Fibroblasts Influence Metastatic Melanoma Cell Sensitivity to Combined BRAF and MEK Inhibition
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell and Tissue Models
2.1.1. Culture Conditions of Established Cell Lines
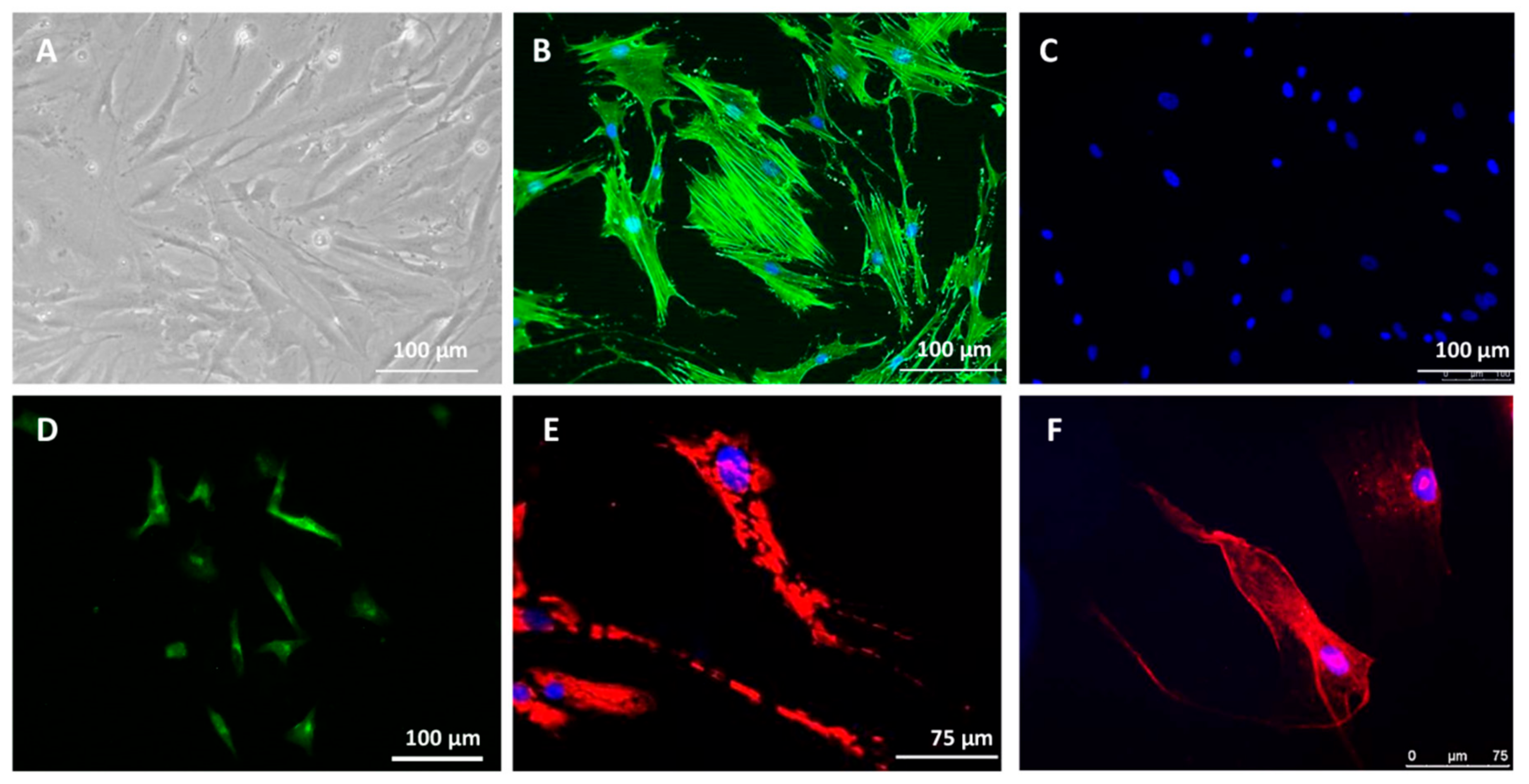
2.1.2. Isolation and Culture of MM Cancer-Associated Fibroblasts (CAFs)
2.1.3. Monolayer Cell (2D) Treatment
2.1.4. Dermal Equivalent Model and 3D Coculture Treatment
2.1.5. Conditioned Media Preparation
2.2. Cell Proliferation Assays
2.2.1. Cell Metabolic Activity Assay
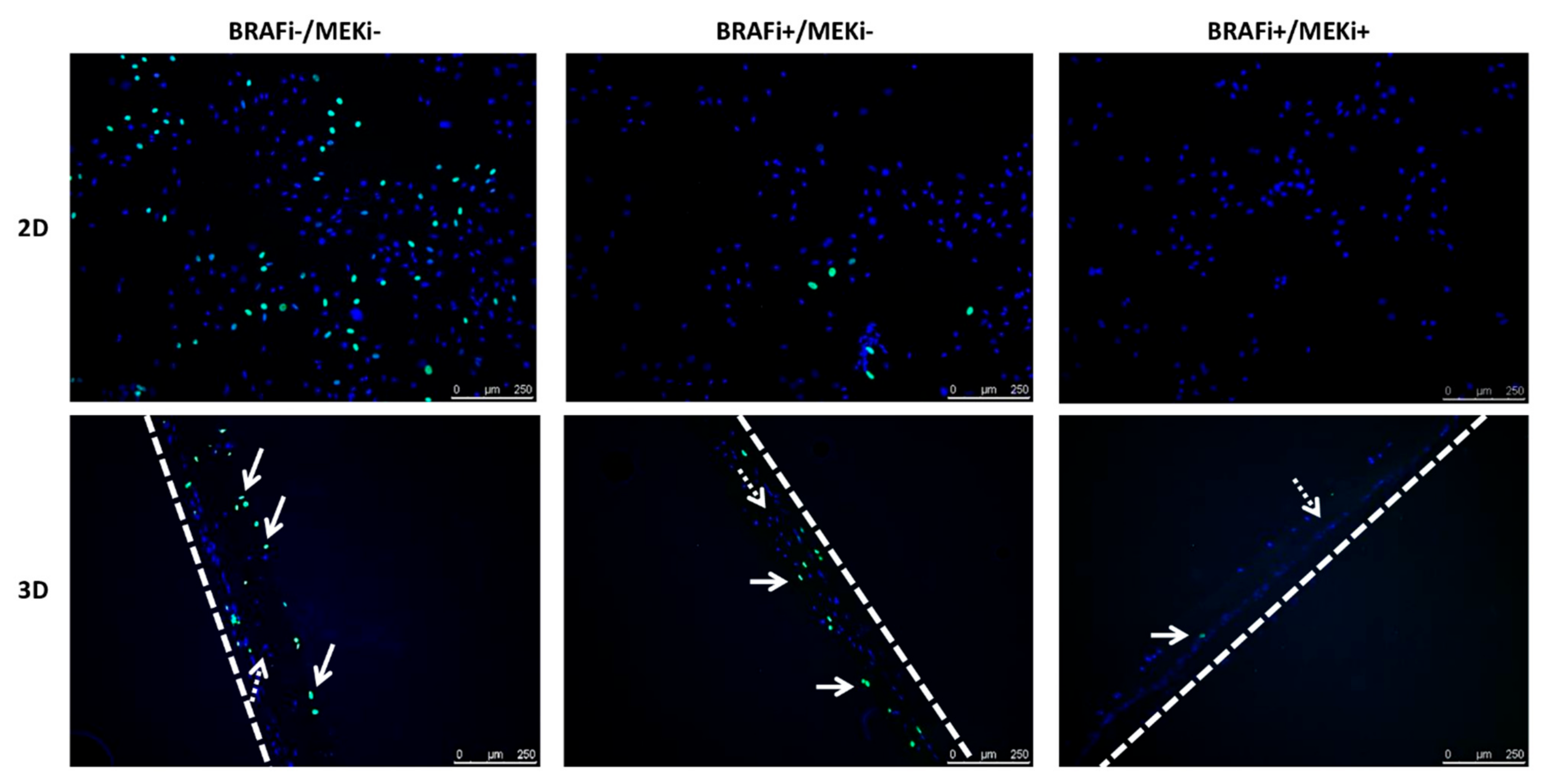
2.2.2. EdU Labelling
2.2.3. Cell Proliferative Index
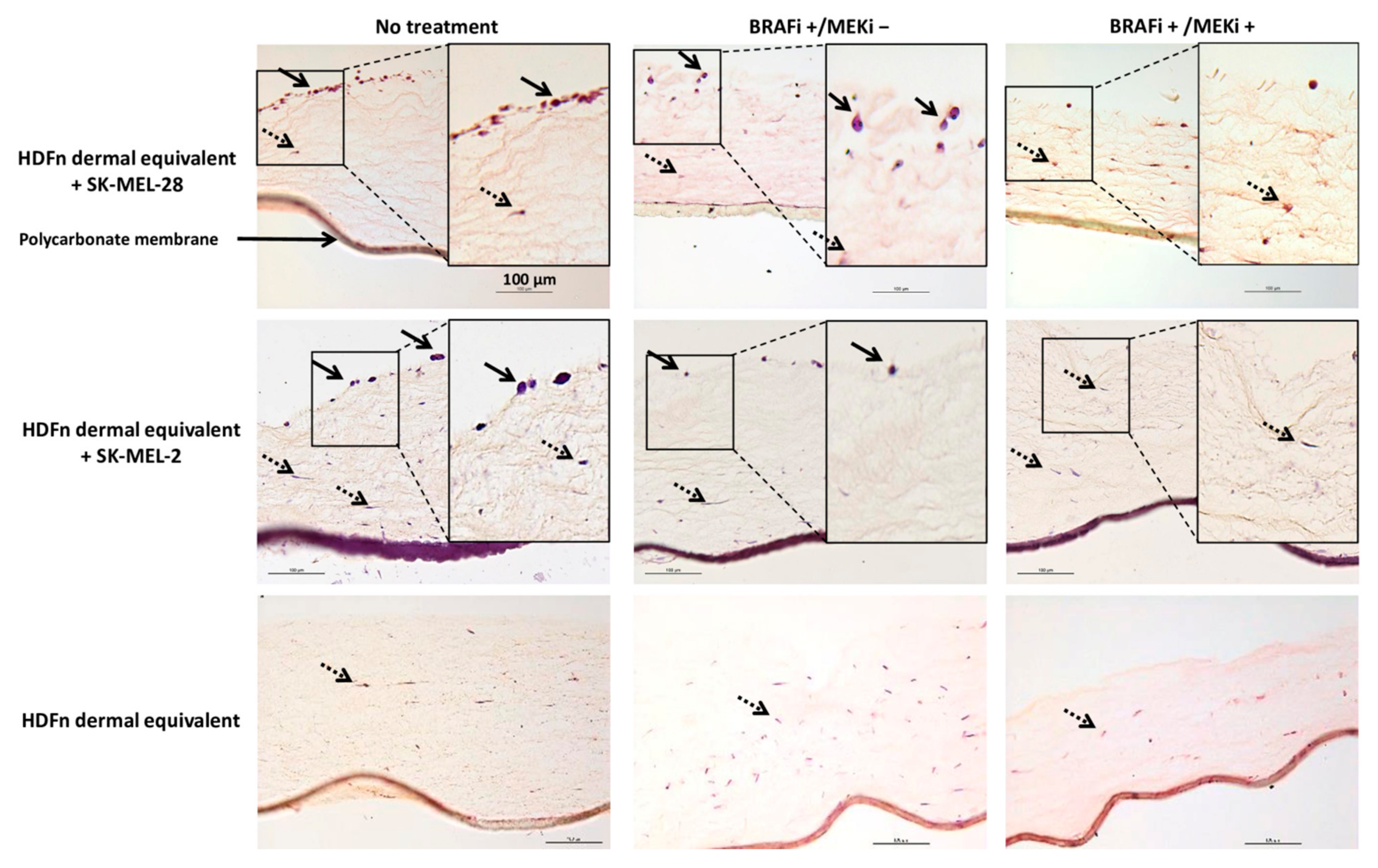
2.3. Histological Observation of the 3D Coculture Models
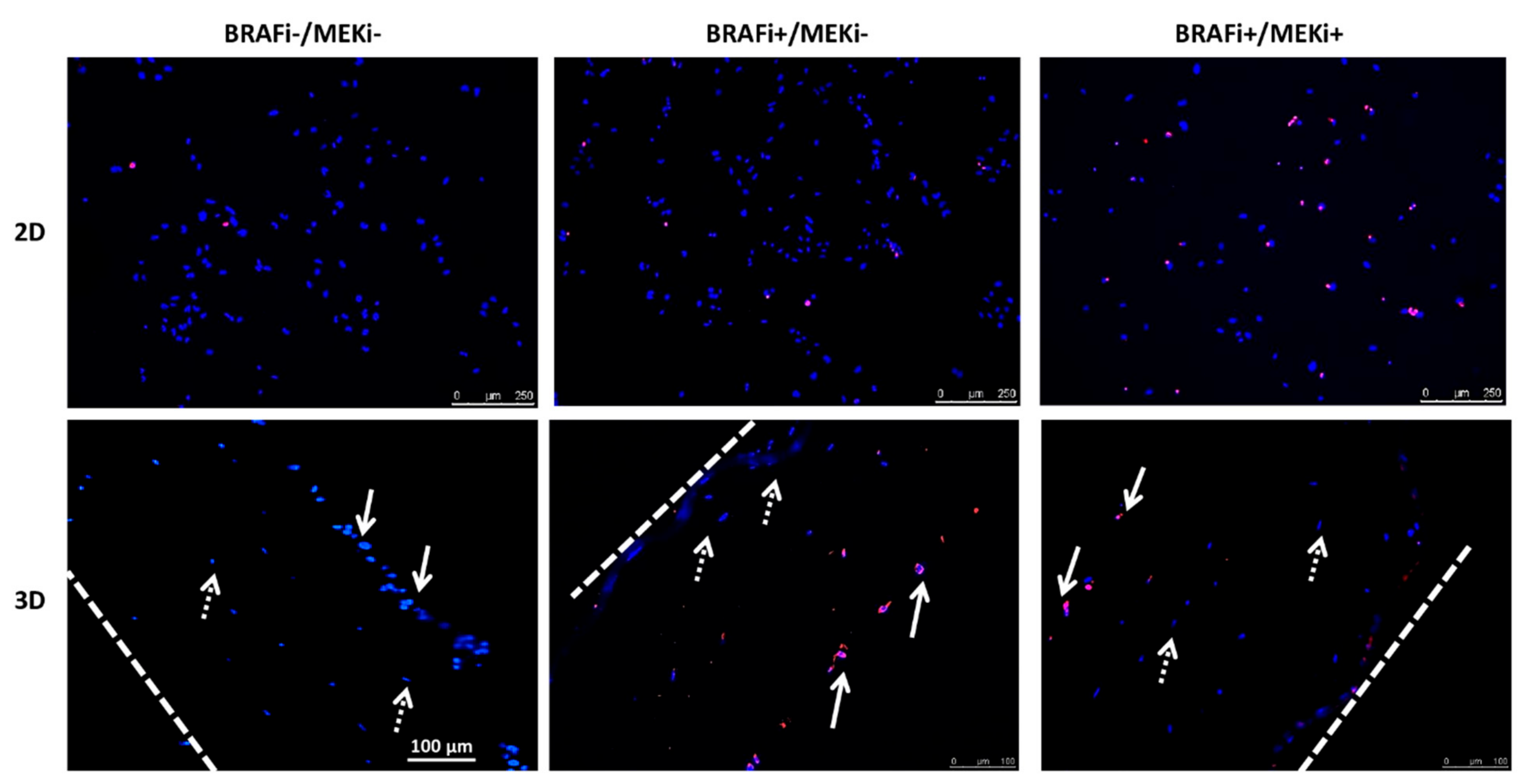
2.4. Apoptosis Detection
2.5. Statistical Analysis
3. Results
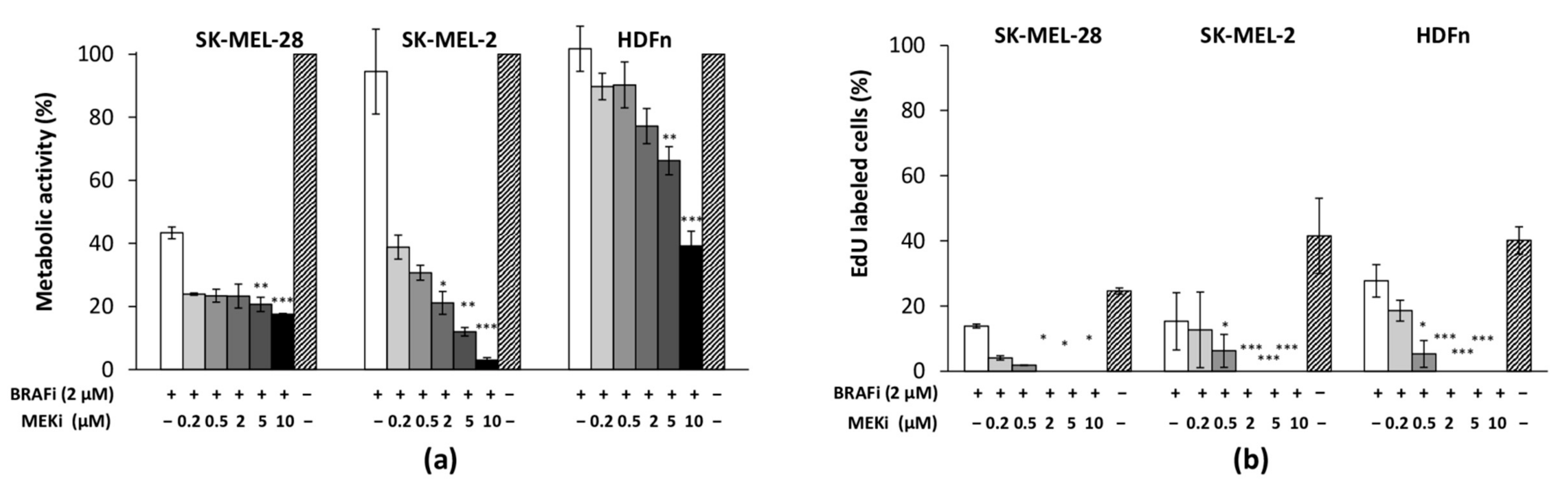
3.1. The Drug Combination Strongly Inhibits the Proliferation of MM Cell Lines Cultured as Monolayer Regardless of Their BRAF Status
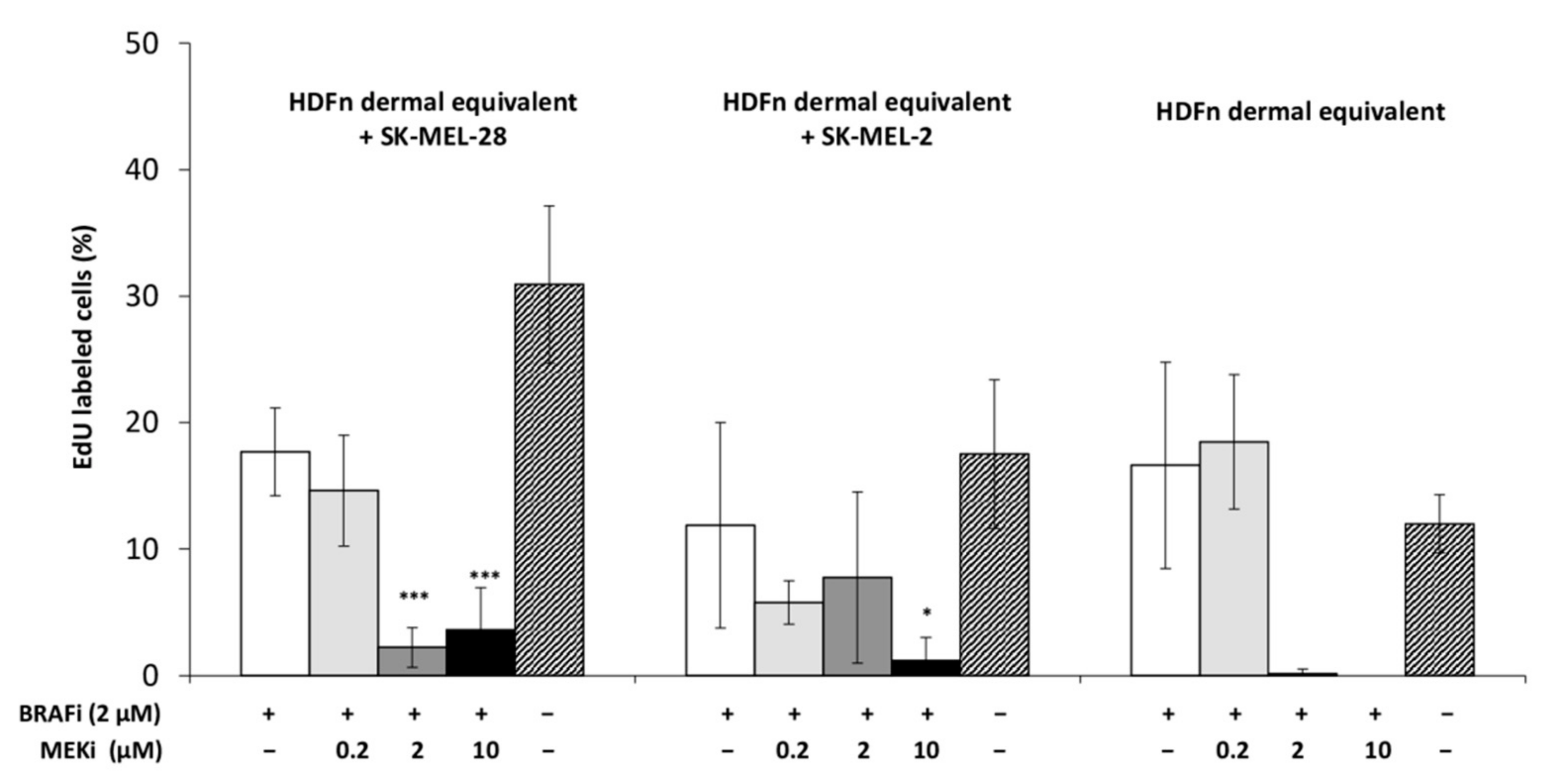
3.2. The Drug Combination Inhibits the Proliferation of MM Cells Cocultured on Dermal Equivalents (HDFn)
3.3. The Drug Combination Is Cytotoxic for MM Cells Cultured as a Monolayer or in a 3D Coculture Model
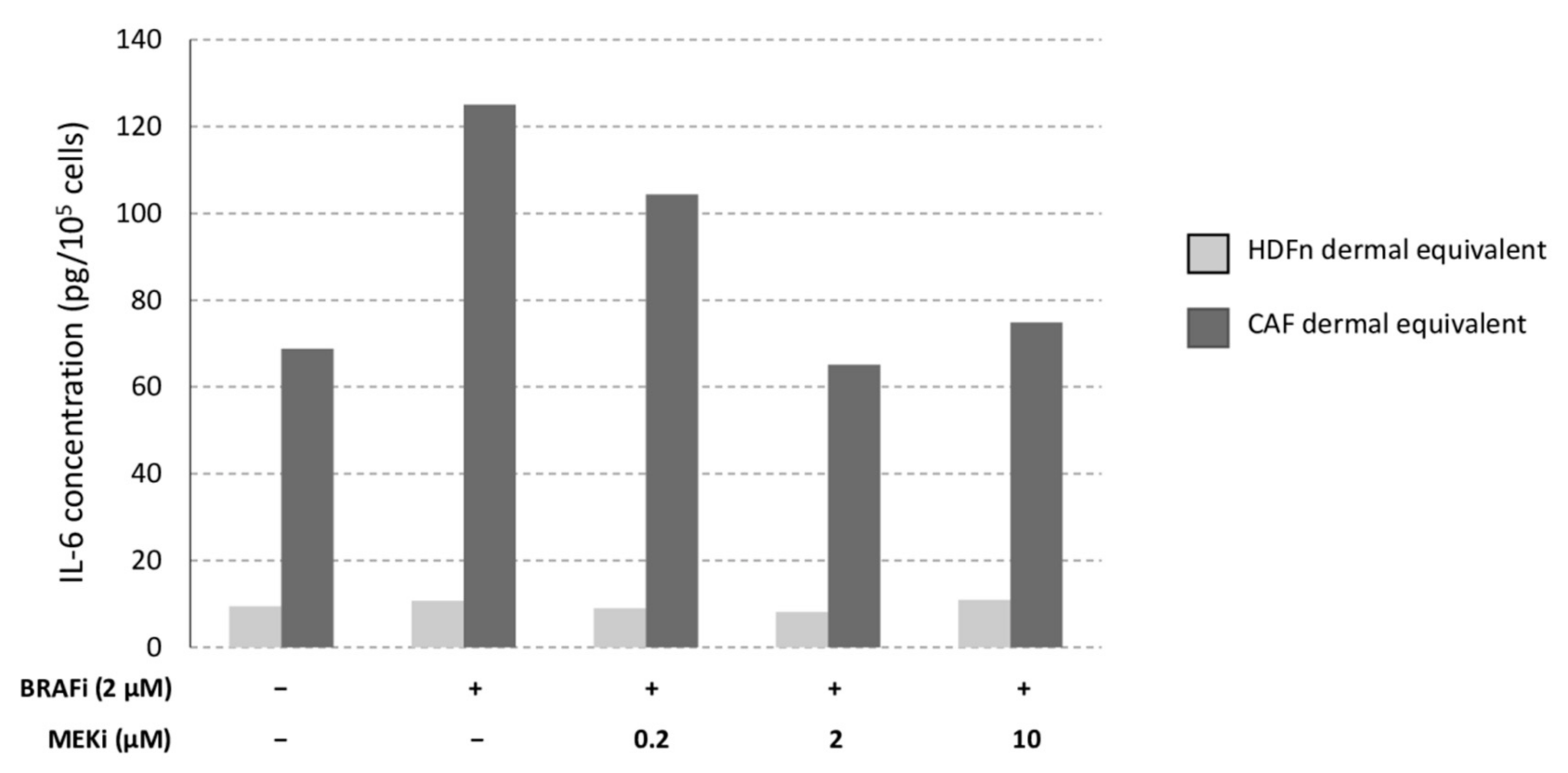
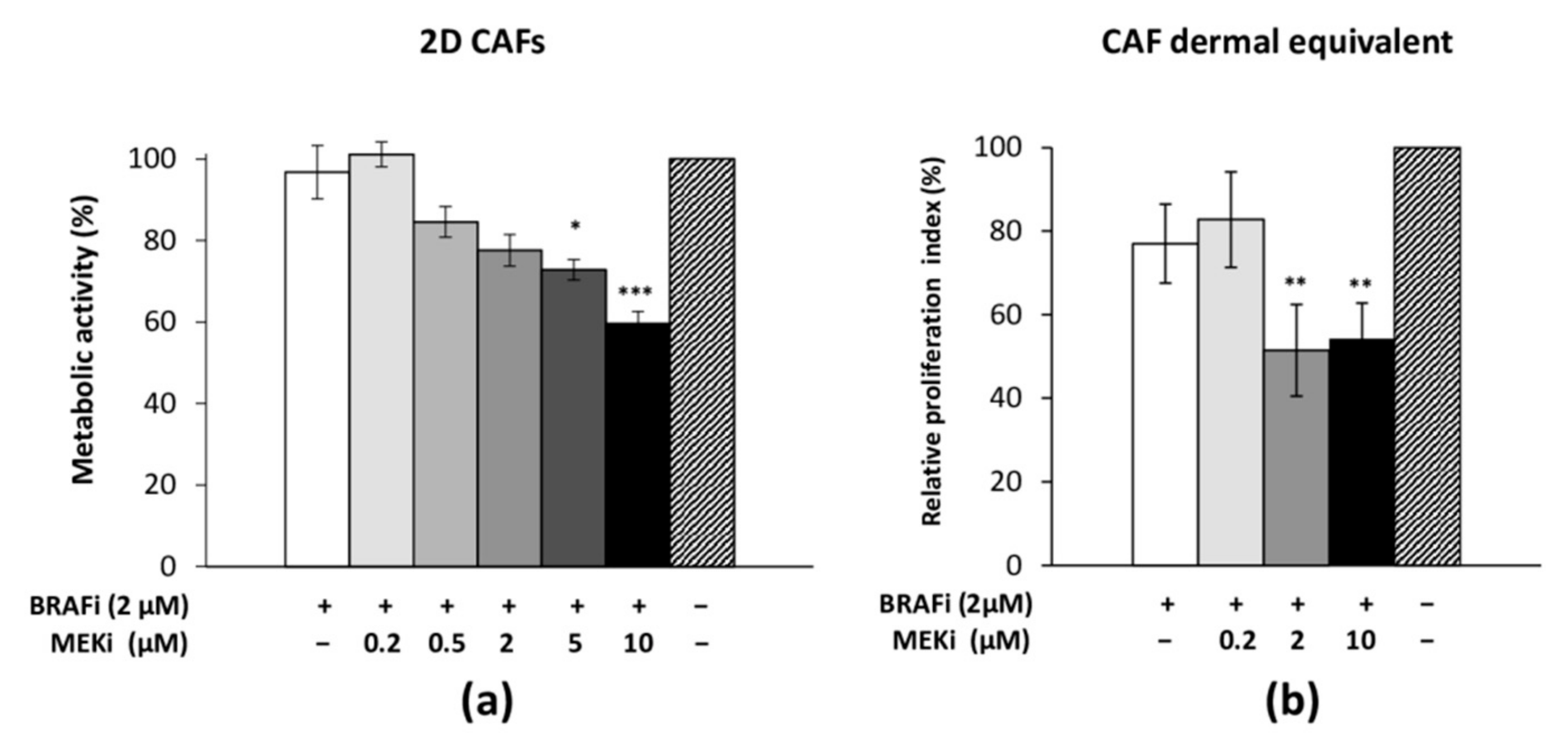
3.4. The Drug Combination Inhibits the Proliferation of Cancer-Associated Fibroblasts
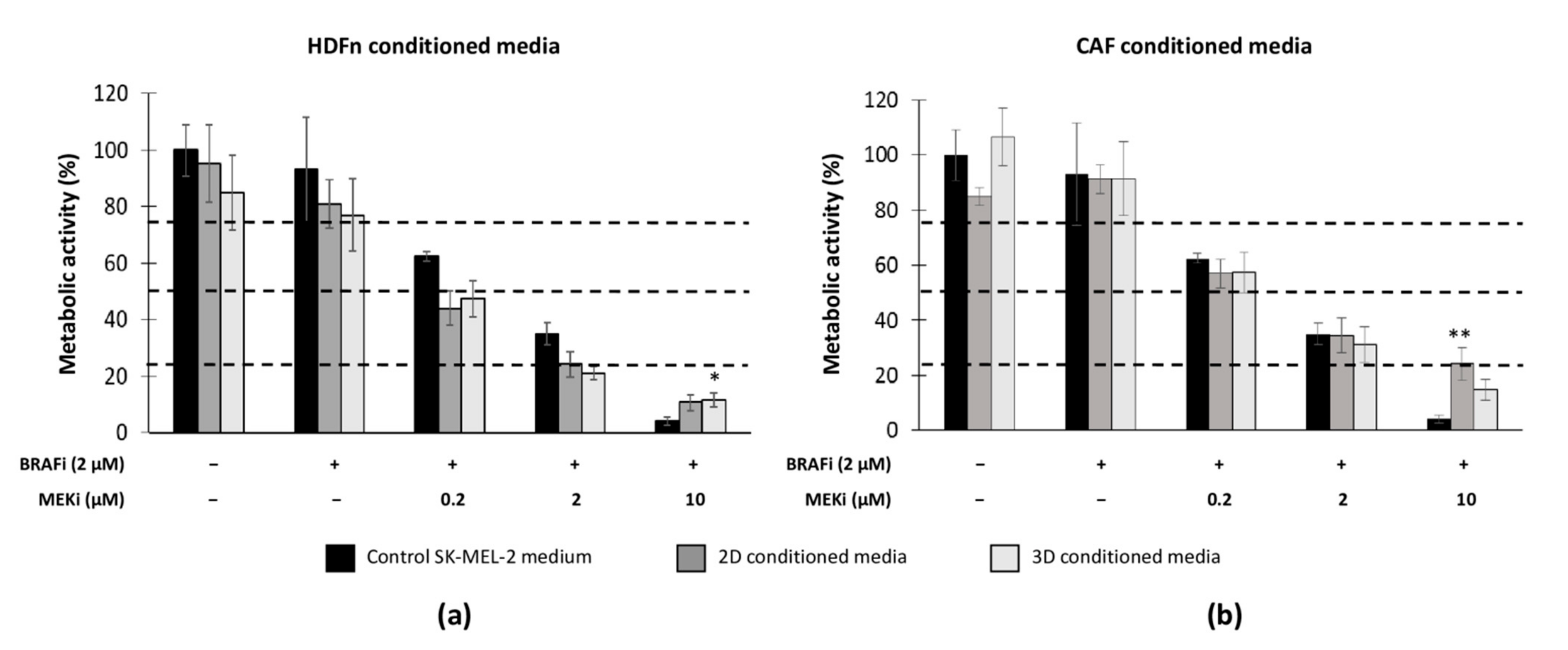
3.5. The Microenvironment (Healthy Fibroblasts vs. CAFs) Influences MM Sensitivity to the Drug Combination
4. Discussion
4.1. The Drug Combination Efficiently Inhibits 2D and 3D MM Cell Proliferation Regardless of BRAF Status
4.2. Healthy or Cancer-Associated Fibroblasts Have a Different Impact on the Sensitivity of MM Cells to Targeted Therapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Inhibitory Concentrations | SK-MEL-2 Control | HDFn CM | CAF CM | ||
|---|---|---|---|---|---|
| 2D | 3D | 2D | 3D | ||
| IC25 (µM) | 0.12 | 0.04 | 0.03 | 0.1 | 0.1 |
| IC50 (µM) | 0.42 | 0.15 | 0.17 | 0.4 | 0.7 |
| IC75 (µM) | 4.4 | 1.85 | 1.7 | 8.8 | 5 |
References
- Donia, M.; Ellebaek, E.; Øllegaard, T.H.; Duval, L.; Aaby, J.B.; Hoejberg, L.; Køhler, U.H.; Schmidt, H.; Bastholt, L.; Svane, I.M. The real-world impact of modern treatments on the survival of patients with metastatic melanoma. Eur. J. Cancer 2019, 108, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Ascierto, P.A.; Dréno, B.; Atkinson, V.; Liszkay, G.; Maio, M.; Mandalà, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Combined Vemurafenib and Cobimetinib in BRAF-mutated melanoma. N. Engl. J. Med. 2014, 371, 1867–1876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, G.V.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; de Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; Grob, J.J.; et al. Combined BRAF and MEK Inhibition versus BRAF Inhibition Alone in Melanoma. N. Engl. J. Med. 2014, 371, 1877–1888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robert, C.; Karaszewska, B.; Schachter, J.; Rutkowski, P.; Mackiewicz, A.; Stroiakovski, D.; Lichinitser, M.; Dummer, R.; Grange, F.; Mortier, L.; et al. Improved overall survival in melanoma with combined Dabrafenib and Trametinib. N. Engl. J. Med. 2015, 372, 30–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flaherty, K.T.; Puzanov, I.; Kim, K.B.; Ribas, A.; McArthur, G.A.; Sosman, J.A.; O’Dwyer, P.J.; Lee, R.J.; Grippo, J.F.; Nolop, K.; et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N. Engl. J. Med. 2010, 363, 809–819. [Google Scholar] [CrossRef] [Green Version]
- Hauschild, A.; Grob, J.-J.; Demidov, L.V.; Jouary, T.; Gutzmer, R.; Millward, M.; Rutkowski, P.; Blank, C.U.; Miller, W.H.; Kaempgen, E.; et al. Dabrafenib in BRAF-mutated metastatic melanoma: A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2021, 380, 358–365. [Google Scholar] [CrossRef]
- Sosman, J.A.; Kim, K.B.; Schuchter, L.; Gonzalez, R.; Pavlick, A.C.; Weber, J.S.; McArthur, G.A.; Hutson, T.E.; Moschos, S.J.; Flaherty, K.T.; et al. Survival in BRAF V600–mutant advanced melanoma treated with Vemurafenib. N. Engl. J. Med. 2021, 366, 707–714. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Wang, L.; Huang, S.; Heynen, G.J.; Prahallad, A.; Robert, C.; Haanen, J.; Blank, C.; Wesseling, J.; Willems, S.M.; et al. Reversible and adaptive resistance to BRAF(V600E) inhibition in melanoma. Nature 2014, 508, 118–122. [Google Scholar] [CrossRef]
- Beaumont, K.A.; Hill, D.S.; Daignault, S.M.; Lui, G.Y.L.; Sharp, D.M.; Gabrielli, B.; Weninger, W.; Haass, N.K. Cell cycle phase-specific drug resistance as an escape mechanism of melanoma cells. J. Investig. Dermatol. 2016, 136, 1479–1489. [Google Scholar] [CrossRef] [Green Version]
- Tiago, M.; de Oliveira, E.M.; Brohem, C.A.; Pennacchi, P.C.; Paes, R.D.; Haga, R.B.; Campa, A.; de Moraes Barros, S.B.; Smalley, K.S.; Maria-Engler, S.S. Fibroblasts protect melanoma cells from the cytotoxic effects of doxorubicin. Tissue Eng. Part A 2014, 20, 2412–2421. [Google Scholar] [CrossRef] [Green Version]
- Morales, D.; Lombart, F.; Truchot, A.; Maire, P.; Hussein, M.; Hamitou, W.; Vigneron, P.; Galmiche, A.; Lok, C.; Vayssade, M. 3D Coculture models underline metastatic melanoma cell sensitivity to vemurafenib. Tissue Eng. Part A 2019, 25, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Ping, Q.; Yan, R.; Cheng, X.; Wang, W.; Zhong, Y.; Hou, Z.; Shi, Y.; Wang, C.; Li, R. Cancer-associated fibroblasts: Overview, progress, challenges, and directions. Cancer Gene Ther. 2021, 12, 984–999. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, R.; Herlyn, M.; Wagner, S.N. The role of tumor microenvironment in melanoma therapy resistance. Melanoma Manag. 2016, 3, 23–32. [Google Scholar] [CrossRef]
- Romano, V.; Belviso, I.; Venuta, A.; Ruocco, M.R.; Masone, S.; Aliotta, F.; Fiume, G.; Montagnani, S.; Avagliano, A.; Arcucci, A. Influence of tumor microenvironment and fibroblast population plasticity on melanoma growth, therapy resistance and immunoescape. Int. J. Mol. Sci. 2021, 22, 5283. [Google Scholar] [CrossRef] [PubMed]
- Hirata, E.; Girotti, M.R.; Viros, A.; Hooper, S.; Spencer-Dene, B.; Matsuda, M.; Larkin, J.; Marais, R.; Sahai, E. Intravital imaging reveals how BRAF inhibition generates drug-tolerant microenvironments with high integrin β1/FAK signaling. Cancer Cell 2015, 27, 574–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Érsek, B.; Silló, P.; Cakir, U.; Molnár, V.; Bencsik, A.; Mayer, B.; Mezey, E.; Kárpáti, S.; Pós, Z.; Németh, K. Melanoma-associated fibroblasts impair CD8+ T cell function and modify expression of immune checkpoint regulators via increased arginase activity. Cell. Mol. Life Sci. 2021, 78, 661–673. [Google Scholar] [CrossRef] [Green Version]
- Material not intended for publication: Morales, D. (Université de technologie de Compiègne, CNRS, Biomechanics and Bioengineering, Compiègne, France). Cells were stained with Trypan blue and cell viability was determined as the ratio between living cells and the number of total cells, 2018.
- Material not intended for publication: Vigneron, P. (Université de technologie de Compiègne, CNRS, Biomechanics and Bioengineering, Compiègne, France). Cells were stained with Trypan blue and cell viability was determined as the ratio between living cells and the number of total cells, 2018.
- Schvartsman, G.; Taranto, P.; Glitza, I.C.; Agarwala, S.S.; Atkins, M.B.; Buzaid, A.C. Management of metastatic cutaneous melanoma: Updates in clinical practice. Ther. Adv. Med. Oncol. 2019, 11, 1758835919851663. [Google Scholar] [CrossRef] [PubMed]
- Wada, M.; Horinaka, M.; Yamazaki, T.; Katoh, N.; Sakai, T. The dual RAF/MEK inhibitor CH5126766/RO5126766 may be a potential therapy for RAS-mutated tumor cells. PLoS ONE 2014, 9, e113217. [Google Scholar] [CrossRef]
- Posch, C.; Cholewa, B.D.; Vujic, I.; Sanlorenzo, M.; Ma, J.; Kim, S.T.; Kleffel, S.; Schatton, T.; Rappersberger, K.; Gutteridge, R.; et al. Combined Inhibition of MEK and Plk1 Has Synergistic Antitumor Activity in NRAS Mutant Melanoma. J. Investig. Dermatol. 2015, 135, 2475–2483. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.F.; Jiang, C.C.; Kiejda, K.A.; Gillespie, S.; Zhang, X.D.; Hersey, P. Apoptosis induction in human melanoma cells by inhibition of MEK Is caspase-independent and mediated by the Bcl-2 family members PUMA, Bim, and Mcl-1. Clin. Cancer Res. 2007, 13, 4934–4942. [Google Scholar] [CrossRef] [Green Version]
- Niessner, H.; Sinnberg, T.; Kosnopfel, C.; Smalley, K.S.M.; Beck, D.; Praetorius, C.; Mai, M.; Beissert, S.; Kulms, D.; Schaller, M.; et al. BRAF Inhibitors amplify the proapoptotic activity of MEK inhibitors by inducing ER stress in NRAS-mutant melanoma. Clin. Cancer Res. 2019, 23, 6203–6214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ascierto, P.A.; Schadendorf, D.; Berking, C.; Agarwala, S.S.; van Herpen, C.M.; Queirolo, P.; Blank, C.U.; Hauschild, A.; Beck, J.T.; St-Pierre, A.; et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: A non-randomised, open-label phase 2 study. Lancet Oncol. 2013, 14, 249–256. [Google Scholar] [CrossRef]
- Yan, J.; Wu, X.; Yu, J.; Yu, H.; Xu, T.; Brown, K.M.; Bai, X.; Dai, J.; Ma, M.; Tang, H.; et al. Analysis of NRAS gain in 657 patients with melanoma and evaluation of its sensitivity to a MEK inhibitor. Eur. J. Cancer 2018, 89, 90–101. [Google Scholar] [CrossRef]
- Murali, V.S.; Chang, B.J.; Fiolka, R.; Danuser, G.; Cobanoglu, M.C.; Welf, E.S. An image-based assay to quantify changes in proliferation and viability upon drug treatment in 3D microenvironments. BMC Cancer 2019, 19, 502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, H.; Enomoto, A.; Woods, S.L.; Burt, A.D.; Takahashi, M.; Worthley, D.L. Cancer-associated fibroblasts in gastrointestinal cancer. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 282–295. [Google Scholar] [CrossRef]
- Li, Z.; Sun, C.; Qin, Z. Metabolic reprogramming of cancer-associated fibroblasts and its effect on cancer cell reprogramming. Theranostics 2021, 11, 8322–8336. [Google Scholar] [CrossRef]
- Micke, P.; Östman, A. Exploring the tumour environment: Cancer-associated fibroblasts as targets in cancer therapy. Expert Opin. Ther. Targets 2005, 9, 1217–1233. [Google Scholar] [CrossRef]
- Gaggioli, C.; Sahai, E. Melanoma invasion—Current knowledge and future directions. Pigment. Cell Res. 2007, 20, 161–172. [Google Scholar] [CrossRef]
- Meads, M.B.; Gatenby, R.A.; Dalton, W.S. Environment-mediated drug resistance: A major contributor to minimal residual disease. Nat. Rev. Cancer 2009, 9, 665–674. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, K.; Andl, T.; Wickett, R.R.; Zhang, Y. Perspective of Targeting Cancer-Associated Fibroblasts in Melanoma. J. Cancer 2015, 6, 717–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kodet, O.; Kučera, J.; Strnadová, K.; Dvořánková, B.; Štork, J.; Lacina, L.; Smetana, K., Jr. Cutaneous melanoma dissemination is dependent on the malignant cell properties and factors of intercellular crosstalk in the cancer microenvironment. Int. J. Oncol. 2020, 57, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Cheteh, E.H.; Sarne, V.; Ceder, S.; Bianchi, J.; Augsten, M.; Rundqvist, H.; Egevad, L.; Östman, A.; Wiman, K.G. Interleukin-6 derived from cancer-associated fibroblasts attenuates the p53 response to doxorubicin in prostate cancer cells. Cell Death Discov. 2020, 6, 42. [Google Scholar] [CrossRef]
- Brábek, J.; Jakubek, M.; Vellieux, F.; Novotný, J.; Kolář, M.; Lacina, L.; Szabo, P.; Strnadová, K.; Rösel, D.; Dvořánková, B.; et al. Interleukin-6: Molecule in the intersection of cancer, ageing and COVID-19. Int. J. Mol. Sci. 2020, 21, 7937. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, B.R.; Domingos, C.; Stefanini, A.C.B.; Henrique, T.; Polachini, G.M.; Castelo-Branco, P.; Tajara, E.H. Cellular Interactions in the Tumor Microenvironment: The Role of Secretome. J. Cancer 2019, 10, 4574–4587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jobe, N.P.; Rösel, D.; Dvořánková, B.; Kodet, O.; Lacina, L.; Mateu, R.; Smetana, K.; Brábek, J. Simultaneous blocking of IL-6 and IL-8 is sufficient to fully inhibit CAF-induced human melanoma cell invasiveness. Histochem. Cell Biol. 2016, 146, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Avagliano, A.; Ruocco, M.R.; Nasso, R.; Aliotta, F.; Sanità, G.; Iaccarino, A.; Bellevicine, C.; Calì, G.; Fiume, G.; Masone, S.; et al. Development of a stromal microenvironment experimental model containing proto-myofibroblast like cells and analysis of its crosstalk with melanoma cells: A new tool to potentiate and stabilize tumor suppressor phenotype of dermal myofibroblasts. Cells 2019, 8, 1435. [Google Scholar] [CrossRef] [Green Version]
| Cell Lines | Culture Conditions | No Treatment | BRAFi+/MEKi− | BRAFi+/MEKi+ |
|---|---|---|---|---|
| SK-MEL-28 | 2D monoculture | 0.4% ± 0.4 | 1.6% ± 1.2 | 7.0% ± 2.2 *** |
| 3D coculture | 1.3% ± 1.3 | 2.5% ± 1.7 | 28.5% ± 10 *** | |
| SK-MEL-2 | 2D monoculture | 4.1% ± 3.4 | 6.4% ± 3.2 *** | 25% ± 8.8 *** |
| 3D coculture | 1.9% ± 1.2 | 12.8% ± 3.4 *** | 17.4% ± 8.6 *** | |
| HDFn | 2D monoculture | 0.3% ± 0.3 | 0.5% ± 0.1 | 6.6% ± 3.2 *** |
| 3D monoculture | 0.5% ± 0.7 | 1.5% ± 1.5 | 12.1% ± 9.5 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales, D.; Vigneron, P.; Ferreira, I.; Hamitou, W.; Magnano, M.; Mahenthiran, L.; Lok, C.; Vayssade, M. Fibroblasts Influence Metastatic Melanoma Cell Sensitivity to Combined BRAF and MEK Inhibition. Cancers 2021, 13, 4761. https://doi.org/10.3390/cancers13194761
Morales D, Vigneron P, Ferreira I, Hamitou W, Magnano M, Mahenthiran L, Lok C, Vayssade M. Fibroblasts Influence Metastatic Melanoma Cell Sensitivity to Combined BRAF and MEK Inhibition. Cancers. 2021; 13(19):4761. https://doi.org/10.3390/cancers13194761
Chicago/Turabian StyleMorales, Delphine, Pascale Vigneron, Ines Ferreira, Warda Hamitou, Mikael Magnano, Laxsika Mahenthiran, Catherine Lok, and Muriel Vayssade. 2021. "Fibroblasts Influence Metastatic Melanoma Cell Sensitivity to Combined BRAF and MEK Inhibition" Cancers 13, no. 19: 4761. https://doi.org/10.3390/cancers13194761
APA StyleMorales, D., Vigneron, P., Ferreira, I., Hamitou, W., Magnano, M., Mahenthiran, L., Lok, C., & Vayssade, M. (2021). Fibroblasts Influence Metastatic Melanoma Cell Sensitivity to Combined BRAF and MEK Inhibition. Cancers, 13(19), 4761. https://doi.org/10.3390/cancers13194761







