Comparative Analysis of Vascular Mimicry in Head and Neck Squamous Cell Carcinoma: In Vitro and In Vivo Approaches
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Samples
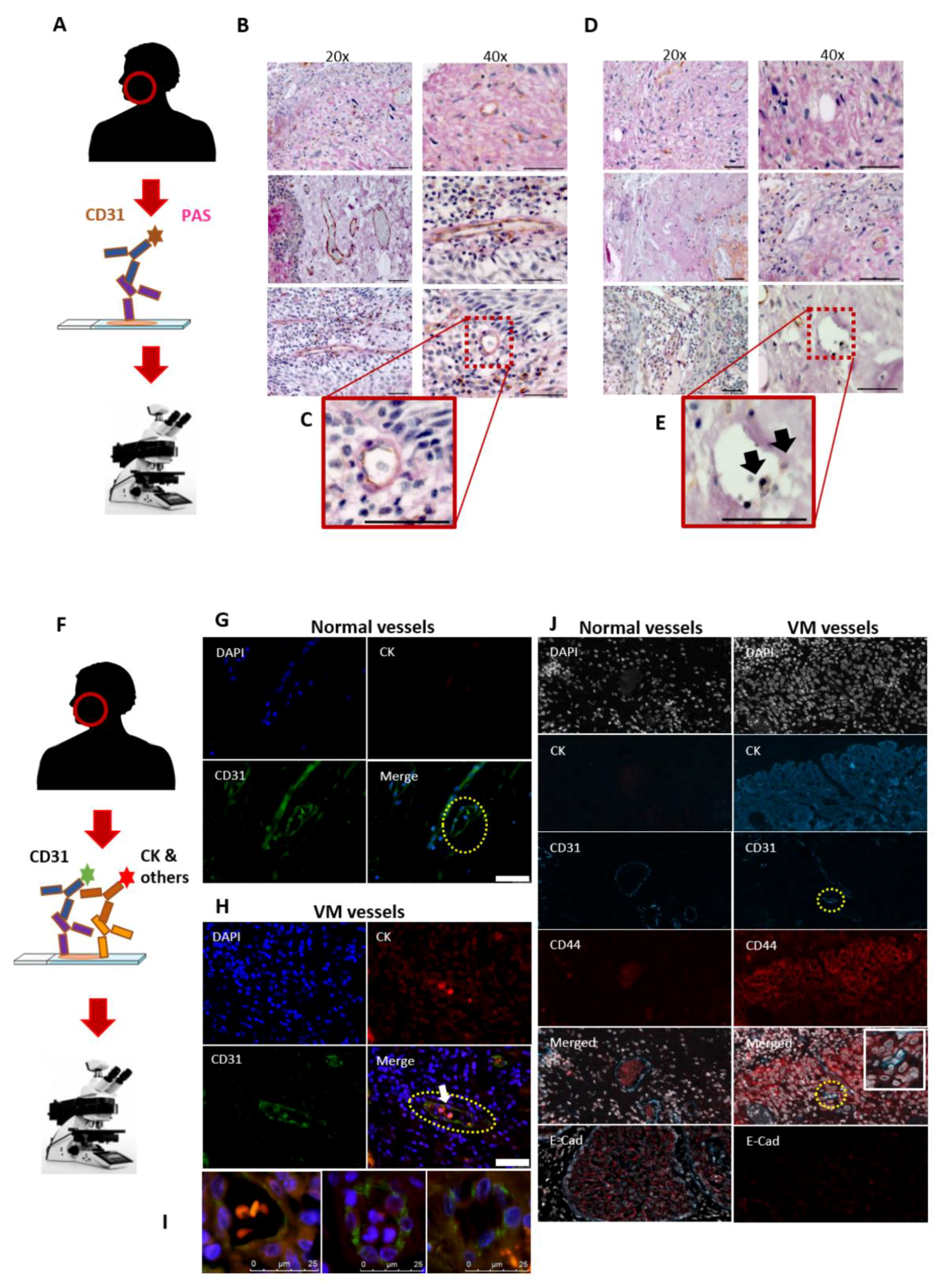
2.2. CD31 and PAS Double Staining
2.3. Double-Labelling Immunofluorescence (IF)
2.4. Cell Line and Culture
2.5. Murine and Human-Derived 3D Matrices
2.6. In Vitro Tube Formation Assay
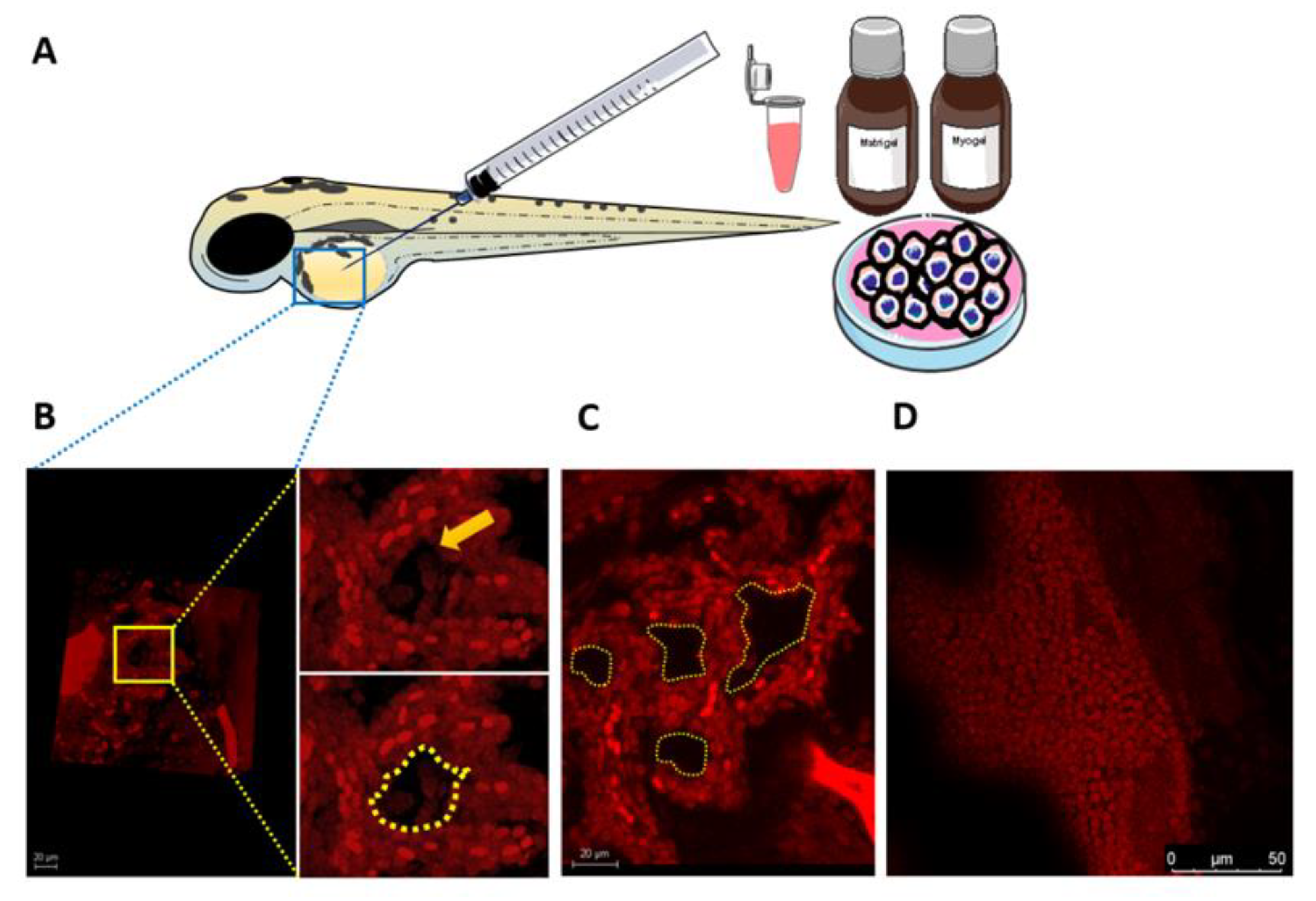
2.7. Zebrafish Larvae Assays
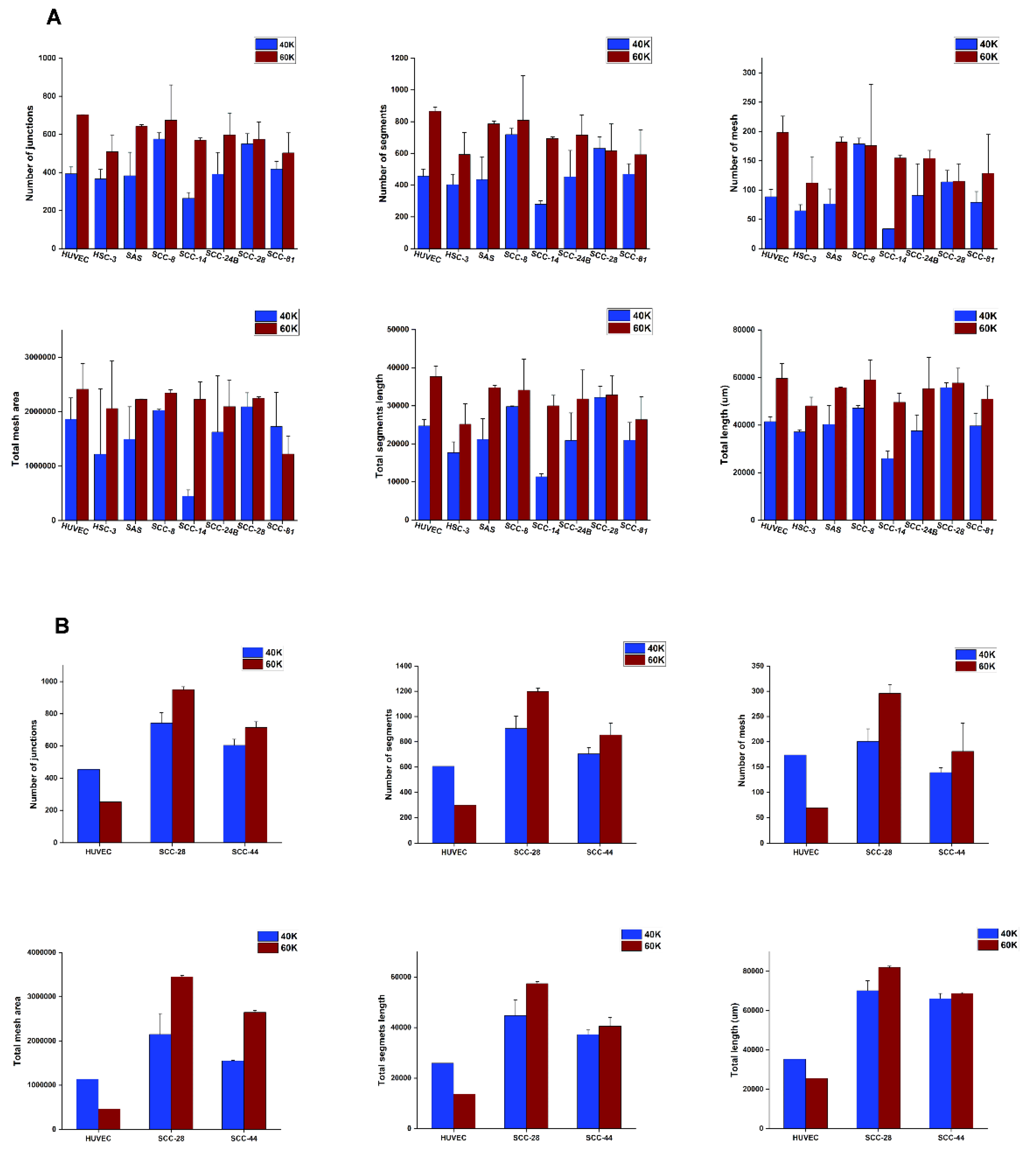
2.8. Imaging and Tube Formation Analysis
3. Results
3.1. Utility of the CD31-ve/PAS+ve Reaction in Identifying the VM in HNSCC Patients
3.2. The Mosaic VM Pattern Reveals Tumour Cell Plasticity
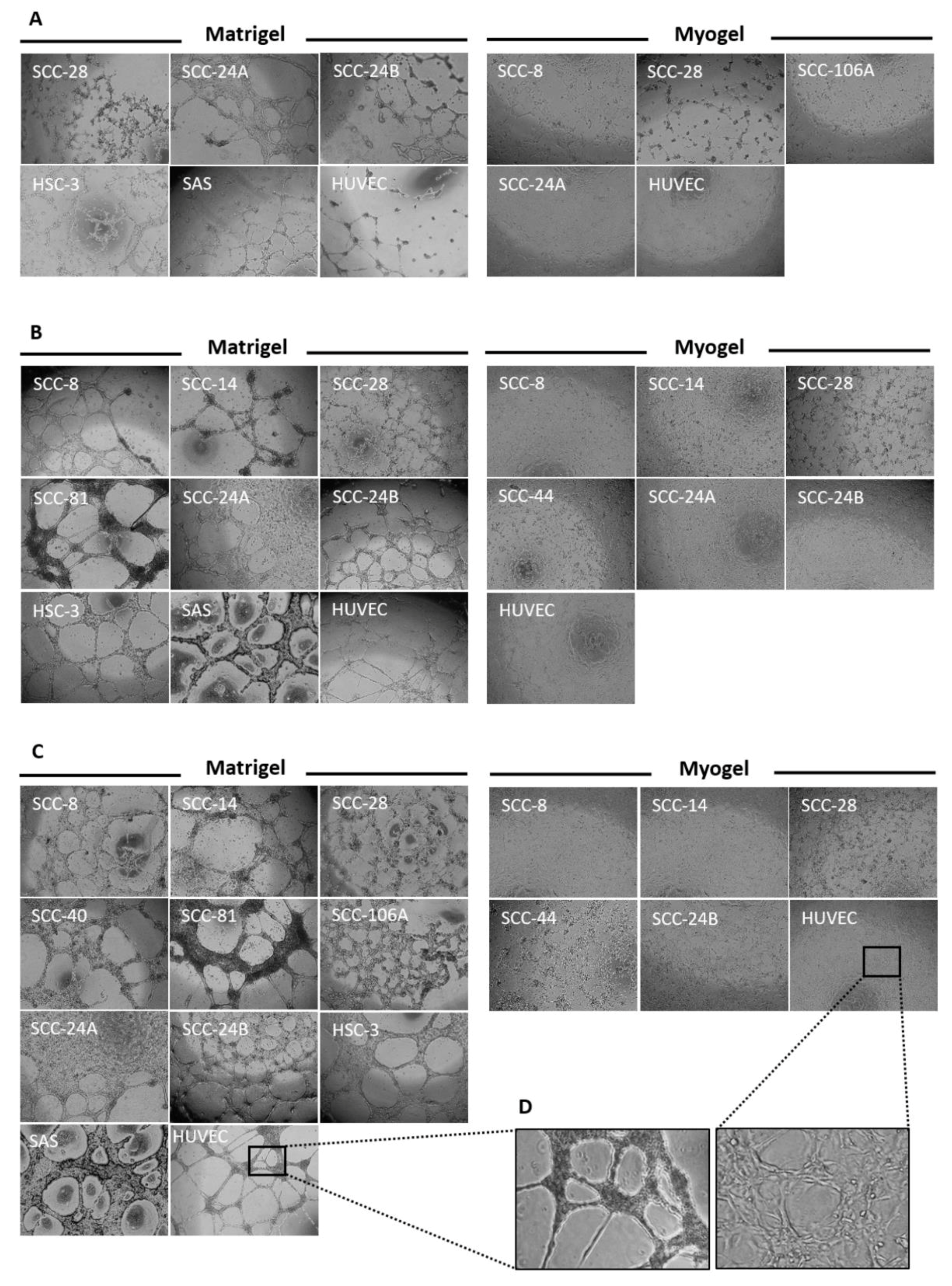
3.3. Metastatic HNSCC Cells Preferentially form VM in Matrigel
3.4. Tumour Cell Density Influences VM Formation In Vitro
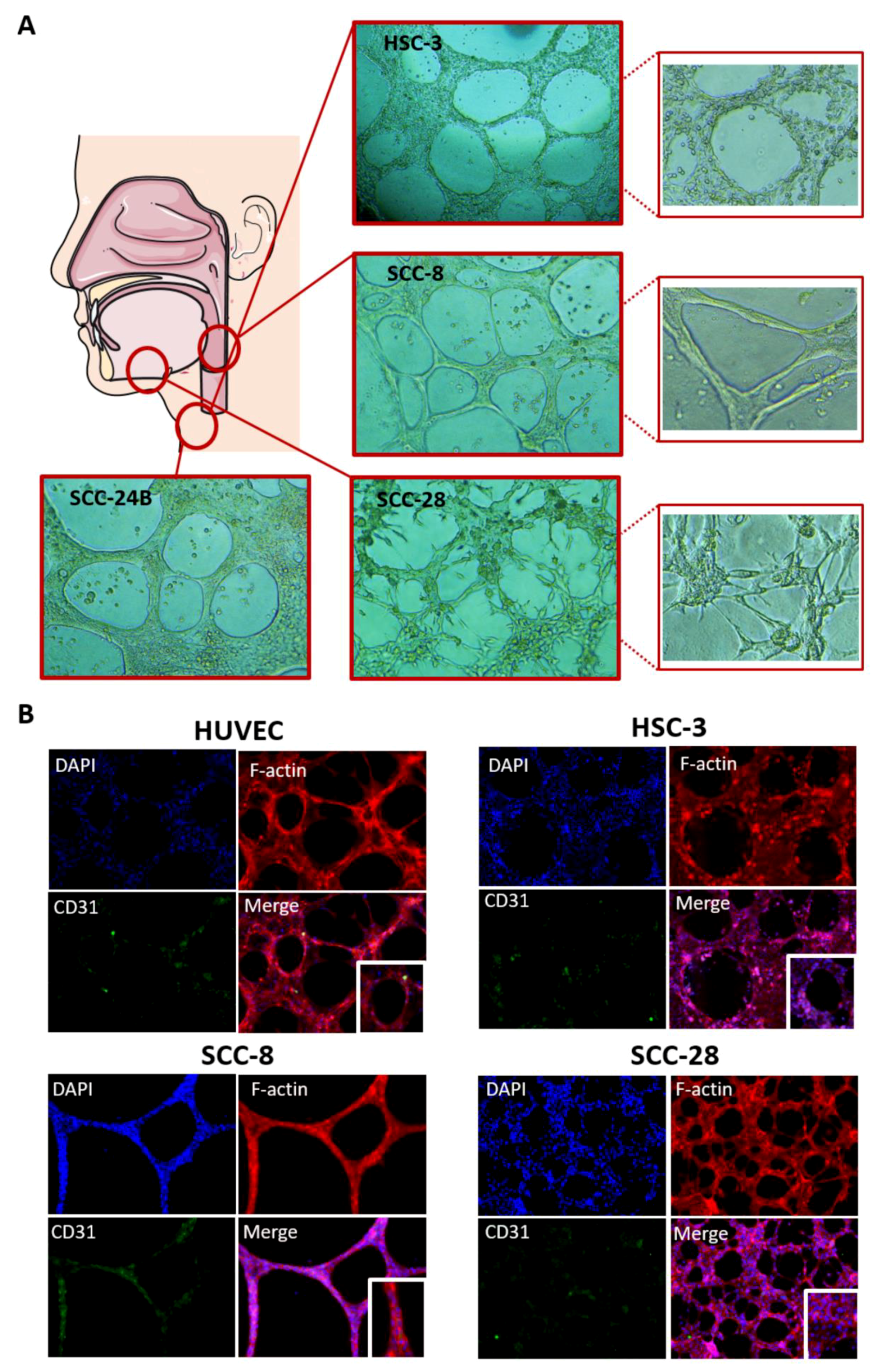
3.5. In Vitro VM Networks Reveal Different Morphological Patterns
3.6. In Vitro VM Networks Express Endothelial Cell Marker
3.7. Larval Zebrafish as a Novel In Vivo Model for VM Formation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marur, S.; Forastiere, A.A. Head and Neck Squamous Cell Carcinoma: Update on Epidemiology, Diagnosis, and Treatment. Mayo Clin. Proc. 2016, 91, 386–396. [Google Scholar] [CrossRef] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer, J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Pisani, P.; Airoldi, M.; Allais, A.; Aluffi Valletti, P.; Battista, M.; Benazzo, M.; Briatore, R.; Cacciola, S.; Cocuzza, S.; Colombo, A.; et al. Metastatic disease in head & neck oncology. Acta Otorhinolaryngol. Ital. 2020, 40, S1–S86. [Google Scholar] [CrossRef]
- Ferlito, A.; Shaha, A.R.; Silver, C.E.; Rinaldo, A.; Mondin, V. Incidence and sites of distant metastases from head and neck cancer. ORL J. Otorhinolaryngol. Relat. Spec. 2001, 63, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Maniotis, A.J.; Folberg, R.; Hess, A.; Seftor, E.A.; Gardner, L.M.; Pe’er, J.; Trent, J.M.; Meltzer, P.S.; Hendrix, M.J. Vascular channel formation by human melanoma cells in vivo and in vitro: Vasculogenic mimicry. Am. J. Pathol. 1999, 155, 739–752. [Google Scholar] [CrossRef] [Green Version]
- Hendrix, M.J.C.; Seftor, E.A.; Hess, A.R.; Seftor, R.E.B. Vasculogenic mimicry and tumour-cell plasticity: Lessons from melanoma. Nat. Rev. Cancer 2003, 3, 411–421. [Google Scholar] [CrossRef]
- Salem, A.; Salo, T. Vasculogenic Mimicry in Head and Neck Squamous Cell Carcinoma—Time to Take Notice. Front. Oral. Health 2021, 2, 666895. [Google Scholar] [CrossRef]
- Hujanen, R.; Almahmoudi, R.; Karinen, S.; Nwaru, B.I.; Salo, T.; Salem, A. Vasculogenic Mimicry: A Promising Prognosticator in Head and Neck Squamous Cell Carcinoma and Esophageal Cancer? A Systematic Review and Meta-Analysis. Cells 2020, 9, 507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendrix, M.J.; Seftor, E.A.; Seftor, R.E.; Chao, J.T.; Chien, D.S.; Chu, Y.W. Tumor cell vascular mimicry: Novel targeting opportunity in melanoma. Pharmacol. Ther. 2016, 159, 83–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagenblast, E.; Soto, M.; Gutiérrez-Ángel, S.; Hartl, C.A.; Gable, A.L.; Maceli, A.R.; Erard, N.; Williams, A.M.; Kim, S.Y.; Dickopf, S.; et al. A model of breast cancer heterogeneity reveals vascular mimicry as a driver of metastasis. Nature 2015, 520, 358–362. [Google Scholar] [CrossRef] [Green Version]
- Ren, H.Y.; Shen, J.X.; Mao, X.M.; Zhang, X.Y.; Zhou, P.; Li, S.Y.; Zheng, Z.W.; Shen, D.Y.; Meng, J.R. Correlation Between Tumor Vasculogenic Mimicry and Poor Prognosis of Human Digestive Cancer Patients: A Systematic Review and Meta-Analysis. Pathol. Oncol. Res. 2019, 25, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, J.; Zhou, H.; Fan, G.; Li, Q. Molecular Mechanisms and Anticancer Therapeutic Strategies in Vasculogenic Mimicry. J. Cancer 2019, 18, 6327–6340. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.P.; Liao, Y.D.; Mai, D.M.; Xie, P.; Qiang, Y.Y.; Zheng, L.S.; Wang, M.Y.; Mei, Y.; Meng, D.F.; Xu, L.; et al. Tumor vasculogenic mimicry predicts poor prognosis in cancer patients: A meta-analysis. Angiogenesis 2016, 19, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Valdivia, A.; Mingo, G.; Aldana, V.; Pinto, M.P.; Ramirez, M.; Retamal, C.; Gonzalez, A.; Nualart, F.; Corvalan, A.H.; Owen, G.I. Fact or Fiction, It Is Time for a Verdict on Vasculogenic Mimicry? Front. Oncol. 2019, 2, 680. [Google Scholar] [CrossRef] [Green Version]
- Ayala-Domínguez, L.; Olmedo-Nieva, L.; Muñoz-Bello, J.O.; Contreras-Paredes, A.; Manzo-Merino, J.; Martínez-Ramírez, I.; Lizano, M. Mechanisms of Vasculogenic Mimicry in Ovarian Cancer. Front. Oncol. 2019, 27, 998. [Google Scholar] [CrossRef] [Green Version]
- Racordon, D.; Valdivia, A.; Mingo, G.; Erices, R.; Aravena, R.; Santoro, F. Structural and functional identification of vasculogenic mimicry in vitro. Sci. Rep. 2017, 7, 6985. [Google Scholar] [CrossRef] [Green Version]
- Blom, S.; Paavolainen, L.; Bychkov, D.; Turkki, R.; Mäki-Teeri, P.; Hemmes, A.; Välimäki, K.; Lundin, J.; Kallioniemi, O.; Pellinen, T. Systems pathology by multiplexed immunohistochemistry and whole-slide digital image analysis. Sci. Rep. 2017, 14, 15580. [Google Scholar] [CrossRef]
- Salo, T.; Sutinen, M.; Hoque Apu, E.; Sundquist, E.; Cervigne, N.K.; de Oliveira, C.E. A novel human leiomyoma tissue derived matrix for cell culture studies. BMC Cancer 2015, 16, 981. [Google Scholar] [CrossRef] [Green Version]
- Salo, T.; Dourado, M.R.; Sundquist, E.; Apu, E.H.; Alahuhta, I.; Tuomainen, K.; Vasara, J.; Al-Samadi, A. Organotypic three-dimensional assays based on human leiomyoma-derived matrices. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 5, 20160482. [Google Scholar] [CrossRef]
- Francescone, R.A.3rd; Faibish, M.; Shao, R. A Matrigel-based tube formation assay to assess the vasculogenic activity of tumor cells. J. Vis. Exp. 2011, 7, 3040. [Google Scholar] [CrossRef]
- Almahmoudi, R.; Salem, A.; Hadler-Olsen, E.; Svineng, G.; Salo, T.; Al-Samadi, A. The effect of interleukin-17F on vasculogenic mimicry in oral tongue squamous cell carcinoma. Cancer Sci. 2021, 112, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fu, C.; Zhang, Q.; He, C.; Zhang, F.; Wei, Q. The role of CD44 in pathological angiogenesis. FASEB J. 2020, 34, 13125–13139. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, J.; Zhao, W.; Peng, Z.; Liu, X.; Li, B.; Zhang, H.; Shan, B.; Zhang, C.; Duan, C. Vasculogenic mimicry in carcinogenesis and clinical applications. J. Hematol. Oncol. 2020, 14, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wechman, S.L.; Emdad, L.; Sarkar, D.; Das, S.K.; Fisher, P.B. Vascular mimicry: Triggers, molecular interactions and in vivo models. Adv. Cancer Res. 2020, 148, 27–67. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, G.; Berndt, S.; Ferratge, S.; Rasband, W.; Cuendet, M.; Uzan, G.; Albanese, P. Angiogenesis Analyzer for ImageJ —A comparative morphometric analysis of “Endothelial Tube Formation Assay” and “Fibrin Bead Assay”. Sci. Rep. 2020, 14, 11568. [Google Scholar] [CrossRef]
- Ricci-Vitiani, L.; Pallini, R.; Biffoni, M.; Todaro, M.; Invernici, G.; Cenci, T.; Maira, G.; Parati, E.A.; Stassi, G.; Larocca, L.M.; et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 2010, 9, 468, 824–828. [Google Scholar] [CrossRef]
- Chang, Y.S.; di Tomaso, E.; McDonald, D.M.; Jones, R.; Jain, R.K.; Munn, L.L. Mosaic blood vessels in tumors: Frequency of cancer cells in contact with flowing blood. Proc. Natl. Acad. Sci. USA 2000, 19, 14608–14613. [Google Scholar] [CrossRef] [Green Version]
- El Hallani, S.; Boisselier, B.; Peglion, F.; Rousseau, A.; Colin, C.; Idbaih, A. A new alternative mechanism in glioblastoma vascularization: Tubular vasculogenic mimicry. Brain 2010, 133, 973–982. [Google Scholar] [CrossRef] [Green Version]
- Tong, M.; Han, B.B.; Holpuch, A.S.; Pei, P.; He, L.; Mallery, S.R. Inherent phenotypic plasticity facilitates progression of head and neck cancer: Endotheliod characteristics enable angiogenesis and invasion. Exp. Cell Res. 2013, 319, 1028–1042. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Won, Y.J.; Shim, J.H.; Kim, H.J.; Kim, J.; Hong, H.N.; Kim, B.S. Morphological characteristics of vasculogenic mimicry and its correlation with EphA2 expression in gastric adenocarcinoma. Sci. Rep. 2019, 49, 3414. [Google Scholar] [CrossRef]
- Paulis, Y.W.; Huijbers, E.J.; van der Schaft, D.W.; Soetekouw, P.M.; Pauwels, P.; Tjan-Heijnen, V.C.; Griffioen, A.W. CD44 enhances tumor aggressiveness by promoting tumor cell plasticity. Oncotarget 2015, 14, 19634–19646. [Google Scholar] [CrossRef] [Green Version]
- Chin, V.L.; Lim, C.L. Epithelial-mesenchymal plasticity-engaging stemness in an interplay of phenotypes. Stem. Cell Investig. 2019, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Tuomainen, K.; Al-Samadi, A.; Potdar, S.; Turunen, L.; Turunen, M.; Karhemo, P.R. Human Tumor-Derived Matrix Improves the Predictability of Head and Neck Cancer Drug Testing. Cancers 2019, 12, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundahl, N.; Duprez, F.; Ost, P.; De Neve, W.; Mareel, M. Effects of radiation on the metastatic process. Mol. Med. 2018, 24, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montero, P.H.; Patel, S.G. Cancer of the oral cavity. Surg. Oncol. Clin. N. Am. 2015, 24, 491–508. [Google Scholar] [CrossRef] [Green Version]
- Karinen, S.; Juurikka, K.; Hujanen, R.; Wahbi, W.; Hadler-Olsen, E.; Svineng, G.; Eklund, K.K.; Salo, T.; Åström, P.; Salem, A. Tumour cells express functional lymphatic endothelium-specific hyaluronan receptor in vitro and in vivo: Lymphatic mimicry promotes oral oncogenesis? Oncogenesis 2021, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Huang, J.; Ye, J. A fresh look at zebrafish from the perspective of cancer research. J. Exp. Clin. Cancer Res. 2015, 34, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fazio, M.; Ablain, J.; Chuan, Y.; Langenau, D.M.; Zon, L.I. Zebrafish patient avatars in cancer biology and precision cancer therapy. Nat. Rev. Cancer 2020, 20, 263–273. [Google Scholar] [CrossRef]
| Matrigel | ||||
|---|---|---|---|---|
| Cell density 1 | SCC-8 | SCC-14 | SCC-28 | SCC-40 |
| A | - | - | + | - |
| B | ++ | ++ | ++ | - |
| C | ++ | +++ | +++ | ++ |
| SCC-44 | SCC-73 | SCC-81 | SCC-106A | |
| A | - | - | - | - |
| B | - | - | ++ | - |
| C | - | - | ++ | ++ |
| SCC-25 | SCC-24A | SCC-24B | HSC-3 | |
| A | - | + | + | + |
| B | - | + | ++ | ++ |
| C | - | + | +++ | +++ |
| SAS | HUVEC | |||
| A | + | + | ||
| B | ++ | ++ | ||
| C | +++ | +++ | ||
| Myogel | ||||
| SCC-8 | SCC-14 | SCC-28 | SCC-40 | |
| A | + | - | + | - |
| B | + | + | ++ | - |
| C | + | + | +++ | - |
| SCC-44 | SCC-73 | SCC-81 | SCC-106A | |
| A | - | - | - | + |
| B | ++ | - | - | - |
| C | +++ | - | - | - |
| SCC-25 | SCC-24A | SCC-24B | HSC-3 | |
| A | - | + | - | - |
| B | - | + | + | - |
| C | - | - | + | - |
| SAS | HUVEC | |||
| A | - | + | ||
| B | - | ++ | ||
| C | - | +++ | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hujanen, R.; Almahmoudi, R.; Salo, T.; Salem, A. Comparative Analysis of Vascular Mimicry in Head and Neck Squamous Cell Carcinoma: In Vitro and In Vivo Approaches. Cancers 2021, 13, 4747. https://doi.org/10.3390/cancers13194747
Hujanen R, Almahmoudi R, Salo T, Salem A. Comparative Analysis of Vascular Mimicry in Head and Neck Squamous Cell Carcinoma: In Vitro and In Vivo Approaches. Cancers. 2021; 13(19):4747. https://doi.org/10.3390/cancers13194747
Chicago/Turabian StyleHujanen, Roosa, Rabeia Almahmoudi, Tuula Salo, and Abdelhakim Salem. 2021. "Comparative Analysis of Vascular Mimicry in Head and Neck Squamous Cell Carcinoma: In Vitro and In Vivo Approaches" Cancers 13, no. 19: 4747. https://doi.org/10.3390/cancers13194747
APA StyleHujanen, R., Almahmoudi, R., Salo, T., & Salem, A. (2021). Comparative Analysis of Vascular Mimicry in Head and Neck Squamous Cell Carcinoma: In Vitro and In Vivo Approaches. Cancers, 13(19), 4747. https://doi.org/10.3390/cancers13194747







