Adolescent and Young Adult (AYA) Cancer Survivorship Practices: An Overview
Abstract
Simple Summary
Abstract
1. Introduction
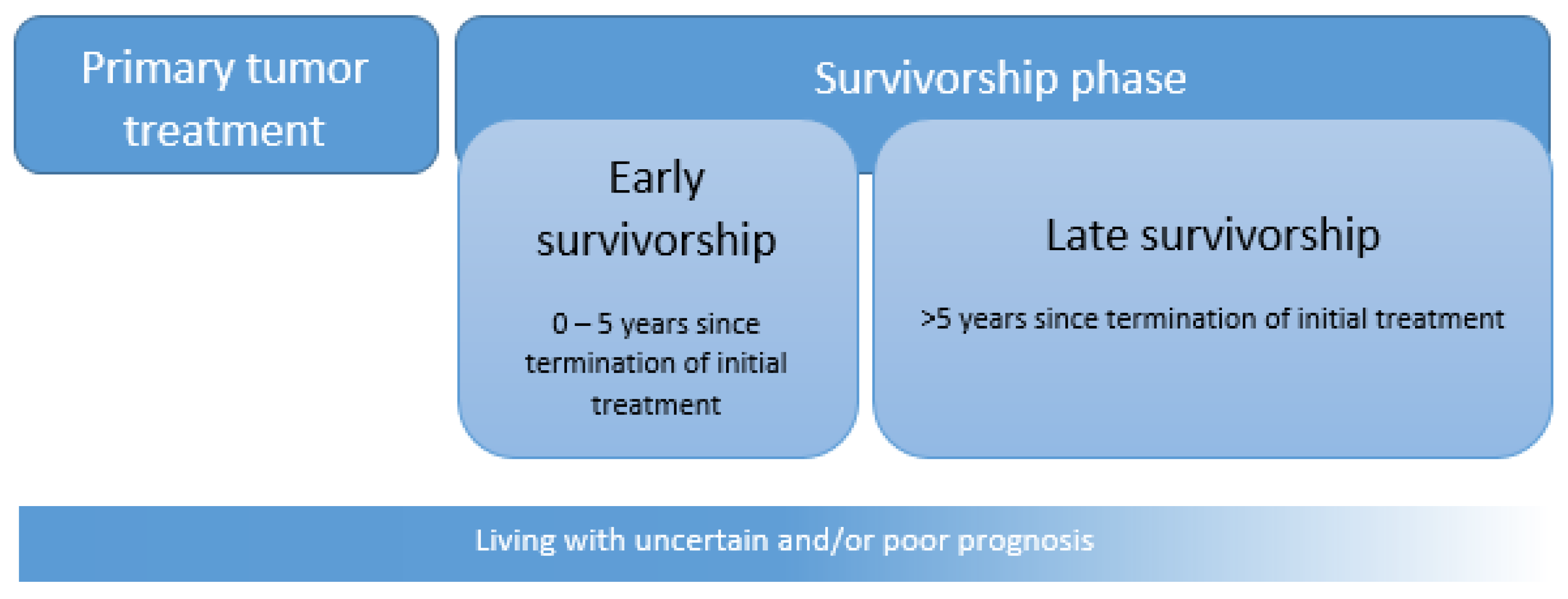
2. AYA Cancer Survivorship: Epidemiology
3. What Sets AYA Cancer Survivors Apart?
- The percentage of AYAs with cancer who carry pathogenic variants in genes that predispose to cancer is significant [18], with important consequences for an individuals’ surveillance strategy.
- In contrast to the emphasis put on late effects of treatment in pediatric cancer patients, for AYAs hardly any late effect clinics are in place, and the lasting (physical) impact of treatments at this age is only known to a limited degree [26].
- Adolescence, emerging, and young adulthood are complex, unique phases of life due to the many physical, emotional, cognitive, and social transitions [27]. A cancer diagnosis during the AYA life stage exacerbates typical developmental challenges and interferes with the attainment of important age-specific milestones [16], including identity-forming, establishing autonomy, responsibility and independence, finishing education and starting a career, obtaining romantic relationships and starting a family [27]. In addition, the type of informal caregivers may differ among cancer patients with varying ages and life courses [28]. The way in which AYA cancer patients adjust to their cancer experience might have life-long implications for the quality of their survival time [29,30,31,32,33].
4. What Do We Know about Long-Term and Late Effects among AYA Cancer Survivors?
4.1. Physical Issues
4.1.1. Secondary Malignancies
4.1.2. Cardiovascular Disease
4.1.3. Endocrine Dysfunction
4.1.4. Neurocognitive Deficits
4.1.5. Fertility
4.1.6. Sexual Dysfunction
4.1.7. Body Disfigurement
4.1.8. Physical Condition
4.2. Psychological Issues
4.3. Social Issues
4.3.1. Education, Employment, and Financial Challenges
4.3.2. Relationships
5. Challenges and Models of AYA Survivorship Care
5.1. How Is Survivorship Care Organized for Children and Older Adults?
5.1.1. Survivorship Care for Children
5.1.2. Survivorship Care for Older Adults (>40 Years)
5.2. Challenges for AYA Survivorship Care
5.3. Models of AYA Survivorship Care and Services Needed
5.4. Examples of AYA Survivorship Care
6. Future Directions
6.1. AYA Cancer Survivorship Research
6.2. AYA Cancer Survivorship Care
- Leading to improved information provision and communication among AYA cancer survivors and HCPs. Although the use of SCPs among cancer survivors in general was limited, they may serve their purpose among AYA cancer survivors, as Hydeman and colleagues highlight the desire of AYAs for more information regarding late and long-term biopsychosocial issues with which they are dealing [118]. Many AYA cancer survivors do not adhere to recommended tumor-specific survivorship care, which may be due to poor knowledge of the possible late effects, treatment, and health maintenance, underinsurance, costs, or reluctance to go back to the hospital [12,25,118]. AYAs should be empowered to assume ownership for their care and to have support for personal transitions (e.g., school, employment, and family planning) and practical issues (e.g., insurances and finances) [43]. As there is a lack of communication around AYA-specific issues, the SCP as part of standard care may facilitate key information provision [118]. An SCP may be beneficial for AYA cancer survivors to transition back to ‘normal life,’ e.g., by providing education and information regarding their treatments’ long-term and late effects and lifestyle recommendations [12,126]. It is, however, important to tailor SCPs to the developmental phase of AYAs [118,161].
- Gaining knowledge about specific (sub) groups at risk may lead to the development of risk-stratified AYA survivorship programs, guidelines, and surveillance strategies. Within pediatric oncology, survivorship care has been associated with improvements on both the patient level (e.g., improved patients’ knowledge regarding treatment and risks of late effects) as well as on a system level (e.g., fewer emergency room visits, hospitalizations, and better surveillance practices) [149]. The potential to mitigate the severity of late effects with early detection and management through survivorship care programs offers a great financial opportunity for healthcare systems [155]. A core outcome set, as described previously, can promote the development of quality care standards and guidelines and improves AYA-specific healthcare. Performance indicators, which can be part of an AYA-specific core outcome set, can improve quality of care but can also serve as a benchmark to achieve outcome-improvement goals and provide the information needed to make policy-related decisions and resources allocation. In Canada, besides an AYA-specific survivorship program, AYA-specific system performance indicators have been established by a national, multidisciplinary panel of AYA oncology experts [73,155]. Identifying system performance indicators specifically for AYAs may be applied internationally to monitor, evaluate, and benchmark the progress of (inter)national cancer care programs and institutions. Adequately resourced AYA care programs are, on a system level, more sustainable when ensured by a governmental policy [12,123,137], with a clear model of care with key performance indicators [124,126,162]. Table 2 provides a summarizing overview of the key points of AYA-specific survivorship care.
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A. Methods Used to Calculate the Age-Standardized 5- and 10-Year Relative Survival
Appendix B. Tumor Type Categorization of Table 1 Explained, Based on Site Recode ICD-O-3/WHO 2008 and ICD-O-3 Histology/Behavior, Malignant
References
- Adolescent; Young Adult Oncology Progress Review Group. Closing the Gap: Research and Care Imperatives for Adolescents and Young Adults with Cancer. 2006. Available online: https://www.livestrong.org/content/closing-gap-research-and-care-imperatives-adolescents-and-young-adults-cancer (accessed on 21 April 2020).
- Barr, R.D.J.C.T.R. Common cancers in adolescents. Cancer Treat. Rev. 2007, 33, 597–602. [Google Scholar] [CrossRef]
- Smith, A.W.; Seibel, N.L.; Lewis, D.R.; Albritton, K.H.; Blair, D.F.; Blanke, C.D.; Bleyer, W.A.; Freyer, D.R.; Geiger, A.M.; Hayes-Lattin, B.; et al. Next steps for adolescent and young adult oncology workshop: An update on progress and recommendations for the future. Cancer 2016, 122, 988–999. [Google Scholar] [CrossRef]
- Lewis, D.R.; Seibel, N.L.; Smith, A.W.; Stedman, M.R. Adolescent and Young Adult Cancer Survival. J. Natl. Cancer Inst. Monogr. 2014, 2014, 228–235. [Google Scholar] [CrossRef]
- Meeneghan, M.R.; Wood, W.A. Challenges for cancer care delivery to adolescents and young adults: Present and future. Acta. Haematol. 2014, 132, 414–422. [Google Scholar] [CrossRef]
- Surveillance, Epidemiology and End Results Program. All Cancer Sites Combined Recent Trends in SEER Age-Adjusted Incidence Rates. Available online: https://seer.cancer.gov/explorer/application.html?site=1&data_type=1&graph_type=2&compareBy=age_range&chk_age_range_62=62&sex=1&race=1&hdn_stage=101&rate_type=1&advopt_precision=1&advopt_display=2#graphArea (accessed on 14 July 2020).
- Close, A.G.; Dreyzin, A.; Mph, K.D.M.; Seynnaeve, B.K.; Rapkin, L.B. Adolescent and young adult oncology—Past, present, and future. CA Cancer J. Clin. 2019, 69, 485–496. [Google Scholar] [CrossRef]
- Van Der Meer, D.J.; Karim-Kos, H.E.; Van Der Mark, M.; Aben, K.K.H.; Bijlsma, R.M.; Rijneveld, A.W.; Van Der Graaf, W.T.A.; Husson, O. Incidence, Survival, and Mortality Trends of Cancers Diagnosed in Adolescents and Young Adults (15–39 Years): A Population-Based Study in The Netherlands. Cancers 2020, 12, 3421. [Google Scholar] [CrossRef]
- Adams, S.C.; Herman, J.; Lega, I.C.; Mitchell, L.; Hodgson, D.; Edelstein, K.; Travis, L.B.; Sabiston, C.M.; Thavendiranathan, P.; Gupta, A.A. Young Adult Cancer Survivorship: Recommendations for Patient Follow-up, Exercise Therapy, and Research. JNCI Cancer Spectr. 2021, 5, pkaa099. [Google Scholar] [CrossRef]
- Kinahan, K.E.; Sanford, S.; Sadak, K.T.; Salsman, J.M.; Danner-Koptik, K.; Didwania, A. Models of Cancer Survivorship Care for Adolescents and Young Adults. Semin. Oncol. Nurs. 2015, 31, 251–259. [Google Scholar] [CrossRef][Green Version]
- ACS. Cancer Treatment and Survivorship Facts & Figures 2012-American Cancer Society. 2012. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-treatment-and-survivorship-facts-and-figures/cancer-treatment-and-survivorship-facts-and-figures-2012-2013.pdf (accessed on 9 December 2020).
- Osborn, M.; Johnson, R.; Thompson, K.; Anazodo, A.; Albritton, K.; Ferrari, A.; Stark, D. Models of care for adolescent and young adult cancer programs. Pediatr. Blood Cancer 2019, 66, e27991. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Gupta, S.; Harper, A.; Ruan, Y.; Barr, R.; Frazier, A.L.; Ferlay, J.; Steliarova-Foucher, E.; Fidler-Benaoudia, M.M. International Trends in the Incidence of Cancer among Adolescents and Young Adults. J. Natl. Cancer Inst. 2020, 112, 1105–1117. [Google Scholar] [CrossRef]
- Miller, K.D.; Fidler-Benaoudia, M.; Keegan, T.H.; Hipp, H.S.; Jemal, A.; Siegel, R.L. Cancer statistics for adolescents and young adults. CA Cancer J. Clin. 2020, 70, 443–459. [Google Scholar] [CrossRef]
- Perez, G.K.; Salsman, J.M.; Fladeboe, K.; Kirchhoff, A.C.; Park, E.R.; Rosenberg, A.R. Taboo Topics in Adolescent and Young Adult Oncology: Strategies for Managing Challenging but Important Conversations Central to Adolescent and Young Adult Cancer Survivorship. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, e171–e185. [Google Scholar] [CrossRef]
- Burgers, V.W.; van der Graaf, W.T.; van der Meer, D.J.; McCabe, M.G.; Rijneveld, A.W.; Bent, M.J.V.D.; Husson, O. Adolescents and Young Adults Living With an Uncertain or Poor Cancer Prognosis: The “New” Lost Tribe. J. Natl. Compr. Cancer Netw. 2021, 19, 240–246. [Google Scholar] [CrossRef]
- Ferrari, A.; Stark, D.; Peccatori, F.; Fern, L.; Laurence, V.; Gaspar, N.; Bozovic-Spasojevic, I.; Smith, O.; De Munter, J.; Derwich, K.; et al. Adolescents and young adults (AYA) with cancer: A position paper from the AYA Working Group of the European Society for Medical Oncology (ESMO) and the European Society for Paediatric Oncology (SIOPE). ESMO Open 2021, 6, 100096. [Google Scholar] [CrossRef]
- Dommett, R.M.; Redaniel, T.; Stevens, M.C.G.; Hamilton, W.; Martin, R.M. Features of cancer in teenagers and young adults in primary care: A population-based nested case–control study. Br. J. Cancer 2013, 108, 2329–2333. [Google Scholar] [CrossRef]
- Fern, L.A.; Birch, R.; Whelan, J.; Cooke, M.; Sutton, S.; Neal, R.D.; Gerrand, C.; Hubbard, G.; Smith, S.; Lethaby, C. Why can’t we improve the timeliness of cancer diagnosis in children, teenagers, and young adults? BMJ 2013, 347, f6493. [Google Scholar] [CrossRef]
- Tricoli, J.V.; Bleyer, A. Adolescent and Young Adult Cancer Biology. Cancer J. 2018, 24, 267–274. [Google Scholar] [CrossRef]
- Sender, L.; Zabokrtsky, K.B. Adolescent and young adult patients with cancer: A milieu of unique features. Nat. Rev. Clin. Oncol. 2015, 12, 465–480. [Google Scholar] [CrossRef]
- Bleyer, A.; on behalf of the Biology and Clinical Trials Subgroups of the US National Cancer Institute Progress Review Group in Adolescent and Young Adult Oncology; Barr, R.; Hayes-Lattin, B.; Thomas, D.; Ellis, C.; Anderson, B. The distinctive biology of cancer in adolescents and young adults. Nat. Rev. Cancer 2008, 8, 288–298. [Google Scholar] [CrossRef]
- Potosky, A.L.; Harlan, L.C.; Albritton, K.; Cress, R.D.; Friedman, D.L.; Hamilton, A.S.; Kato, I.; Keegan, T.H.; Keel, G.; Schwartz, S.M.; et al. Use of Appropriate Initial Treatment Among Adolescents and Young Adults With Cancer. J. Natl. Cancer Inst. 2014, 106, 300. [Google Scholar] [CrossRef]
- Fardell, J.E.; Patterson, P.; Wakefield, C.E.; Signorelli, C.; Cohn, R.; Anazodo, A.; Zebrack, B.; Sansom-Daly, U. A Narrative Review of Models of Care for Adolescents and Young Adults with Cancer: Barriers and Recommendations. J. Adolesc. Young-Adult Oncol. 2018, 7, 148–152. [Google Scholar] [CrossRef]
- Stoneham, S.J. AYA survivorship: The next challenge. Cancer 2020, 126, 2116–2119. [Google Scholar] [CrossRef] [PubMed]
- Zebrack, B.J. Psychological, social, and behavioral issues for young adults with cancer. Cancer 2011, 117, 2289–2294. [Google Scholar] [CrossRef] [PubMed]
- Kent, E.E.; Mollica, M.A.; Buckenmaier, S.; Smith, A.W. The Characteristics of Informal Cancer Caregivers in the United States. Semin. Oncol. Nurs. 2019, 35, 328–332. [Google Scholar] [CrossRef]
- Greup, S.R.; Kaal, S.E.J.; Jansen, R.; Manten-Horst, E.; Thong, M.S.Y.; Van Der Graaf, W.T.A.; Prins, J.B.; Husson, O. Post-Traumatic Growth and Resilience in Adolescent and Young Adult Cancer Patients: An Overview. J. Adolesc. Young-Adult Oncol. 2018, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Harju, E.; Roser, K.; Dehler, S.; Michel, G. Health-related quality of life in adolescent and young adult cancer survivors. Support. Care Cancer 2018, 26, 3099–3110. [Google Scholar] [CrossRef]
- Husson, O.; Huijgens, P.C.; Van Der Graaf, W.T.A. Psychosocial challenges and health-related quality of life of adolescents and young adults with hematologic malignancies. Blood 2018, 132, 385–392. [Google Scholar] [CrossRef]
- Husson, O.; Zebrack, B.J.; Aguilar, C.; Hayes-Lattin, B.; Cole, S. Cancer in adolescents and young adults: Who remains at risk of poor social functioning over time? Cancer 2017, 123, 2743–2751. [Google Scholar] [CrossRef]
- Schulte, F.S.M.; Chalifour, K.; Eaton, G.; Garland, S.N. Quality of life among survivors of adolescent and young adult cancer in Canada: A Young Adults with Cancer in Their Prime (YACPRIME) study. Cancer 2021, 127, 1325–1333. [Google Scholar] [CrossRef]
- Robison, L.L.; Hudson, M.M. Survivors of childhood and adolescent cancer: Life-long risks and responsibilities. Nat. Rev. Cancer 2014, 14, 61–70. [Google Scholar] [CrossRef]
- Robison, L.L.; Mertens, A.C.; Boice, J.D.; Breslow, N.E.; Donaldson, S.S.; Green, D.M.; Li, F.P.; Meadows, A.T.; Mulvihill, J.J.; Neglia, J.; et al. Study design and cohort characteristics of the childhood cancer survivor study: A multi-institutional collaborative project. Med. Pediatr. Oncol. 2002, 38, 229–239. [Google Scholar] [CrossRef]
- Travis, L.B.; Beard, C.; Allan, J.; Dahl, A.A.; Feldman, D.; Oldenburg, J.; Daugaard, G.; Kelly, J.L.; Dolan, M.E.; Hannigan, R.; et al. Testicular Cancer Survivorship: Research Strategies and Recommendations. J. Natl. Cancer Inst. 2010, 102, 1114–1130. [Google Scholar] [CrossRef]
- Steininger, J.; Gellrich, F.; Schulz, A.; Westphal, D.; Beissert, S.; Meier, F. Systemic Therapy of Metastatic Melanoma: On the Road to Cure. Cancers 2021, 13, 1430. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Blank, C.U.; Mandalà, M.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M.; Meshcheryakov, A.; Khattak, A.; Carlino, M.S.; et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): Distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 643–654. [Google Scholar] [CrossRef]
- Bottomley, A.; Coens, C.; Mierzynska, J.; Blank, C.U.; Mandalà, M.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M.; Meshcheryakov, A. Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): Health-related quality-of-life results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 655–664. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Coccia, P.F.; Pappo, A.S.; Beaupin, L.; Borges, V.F.; Borinstein, S.C.; Chugh, R.; Dinner, S.; Folbrecht, J.; Frazier, A.L.; Goldsby, R.; et al. Adolescent and Young Adult Oncology, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 66–97. [Google Scholar] [CrossRef]
- Bleyer, W.A.; Barr, R.D.; Ries, L.; Whelan, J.; Ferrari, A. Cancer in Adolescents and Young Adults; Springer: Berlin, Germany, 2007. [Google Scholar]
- Patterson, P.; McDonald, F.E.; Zebrack, B.; Medlow, S. Emerging Issues Among Adolescent and Young Adult Cancer Survivors. Semin. Oncol. Nurs. 2015, 31, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.; Bhatia, S.; Xu, L.; Cannavale, K.L.; Wong, F.L.; Huang, P.-Y.S.; Cooper, R.; Armenian, S.H. Chronic Comorbidities Among Survivors of Adolescent and Young Adult Cancer. J. Clin. Oncol. 2020, 38, 3161–3174. [Google Scholar] [CrossRef]
- Abdelhadi, O.A.; Joseph, J.; Pollock, B.H.; Keegan, T.H.M. Additional medical costs of chronic conditions among adolescent and young adult cancer survivors. J. Cancer Surviv. 2021, 1–10. [Google Scholar] [CrossRef]
- Fidler, M.M.; Frobisher, C.; Hawkins, M.M.; Nathan, P.C. Challenges and opportunities in the care of survivors of adolescent and young adult cancers. Pediatr. Blood Cancer 2019, 66, e27668. [Google Scholar] [CrossRef] [PubMed]
- Bright, C.J.; Reulen, R.C.; Winter, D.L.; Stark, D.P.; McCabe, M.G.; Edgar, A.B.; Frobisher, C.; Hawkins, M.M. Risk of subsequent primary neoplasms in survivors of adolescent and young adult cancer (Teenage and Young Adult Cancer Survivor Study): A population-based, cohort study. Lancet Oncol. 2019, 20, 531–545. [Google Scholar] [CrossRef]
- Lee, J.S.; DuBois, S.G.; Coccia, P.F.; Bleyer, A.; Olin, R.L.; Goldsby, R.E. Increased risk of second malignant neoplasms in adolescents and young adults with cancer. Cancer 2016, 122, 116–123. [Google Scholar] [CrossRef]
- Chao, C.; Bhatia, S.; Xu, L.; Cannavale, K.L.; Wong, F.L.; Huang, P.-Y.S.; Cooper, R.; Armenian, S.H. Incidence, Risk Factors, and Mortality Associated With Second Malignant Neoplasms Among Survivors of Adolescent and Young Adult Cancer. JAMA Netw. Open 2019, 2, e195536. [Google Scholar] [CrossRef]
- Van Leeuwen, F.E.; Ng, A.K. Long-term risk of second malignancy and cardiovascular disease after Hodgkin lymphoma treatment. Hematology Am. Soc. Hematol. Educ. Program 2016, 2016, 323–330. [Google Scholar] [CrossRef]
- Schaapveld, M.; Aleman, B.M.P.; Van Eggermond, A.M.; Janus, C.P.M.; Krol, S.; Van Der Maazen, R.W.M.; Roesink, J.M.; Raemaekers, J.M.M.; De Boer, J.P.; Zijlstra, J.M.; et al. Second Cancer Risk Up to 40 Years after Treatment for Hodgkin’s Lymphoma. N. Engl. J. Med. 2015, 373, 2499–2511. [Google Scholar] [CrossRef]
- Hauptmann, M.; Fossa, S.D.; Stovall, M.; Van Leeuwen, F.E.; Johannesen, T.B.; Rajaraman, P.; Gilbert, E.S.; Smith, S.A.; Weathers, R.E.; Aleman, B.M.P.; et al. Increased stomach cancer risk following radiotherapy for testicular cancer. Br. J. Cancer 2014, 112, 44–51. [Google Scholar] [CrossRef]
- Keegan, T.H.M.; Kushi, L.H.; Li, Q.; Brunson, A.; Chawla, X.; Chew, H.K.; Malogolowkin, M.; Wun, T. Cardiovascular disease incidence in adolescent and young adult cancer survivors: A retrospective cohort study. J. Cancer Surviv. 2018, 12, 388–397. [Google Scholar] [CrossRef]
- Meinardi, M.; Gietema, J.; Van Der Graaf, W.A.; Van Veldhuisen, D.; Runne, M.; Sluiter, W.; de Vries, E.; Willemse, P.H.; Mulder, N.; Berg, M.V.D.; et al. Cardiovascular Morbidity in Long-Term Survivors of Metastatic Testicular Cancer. J. Clin. Oncol. 2000, 18, 1725–1732. [Google Scholar] [CrossRef]
- Belt-Dusebout, A.W.V.D.; de Wit, R.; Gietema, J.A.; Horenblas, S.; Louwman, M.W.; Ribot, J.G.; Hoekstra, H.J.; Ouwens, G.M.; Aleman, B.M.; van Leeuwen, F.E. Treatment-Specific Risks of Second Malignancies and Cardiovascular Disease in 5-Year Survivors of Testicular Cancer. J. Clin. Oncol. 2007, 25, 4370–4378. [Google Scholar] [CrossRef] [PubMed]
- Tai, E.; Buchanan, N.; Ms, J.T.; Fairley, T.; Moore, A.; Richardson, L.C. Health status of adolescent and young adult cancer survivors. Cancer 2012, 118, 4884–4891. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.V.; Rugbjerg, K.; de Fine Licht, S.; Johansen, C.; Schmiegelow, K.; Andersen, K.K.; Winther, J.F. Endocrine late effects in survivors of cancer in adolescence and young adulthood: A Danish population-based cohort study. JAMA Netw. Open 2018, 1, e180349. [Google Scholar] [CrossRef] [PubMed]
- Jim, H.S.; Jennewein, S.L.; Quinn, G.; Reed, D.R.; Small, B.J. Cognition in Adolescent and Young Adults Diagnosed With Cancer: An Understudied Problem. J. Clin. Oncol. 2018, 36, 2752–2754. [Google Scholar] [CrossRef] [PubMed]
- Dewar, E.O.; Ahn, C.; Eraj, S.; Mahal, B.A.; Sanford, N.N. Psychological distress and cognition among long-term survivors of adolescent and young adult cancer in the USA. J. Cancer Surviv. 2021, 15, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Schover, L.R.; Partridge, A.H.; Patrizio, P.; Wallace, W.H.; Hagerty, K.; Beck, L.N.; Brennan, L.V.; Oktay, K. American Society of Clinical Oncology Recommendations on Fertility Preservation in Cancer Patients. J. Clin. Oncol. 2006, 24, 2917–2931. [Google Scholar] [CrossRef]
- Murphy, D.; Orgel, E.; Termuhlen, A.; Shannon, S.; Warren, K.; Quinn, G.P. Why Healthcare Providers Should Focus on the Fertility of AYA Cancer Survivors: It’s Not Too Late! Front. Oncol. 2013, 3, 248. [Google Scholar] [CrossRef]
- Haugnes, H.S.; Bosl, G.; Boer, H.; Gietema, J.; Brydøy, M.; Oldenburg, J.; Dahl, A.A.; Bremnes, R.M.; Fosså, S.D. Long-Term and Late Effects of Germ Cell Testicular Cancer Treatment and Implications for Follow-Up. J. Clin. Oncol. 2012, 30, 3752–3763. [Google Scholar] [CrossRef]
- Teenage Cancer Trust. A Blueprint of Care for Teenagers and Young Adults with Cancer. 2012. Available online: https://www.teenagecancertrust.org/sites/default/files/Blueprint-of-Care.pdf (accessed on 3 April 2020).
- Barr, R.D.; Ferrari, A.; Ries, L.; Whelan, J.; Bleyer, W.A. Cancer in adolescents and young adults: A narrative review of the current status and a view of the future. JAMA Pediatr. 2016, 170, 495–501. [Google Scholar] [CrossRef]
- Loren, A.W. Fertility issues in patients with hematologic malignancies. Hematology 2015, 2015, 138–145. [Google Scholar] [CrossRef]
- Barnett, M.; McDonnell, G.; DeRosa, A.; Schuler, T.; Philip, E.; Peterson, L.; Touza, K.; Jhanwar, S.; Atkinson, T.M.; Ford, J.S. Psychosocial outcomes and interventions among cancer survivors diagnosed during adolescence and young adulthood (AYA): A systematic review. J. Cancer Surviv. 2016, 10, 814–831. [Google Scholar] [CrossRef]
- Benedict, C.; Thom, B.; Kelvin, J. Fertility preservation and cancer: Challenges for adolescent and young adult patients. Curr. Opin. Support. Palliat. Care. 2016, 10, 87. [Google Scholar] [CrossRef]
- Russell, A.M.; Galvin, K.M.; Harper, M.M.; Clayman, M.L. A comparison of heterosexual and LGBTQ cancer survivors’ outlooks on relationships, family building, possible infertility, and patient-doctor fertility risk communication. J. Cancer Surviv. 2016, 10, 935–942. [Google Scholar] [CrossRef]
- Su, H.I.; Lee, Y.T.; Barr, R. Oncofertility: Meeting the fertility goals of adolescents and young adults with cancer. Cancer J. 2018, 24, 328. [Google Scholar]
- Zhou, E.S.; Falk, S.J.; Bober, S.L. Managing premature menopause and sexual dysfunction. Curr. Opin. Support. Palliat. Care 2015, 9, 294–300. [Google Scholar] [CrossRef]
- Bolte, S.; Zebrack, B. Sexual Issues in Special Populations: Adolescents and Young Adults. Semin. Oncol. Nurs. 2008, 24, 115–119. [Google Scholar] [CrossRef]
- Olsson, M.; Enskär, K.; Steineck, G.; Wilderäng, U.; Jarfelt, M. Self-Perceived Physical Attractiveness in Relation to Scars Among Adolescent and Young Adult Cancer Survivors: A Population-Based Study. J. Adolesc. Young-Adult Oncol. 2018, 7, 358–366. [Google Scholar] [CrossRef]
- Pt, M.L.M.; Med, J.K.P.; Jones, J.M.; Amin, L.; Chang, E.; Korenblum, C.; Mina, D.S.; McCabe, L.; Mitchell, L.; Giuliani, M. Reimagining care for adolescent and young adult cancer programs: Moving with the times. Cancer 2016, 122, 1038–1046. [Google Scholar] [CrossRef]
- Brierley, M.E.E.; Sansom-Daly, U.M.; Baenziger, J.; McGill, B.; Wakefield, C.E. Impact of physical appearance changes reported by adolescent and young adult cancer survivors: A qualitative analysis. Eur. J. Cancer Care 2019, 28, e13052. [Google Scholar] [CrossRef] [PubMed]
- Sun, V.; Grant, M.; Ms, C.S.W.; McMullen, C.K.; Bulkley, J.E.; Altschuler, A.; Ramirez, M.; Baldwin, C.; Herrinton, L.J.; Hornbrook, M.C.; et al. Dietary and Behavioral Adjustments to Manage Bowel Dysfunction After Surgery in Long-Term Colorectal Cancer Survivors. Ann. Surg. Oncol. 2015, 22, 4317–4324. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.N.; Arshad, O.; Kwok, J.; Tran, E.; Howard, A.F.; Serrano, I.; Goddard, K. Documentation and incidence of late effects and screening recommendations for adolescent and young adult head and neck cancer survivors treated with radiotherapy. Support. Care Cancer 2018, 27, 2609–2616. [Google Scholar] [CrossRef]
- CanTeen. Exploring Survivorship Care for Adolescent and Young Adult Cancer Survivors in Australia. Sydney, Australia: CanTeen Australia. 2015. Available online: https://www.canteen.org.au/wp-content/uploads/2016/09/Adolescent-and-Young-Adult-Cancer-Survivorship-Report.pdf (accessed on 4 July 2021).
- Ketterl, T.G.; Syrjala, K.L.; Casillas, J.; Jacobs, L.A.; Palmer, S.C.; McCabe, M.S.; Ganz, P.A.; Overholser, L.; Partridge, A.; Rajotte, E.J.; et al. Lasting effects of cancer and its treatment on employment and finances in adolescent and young adult cancer survivors. Cancer 2019, 125, 1908–1917. [Google Scholar] [CrossRef]
- Zebrack, B.J.; Corbett, V.; Embry, L.; Aguilar, C.; Meeske, K.A.; Hayes-Lattin, B.; Block, R.; Zeman, D.T.; Cole, S. Psychological distress and unsatisfied need for psychosocial support in adolescent and young adult cancer patients during the first year following diagnosis. Psycho-Oncology 2014, 23, 1267–1275. [Google Scholar] [CrossRef]
- Jones, J.M.; Fitch, M.; Bongard, J.; Maganti, M.; Gupta, A.; D’Agostino, N.; Korenblum, C. The Needs and Experiences of Post-Treatment Adolescent and Young Adult Cancer Survivors. J. Clin. Med. 2020, 9, 1444. [Google Scholar] [CrossRef]
- De, R.; Sutradhar, R.; Kurdyak, P.; Aktar, S.; Pole, J.D.; Baxter, N.; Nathan, P.C.; Gupta, S. Incidence and Predictors of Mental Health Outcomes Among Survivors of Adolescent and Young Adult Cancer: A Population-Based Study Using the IMPACT Cohort. J. Clin. Oncol. 2021, 39, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Zebrack, B.; Isaacson, S. Psychosocial care of adolescent and young adult patients with cancer and survivors. J. Clin. Oncol. 2012, 30, 1221–1226. [Google Scholar] [CrossRef]
- Kahl, K.G.; Winter, L.; Schweiger, U. The third wave of cognitive behavioural therapies: What is new and what is effective? Current opinion in psychiatry. Curr. Opin. Psychiatry 2012, 25, 522–528. [Google Scholar] [CrossRef]
- Hulbert-Williams, N.J.; Storey, L.; Wilson, K.G. Psychological interventions for patients with cancer: Psychological flexibility and the potential utility of Acceptance and Commitment Therapy. Eur. J. Cancer Care 2014, 24, 15–27. [Google Scholar] [CrossRef]
- Keegan, T.H.M.; Lichtensztajn, D.; Kato, I.; Kent, E.E.; Wu, X.-C.; West, M.M.; Hamilton, A.S.; Zebrack, B.; Bellizzi, K.M.; et al.; the AYA HOPE Study Collaborative Group Unmet adolescent and young adult cancer survivors information and service needs: A population-based cancer registry study. J. Cancer Surviv. 2012, 6, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.; Zebrack, B.J.; Meeske, K.A.; Embry, L.; Aguilar, C.; Block, R.; Hayes-Lattin, B.; Li, Y.; Butler, M.; Cole, S. Trajectories of Psychological Distress in Adolescent and Young Adult Patients with Cancer: A 1-Year Longitudinal Study. J. Clin. Oncol. 2013, 31, 2160–2166. [Google Scholar] [CrossRef] [PubMed]
- Thewes, B.; Kaal, S.E.J.; Custers, J.A.E.; Manten-Horst, E.; Jansen, R.; Servaes, P.; Van Der Graaf, W.T.A.; Prins, J.B.; Husson, O. Prevalence and correlates of high fear of cancer recurrence in late adolescents and young adults consulting a specialist adolescent and young adult (AYA) cancer service. Support. Care Cancer 2017, 26, 1479–1487. [Google Scholar] [CrossRef]
- Shay, L.A.; Carpentier, M.Y.; Vernon, S.W. Prevalence and correlates of fear of recurrence among adolescent and young adult versus older adult post-treatment cancer survivors. Support. Care Cancer 2016, 24, 4689–4696. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, W.; Wen, Y.; Wang, H.; Sun, H.; Liang, W.; Zhang, B.; Humphris, G. Fear of cancer recurrence in adolescent and young adult cancer survivors: A systematic review of the literature. Psycho-Oncology 2019, 28, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Zebrack, B.; Santacroce, S.J.; Patterson, P.; Gubin, A. Adolescents and Young Adults with Cancer: A Biopsychosocial Approach. In Pediatric Psychosocial Oncology: Textbook for Multidisciplinary Care; Springer Science and Business Media LLC: Berlin, Germany, 2016; pp. 199–217. [Google Scholar]
- McCarthy, M.C.; McNeil, R.; Drew, S.; Dunt, D.; Kosola, S.; Orme, L.; Sawyer, S.M. Psychological Distress and Posttraumatic Stress Symptoms in Adolescents and Young Adults with Cancer and Their Parents. J. Adolesc. Young-Adult Oncol. 2016, 5, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, A.R.; Yi-Frazier, J.P.; Wharton, C.; Gordon, K.; Jones, B. Contributors and Inhibitors of Resilience Among Adolescents and Young Adults with Cancer. J. Adolesc. Young-Adult Oncol. 2014, 3, 185–193. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, G.A.; Pope, A.W.; Schuler, T.A.; Ford, J.S. The relationship between cancer-related worry and posttraumatic growth in adolescent and young adult cancer survivors. Psycho-Oncology 2018, 27, 2155–2164. [Google Scholar] [CrossRef]
- Lidington, E.; Vlooswijk, C.; Stallard, K.; Travis, E.; Younger, E.; Edwards, P.; Nandhabalan, M.; Hunter, N.; Sarpal, N.; Flett, D.; et al. ‘This is not part of my life plan’: A qualitative study on the psychosocial experiences and practical challenges in young adults with cancer age 25 to 39 years at diagnosis. Eur. J. Cancer Care 2021, 30, e13458. [Google Scholar] [CrossRef]
- D’Agostino, N.M.; Penney, A.; Zebrack, B. Providing developmentally appropriate psychosocial care to adolescent and young adult cancer survivors. Cancer 2011, 117, 2329–2334. [Google Scholar] [CrossRef]
- Carpentier, M.Y.; Fortenberry, J.D.; Ott, M.; Brames, M.J.; Einhorn, L.H. Perceptions of masculinity and self-image in adolescent and young adult testicular cancer survivors: Implications for romantic and sexual relationships. Psycho-Oncology 2010, 20, 738–745. [Google Scholar] [CrossRef]
- Warner, E.L.; Kent, E.E.; Trevino, K.M.; Parsons, H.M.; Zebrack, B.J.; Kirchhoff, A.C. Social well-being among adolescents and young adults with cancer: A systematic review. Cancer 2016, 122, 1029–1037. [Google Scholar] [CrossRef]
- Grinyer, A. The biographical impact of teenage and adolescent cancer. Chronic Illn. 2007, 3, 265–277. [Google Scholar] [CrossRef]
- Bellizzi, K.M.; Smith, A.; Schmidt, S.; Keegan, T.H.M.; Zebrack, B.; Lynch, C.F.; Deapen, D.; Shnorhavorian, M.; Tompkins, B.J.; Simon, M.; et al. Positive and negative psychosocial impact of being diagnosed with cancer as an adolescent or young adult. Cancer 2012, 118, 5155–5162. [Google Scholar] [CrossRef] [PubMed]
- Herbertson, R.; Hancock, B. Hodgkin Lymphoma in adolescents. Cancer Treat. Rev. 2005, 31, 339–360. [Google Scholar] [CrossRef]
- Guy, G.P.; Yabroff, K.R.; Ekwueme, D.U.; Smith, A.W.; Dowling, E.C.; Rechis, R.; Nutt, S.; Richardson, L.C. Estimating The Health And Economic Burden Of Cancer Among Those Diagnosed As Adolescents And Young Adults. Health Aff. 2014, 33, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Husson, O.; Prins, J.B.; Kaal, S.E.J.; Oerlemans, S.; Stevens, W.B.; Zebrack, B.; Van Der Graaf, W.T.A.; Van De Poll-Franse, L.V. Adolescent and young adult (AYA) lymphoma survivors report lower health-related quality of life compared to a normative population: Results from the PROFILES registry. Acta Oncol. 2017, 56, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Goodall, S.; King, M.; Ewing, J.; Smith, N.; Kenny, P. Preferences for support services among adolescents and young adults with cancer or a blood disorder: A discrete choice experiment. Heal. Policy 2012, 107, 304–311. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Elsbernd, A.; Pedersen, K.J.; Boisen, K.A.; Midtgaard, J.; Larsen, H.B. “On Your Own”: Adolescent and Young Adult Cancer Survivors’ Experience of Managing Return to Secondary or Higher Education in Denmark. J. Adolesc. Young-Adult Oncol. 2018, 7, 618–625. [Google Scholar] [CrossRef]
- Yanez, B.; Garcia, S.F.; Victorson, D.; Salsman, J.M. Distress among young adult cancer survivors: A cohort study. Support. Care Cancer 2013, 21, 2403–2408. [Google Scholar] [CrossRef]
- Kent, E.E.; Forsythe, L.P.; Yabroff, K.R.; Weaver, K.E.; De Moor, J.S.; Rodriguez, J.L.; Rowland, J.H. Are survivors who report cancer-related financial problems more likely to forgo or delay medical care? Cancer 2013, 119, 3710–3717. [Google Scholar] [CrossRef]
- Kirchhoff, A.C.; Lyles, C.R.; Fluchel, M.; Wright, J.; Leisenring, W. Limitations in health care access and utilization among long-term survivors of adolescent and young adult cancer. Cancer 2012, 118, 5964–5972. [Google Scholar] [CrossRef]
- Leuteritz, K.; Friedrich, M.; Sender, A.; Richter, D.; Mehnert-Theuerkauf, A.; Sauter, S.; Geue, K. Return to Work and Employment Situation of Young Adult Cancer Survivors: Results from the Adolescent and Young Adult-Leipzig Study. J. Adolesc. Young-Adult Oncol. 2021, 10, 226–233. [Google Scholar] [CrossRef]
- Vetsch, J.; Wakefield, C.E.; McGill, B.C.; Cohn, R.; Ellis, S.J.; Stefanic, N.; Sawyer, S.M.; Zebrack, B.; Sansom-Daly, U.M. Educational and vocational goal disruption in adolescent and young adult cancer survivors. Psycho-Oncology 2018, 27, 532–538. [Google Scholar] [CrossRef]
- Kent, E.E.; Parry, C.; Montoya, M.J.; Sender, L.S.; Morris, R.A.; Anton-Culver, H. “You’re too young for this”: Adolescent and young adults’ perspectives on cancer survivorship. J. Psychosoc. Oncol. 2012, 30, 260–279. [Google Scholar] [CrossRef]
- Kent, E.E.; Smith, A.W.; Keegan, T.H.M.; Lynch, C.F.; Wu, X.-C.; Hamilton, A.S.; Kato, I.; Schwartz, S. Talking About Cancer and Meeting Peer Survivors: Social Information Needs of Adolescents and Young Adults Diagnosed with Cancer. J. Adolesc. Young-Adult Oncol. 2013, 2, 44–52. [Google Scholar] [CrossRef]
- Kirchhoff, A.C.; Yi, J.; Wright, J.; Warner, E.L.; Smith, K.R. Marriage and divorce among young adult cancer survivors. J. Cancer Surviv. 2012, 6, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Borstelmann, N.A.; Rosenberg, S.M.; Ruddy, K.J.; Tamimi, R.M.; Gelber, S.; Schapira, L.; Come, S.; Borges, V.; Morgan, E.; Partridge, A.H. Partner support and anxiety in young women with breast cancer. Psycho-Oncology 2015, 24, 1679–1685. [Google Scholar] [CrossRef] [PubMed]
- Fosså, S.D.; Dahl, A.A. Fertility and Sexuality in Young Cancer Survivors Who Have Adult-Onset Malignancies. Hematol. Clin. N. Am. 2008, 22, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Olsen, P.R.; Lorenzo, R. Supportive Care. Prog. Tumor Res. 2016, 43, 16–26. [Google Scholar] [CrossRef]
- Mellblom, A.V.; Kiserud, C.E.; Rueegg, C.S.; Ruud, E.; Loge, J.H.; Fosså, S.D.; Lie, H.C. Self-reported late effects and long-term follow-up care among 1889 long-term Norwegian Childhood, Adolescent, and Young Adult Cancer Survivors (the NOR-CAYACS study). Support. Care Cancer 2021, 29, 2947–2957. [Google Scholar] [CrossRef] [PubMed]
- Hauken, M.A.; Larsen, T.M.B.; Holsen, I. Meeting reality: Young adult cancer survivors’ experiences of reentering everyday life after cancer treatment. Cancer Nurs. 2013, 36, E17–E26. [Google Scholar] [CrossRef]
- Hydeman, J.A.; Uwazurike, O.C.; Adeyemi, E.I.; Beaupin, L.K. Survivorship needs of adolescent and young adult cancer survivors: A concept mapping analysis. J. Cancer Surviv. 2019, 13, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.; Smitherman, A.; Nichols, H.B. Conditional relative survival among long-term survivors of adolescent and young adult cancers. Cancer 2018, 124, 3037–3043. [Google Scholar] [CrossRef]
- Adolescent and Young Adult Oncology Review Group. Closing the Gap: Research and Care Imperaives for Adolescents and Young Adults with Cancer; National Institute of Health, National Cancer Institute, and Livestrong Young Ault Alliance: Bethesda, MD, USA, 2006. [Google Scholar]
- Albritton, K.; Bleyer, W. The management of cancer in the older adolescent. Eur. J. Cancer 2003, 39, 2584–2599. [Google Scholar] [CrossRef] [PubMed]
- Lewis, I.; Morgan, S. Models of Care and Specialized Units. In Cancer in Adolescents and Young Adults; Springer Science: Berlin/Heidelberg, Germany, 2007; pp. 341–352. [Google Scholar]
- Husson, O.; Manten-Horst, E.; van der Graaf, W. Collaboration and Networking. Prog. Tumor Res. 2016, 43, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Cancer Australia; CanTeen. National Service Delivery Framework for Adolescents and Young Adults with Cancer. Cranberra Australia. 2008. Available online: https://www.canceraustralia.gov.au/publications-and-resources/cancer-australia-publications/national-service-delivery-framework-adolescents-and-young-adults-cancer (accessed on 3 April 2020).
- Ferrari, A.; Thomas, D.; Franklin, A.R.; Hayes-Lattin, B.M.; Mascarin, M.; van der Graaf, W.; Albritton, K.H. Starting an Adolescent and Young Adult Program: Some Success Stories and Some Obstacles to Overcome. J. Clin. Oncol. 2010, 28, 4850–4857. [Google Scholar] [CrossRef]
- Ferrari, A.; Albritton, K.; Osborn, M.; Barr, R.; Johnson, R.H.; Stark, D.; Whelan, J. Access and Models of Care. In Pediatric Oncology; Springer Science and Business Media LLC: Berlin, Germany, 2017; pp. 509–547. [Google Scholar]
- Reed, D.; Block, R.G.; Johnson, R. Creating an Adolescent and Young Adult Cancer Program: Lessons Learned From Pediatric and Adult Oncology Practice Bases. J. Natl. Compr. Cancer Netw. 2014, 12, 1409–1415. [Google Scholar] [CrossRef]
- Bradford, N.K.; Greenslade, R.; Edwards, R.M.; Orford, R.; Roach, J.; Henney, R. Educational Needs of Health Professionals Caring for Adolescents and Young Adults with Cancer. J. Adolesc. Young-Adult Oncol. 2018, 7, 298–305. [Google Scholar] [CrossRef]
- Linendoll, N.; Murphy-Banks, R.; Barthel, E.; Bartucca, L.; Boehm, L.; Welch, M.; Weidner, R.A.; Parsons, S.K. The Creation of a Comprehensive Adolescent and Young Adult Cancer Survivorship Program: “Lost in Transition” No More. J. Adolesc. Young-Adult Oncol. 2020, 10, 397–403. [Google Scholar] [CrossRef]
- Gupta, S.; Pole, J.D.; Baxter, N.N.; Sutradhar, R.; Lau, C.; Nagamuthu, C.; Nathan, P.C. The effect of adopting pediatric protocols in adolescents and young adults with acute lymphoblastic leukemia in pediatric vs. adult centers: An IMPACT Cohort study. Cancer Med. 2019, 8, 2095–2103. [Google Scholar] [CrossRef]
- Ramphal, R.; Meyer, R.; Schacter, B.; Rogers, P.; Pinkerton, R. Active therapy and models of care for adolescents and young adults with cancer. Cancer 2011, 117, 2316–2322. [Google Scholar] [CrossRef]
- Stark, D.; Bielack, S.; Brugieres, L.; Dirksen, U.; Duarte, X.; Dunn, S.; Erdelyi, D.; Grew, T.; Hjorth, L.; Jazbec, J.; et al. Teenagers and young adults with cancer in Europe: From national programmes to a European integrated coordinated project. Eur. J. Cancer Care 2015, 25, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Muffly, L.; Alvarez, E.; Lichtensztajn, D.; Abrahão, R.; Gomez, S.L.; Keegan, T. Patterns of care and outcomes in adolescent and young adult acute lymphoblastic leukemia: A population-based study. Blood Adv. 2018, 2, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Saloustros, E.; Stark, D.P.; Michailidou, K.; Mountzios, G.; Brugieres, L.; Peccatori, F.; Jezdic, S.; Essiaf, S.; Douillard, J.-Y.; Bielack, S. The care of adolescents and young adults with cancer: Results of the ESMO/SIOPE survey. ESMO Open 2017, 2, e000252. [Google Scholar] [CrossRef] [PubMed]
- Li, C.K.; Dalvi, R.; Yonemori, K.; Ariffin, H.; Lyu, C.J.; Farid, M.; Gonzales-Santos, J.R.N.; Zhou, Q.; Bielack, S.; Brugieres, L.; et al. Care of adolescents and young adults with cancer in Asia: Results of an ESMO/SIOPE/SIOP Asia survey. ESMO Open 2019, 4, e000467. [Google Scholar] [CrossRef]
- Guirguis, S.; Fitch, M.; Maganti, M.; Gupta, A.; D’Agostino, N.; Korenblum, C.; Jones, J. Biopsychosocial Factors Associated with Supportive Care Needs in Canadian Adolescent and Young Adult Cancer Survivors. J. Clin. Med. 2021, 10, 2628. [Google Scholar] [CrossRef]
- Baird, H.; Patterson, P.; Medlow, S.; Allison, K.R. Understanding and Improving Survivorship Care for Adolescents and Young Adults with Cancer. J. Adolesc. Young-Adult Oncol. 2019, 8, 581–586. [Google Scholar] [CrossRef]
- Phillips, S.M.; Padgett, L.S.; Leisenring, W.; Stratton, K.K.; Bishop, K.; Krull, K.R.; Alfano, C.M.; Gibson, T.M.; De Moor, J.S.; Hartigan, D.B.; et al. Survivors of Childhood Cancer in the United States: Prevalence and Burden of Morbidity. Cancer Epidemiol. Biomark. Prev. 2015, 24, 653–663. [Google Scholar] [CrossRef]
- Chemaitilly, W.; Cohen, L.E.; Mostoufi-Moab, S.; Patterson, B.; Simmons, J.; Meacham, L.R.; van Santen, H.M.; Sklar, C.A. Endocrine Late Effects in Childhood Cancer Survivors. J. Clin. Oncol. 2018, 36, 2153–2159. [Google Scholar] [CrossRef]
- Shapiro, C.L. Cancer Survivorship. N. Engl. J. Med. 2018, 379, 2438–2450. [Google Scholar] [CrossRef]
- Oeffinger, K.C.; Mertens, A.C.; Sklar, C.A.; Kawashima, T.; Hudson, M.M.; Meadows, A.T.; Friedman, D.L.; Marina, N.; Hobbie, W.; Kadan-Lottick, N.; et al. Chronic Health Conditions in Adult Survivors of Childhood Cancer. N. Engl. J. Med. 2006, 355, 1572–1582. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; DeSantis, C.; Virgo, K.; Stein, K.; Mariotto, A.; Smith, T.; Cooper, D.; Gansler, T.; Lerro, C.; Fedewa, S.; et al. Cancer treatment and survivorship statistics. CA Cancer J. Clin. 2012, 62, 220–241. [Google Scholar] [CrossRef]
- Landier, W.; Armenian, S.; Bhatia, S. Late Effects of Childhood Cancer and Its Treatment. Pediatr. Clin. N. Am. 2015, 62, 275–300. [Google Scholar] [CrossRef]
- Children’s Oncology Group. Long-Term Follow-Up Guidelines, Version 5.0. Available online: http://www.survivorshipguidelines.org/pdf/2018/COG_LTFU_Guidelines_v5.pdf (accessed on 9 July 2020).
- Michel, G.; Mulder, R.L.; Van Der Pal, H.J.H.; Skinner, R.; Bárdi, E.; Brown, M.C.; Vetsch, J.; Frey, E.; Windsor, R.; Kremer, L.C.M.; et al. Evidence-based recommendations for the organization of long-term follow-up care for childhood and adolescent cancer survivors: A report from the PanCareSurFup Guidelines Working Group. J. Cancer Surviv. 2019, 13, 759–772. [Google Scholar] [CrossRef]
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef]
- Denlinger, C.S.; Sanft, T.; Baker, K.S.; Broderick, G.; Demark-Wahnefried, W.; Friedman, D.L.; Goldman, M.; Hudson, M.; Khakpour, N.; King, A.; et al. Survivorship, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 1216–1247. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practical Guidelines in Oncology. Breast Cancer, Version 2. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx (accessed on 9 December 2020).
- Tonorezos, E.S.; Barnea, D.; Cohn, R.; Cypriano, M.S.; Fresneau, B.C.; Haupt, R.; Hjorth, L.; Ishida, Y.; Kruseova, J.; Kuehni, C.E.; et al. Models of Care for Survivors of Childhood Cancer from Across the Globe: Advancing Survivorship Care in the Next Decade. J. Clin. Oncol. 2018, 36, 2223–2230. [Google Scholar] [CrossRef]
- Tawfik, B.; Jaffe, S.A.; Mohler, L.; Oomen-Hajagos, J.; Gil, I.S.; Chamberlain, R.; Gagnon, S.; Kano, M.; Gundelach, A.; Ryan, S.R.; et al. Developing a survivorship care plan (SCP) delivery process for patients and primary care providers serving poor, rural, and minority patients with cancer. Support. Care Cancer 2021, 29, 5021–5028. [Google Scholar] [CrossRef]
- Hill, R.E.; Wakefield, C.E.; Cohn, R.J.; Fardell, J.E.; Brierley, M.E.; Kothe, E.; Jacobsen, P.B.; Hetherington, K.; Mercieca-Bebber, R. Survivorship Care Plans in Cancer: A Meta-Analysis and Systematic Review of Care Plan Outcomes. Oncology 2019, 25, 351. [Google Scholar] [CrossRef] [PubMed]
- Haggstrom, D.A.; Kahn, K.L.; Klabunde, C.N.; Gray, S.W.; Keating, N.L. Oncologists’ perceptions of the usefulness of cancer survivorship care plan components. Support. Care Cancer 2021, 29, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, P.; DeRosa, A.P.; Henderson, T.O.; Mayer, D.K.; Moskowitz, C.S.; Paskett, E.D.; Rowland, J.H. Systematic Review of the Impact of Cancer Survivorship Care Plans on Health Outcomes and Health Care Delivery. J. Clin. Oncol. 2018, 36, 2088–2100. [Google Scholar] [CrossRef] [PubMed]
- Nass, S.J.; Beaupin, L.K.; Demark-Wahnefried, W.; Fasciano, K.; Ganz, P.A.; Hayes-Lattin, B.; Hudson, M.M.; Nevidjon, B.; Oeffinger, K.C.; Rechis, R.; et al. Identifying and Addressing the Needs of Adolescents and Young Adults with Cancer: Summary of an Institute of Medicine Workshop. Oncologist 2015, 20, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Marjerrison, S.; Barr, R.D. Unmet survivorship care needs of adolescent and young adult cancer survivors. JAMA Netw. Open. 2018, 1, e180350. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, L.; Tam, S.; Lewin, J.; Srikanthan, A.; Heck, C.; Hodgson, D.; Vakeesan, B.; Sim, H.-W.; Gupta, A. Measuring the Impact of an Adolescent and Young Adult Program on Addressing Patient Care Needs. J. Adolesc. Young-Adult Oncol. 2018, 7, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Pollock, B.H. What’s missing in the assessment of adolescent and young adult (AYA) cancer outcomes? J. Natl. Cancer Inst. 2020, 112, 975–976. [Google Scholar] [CrossRef] [PubMed]
- Husson, O.; Ligtenberg, M.; van de Poll-Franse, L.; Prins, J.; Bent, M.V.D.; van Eenbergen, M.; Fles, R.; Manten-Horst, E.; Gietema, J.; van der Graaf, W. Comprehensive Assessment of Incidence, Risk Factors, and Mechanisms of Impaired Medical and Psychosocial Health Outcomes among Adolescents and Young Adults with Cancer: Protocol of the Prospective Observational COMPRAYA Cohort Study. Cancers 2021, 13, 2348. [Google Scholar] [CrossRef] [PubMed]
- Salsman, J.M.; Danhauer, S.C.; Moore, J.B.; Canzona, M.R.; Victorson, D.E.; Zebrack, B.J.; Reeve, B.B. Optimizing the measurement of health-related quality of life in adolescents and young adults with cancer. Cancer 2020, 126, 4818–4824. [Google Scholar] [CrossRef]
- Kaal, S.; Lidington, E.; Prins, J.; Jansen, R.; Manten-Horst, E.; Servaes, P.; van der Graaf, W.; Husson, O. Health-Related Quality of Life Issues in Adolescents and Young Adults with Cancer: Discrepancies with the Perceptions of Health Care Professionals. J. Clin. Med. 2021, 10, 1833. [Google Scholar] [CrossRef]
- Siembida, E.J.; Reeve, B.B.; Zebrack, B.J.; Snyder, M.A.; Salsman, J.M. Measuring health-related quality of life in adolescent and young adult cancer survivors with the National Institutes of Health Patient-Reported Outcomes Measurement Information System®: Comparing adolescent, emerging adult, and young adult survivor perspectives. Psycho-Oncology 2021, 30, 303–311. [Google Scholar] [CrossRef]
- CanTeen Australia. Australian Youth Cancer Framework for Adolescents and Young Adults with Cancer. Available online: https://www.canteen.org.au/wp-content/uploads/2017/09/Australian_Youth_Cancer_Framework_2017.pdf (accessed on 4 December 2020).
- Dickman, P.W.; Coviello, E. Estimating and Modeling Relative Survival. Stata J. Promot. Commun. Stat. Stata 2015, 15, 186–215. [Google Scholar] [CrossRef]
- Surveillance Epidemiology and End Results (SEER) Program. Expected Survival Life Tables. Available online: https://seer.cancer.gov/expsurvival/ (accessed on 9 December 2020).
- Surveillance Epidemiology and End Results (SEER) Program. Age Standards for Survival. Available online: https://seer.cancer.gov/stdpopulations/survival.html (accessed on 9 December 2020).
| Cancer Type | n at Risk | 5-Year % RS (95%CI) | 10-Year % RS (95%CI) |
|---|---|---|---|
| 10-year survival > 80% | |||
| Thyroid | 43,504 | 99.8 (99.7–99.8) | 99.7 (99.5–99.8) |
| Appendix (NET) a | 971 | 98.4 (96.9–99.2) | 99.1 (97.1–99.7) |
| Lung and Bronchus (NET) b | 920 | 97.9 (96.7–98.6) | 96.2 (94.7–97.3) |
| Testis | 25,835 | 95.9 (95.7–96.1) | 95.6 (95.3–95.8) |
| Melanoma of the Skin | 29,489 | 95.4 (95.2–95.6) | 93.8 (93.5–94.1) |
| Salivary Gland | 1736 | 94.9 (93.9–95.7) | 92.4 (91.2–93.5) |
| Hodgkin’s lymphoma c | 17,494 | 94.0 (93.6–94.2) | 91.8 (91.4–92.2) |
| Ovary, non-epithelial d | 2250 | 92.5 (91.6–93.4) | 91.1 (89.9–92.1) |
| Uterus e | 6318 | 89.5 (87.9–91.0) | 87.5 (85.8–89.1) |
| Larynx | 513 | 90.2 (87.4–92.5) | 85.5 (80.6–89.2) |
| Vulva vaginal f | 666 | 83.4 (79.4–86.7) | 80.5 (76.2–84.2) |
| Cervix Uteri | 14,702 | 81.8 (80.6–83.0) | 80.5 (79.2–81.7) |
| 10-year survival 60–80% | |||
| Pancreas (NET) g | 428 | 86.7 (82.7–89.8) | 79.2 (73.3–83.9) |
| Kidney h | 7359 | 82.0 (80.8–83.2) | 79.2 (77.8–80.6) |
| Non-Hodgkin Lymphoma i | 17,403 | 81.1 (80.6–81.6) | 79.1 (78.5–79.6) |
| Tonsil ** | 492 | 82.0 (74.9–87.2) | 78.5 (71.0–84.3) |
| Appendix (non-NET) j | 818 | 84.0 (81.7–86.1) | 75.7 (72.2–78.8) |
| Small intestine | 935 | 79.2 (76.2–81.9) | 74.4 (71.2–77.3) |
| Breast | 42,967 | 82.0 (80.8–83.1) | 73.6 (72.3–74.9) |
| Bladder | 759 | 75.2 (71.2–78.7) | 71.5 (67.1–75.4) |
| Penis ** | 204 | 69.5 (59.9–77.2) | 69.0 (59.3–76.8) |
| Ovary, epithelial k | 3544 | 74.4 (72.9–75.9) | 68.0 (66.2–69.7) |
| Tongue | 1439 | 69.0 (66.1–71.6) | 67.5 (64.6–70.3) |
| Bones * | 4061 | 72.3 (71.1–73.5) | 67.3 (65.9–68.6) |
| Nasopharynx ** | 1229 | 74.1 (71.7–76.2) | 66.2 (63.5–68.8) |
| Soft tissue sarcoma l | 6876 | 69.2 (68.3–70.1) | 64.6 (63.6–65.5) |
| Prostate | 473 | 64.1 (55.2–71.7) | 63.8 (54.9–71.5) |
| Multiple Myeloma | 1193 | 73.0 (68.5–76.9) | 63.3 (58.4–67.7) |
| Rectum | 4455 | 68.4 (66.5–70.3) | 62.1 (60.0–64.2) |
| Leukemia m ** | 13,285 | 65.3 (64.6–66.0) | 61.0 (60.3–61.7) |
| Anal n | 669 | 62.9 (55.1–69.8) | - * |
| 10-year survival 40–60% | |||
| Colon o | 4450 | 61.8 (60.0–63.5) | 57.7 (55.8–59.5) |
| Brain | 11,825 | 68.7 (68.0–69.3) | 56.6 (55.7–57.4) |
| Sigmoid p | 2945 | 61.1 (58.4–63.7) | 54.8 (51.9–57.6) |
| Rectosigmoid Junction | 1368 | 60.9 (57.2–64.4) | 54.1 (50.0–58.1) |
| 10-year survival < 40% | |||
| Pancreas (non-NET) q | 1369 | 38.8 (35.3–42.3) | 32.1 (28.5–35.7) |
| Lung and Bronchus (non-NET) r | 3783 | 34.7 (32.6–36.8) | 31.3 (29.1–33.6) |
| Stomach | 3148 | 31.9 (29.9–34.0) | 28.7 (26.6–30.8) |
| Liver | 1346 | 33.8 (31.5–36.1) | 27.3 (25.0–29.7) |
| Esophagus | 519 | 23.2 (18.7–28.0) | 21.2 (16.8–26.0) |
| Intrahepatic Bile Duct | 236 | 19.5 (14.7–24.9) | - * |
| Focus on AYA-Specific Survivorship Issues Physical, e.g.,
|
AYA Cancer Survivorship Research:
|
AYA Cancer Survivorship Care:
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janssen, S.H.M.; van der Graaf, W.T.A.; van der Meer, D.J.; Manten-Horst, E.; Husson, O. Adolescent and Young Adult (AYA) Cancer Survivorship Practices: An Overview. Cancers 2021, 13, 4847. https://doi.org/10.3390/cancers13194847
Janssen SHM, van der Graaf WTA, van der Meer DJ, Manten-Horst E, Husson O. Adolescent and Young Adult (AYA) Cancer Survivorship Practices: An Overview. Cancers. 2021; 13(19):4847. https://doi.org/10.3390/cancers13194847
Chicago/Turabian StyleJanssen, Silvie H. M., Winette T. A. van der Graaf, Daniël J. van der Meer, Eveliene Manten-Horst, and Olga Husson. 2021. "Adolescent and Young Adult (AYA) Cancer Survivorship Practices: An Overview" Cancers 13, no. 19: 4847. https://doi.org/10.3390/cancers13194847
APA StyleJanssen, S. H. M., van der Graaf, W. T. A., van der Meer, D. J., Manten-Horst, E., & Husson, O. (2021). Adolescent and Young Adult (AYA) Cancer Survivorship Practices: An Overview. Cancers, 13(19), 4847. https://doi.org/10.3390/cancers13194847









