Advances in Functional Imaging of Differentiated Thyroid Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Natural History of Differentiated Thyroid Cancer
3. Radiopharmaceuticals for DTC Patients
3.1. Available Radioactive Isotopes of Iodine for Clinical Use Include 123I, 131I and 124I
3.2. Physical Characteristics of Radioactive Iodine Isotopes
3.3. Methods Used for Functional Imaging with 131I: WBS and SPECT/CT
3.4. 18F-FDG
3.5. Other Tracers
4. PET Imaging Methods
4.1. PET/CT
4.2. PET/MR
5. Indications for the Use of Radioactive Iodine
5.1. Post-Operative Administration of RAI
- The current definition of excellent response to treatment (and of complete ablation in case of post-operative administration of 131I) at 6–12 months is based, in the absence of a control radioiodine WBS [99,100], on undetectable serum Tg level in the absence of Tg-Ab (either < 1ng/mL following rhTSH when using a traditional assay or < 0.2 ng/mL on L-T4 treatment when using a sensitive assay) and a neck ultrasound (US) without any abnormal findings [4,9,101].
- In two prospective randomized non-inferiority trials in low-risk patients, the percentage of complete ablation was similar when using for preparation either rhTSH injections on L-T4 treatment or withdrawal of thyroid hormone treatment and administration of either 1100 or 3700 MBq 131I [52,53]. Indeed, with rhTSH there was no hypothyroid symptom, and the quality of life was maintained [49]. With a 5-year follow-up, only few patients (<5%) received further treatments and the same favorable outcome was observed regardless of the initial ablation protocol used [102,103]. In conclusion, when indicated in low-risk patients a post-operative administration of 131I should consist in the administration of 1100 MBq following rhTSH injections.
- Less than 5% of these low-risk patients had persistent disease, and this suggests that 131I might be not beneficial and may represent over-treatment in the other 95% [4,47]; low-risk patients might be selected for ablation on the basis of the post-operative neck US and serum Tg level, the risk of persistent disease being low in patients with undetectable Tg and increases with higher serum Tg levels [101,102,103,104]. It is, however, still unclear whether the post-operative 131I administration may improve the outcome of these patients with detectable post-operative Tg levels. This is being evaluated within two ongoing prospective randomized non-inferiority trials (ESTIMABL2 and ION) that compare the outcome of low-risk patients treated either with 1100 MBq following rhTSH or followed-up with no post-operative 131I.
5.2. Use of Radioiodine for Diagnosis
6. Comparison between 131I and 124I
7. Use of Radioactive Iodine for Treatment of Distant Metastases
8. Use of 18F-FDG PET/CT in Clinical Practice
8.1. 18F-FDG PET/CT for the Post-Operative Staging of Aggressive Disease
8.2. 18F-FDG PET/CT for Elevated Serum Tg and No Other Evidence of Disease
8.3. 18F-FDG PET/CT in Patients with Structural Disease
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ATA | American Thyroid Association |
| CT | Computed tomography |
| DTC | Differentiated thyroid cancer |
| 18F-FDG | 18F-fluorodeoxyglucose |
| 18F-TFB | 18F-tetrafluoroborate |
| GLUT | Glucose Transporter |
| GLUT-1 | Glucose Transporter-1 |
| MR | Magnetic resonance |
| NIS | Natrium/Iodide Symporter |
| PET | Positron emission tomography |
| PSMA | Prostate specific membrane antigen |
| RAI | Radioactive iodine |
| SPECT | Single photon emission tomography |
| Tg | Thyroglobulin |
| TPO | Thyroid peroxidase |
| TSH | Thyroid stimulating hormone |
| US | Ultrasound |
| WBS | Whole-body scan |
| d-WBS | Diagnostic-WBS |
References
- Hertz, S.; Roberts, A. Radioactive Iodine as an Indicator in Thyroid Physiology. V. The Use of Radioactive Iodine in the Differential Diagnosis of Two Types of Graves’ Disease. J. Clin. Investig. 1942, 21, 31–32. [Google Scholar] [CrossRef] [PubMed]
- Hertz, S.; Roberts, A.; Salter, W.T. Radioactive Iodine as an Indicator in Thyroid Physiology. IV. The Metabolism of Iodine in Graves’ Disease. J. Clin. Investig. 1942, 21, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Keston, A.S.; Ball, R.P.; Frantz, V.K.; Palmer, W.W. Storage of Radioactive Iodine in a Metastasis from Thyroid Carcinoma. Science 1942, 95, 362–363. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Schlumberger, M.; Lacroix, L.; Russo, D.; Filetti, S.; Bidart, J.M. Defects in iodide metabolism in thyroid cancer and implications for the follow-up and treatment of patients. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 260–269. [Google Scholar] [CrossRef]
- Lazar, V.; Bidart, J.M.; Caillou, B.; Mahé, C.; Lacroix, L.; Filetti, S.; Schlumberger, M. Expression of the Na+/I- symporter gene in human thyroid tumors: A comparison study with other thyroid-specific genes. J. Clin. Endocrinol. Metab. 1999, 84, 3228–3234. [Google Scholar] [CrossRef]
- Durski, J.M.; Hruska, C.B.; Bogsrud, T.V.; Ryder, M.; Johnson, G.B. 123I Scan With Whole-Body Retention Measurement at 48 Hours for Simplified Dosimetry Before 131I Treatment of Metastatic Thyroid Cancer. Clin. Nucl. Med. 2021, 46, e151–e153. [Google Scholar] [CrossRef]
- Mazzaferri, E.L.; Jhiang, S.M. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am. J. Med. 1994, 97, 418–428, Erratum in 1995, 98, 215. [Google Scholar] [CrossRef]
- Filetti, S.; Durante, C.; Hartl, D.; Leboulleux, S.; Locati, L.D.; Newbold, K.; Papotti, M.G.; Berruti, A.; ESMO Guidelines Committee. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann. Oncol. 2019, 30, 1856–1883. [Google Scholar] [CrossRef]
- Avram, A.M.; Dewaraja, Y.K. Thyroid Cancer Radiotheragnostics: The case for activity adjusted 131I therapy. Clin. Transl. Imaging 2018, 6, 335–346. [Google Scholar] [CrossRef]
- Ciappuccini, R.; Heutte, N.; Lasne-Cardon, A.; Saguet-Rysanek, V.; Leroy, C.; Le Hénaff, V.; Vaur, D.; Babin, E.; Bardet, S. Tumor burden of persistent disease in patients with differentiated thyroid cancer: Correlation with postoperative risk-stratification and impact on outcome. BMC Cancer 2020, 20, 765. [Google Scholar] [CrossRef] [PubMed]
- Lodi Rizzini, E.; Repaci, A.; Tabacchi, E.; Zanoni, L.; Vicennati, V.; Cavicchi, O.; Pagotto, U.; Morganti, A.G.; Fanti, S.; Monari, F. Impact of 18F-FDG PET/CT on Clinical Management of Suspected Radio-Iodine Refractory Differentiated Thyroid Cancer (RAI-R-DTC). Diagnostics 2021, 11, 1430. [Google Scholar] [CrossRef] [PubMed]
- Freudenberg, L.S.; Antoch, G.; Jentzen, W.; Pink, R.; Knust, J.; Görges, R.; Müller, S.P.; Bockisch, A.; Debatin, J.F.; Brandau, W. Value of (124)I-PET/CT in staging of patients with differentiated thyroid cancer. European radiology. Eur. Radiol. 2004, 14, 2092–2098. [Google Scholar] [CrossRef] [PubMed]
- D’Avanzo, A.; Treseler, P.; Ituarte, P.H.; Wong, M.; Streja, L.; Greenspan, F.S.; Siperstein, A.E.; Duh, Q.Y.; Clark, O.H. Follicular thyroid carcinoma: Histology and prognosis. Cancer 2004, 100, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Ibrahimpasic, T.; Ghossein, R.; Shah, J.P.; Ganly, I. Poorly Differentiated Carcinoma of the Thyroid Gland: Current Status and Future Prospects. Thyroid 2019, 29, 311–321. [Google Scholar] [CrossRef]
- Kim, K.; Kim, J.K.; Lee, C.R.; Kang, S.W.; Lee, J.; Jeong, J.J.; Nam, K.H.; Chung, W.Y. Comparison of long-term prognosis for differentiated thyroid cancer according to the 7th and 8th editions of the AJCC/UICC TNM staging system. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820921019. [Google Scholar] [CrossRef]
- Simões-Pereira, J.; Mourinho, N.C.; Ferreira, T.; Limbert, E.; Cavaco, B.M.; Leite, V. Avidity and outcomes of radioiodine therapy for distant metastasis of distinct types of differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 2021, dgab436. [Google Scholar] [CrossRef]
- Avram, A.M.; Zukotynski, K.; Nadel, H.R.; Giovanella, L.M. Management Of Differentiated Thyroid Cancer: The Standard Of Care. J. Nucl. Med. 2021, 121, 262402. [Google Scholar] [CrossRef]
- Fernández, L.P.; López-Márquez, A.; Santisteban, P. Thyroid transcription factors in development, differentiation and disease. Nat. Rev. Endocrinol. 2015, 11, 29–42. [Google Scholar] [CrossRef]
- Fabbro, D.; Di Loreto, C.; Beltrami, C.A.; Belfiore, A.; Di Lauro, R.; Damante, G. Expression of thyroid-specific transcription factors TTF-1 and PAX-8 in human thyroid neoplasms. Cancer Res. 1994, 54, 4744–4749. [Google Scholar]
- Ravera, S.; Reyna-Neyra, A.; Ferrandino, G.; Amzel., L.M.; Carrasco, N. The Sodium/Iodide Symporter (NIS): Molecular Physiology and Preclinical and Clinical Applications. Annu. Rev. Physiol. 2017, 79, 261–289. [Google Scholar] [CrossRef] [PubMed]
- Dohán, O.; Baloch, Z.; Bánrévi, Z.; Livolsi, V.; Carrasco, N. Rapid communication: Predominant intracellular overexpression of the Na(+)/I(-) symporter (NIS) in a large sampling of thyroid cancer cases. J. Clin. Endocrinol. Metab. 2001, 86, 2697–2700. [Google Scholar] [CrossRef] [PubMed]
- Fragu, P.; Nataf, B.M. Human thyroid peroxidase activity in benign and malign thyroid disorders. J. Clin. Endocrinol. Metab. 1997, 45, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Di Cristofaro, J.; Silvy, M.; Lanteaume, A.; Marcy, M.; Carayon, P.; De Micco, C. Expression of tpo mRNA in thyroid tumors: Quantitative PCR analysis and correlation with alterations of RET, BRAF, RAS and PAX8 genes. Endocr.-Relat. Cancer 2006, 13, 485–495. [Google Scholar] [CrossRef]
- Schlumberger, M.; Charbord, P.; Fragu, P.; Lumbroso, J.; Parmentier, C.; Tubiana, M. Circulating thyroglobulin and thyroid hormones in patients with metastases of differentiated thyroid carcinoma: Relationship to serum thyrotropin levels. J. Clin. Endocrinol. Metab. 1980, 51, 513–519. [Google Scholar] [CrossRef]
- Schlumberger, M.; Charbord, P.; Fragu, P.; Gardet, P.; Lumbroso, J.; Parmentier, C.; Tubiana, M. Relationship between thyrotropin stimulation and radioiodine uptake in lung metastases of differentiated thyroid carcinoma. J. Clin. Endocrinol. Metab. 1983, 57, 148–151. [Google Scholar] [CrossRef]
- Xing, M.; Alzahrani, A.S.; Carson, K.A.; Viola, D.; Elisei, R.; Bendlova, B.; Yip, L.; Mian, C.; Vianello, F.; Tuttle, R.M.; et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA 2013, 309, 1493–1501. [Google Scholar] [CrossRef]
- Xing, M.; Alzahrani, A.S.; Carson, K.A.; Shong, Y.K.; Kim, T.Y.; Viola, D.; Elisei, R.; Bendlová, B.; Yip, L.; Mian, C.; et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J. Clin. Oncol. 2015, 33, 42–50. [Google Scholar] [CrossRef]
- Yoo, S.K.; Lee, S.; Kim, S.J.; Jee, H.G.; Kim, B.A.; Cho, H.; Song, Y.S.; Cho, S.W.; Won, J.K.; Shin, J.Y.; et al. Comprehensive analysis of the transcriptional and mutational landscape of follicular and papillary thyroid cancers. PLoS Genet. 2016, 12, e1006239. [Google Scholar] [CrossRef]
- Pozdeyev, N.; Gay, L.M.; Sokol, E.S.; Hartmaier, R.; Deaver, K.E.; Davis, S.; French, J.D.; Borre, P.V.; LaBarbera, D.V.; Tan, A.C.; et al. Genetic analysis of 779 advanced differentiated and anaplastic thyroid cancers. Clin. Cancer Res. 2018, 24, 3059–3068. [Google Scholar] [CrossRef]
- Ganly, I.; Makarov, V.; Deraje, S.; Dong, Y.; Reznik, E.; Seshan, V.; Nanjangud, G.; Eng, S.; Bose, P.; Kuo, F.; et al. Integrated genomic analysis of Hürthle cell cancer reveals oncogenic drivers, recurrent mitochondrial mutations, and unique chromosomal landscapes. Cancer Cell 2018, 34, 256–270.e5. [Google Scholar] [CrossRef]
- Landa, I.; Ibrahimpasic, T.; Boucai, L.; Sinha, R.; Knauf, J.A.; Shah, R.H.; Dogan, S.; Ricarte-Filho, J.C.; Krishnamoorthy, G.P.; Xu, B.; et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J. Clin. Investig. 2016, 126, 1052–1066. [Google Scholar] [CrossRef]
- Knauf, J.A.; Ma, X.; Smith, E.P.; Zhang, L.; Mitsutake, N.; Liao, X.H.; Refetoff, S.; Nikiforov, Y.E.; Fagin, J.A. Targeted expression of BRAFV600E in thyroid cells of transgenic mice results in papillary thyroid cancers that undergo dedifferentiation. Cancer Res. 2005, 65, 4238–4245. [Google Scholar] [CrossRef]
- Chakravarty, D.; Santos, E.; Ryder, M.; Knauf, J.A.; Liao, X.H.; West, B.L.; Bollag, G.; Kolesnick, R.; Thin, T.H.; Rosen, N.; et al. Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. J. Clin. Investig. 2011, 121, 4700–4711. [Google Scholar] [CrossRef] [PubMed]
- Durante, C.; Puxeddu, E.; Ferretti, E.; Morisi, R.; Moretti, S.; Bruno, R.; Barbi, F.; Avenia, N.; Scipioni, A.; Verrienti, A.; et al. BRAF mutations in papillary thyroid carcinomas inhibit genes involved in iodine metabolism. J. Clin. Endocrinol. Metab. 2007, 92, 2840–2843. [Google Scholar] [CrossRef]
- Romei, C.; Ciampi, R.; Faviana, P.; Agate, L.; Molinaro, E.; Bottici, V.; Basolo, F.; Miccoli, P.; Pacini, F.; Pinchera, A.; et al. BRAFV600E mutation; but not RET/PTC rearrangements, is correlated with a lower expression of both thyroperoxidase and sodium iodide symporter genes in papillary thyroid cancer. Endocr.-Relat. Cancer 2008, 15, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, R.; Shen, X.; Zhu, G.; Li, B.; Xing, M. The genetic duet of BRAF V600E and TERT promoter mutations robustly predicts loss of radioiodine avidity in recurrent papillary thyroid cancer. J. Nucl. Med. 2020, 61, 177–182. [Google Scholar] [CrossRef]
- Sabra, M.M.; Dominguez, J.M.; Grewal, R.K.; Larson, S.M.; Ghossein, R.A.; Tuttle, R.M.; Fagin, J.A. Clinical outcomes and molecular profile of differentiated thyroid cancers with radioiodine-avid distant metastases. J. Clin. Endocrinol. Metab. 2013, 98, E829–E836. [Google Scholar] [CrossRef]
- Yoon, M.; Jung, S.J.; Kim, T.H.; Ha, T.K.; Urm, S.H.; Park, J.S.; Lee, S.M.; Bae, S.K. Relationships between transporter expression and the status of BRAF V600E mutation and F-18 FDG uptake in papillary thyroid carcinomas. Endocr. Res. 2016, 41, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Grabellus, F.; Worm, K.; Schmid, K.W.; Sheu, S.Y. The BRAF V600E mutation in papillary thyroid carcinoma is associated with glucose transporter 1 overexpression. Thyroid 2012, 22, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Giovanella, L.; Avram, A.M.; Iakovou, I.; Kwak, J.; Lawson, S.A.; Lulaj, E.; Luster, M.; Piccardo, A.; Schmidt, M.; Tulchinsky, M.; et al. EANM practice guideline/SNMMI procedure standard for RAIU and thyroid scintigraphy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2514–2525. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, P.S.; Gupta, M. Differentiated thyroid cancer theranostics: Radioiodine and beyond. Br. J. Radiol. 2018, 91, 20180136. [Google Scholar] [CrossRef]
- Santhanam, P.; Taieb, D.; Solnes, L.; Marashdeh, W.; Ladenson, P.W. Utility of I-124 PET/CT in identifying radioiodine avid lesions in differentiated thyroid cancer: A systematic review and meta-analysis. Clin. Endocrinol. 2017, 86, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, G.; Kolbert, K.S.; Sheikh, A.; Pentlow, K.S.; Mun, E.F.; Barth, A.; Robbins, R.J.; Larson, S.M. Patient-specific dosimetry for 131I thyroid cancer therapy using 124I PET and 3-dimensional-internal dosimetry (3D-ID) software. J. Nucl. Med. 2004, 45, 1366–1372. [Google Scholar] [PubMed]
- Lassmann, M.; Reiners, C.; Luster, M. Dosimetry and thyroid cancer: The individual dosage of radioiodine. Endocr.-Relat. Cancer 2010, 17, R161–R172. [Google Scholar] [CrossRef] [PubMed]
- Klubo-Gwiezdzinska, J.; Van Nostrand, D.; Atkins, F.; Burman, K.; Jonklaas, J.; Mete, M.; Wartofsky, L. Efficacy of dosimetric versus empiric prescribed activity of 131I for therapy of differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 2011, 96, 3217–3225. [Google Scholar] [CrossRef]
- Wierts, R.; Brans, B.; Havekes, B.; Kemerink, G.J.; Halders, S.G.; Schaper, N.N.; Backes, W.H.; Mottaghy, F.M.; Jentzen, W. Dose-response relationship in differentiated thyroid cancer patients undergoing radioiodine treatment assessed by means of 124I PET/CT. J. Nucl. Med. 2016, 57, 1027–1032. [Google Scholar] [CrossRef]
- Luster, M.; Clarke, S.E.; Dietlein, M.; Lassmann, M.; Lind, P.; Oyen, W.J.; Tennvall, J.; Bombardieri, E. Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1941–1959. [Google Scholar] [CrossRef]
- Borget, I.; Bonastre, J.; Catargi, B.; Déandréis, D.; Zerdoud, S.; Rusu, D.; Bardet, S.; Leenhardt, L.; Bastie, D.; Schvartz, C.; et al. Quality of life and cost-effectiveness assessment of radioiodine ablation strategies in patients with thyroid cancer: Results from the randomized phase III ESTIMABL trial. J. Clin. Oncol. 2015, 33, 2885–2892. [Google Scholar] [CrossRef]
- Haugen, B.R.; Pacini, F.; Reiners, C.; Schlumberger, M.; Ladenson, P.W.; Sherman, S.; Cooper, D.S.; Graham, K.E.; Braverman, L.E.; Skarulis, M.C.; et al. A comparison of recombinant human thyrotropin and thyroid hormone withdrawal for the detection of thyroid remnant or cancer. J. Clin. Endocrinol. Metab. 1999, 84, 3877–3885. [Google Scholar] [CrossRef]
- Pacini, F.; Ladenson, P.W.; Schlumberger, M.; Driedger, A.; Luster, M.; Kloos, R.T.; Sherman, S.; Haugen, B.; Corone, C.; Molinaro, E.; et al. Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin in differentiated thyroid carcinoma: Results of an international, randomized, controlled study. J. Clin. Endocrinol. Metab. 2006, 91, 926–932. [Google Scholar] [CrossRef]
- Schlumberger, M.; Catargi, B.; Borget, I.; Deandreis, D.; Zerdoud, S.; Bridji, B.; Bardet, S.; Leenhardt, L.; Bastie, D.; Schvartz, C.; et al. Tumeurs de la thyroïde refractaires network for the essai stimulation ablation equivalence trial. Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N. Engl. J. Med. 2012, 366, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
- Mallick, U.; Harmer, C.; Yap, B.; Wadsley, J.; Clarke, S.; Moss, L.; Nicol, A.; Clark, P.M.; Farnell, K.; McCready, R.; et al. Ablation with low-dose radioiodine and thyrotropin alfa in thyroid cancer. N. Engl. J. Med. 2012, 366, 1674–1685. [Google Scholar] [CrossRef]
- Pötzi, C.; Moameni, A.; Karanikas, G.; Preitfellner, J.; Becherer, A.; Pirich, C.; Dudczak, R. Comparison of iodine uptake in tumour and nontumour tissue under thyroid hormone deprivation and with recombinant human thyrotropin in thyroid cancer patients. Clin. Endocrinol. 2006, 65, 519–523. [Google Scholar] [CrossRef]
- Plyku, D.; Hobbs, R.F.; Huang, K.; Atkins, F.; Garcia, C.; Sgouros, G.; Van Nostrand, D. Recombinant human thyroid-stimulating hormone versus thyroid hormone withdrawal in 124I PET/CT-based dosimetry for 131I therapy of metastatic differentiated thyroid cancer. J. Nucl. Med. 2017, 58, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Padovani, R.P.; Kasamatsu, T.S.; Nakabashi, C.C.; Camacho, C.P.; Andreoni, D.M.; Malouf, E.Z.; Marone, M.M.; Maciel, R.M.; Biscolla, R.P. One month is sufficient for urinary iodine to return to its baseline value after the use of water-soluble iodinated contrast agents in post-thyroidectomy patients requiring radioiodine therapy. Thyroid 2012, 22, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Sacks, W.; Fung, C.H.; Chang, J.T.; Waxman, A.; Braunstein, G.D. The effectiveness of radioactive iodine for treatment of low-risk thyroid cancer: A systematic analysis of the peer-reviewed literature from 1966 to April 2008. Thyroid 2010, 20, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Yap, B.K.; Murby, B. No adverse effect in clinical outcome using low preablation diagnostic (131) I activity in differentiated thyroid cancer: Refuting thyroid-stunning effect. J. Clin. Endocrinol. Metab. 2014, 99, 2433–2440. [Google Scholar] [CrossRef][Green Version]
- Leger, F.A.; Izembart, M.; Dagousset, F.; Barritault, L.; Baillet, G.; Chevalier, A.; Clerc, J. Decreased uptake of therapeutic doses of iodine-131 after 185-MBq iodine-131 diagnostic imaging for thyroid remnants in differentiated thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging 1998, 25, 242–246. [Google Scholar] [CrossRef]
- Schlumberger, M.; Mancusi, F.; Baudin, E.; Pacini, F. 131I therapy for elevated thyroglobulin levels. Thyroid 1997, 7, 273–276. [Google Scholar] [CrossRef]
- Aide, N.; Heutte, N.; Rame, J.P.; Rousseau, E.; Loiseau, C.; Henry-Amar, M.; Bardet, S. Clinical relevance of single-photon emission computed tomography/computed tomography of the neck and thorax in postablation (131) I scintigraphy for thyroid cancer. J. Clin. Endocrinol. Metab. 2009, 94, 2075–2084. [Google Scholar] [CrossRef] [PubMed]
- Grewal, R.K.; Tuttle, R.M.; Fox, J.; Borkar, S.; Chou, J.F.; Gonen, M.; Strauss, H.W.; Larson, S.M.; Schöder, H. The effect of posttherapy 131I SPECT/CT on risk classification and management of patients with differentiated thyroid cancer. J. Nucl. Med. 2010, 51, 1361–1367. [Google Scholar] [CrossRef]
- Wong, K.K.; Sisson, J.C.; Koral, K.F.; Frey, K.A.; Avram, A.M. Staging of differentiated thyroid carcinoma using diagnostic 131I SPECT/CT. AJR Am. J. Roentgenol. 2010, 195, 730–736. [Google Scholar] [CrossRef]
- Nakada, K.; Ishibashi, T.; Takei, T.; Hirata, K.; Shinohara, K.; Katoh, S.; Zhao, S.; Tamaki, N.; Noguchi, Y.; Noguchi, S. Does lemon candy decrease salivary gland damage after radioiodine therapy for thyroid cancer? J. Nucl. Med. 2005, 46, 261–266. [Google Scholar] [PubMed]
- Carlisle, M.R.; Lu, C.; McDougall, I.R. The interpretation of 131I scans in the evaluation of thyroid cancer; with an emphasis on false positive findings. Nucl. Med. Commun. 2003, 24, 715–735. [Google Scholar] [CrossRef] [PubMed]
- Durante, C.; Haddy, N.; Baudin, E.; Leboulleux, S.; Hartl, D.; Travagli, J.P.; Caillou, B.; Ricard, M.; Lumbroso, J.D.; De Vathaire, F.; et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: Benefits and limits of radioiodine therapy. J. Clin. Endocrinol. Metab. 2006, 91, 2892–2899. [Google Scholar] [CrossRef] [PubMed]
- Gallamini, A.; Zwarthoed, C.; Borra, A. Positron Emission Tomography (PET) in Oncology. Cancers 2014, 6, 1821–1889. [Google Scholar] [CrossRef] [PubMed]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. European Association of Nuclear Medicine (EANM) FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Schönberger, J.; Rüschoff, J.; Grimm, D.; Marienhagen, J.; Rümmele, P.; Meyringer, R.; Kossmehl, P.; Hofstaedter, F.; Eilles, C. Glucose transporter 1 gene expression is related to thyroid neoplasms with an unfavorable prognosis: An immunohistochemical study. Thyroid 2002, 12, 747–754. [Google Scholar] [CrossRef]
- Leboulleux, S.; Schroeder, P.R.; Schlumberger, M.; Ladenson, P.W. The role of PET in follow-up of patients treated for differentiated epithelial thyroid cancers. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 112–121. [Google Scholar] [CrossRef]
- Giovanella, L.; Trimboli, P.; Verburg, F.A.; Treglia, G.; Piccardo, A.; Foppiani, L.; Ceriani, L. Thyroglobulin levels and thyroglobulin doubling time independently predict a positive 18F-FDG PET/CT scan in patients with biochemical recurrence of differentiated thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 874–880. [Google Scholar] [CrossRef]
- Marcus, C.; Whitworth, P.W.; Surasi, D.S.; Pai, S.I.; Subramaniam, R.M. PET/CT in the management of thyroid cancers. AJR Am. J. Roentgenol. 2014, 202, 1316–1329. [Google Scholar] [CrossRef] [PubMed]
- Salvatori, M.; Biondi, B.; Rufini, V. Imaging in endocrinology: 2-[18F]-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography in differentiated thyroid carcinoma: Clinical indications and controversies in diagnosis and follow-up. Eur. J. Endocrinol. 2015, 173, R115–R130. [Google Scholar] [CrossRef]
- Deandreis, D.; Al Ghuzlan, A.; Leboulleux, S.; Lacroix, L.; Garsi, J.P.; Talbot, M.; Lumbroso, J.; Baudin, E.; Caillou, B.; Bidart, J.M.; et al. Do histological, immunohistochemical, and metabolic (radioiodine and fluorodeoxyglucose uptakes) patterns of metastatic thyroid cancer correlate with patient outcome? Endocr.-Relat. Cancer 2011, 18, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum-Krumme, S.J.; Görges, R.; Bockisch, A.; Binse, I. 18F-FDG PET/CT changes therapy management in high-risk DTC after first radioiodine therapy. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Grewal, R.K.; Ho, A.; Schöder, H. Novel approaches to thyroid cancer treatment and response assessment. Semin. Nucl. Med. 2016, 46, 109–118. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Robbins, R.J.; Wan, Q.; Grewal, R.K.; Reibke, R.; Gonen, M.; Strauss, H.W.; Tuttle, R.M.; Drucker, W.; Larson, S.M. Real-time prognosis for metastatic thyroid carcinoma based on 2-[18F] fluoro-2-deoxy-D-glucose-positron emission tomography scanning. J. Clin. Endocrinol. Metab. 2006, 91, 498–505. [Google Scholar] [CrossRef]
- Samnick, S.; Al-Momani, E.; Schmid, J.S.; Mottok, A.; Buck, A.K.; Lapa, C. Initial clinical investigation of [18F] Tetrafluoroborate PET/CT in comparison to [124I] Iodine PET/CT for imaging thyroid cancer. Clin. Nucl. Med. 2018, 43, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; DeGrado, T.R. [18F] Tetrafluoroborate ([18F] TFB) and its analogs for PET imaging of the sodium/iodide symporter. Theranostics 2018, 8, 3918–3931. [Google Scholar] [CrossRef] [PubMed]
- Dittmann, M.; Gonzalez Carvalho, J.M.; Rahbar, K.; Schäfers, M.; Claesener, M.; Riemann, B.; Seifert, R. Incremental diagnostic value of [18F] tetrafluoroborate PET-CT compared to [131I] iodine scintigraphy in recurrent differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2639–2646. [Google Scholar] [CrossRef]
- Lawhn-Heath, C.; Yom, S.S.; Liu, C.; Villanueva-Meyer, J.E.; Aslam, M.; Smith, R.; Narwal, M.; Juarez, R.; Behr, S.C.; Pampaloni, M.H.; et al. Gallium-68 prostate-specific membrane antigen ([68Ga] Ga-PSMA-11) PET for imaging of thyroid cancer: A feasibility study. EJNMMI Res. 2020, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- De Vries, L.H.; Lodewijk, L.; Braat, A.; Krijger, G.C.; Valk, G.D.; Lam, M.; Borel Rinkes, I.; Vriens, M.R.; de Keizer, B. 68Ga-PSMA PET/CT in radioactive iodine-refractory differentiated thyroid cancer and first treatment results with 177Lu-PSMA-617. EJNMMI Res. 2020, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Schirrmeister, H.; Buck, A.; Guhlmann, A.; Reske, S.N. Anatomical distribution and sclerotic activity of bone metastases from thyroid cancer assessed with F-18 sodium fluoride positron emission tomography. Thyroid 2001, 11, 677–683. [Google Scholar] [CrossRef]
- Deandreis, D.; Maillard, A.; Zerdoud, S.; Bournaud, C.; Vija, L.; Sajous, C.; Terroir, M.; Leenhardt, L.; Schlumberger, M.; Borget, I.; et al. RADTHYR: An open-label, single-arm, prospective multicenter phase II trial of Radium-223 for the treatment of bone metastases from radioactive iodine refractory differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3238–3249. [Google Scholar] [CrossRef] [PubMed]
- Brink, J.A. PET/CT unplugged: The merging technologies of PET and CT imaging. AJR Am. J. Roentgenol. 2005, 184, S135–S137. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.C.; Visvikis, D.; Crosdale, I.; Pigden, I.; Townsend, C.; Bomanji, J.; Prvulovich, E.; Lonn, A.; Ell, P.J. Positron emission and computed X-ray tomography: A coming together. Nucl. Med. Commun. 2003, 24, 351–358. [Google Scholar] [CrossRef]
- Shammas, A.; Degirmenci, B.; Mountz, J.M.; McCook, B.M.; Branstetter, B.; Bencherif, B.; Joyce, J.M.; Carty, S.E.; Kuffner, H.A.; Avril, N. 18F-FDG PET/CT in patients with suspected recurrent or metastatic well-differentiated thyroid cancer. J. Nucl. Med. 2007, 48, 221–226. [Google Scholar]
- Vrachimis, A.; Burg, M.C.; Wenning, C.; Allkemper, T.; Weckesser, M.; Schäfers, M.; Stegger, L. [(18)F]FDG PET/CT outperforms [(18)F]FDG PET/MRI in differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Klain, M.; Nappi, C.; Nicolai, E.; Romeo, V.; Piscopo, L.; Giordano, A.; Gaudieri, V.; Zampella, E.; Pace, L.; Carlo, C.; et al. Comparison of simultaneous 18F-2-[18F] FDG PET/MR and PET/CT in the follow-up of patients with differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 3066–3073. [Google Scholar] [CrossRef]
- M’Kacher, R.; Legal, J.D.; Schlumberger, M.; Voisin, P.; Aubert, B.; Gaillard, N.; Parmentier, C. Biological dosimetry in patients treated with iodine-131 for differentiated thyroid carcinoma. J. Nucl. Med. 1996, 37, 1860–1864. [Google Scholar]
- Rubino, C.; De Vathaire, F.; Dottorini, M.E.; Hall, P.; Schvartz, C.; Couette, J.E.; Dondon, M.-G.; Abbas, M.T.; Langlois, C.; Schlumberger, M. Second primary malignancies in thyroid cancer patients. Br. J. Cancer 2003, 89, 1638–1644. [Google Scholar] [CrossRef] [PubMed]
- Garsi, J.P.; Schlumberger, M.; Rubino, C.; Ricard, M.; Labbé, M.; Ceccarelli, C.; Schvartz, C.; Henri-Amar, M.; Bardet, S.; de Vathaire, F. Therapeutic administration of 131I for differentiated thyroid cancer: Radiation dose to ovaries and outcome of pregnancies. J. Nucl. Med. 2008, 49, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Verburg, F.A.; Hoffmann, M.; Iakovou, I.; Konijnenberg, M.W.; Mihailovic, J.; Gabina, P.M.; Ovčariček, P.P.; Reiners, C.; Vrachimis, A.; Zerdoud, S.; et al. Errare humanum est, sed in errare perseverare diabolicum: Methodological errors in the assessment of the relationship between I-131 therapy and possible increases in the incidence of malignancies. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 519–522. [Google Scholar] [CrossRef]
- Hay, I.D.; Johnson, T.R.; Kaggal, S.; Reinalda, M.S.; Iniguez-Ariza, N.M.; Grant, C.S.; Pittock, S.T.; Thompson, G.B. Papillary Thyroid Carcinoma (PTC) in Children and Adults: Comparison of Initial Presentation and Long-Term Postoperative Outcome in 4432 Patients Consecutively Treated at the Mayo Clinic During Eight Decades (1936–2015). World J. Surg. 2018, 42, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Sawka, A.M.; Brierley, J.D.; Tsang, R.W.; Thabane, L.; Rotstein, L.; Gafni, A.; Straus, S.; Goldstein, D.P. An updated systematic review and commentary examining the effectiveness of radioactive iodine remnant ablation in well-differentiated thyroid cancer. Endocrinol. Metab. Clin. North Am. 2008, 37, 457–480. [Google Scholar] [CrossRef]
- Schlumberger, M.; Leboulleux, S. Current practice in patients with differentiated thyroid cancer. Nat. Rev. Endocrinol. 2021, 17, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Schvartz, C.; Bonnetain, F.; Dabakuyo, S.; Gauthier, M.; Cueff, A.; Fieffé, S.; Pochart, J.M.; Cochet, I.; Crevisy, E.; Dalac, A.; et al. Impact on overall survival of radioactive iodine in low-risk differentiated thyroid cancer patients. J. Clin. Endocrinol. Metab. 2012, 97, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Lamartina, L.; Durante, C.; Filetti, S.; Cooper, D.S. Low-risk differentiated thyroid cancer and radioiodine remnant ablation: A systematic review of the literature. J. Clin. Endocrinol. Metab. 2015, 100, 1748–1761. [Google Scholar] [CrossRef]
- Cailleux, A.F.; Baudin, E.; Travagli, J.P.; Ricard, M.; Schlumberger, M. Is diagnostic iodine-131 scanning useful after total thyroid ablation for differentiated thyroid cancer? J. Clin. Endocrinol. Metab. 2000, 85, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Pacini, F.; Capezzone, M.; Elisei, R.; Ceccarelli, C.; Taddei, D.; Pinchera, A. Diagnostic 131-iodine whole-body scan may be avoided in thyroid cancer patients who have undetectable stimulated serum Tg levels after initial treatment. J. Clin. Endocrinol. Metab. 2002, 87, 1499–1501. [Google Scholar] [CrossRef]
- Lamartina, L.; Grani, G.; Durante, C.; Borget, I.; Filetti, S.; Schlumberger, M. Follow-up of differentiated thyroid cancer—What should (and what should not) be done. Nat. Rev. Endocrinol. 2018, 14, 538–551. [Google Scholar] [CrossRef]
- Schlumberger, M.; Leboulleux, S.; Catargi, B.; Deandreis, D.; Zerdoud, S.; Bardet, S.; Rusu, D.; Godbert, Y.; Buffet, C.; Schvartz, C.; et al. Outcome after ablation in patients with low-risk thyroid cancer (ESTIMABL1): 5-year follow-up results of a randomised, phase 3, equivalence trial. Lancet Diabetes Endocrinol. 2018, 6, 618–626. [Google Scholar] [CrossRef]
- Dehbi, H.M.; Mallick, U.; Wadsley, J.; Newbold, K.; Harmer, C.; Hackshaw, A. Recurrence after low-dose radioiodine ablation and recombinant human thyroid-stimulating hormone for differentiated thyroid cancer (HiLo): Long-term results of an open-label, non-inferiority randomised controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 44–51. [Google Scholar] [CrossRef]
- Matrone, A.; Gambale, C.; Piaggi, P.; Viola, D.; Giani, C.; Agate, L.; Bottici, V.; Bianchi, F.; Materazzi, G.; Vitti, P.; et al. Postoperative thyroglobulin and neck ultrasound in the risk restratification and decision to perform 131I ablation. J. Clin. Endocrinol. Metab. 2017, 102, 893–902. [Google Scholar] [CrossRef]
- Gulec, S.A.; Ahuja, S.; Avram, A.M.; Bernet, V.J.; Bourguet, P.; Draganescu, C.; Elisei, R.; Giovanella, L.; Grant, F.; Greenspan, B.; et al. A Joint Statement from the American Thyroid Association, the European Association of Nuclear Medicine, the European Thyroid Association, the Society of Nuclear Medicine and Molecular Imaging on Current Diagnostic and Theranostic Approaches in the Management of Thyroid Cancer. Thyroid 2021, 31, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Grani, G.; Lamartina, L.; Alfò, M.; Ramundo, V.; Falcone, R.; Giacomelli, L.; Biffoni, M.; Filetti, S.; Durante, C. Selective use of radioactive iodine therapy for papillary thyroid cancers with low or lower-intermediate recurrence risk. J. Clin. Endocrinol. Metab. 2021, 106, e1717–e1727. [Google Scholar] [CrossRef] [PubMed]
- Verburg, F.A.; Mäder, U.; Reiners, C.; Hänscheid, H. Long-term survival in differentiated thyroid cancer is worse after low-activity initial post-surgical 131I therapy in both high- and low-risk patients. J. Clin. Endocrinol. Metab. 2014, 99, 4487–4496. [Google Scholar] [CrossRef]
- Gray, K.D.; Bannani, S.; Caillard, C.; Amanat, S.; Ullmann, T.M.; Romanov, P.; Brunaud, L.; Beninato, T.; Fahey, T.J., 3rd; Mirallie, E.; et al. High-dose radioactive iodine therapy is associated with decreased risk of recurrence in high-risk papillary thyroid cancer. Surgery 2019, 165, 37–43. [Google Scholar] [CrossRef]
- Wu, D.; Ylli, D.; Heimlich, S.L.; Burman, K.D.; Wartofsky, L.; Van Nostrand, D. 124I Positron emission tomography/computed tomography versus conventional radioiodine imaging in differentiated thyroid cancer: A review. Thyroid 2019, 29, 1523–1535. [Google Scholar] [CrossRef]
- Hertz, S.; Roberts, A. Radioactive iodine in the study of thyroid physiology; the use of radioactive iodine therapy in hyperthyroidism. J. Am. Med. Assoc. 1946, 131, 81–86. [Google Scholar] [CrossRef]
- Seidlin, S.M.; Marinelli, L.D.; Oshry, E. Radioactive iodine therapy; effect on functioning metastases of adenocarcinoma of the thyroid. J. Am. Med. Assoc. 1946, 132, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Coliez, R.; Tubiana, M.; Sung, S. Disappearance of pulmonary metastases of a thyroid cancer under the action of radioactive iodine 131. J. Radiol. Electrol. Arch. Electr. Med. 1951, 32, 396–399. [Google Scholar]
- Maxon, H.R., 3rd; Smith, H.S. Radioiodine-131 in the diagnosis and treatment of metastatic well differentiated thyroid cancer. Endocrinol. Metab. Clin. N. Am. 1990, 19, 685–718. [Google Scholar] [CrossRef]
- Maxon, H.R.; Thomas, S.R.; Hertzberg, V.S.; Kereiakes, J.G.; Chen, I.W.; Sperling, M.I.; Saenger, E.L. Relation between effective radiation dose and outcome of radioiodine therapy for thyroid cancer. N. Engl. J. Med. 1983, 309, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Deandreis, D.; Rubino, C.; Tala, H.; Leboulleux, S.; Terroir, M.; Baudin, E.; Larson, S.; Fagin, J.A.; Schlumberger, M.; Tuttle, R.M. Comparison of Empiric Versus Whole-Body/-Blood Clearance Dosimetry-Based Approach to Radioactive Iodine Treatment in Patients with Metastases from Differentiated Thyroid Cancer. J. Nucl. Med. 2017, 58, 717–722. [Google Scholar] [CrossRef]
- Dewaraja, Y.K.; Ljungberg, M.; Green, A.J.; Zanzonico, P.B.; Frey, E.C.; SNMMI MIRD Committee; Bolch, W.E.; Brill, A.B.; Dunphy, M.; Fisher, D.R.; et al. MIRD pamphlet No. 24: Guidelines for quantitative 131I SPECT in dosimetry applications. J. Nucl. Med. 2013, 54, 2182–2188. [Google Scholar] [CrossRef]
- Rall, J.E.; Alpers, J.B.; Lewallen, C.G.; Sonenberg, M.; Berman, M.; Rawson, R.W. Radiation pneumonitis and fibrosis: A complication of radioiodine treatment of pulmonary metastases from cancer of the thyroid. J. Clin. Endocrinol. Metab. 1957, 17, 1263–1276. [Google Scholar] [CrossRef]
- Benua, R.S.; Cicale, N.R.; Sonenberg, M.; Rawson, R.W. The relation of radioiodine dosimetry to results and complications in the treatment of metastatic thyroid cancer. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1962, 87, 171–182. [Google Scholar] [PubMed]
- Sgouros, G.; Song, H.; Ladenson, P.W.; Wahl, R.L. Lung toxicity in radioiodine therapy of thyroid carcinoma: Development of a dose-rate method and dosimetric implications of the 80-mCi rule. J. Nucl. Med. 2006, 47, 1977–1984. [Google Scholar]
- Tuttle, R.M.; Leboeuf, R.; Robbins, R.J.; Qualey, R.; Pentlow, K.; Larson, S.M.; Chan, C.Y. Empiric radioactive iodine dosing regimens frequently exceed maximum tolerated activity levels in elderly patients with thyroid cancer. J. Nucl. Med. 2006, 47, 1587–1591. [Google Scholar]
- Schlumberger, M.; Brose, M.; Elisei, R.; Leboulleux, S.; Luster, M.; Pitoia, F.; Pacini, F. Definition and management of radioactive iodine-refractory differentiated thyroid cancer. Lancet Diabetes Endocrinol. 2014, 2, 356–358. [Google Scholar] [CrossRef]
- Wirth, L.J. Still Perfecting Radioiodine in Thyroid Cancer, After All These Years. J. Clin. Endocrinol. Metab. 2019, 104, 1655–1657. [Google Scholar] [CrossRef]
- Jhiang, S.M.; Konda, B.; Sipos, J.A.; Nabhan, F.A. Prospects for redifferentiating agents in the use of radioactive Iodine therapy for thyroid cancer. Thyroid 2020, 30, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.L.; Grewal, R.K.; Leboeuf, R.; Sherman, E.J.; Pfister, D.G.; Deandreis, D.; Pentlow, K.S.; Zanzonico, P.B.; Haque, S.; Gavane, S.; et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N. Engl. J. Med. 2013, 368, 623–632. [Google Scholar] [CrossRef]
- Rothenberg, S.M.; McFadden, D.G.; Palmer, E.L.; Daniels, G.H.; Wirth, L.J. Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib. Clin Cancer Res. 2015, 21, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Leboulleux, S.; Dupuy, C.; Lacroix, L.; Attard, M.; Grimaldi, S.; Corre, R.; Ricard, M.; Nasr, S.; Berdelou, A.; Hadoux, J.; et al. Redifferentiation of a BRAFK601E-mutated poorly differentiated thyroid cancer patient with Dabrafenib and Trametinib treatment. Thyroid 2019, 29, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Lamartina, L.; Anizan, N.; Dupuy, C.; Leboulleux, S.; Schlumberger, M. Redifferentiation-facilitated radioiodine therapy in thyroid cancer. Endocr.-Relat. Cancer 2021, 28, T179–T191. [Google Scholar] [CrossRef]
- Groussin, L.; Clerc, J.; Huillard, O. Larotrectinib-enhanced radioactive iodine uptake in advanced thyroid cancer. N. Engl. J. Med. 2020, 383, 1686–1687. [Google Scholar] [CrossRef] [PubMed]
- Groussin, L.; Bessiene, L.; Arrondeau, J.; Garinet, S.; Cochand-Priollet, B.; Lupo, A.; Zerbit, J.; Clerc, J.; Huillard, O. Selpercatinib-Enhanced Radioiodine Uptake in RET-Rearranged Thyroid Cancer. Thyroid 2021. [Google Scholar] [CrossRef]
- Nascimento, C.; Borget, I.; Al Ghuzlan, A.; Deandreis, D.; Hartl, D.; Lumbroso, J.; Berdelou, A.; Lepoutre-Lussey, C.; Mirghani, H.; Baudin, E.; et al. Postoperative fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography: An important imaging modality in patients with aggressive histology of differentiated thyroid cancer. Thyroid 2015, 25, 437–444. [Google Scholar] [CrossRef]
- Leboulleux, S.; El Bez, I.; Borget, I.; Elleuch, M.; Déandreis, D.; Al Ghuzlan, A.; Chougnet, C.; Bidault, F.; Mirghani, H.; Lumbroso, J.; et al. Postradioiodine treatment whole-body scan in the era of 18-fluorodeoxyglucose positron emission tomography for differentiated thyroid carcinoma with elevated serum thyroglobulin levels. Thyroid 2012, 22, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Lee, S.W.; Pak, K.; Shim, S.R. Diagnostic performance of PET in thyroid cancer with elevated anti-Tg Ab. Endocr. Relat. Cancer 2018, 25, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Lamartina, L.; Borget, I.; Mirghani, H.; Al Ghuzlan, A.; Berdelou, A.; Bidault, F.; Deandreis, D.; Baudin, E.; Travagli, J.-P.; Schlumberger, M.; et al. Surgery for neck recurrence of differentiated thyroid cancer: Outcomes and risk factors. J. Clin. Endocrinol. Metab. 2017, 102, 1020–1031. [Google Scholar] [CrossRef] [PubMed]
- Pacini, F.; Cetani, F.; Miccoli, P.; Mancusi, F.; Ceccarelli, C.; Lippi, F.; Martino, E.; Pinchera, A. Outcome of 309 patients with metastatic differentiated thyroid carcinoma treated with radioiodine. World J. Surg. 1994, 18, 600–604. [Google Scholar] [CrossRef]
- Terroir, M.; Borget, I.; Bidault, F.; Ricard, M.; Deschamps, F.; Hartl, D.; Tselikas, L.; Dercle, L.; Lumbroso, J.; Baudin, E.; et al. The intensity of 18FDG uptake does not predict tumor growth in patients with metastatic differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 638–646. [Google Scholar] [CrossRef]
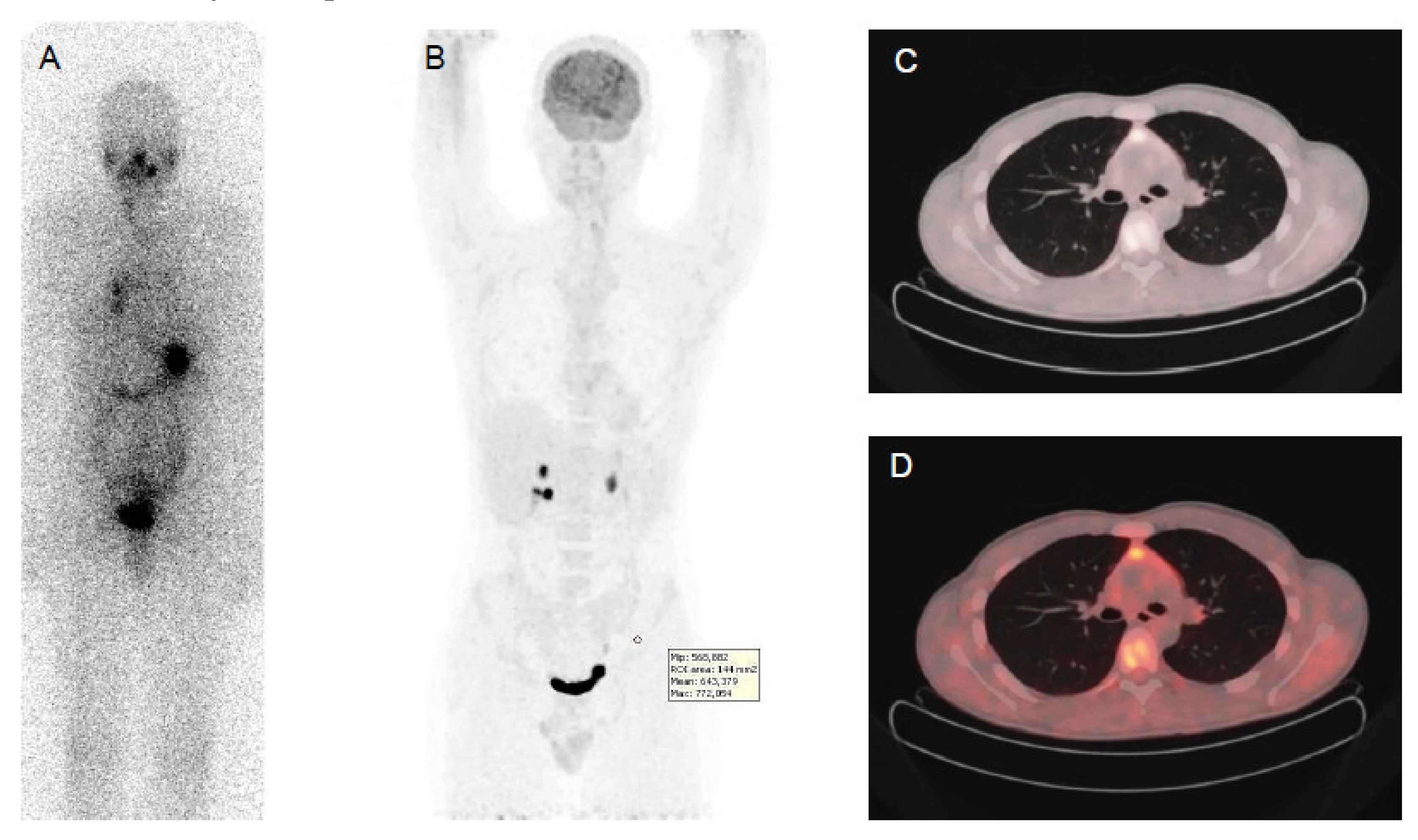
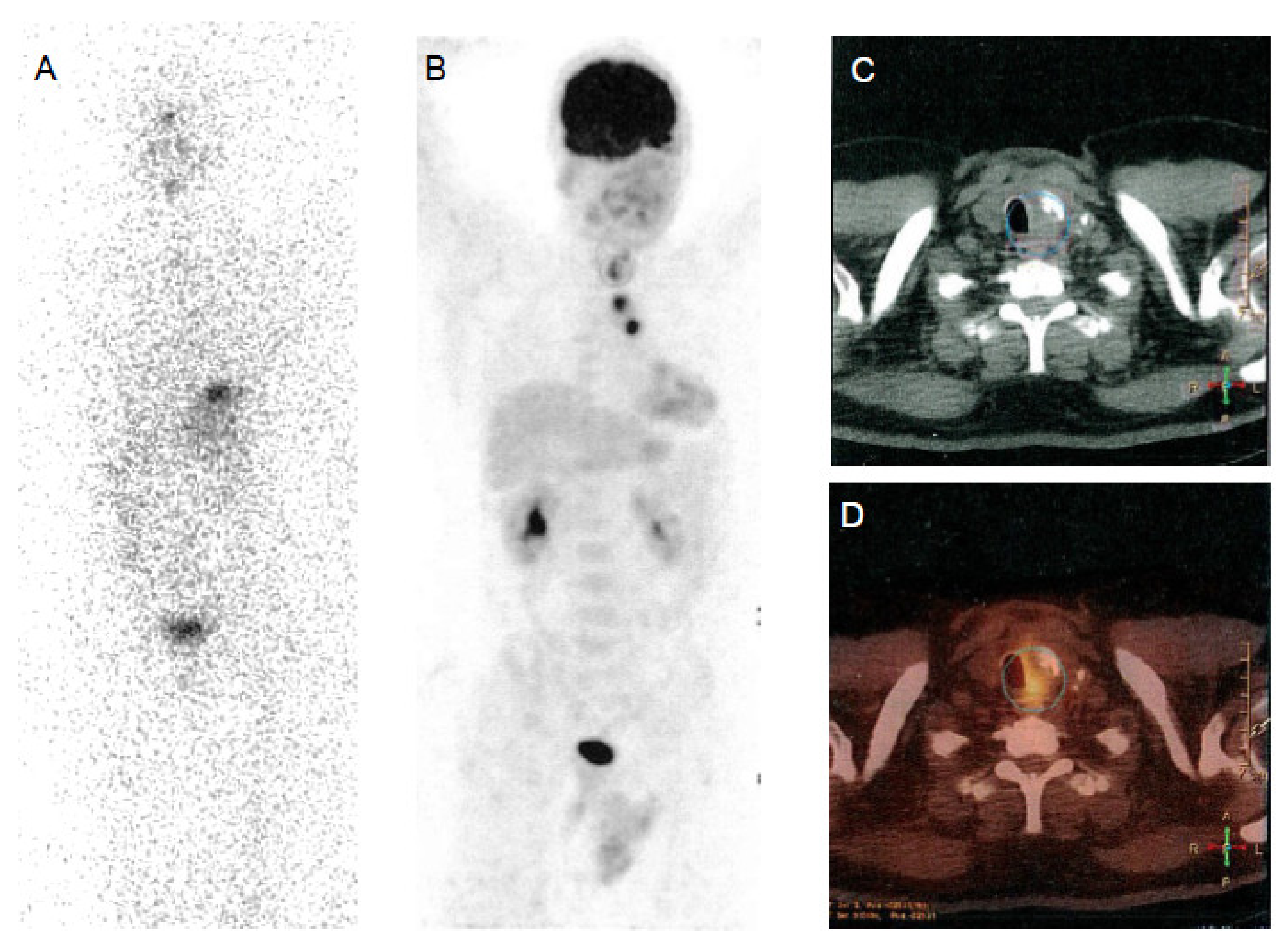
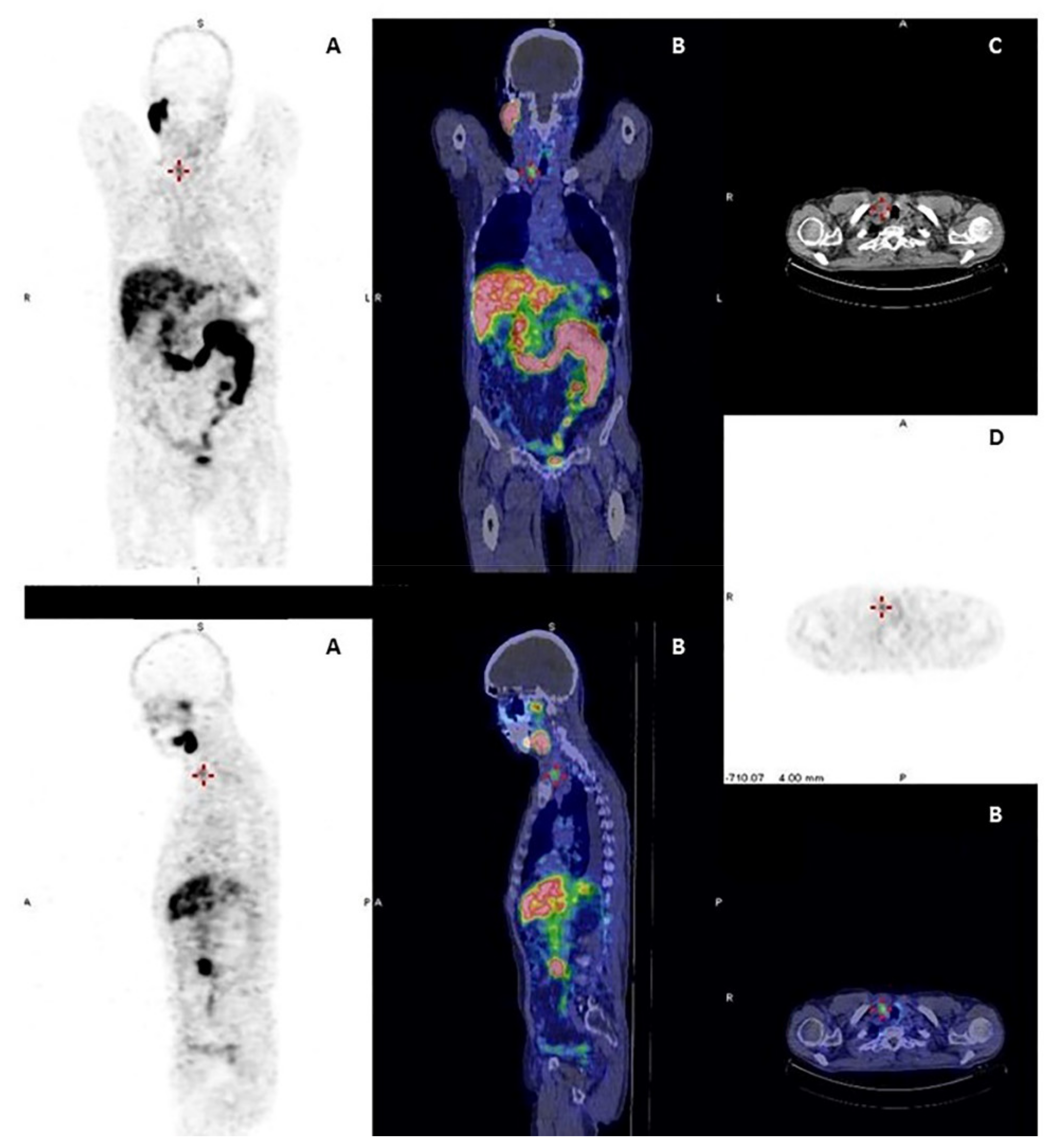
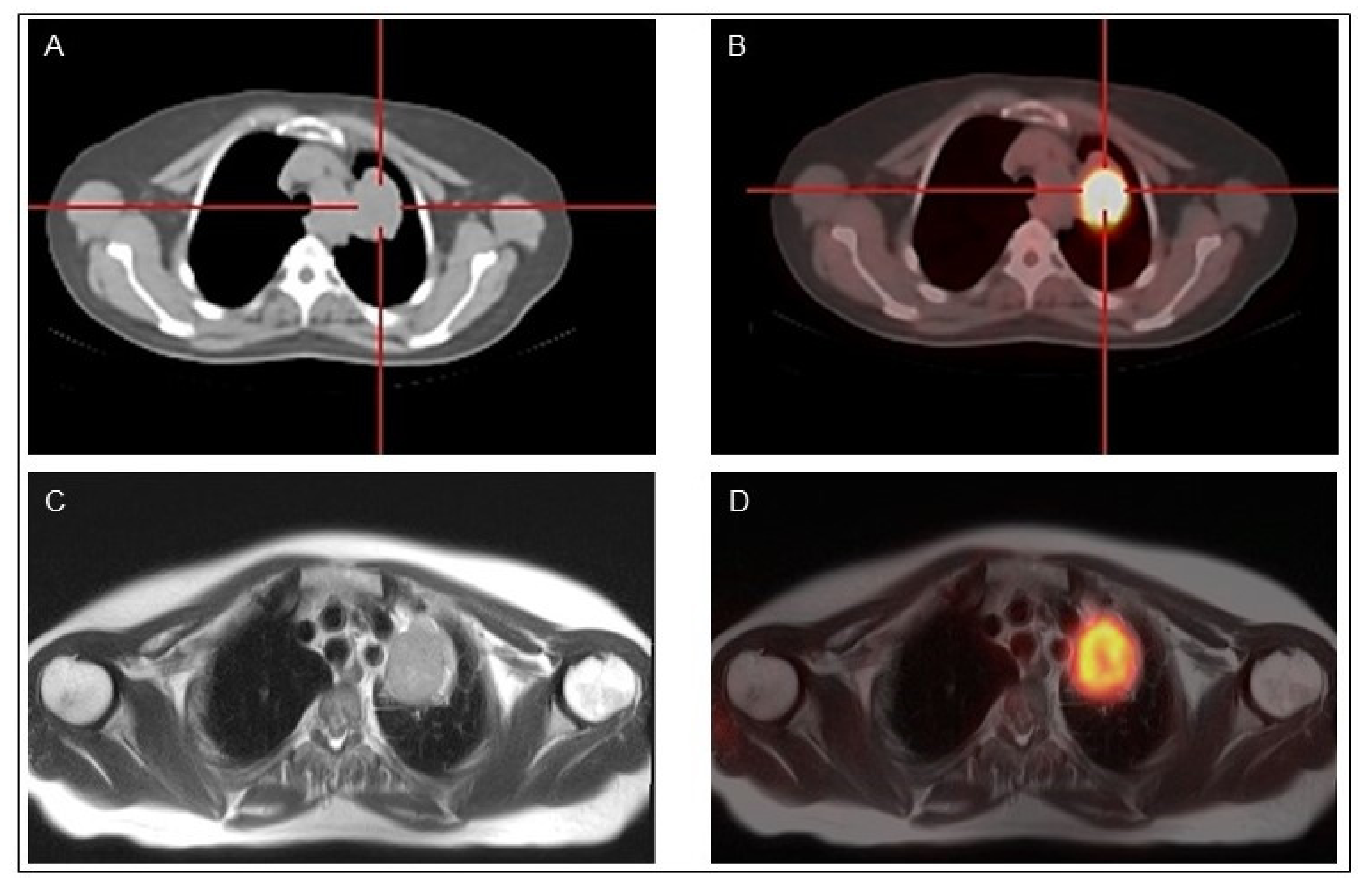
| Nuclide | Production | Decay Mode | Energy (keV) | Half-Life | Applications |
|---|---|---|---|---|---|
| 123I | Cyclotron | Electron capture | 159 | 13.22 hours | SPECT imaging |
| 131I | Nuclear reactor | β- decay γ decay | 606 364 | 8.02 days | Therapy SPECT imaging Dosimetry |
| 124I | Cyclotron | β+ decay | 511 | 4.18 days | PET imaging Dosimetry |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klain, M.; Zampella, E.; Nappi, C.; Nicolai, E.; Ambrosio, R.; Califaretti, E.; Lamartina, L.; Schlumberger, M.; Deandreis, D.; Salvatore, D.; et al. Advances in Functional Imaging of Differentiated Thyroid Cancer. Cancers 2021, 13, 4748. https://doi.org/10.3390/cancers13194748
Klain M, Zampella E, Nappi C, Nicolai E, Ambrosio R, Califaretti E, Lamartina L, Schlumberger M, Deandreis D, Salvatore D, et al. Advances in Functional Imaging of Differentiated Thyroid Cancer. Cancers. 2021; 13(19):4748. https://doi.org/10.3390/cancers13194748
Chicago/Turabian StyleKlain, Michele, Emilia Zampella, Carmela Nappi, Emanuele Nicolai, Raffaele Ambrosio, Elena Califaretti, Livia Lamartina, Martin Schlumberger, Désirée Deandreis, Domenico Salvatore, and et al. 2021. "Advances in Functional Imaging of Differentiated Thyroid Cancer" Cancers 13, no. 19: 4748. https://doi.org/10.3390/cancers13194748
APA StyleKlain, M., Zampella, E., Nappi, C., Nicolai, E., Ambrosio, R., Califaretti, E., Lamartina, L., Schlumberger, M., Deandreis, D., Salvatore, D., & Cuocolo, A. (2021). Advances in Functional Imaging of Differentiated Thyroid Cancer. Cancers, 13(19), 4748. https://doi.org/10.3390/cancers13194748









