer, E.S.; Tamimi, R.M.; Laden, F. Outdoor light at night and breast cancer incidence in the nurses’ health study ii. Environ. Health Perspect. 2017, 125, 087010. [Google Scholar] [CrossRef] [PubMed]
- Hurley, S.; Goldberg, D.; Nelson, D.; Hertz, A.; Horn-Ross, P.L.; Bernstein, L.; Reynolds, P. Light at night and breast cancer risk among california teachers. Epidemiology 2014, 25, 697–706. [Google Scholar] [CrossRef]
- Hill, S.M.; Xiang, S.; Dauchy, R.T.; Wren-Dail, M.; Anbalagan, M.; Rowan, B.; Frasch, T.; Blask, D.E. Abstract 4897: Circadian/melatonin disruption by dim light at night drives human epithelial breast cancer to a metastatic phenotype. Cancer Res. 2017, 77, 4897. [Google Scholar] [CrossRef]
- Fonken, L.K.; Finy, M.S.; Walton, J.C.; Weil, Z.M.; Workman, J.L.; Ross, J.; Nelson, R.J. Influence of light at night on murine anxiety- and depressive-like responses. Behav. Brain Res. 2009, 205, 349–354. [Google Scholar] [CrossRef]
- Tsaras, K.; Papathanasiou, I.V.; Mitsi, D.; Veneti, A.; Kelesi, M.; Zyga, S.; Fradelos, E.C. Assessment of depression and anxiety in breast cancer patients: Prevalence and associated factors. Asian Pac. J. Cancer Prev. 2018, 19, 1661–1669. [Google Scholar]
- Park, E.M.; Gelber, S.; Rosenberg, S.M.; Seah, D.S.E.; Schapira, L.; Come, S.E.; Partridge, A.H. Anxiety and depression in young women with metastatic breast cancer: A cross-sectional study. Psychosomatics 2018, 59, 251–258. [Google Scholar] [CrossRef]
- Pyter, L.M.; Suarez-Kelly, L.P.; Carson, W.E.; Kaur, J.; Bellisario, J.; Bever, S.R. Novel rodent model of breast cancer survival with persistent anxiety-like behavior and inflammation. Behav. Brain Res. 2017, 330, 108–117. [Google Scholar] [CrossRef]
- Watts, S.; Prescott, P.; Mason, J.; McLeod, N.; Lewith, G. Depression and anxiety in ovarian cancer: A systematic review and meta-analysis of prevalence rates. BMJ Open. 2015, 5, e007618. [Google Scholar] [CrossRef]
- Arrieta, Ó.; Angulo, L.P.; Núñez-Valencia, C.; Dorantes-Gallareta, Y.; Macedo, E.O.; Martínez-López, D.; Alvarado, S.; Corona-Cruz, J.F.; Oñate-Ocaña, L.F. Association of depression and anxiety on quality of life, treatment adherence, and prognosis in patients with advanced non-small cell lung cancer. Ann. Surg. Oncol. 2013, 20, 1941–1948. [Google Scholar] [CrossRef]
- Aubrecht, T.G.; Weil, Z.M.; Magalang, U.J.; Nelson, R.J. Dim light at night interacts with intermittent hypoxia to alter cognitive and affective responses. Am. J. Physiol. Integr. Comp. Physiol. 2013, 305, R78–R86. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nelson, R.J. Photoperiodic responsiveness in house mice. Physiol. Behav. 1990, 48, 403–408. [Google Scholar] [CrossRef]
- Yellon, S.M.; Tran, L.T. Photoperiod, reproduction, and immunity in select strains of inbred mice. J. Biol. Rhythms 2002, 17, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Thaker, P.H.; Han, L.Y.; Kamat, A.A.; Arevalo, J.M.; Takahashi, R.; Lu, C.; Jennings, N.B.; Armaiz-Pena, G.; Bankson, J.A.; Ravoori, M.; et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat. Med. 2006, 12, 939–944. [Google Scholar] [CrossRef]
- McEwen, B.S.; Akil, H. Revisiting the stress concept: Implications for affective disorders. J. Neurosci. 2020, 40, 12–21. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walker, W.H., II; Kvadas, R.M.; May, L.E.; Liu, J.A.; Bumgarner, J.R.; Walton, J.C.; DeVries, A.C.; Dauchy, R.T.; Blask, D.E.; Nelson, R.J. Artificial Light at Night Reduces Anxiety-like Behavior in Female Mice with Exacerbated Mammary Tumor Growth. Cancers 2021, 13, 4860. https://doi.org/10.3390/cancers13194860
Walker WH II, Kvadas RM, May LE, Liu JA, Bumgarner JR, Walton JC, DeVries AC, Dauchy RT, Blask DE, Nelson RJ. Artificial Light at Night Reduces Anxiety-like Behavior in Female Mice with Exacerbated Mammary Tumor Growth. Cancers. 2021; 13(19):4860. https://doi.org/10.3390/cancers13194860
Chicago/Turabian StyleWalker, William H., II, Raegan M. Kvadas, Laura E. May, Jennifer A. Liu, Jacob R. Bumgarner, James C. Walton, A. Courtney DeVries, Robert T. Dauchy, David E. Blask, and Randy J. Nelson. 2021. "Artificial Light at Night Reduces Anxiety-like Behavior in Female Mice with Exacerbated Mammary Tumor Growth" Cancers 13, no. 19: 4860. https://doi.org/10.3390/cancers13194860
APA StyleWalker, W. H., II, Kvadas, R. M., May, L. E., Liu, J. A., Bumgarner, J. R., Walton, J. C., DeVries, A. C., Dauchy, R. T., Blask, D. E., & Nelson, R. J. (2021). Artificial Light at Night Reduces Anxiety-like Behavior in Female Mice with Exacerbated Mammary Tumor Growth. Cancers, 13(19), 4860. https://doi.org/10.3390/cancers13194860







